Abstract
Lacticaseibacillus rhamnosus CRL1505 can be used in functional products as a probiotic powder (dried live cells) or as a postbiotic intracellular extract containing inorganic polyphosphate as a functional biopolymer. Thus, the aim of this work was to optimize the production of Lr-CRL1505 depending on the target of the functional product (probiotic or postbiotic). For this purpose, the effect of culture parameters (pH, growth phase) on cell viability, heat tolerance and polyphosphate accumulation by Lacticaseibacillus rhamnosus CRL1505 was evaluated. Fermentations at free pH produced less biomass (0.6 log units) than at controlled pH while the growth phase affected both polyphosphate accumulation and cell heat tolerance. Exponential phase cultures showed 4–15 times greater survival rate against heat shock and 49–62% increased polyphosphate level, compared with the stationary phase. Results obtained allowed setting the appropriate culture conditions for the production of this strain according to its potential application, i.e., as live probiotic cells in powder form or postbiotic. In the first case, running fermentations at pH 5.5 and harvesting the cells at the exponential phase are the best conditions for obtaining a high live biomass yield capable of overcoming heat stress. Whereas the postbiotic formulations production requires fermentations at free pH and harvesting the cells in exponential phase to increase the intracellular polyphosphate level as a first step.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics lactic acid bacteria (LAB) have received considerable attention over the past years as microorganisms with beneficial effects to the host. Although this traditional probiotic definition assumes bacterial cells have to be alive to produce health-promoting effects, much scientific evidence has shown that formulations containing their cellular byproducts can also promote the desired response (Delgado et al. 2020). These compounds are called postbiotics and include exopolysaccharides, peptidoglycans, inorganic polyphosphate (polyP), lipoteichoic acids, bacteriocins, surface-associated proteins and peptides, as well as other metabolites released from lysed cells or during bacterial growth (Żółkiewicz et al. 2020; Ferrucci et al. 2021; Schepler et al. 2021). Thus, postbiotics are an interesting alternative to promote beneficial effects in vulnerable populations by their safety and efficacy (Daniali et al. 2020).
The probiotics and postbiotics supplements market is one of the fastest industry growing segments (Daniali et al. 2020). For the development of probiotic or postbiotic supplements, it is necessary to design a production process appropriate to the final product. Probiotic production still involves the challenge of improving survival rate during the different steps of the bioprocess that includes biomass production, dehydration (freezing, freeze drying, spray drying), and long-term storage of the products (Velly et al. 2014). On the other hand, postbiotic production does not require the maintenance of cell viability, but it is necessary to optimize the production of by-products and the method of concentrating them according to their cellular location (structural cellular component or metabolite) (Żółkiewicz et al. 2020). In both probiotic (live bacterial cells) and postbiotic (cellular by-products) production, growth conditions (culture medium, temperature, pH, incubation time) can affect the physiological state of the cells or the production of the postbiotic.
Lacticaseibacillus (L.) rhamnosus CRL1505 (Lr-CRL1505; Basionym Lactobacillus rhamnosus CRL1505) (Zheng et al. 2020) is a probiotic strain with great scientific and technological support that has been used since 2008 in nutritional programs for vulnerable populations by the government of Tucumán, Argentina (Kitazawa 2014; Salva et al. 2010; Villena et al. 2012a, b, 2016; Zelaya et al. 2016). This probiotic strain is marketed by Sacco S.r.L. (Italy) and is currently incorporated in dairy products by Danone S.A. (France). Lr-CRL1505 administrated as alive cells improved the immune responses in the gut and beyond the gastrointestinal tract (Salva et al. 2010) also being effective against bacterial respiratory pathogens, respiratory syncytial, and influenza virus (Chiba et al. 2013; Herrera et al. 2014; Tomosada et al. 2013; Tonetti et al. 2020; Zelaya et al. 2015; 2016). On the other hand, the intracellular accumulation of polyP granules by Lr-CRL1505 was demonstrated and its intracellular extract containing this biopolymer showed an anti-inflammatory effect when it was nasally administered to a mouse model of acute respiratory inflammation (Correa Deza et al. 2021a). These results support the potential of the polyP from Lr-CRL1505 as a new postbiotic product to complement the treatment of respiratory diseases.
In a recent work, Lr-CRL1505 was subjected to spray drying in order to formulate a probiotic powder supplement showing a low viable cell count after drying (Correa Deza et al. 2021b). Despite this unfavorable result, a direct relationship between polyP accumulation and thermotolerance of Lr-CRL1505 could be demonstrated (Correa Deza et al. 2017). From these results, there is also a need to evaluate other culture conditions to optimize heat tolerance, which will determine their survival to spray drying.
Our results support that Lr-CRL1505 can be used as a probiotic (dried live cell) or as a postbiotic (intracellular extract with polyP). Based on these considerations, our work hypothesized that culture parameters such as pilot-scale production, growth phase and culture pH affect both heat tolerance (spray-drying survival) and polyP accumulation of Lr-CRL-1505. Consequently, both properties can be optimized by selecting one or more culture conditions. Thus, the aim of this work was to evaluate the effect of culture parameters (pH, growth phase) on cell viability, heat tolerance and polyP accumulation by Lr-CRL1505. This study would allow establishing the best conditions to obtain thermotolerant biomass for spray drying (for use as a probiotic powder supplement), and an intracellular extract with high levels of polyP (for use as a postbiotic supplement).
Materials and methods
Microorganism and culture conditions
Lr-CRL1505 was obtained from the stock culture collection of Centro de Referencia para Lactobacilos (CERELA-CONICET, Tucumán, Argentina). The strain was grown in MCM broth (pH = 6.5) with the following composition (g/L): 0.04; MnSO4; 0.05 MgSO4; 5.6 KH2PO4; 3.6 Na2HPO4, 5 peptone, 10 yeast extract, and 20 lactose.
Pilot-scale biomass production
Batch fermentations (28 h) were performed in a 2 L bioreactor (INFORS HT, Switzerland). The MCM media (1.5 L, pH 6.5) was added to the bioreactor and inoculated with Lr-CRL1505 cultures (1%, v/v). Temperature was maintained at 37 °C and the agitation speed at 150 rpm. Batch cultures were performed under controlled and uncontrolled pH. In the first case, pH was maintained at 5.5 and 6.0 by adding 20% (v/v) NH4OH solution. Samples were withdrawn periodically every 2 h to determine cell growth (log CFU mL−1), absorbance of the cultures (A600) and pH.
The maximum specific growth rate [μmax (h−1)] was calculated during the exponential growth phase by applying the following equation:
where N and Ni represent CFU mL−1 at the end and beginning of the exponential phase, respectively, and ΔT the time in which the exponential phase elapsed (h).
The fermentation time was modified according the growth phase in order to evaluate the effect of the exponential and stationary phase on the polyP accumulation, the ratio viability/damage cells (flow cytometry) and heat stress tolerance. Cells obtained from uncontrolled and controlled pH cultures at different growth stages were centrifuged (8000 g for 10 min) and washed with sterile potassium phosphate buffer (10 mM, pH 7.0) for the followings determinations.
Measurements of intracellular polyP level
Intracellular polyP was determined in cell suspensions of Lr-CRL1505 obtained in exponential and stationary growth phase at the different pH conditions. A DAPI (4,6-diamidino-2-phenylindole)-based fluorescence approach (Aschar-Sobbi et al. 2008) was used. The method was adapted from the technique reported by Schurig-Briccio et al. (2009a; b) and Grillo-Puertas et al. (2012) for Gram-negative bacteria (Escherichia coli). Briefly, 17 mM DAPI (Sigma) were added to cells (A600 = 0.02), washed and suspended in buffer T (100 mM Tris–HCl, pH 8) together with sodium dodecyl sulfate (SDS) and chloroform for cell permeabilization. After 15 min agitation at 37 °C, the DAPI fluorescence spectra (excitation, 415 nm; emission, 450–650 nm) were recorded using an ISS PCI spectrofluorometer (Champaign, IL). Fluorescence (in arbitrary units) of the DAPI-polyP complex at 550 nm was used as a measure of intracellular polyP since fluorescence emissions from free DAPI and DAPI-DNA are minimal at this wavelength (Aschar-Sobbi et al. 2008). A non-polyP producing strain [Escherichia coli MC4100 (ppkppx::Km)] (Schurig-Briccio et al. 2009a; b) was assayed only as negative control of polyP-DAPI fluorescence technique instead as a physiological negative control (Supplementary Fig. S1). By DAPI-based fluorescence technique, there is a threshold below which short polyP chains are not detectable, however, the polyP chain length from Lr-CRL1505 was previously reported greater than 45 phosphate residues (Correa Deza et al. 2021a).
Heat tolerance evaluation of Lr-CRL1505
Cells of Lr-CRL1505 obtained under the growth conditions tested were exposed to heat shock (60 °C, 5 min) (Correa Deza et al. 2017) to simulate heat stress conditions during spray drying. Briefly, bottles containing 50 mL of potassium phosphate buffer were inoculated (2%, v/v) with the cell suspension and settled at 60 °C and 37 °C (control) since no changes in cell viability at this temperature was observed. Samples (1 mL) were taken after 5 min of incubation at each temperature and immediately cooled in ice bath for colony counts on LAPTg (Raibaud et al. 1973). The plates (duplicate assays) were incubated at 37 °C for 48 h. The results were expressed as survival to heat shock according to the following equation:
where N is the number of cells at 5 min and Ni represents the initial count, which was obtained from the control at 37 °C.
Determination of cell viability and damage by flow cytometry
The samples of Lr-CRL1505 obtained at the different growth parameters and after heat shock were stained with a cell viability kit (BD™ Biosciences, USA). Dual staining of the cells provides information of the cell membrane functionality. Thiazole orange (TO) is permeant in all cells whereas propidium iodide (PI) enters cells only when the integrity of the membrane has been compromised (Haugland 1992). PI uptake indicates both cell membrane injury and death.
The cells were suspended in phosphate-buffered saline solution to a concentration of ca. 1 × 106 cells mL−1 and incubated at room temperature for 5 min with 420 μM for TO and 48 μM for PI. Samples were analyzed with a FACS Calibur flow cytometer (BD™ Biosciences, USA) equipped with 488 nm air-cooled argon-ion laser excitation. 488 nm is the excitation wavelength of PI and TO. The TO emission fluorescence was detected at 530 nm while the IP emission fluorescence was detected at 650 nm. The BD FACS flow was used as sheath fluid and 10,000 events per sample were counted. Data analysis was performed using low FlowJo Version 10 using the analysis strategy shown in Fig. 3. Results were expressed as the percentage of live, injured, and dead cells.
Statistical analysis
Data obtained corresponded to at least three independent assays, and are reported as mean values with standard deviation. After verification of the normal distribution of data, the general lineal model of ANOVA was applied using the software GraphPad Prism 7. Significant differences between mean values were determined by Tukey’s and Sidak’s tests. Differences were considered significant with a p value < 0.05.
Results
Growth under different pH conditions
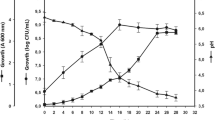
Growth of Lr-CRL1505 under different pH conditions is shown in Fig. 1. At free pH, the lag phase was about 4 h with a μmax of 0.36 ± 0.02 h−1. The stationary phase began after 16 h of fermentation reaching the maximum cell count (9.24 ± 0.04 log CFU mL−1; Fig. 1a) which decreased below 9 log CFU mL−1 at 24 h of fermentation. Different growth kinetics were observed under controlled pH conditions. At pH 5.5 the lag phase was 10 h and the exponential phase elapsed until 24 h with a μmax of 0.30 ± 0.03 h−1. At this point began the stationary phase with the maximum cell count of 9.4 ± 0.2 log CFU mL−1 (Fig. 1b). Similar performance was obtained at pH 6.0 (Fig. 1c).
Lr-CRL1505 cells were harvested at the mean exponential or stationary phase of growth (24 h). Mean exponential phase was considered as the midpoint (h) between the beginning and the end of that phase, which was different according to the growth pH (8 h for free pH and 12 h for controlled pH). These harvested cultures were evaluated for heat tolerance and polyP content.
Effect of the pH and growth phase on the polyP accumulation and heat tolerance
The effect of the pH and growth phase on polyP content is shown in Fig. 2a. At free pH and pH 5.5, the accumulation of polyP was significantly higher (p < 0.001) in exponential phase (17.830 and 14.453 AU, respectively) compared to stationary phase (10.980 and 9.702 AU, respectively) while at pH 6.0 the lowest levels of polyP (approx. 8.600 AU) were obtained, without significant differences (p > 0.05) between growth phase.
PolyP levels (AU, arbitrary units) accumulated by Lr-CRL1505 (a) and survival of Lr-CRL1505 (log N/Ni) to heat shock (b). Lr-CRL1505 was cultured under free and controlled pH (5.5 and 6.0) in MCM, at 37 °C and harvested in exponential and stationary phase of growth. Sidak's multiple comparisons and Tukey tests (95% CI) were used to estimate significant differences between mean values of growth phase and growth pH, which are indicated with asterisks *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significant differences
Figure 2b shows the effect of pH and growth phase on the survival (log N/Ni) of the strain after heat shock. Survival was significantly higher (p < 0.05) in exponential phase than in stationary phase at free pH and pH 5.5 (4.78 times and 15.20 times, respectively). At pH 6.0 there was low survival to heat shock regardless of the growth stage (− 1.71 log N/Ni and − 2.10 log N/Ni in exponential and stationary growth phase, respectively) without significant differences between phase (p > 0.05). The survival values obtained were similar to those obtained in stationary phase at free pH and pH 5.5.
Flow cytometry analysis
The cellular integrity of the strain Lr-CRL1505 in the different growth conditions was evaluated by flow cytometry. According to the results obtained, the cell population was grouped into three regions as it is illustrated in Fig. 3: dead, injured and live cells. Different density plots of Lr-CRL1505 were observed according to the growth phase, pH and heat treatment. Regarding the growth phase, a decrease in the percentage of live cells was observed in stationary phase (30.00%) compared to the exponential phase at free pH (T0 Fig. 3I-a, b). This population shift was smaller at pH 5.5 (5.70% decrease; T0 Fig. 3II-a, b) and pH 6.0 (4.60% decrease; T0 Fig. 3III-a, b).
Cell integrity of Lr-CRL1505 was also assessed after heat shock (T5). Under all growth conditions, heat shock resulted in a decrease in the live cell population, which was most pronounced in stationary phase, regardless of pH. For example, at pH 5.5 the live population in exponential phase was reduced by 35.90% between T0 and T5 (after heat shock) while the decrease was 54.20% in stationary phase (Fig. 3II-a, b). These results are in agreement with those shown in Fig. 2b. In addition, an increase in injured and dead cells was observed, the magnitude of which depended on the physiological state of the bacteria.
Regarding culture pH and its effect on cell heat tolerance, cultures at pH 6.0 in stationary phase (Fig. 3III-b) were the most sensitive to heat shock, showing a 40.64% increase in dead cells after heat treatment. In contrast, cultures at pH free and pH 5.5 in stationary phase showed the smallest increase (2.40 and 2.06%, respectively) in the dead cell population (Fig. 3I-b, II-b).
Discussion
Previous results revealed that Lr-CRL1505 has multiple functions, for instance, as a probiotic can enhance immune responses in the gut (Salva et al. 2010) and provide protection against bacterial respiratory pathogens, respiratory syncytial virus, and influenza virus (Zelaya et al. 2015, 2016; Tonetti 2020). Furthermore, its intracellular extract with polyP (as a postbiotic) was able to prevent the development of local inflammatory response in the respiratory mucosa in a murine model of acute respiratory inflammation (Correa Deza et al. 2021a), a fact that allows new applications of Lr-CRL1505 as a probiotic and postbiotic. In this work, the effect of culture parameters (pilot scale biomass production, pH and growth phase) on cell viability, heat tolerance and polyP accumulation by Lr-CRL1505 was evaluated in order to establish better conditions to obtain, on the one hand, heat-tolerant biomass with a high percentage of live cells and, on the other hand, intracellular extracts with high polyP levels, depending on the destination of the functional product (probiotic or postbiotic).
As the name of the group indicates, LAB produce lactic acid due to their type of metabolism, with a consequent decrease in the pH of the culture. Even though LAB can grow in a wide pH range (3.5–6.5) (McDonald et al. 1990; Russell 1991), pH-controlled fermentations are useful to avoid cell injury and improve biomass yield (Savoie et al. 2007). In this study, a lower biomass yield (0.6 log units) was observed at 24 h of fermentation in pH-free cultures compared to pH-controlled cultures. This result may have been due to the final pH (4.0) reached at this point, the effect of which could have been detrimental to cell viability. Similar outcomes were reported by Polak-Berecka et al. (2011) for L. rhamnosus E/N and other genera belonging to LAB such as Enterococcus faecium CRL1943 and Leuconostoc citreum CRL1945 (Correa Deza et al. 2018). In contrast, different results were obtained for L. bulgaricus Q7 where the average growth rate and the final biomass were higher under free pH conditions (Ai et al. 2017).
Fluorescent probe staining technology has improved procedures for measuring cell function, such as the estimation of live and dead cells (Chitarra et al. 2006) within a population, and further distinguishes between “vigorous, fragile, and injured cells” (Lloyd and Hayes 1995). In this study, flow cytometric determinations revealed the great impact that growth conditions have on the cellular integrity of Lr-CRL1505. A significant decrease (30%) in live cells at free pH was observed in the stationary phase of growth along with an increase in injured and dead cells (7.6% and 2.17%, respectively) compared to the exponential phase. The population of living cells in the stationary phase at pH 5.5 and 6.0 remained unchanged, showing the advantages of using controlled pH fermentations.
Spray drying represents a rising technique in the development of probiotic supplements in powder. However, during the process the bacteria may undergo heat stress and loss of cell viability (Janning and In’t Veld 1994; Perdana et al. 2013). In a previous work, Lr-CRL1505 was subjected to spray drying, obtaining low viable cell counts in the dried product (Correa Deza et al. 2021b). It is likely that the biomass production conditions (standard pilot plant conditions) were not optimal. For this reason, this optimization is described in this manuscript. A heat shock test was used because of its simplicity and because the correlation with the resistance of strain Lr-CRL 1505 to a real spray drying process is quite high (Correa Deza et al. 2021b). The heat tolerance of Lr-CRL1505 was influenced by the culture conditions. At pH 6.0 cultures showed a low heat shock survival regardless the growth phase, which was confirmed by flow cytometry as a high percentage of injured and dead cells was obtained after heat shock. Similar results were reported for some strains of Streptococcus grown at pH 6.0 subjected to heat, oxidative and osmotic stress (Zotta et al. 2008). On the contrary, at free pH and pH 5.5, the growth phase affected the heat tolerance of Lr-CRL1505. The high survival to heat shock was observed in the exponential phase at both pH conditions. This similarity in behavior at free pH and pH 5.5 could be due to the fact that both cultures presented a pH value of 5.5 in this exponential growth phase (Fig. 1), with a high percentage of live heat-tolerant cells (Fig. 3) and a high intracellular polyP content (Fig. 2). It has been shown in a previous work that high levels of polyP in this strain confer greater tolerance to heat shock (Correa Deza et al. 2017). Other studies (Kim et al. 2001; van de Guchte et al. 2002; Zotta et al. 2008; Parente et al. 2010) found high stress tolerance in LAB but during the stationary phase, due to synthesis of stress proteins triggered by the depletion of nutrients and/or the accumulation of toxic products, e.g., lactic acid in the culture medium.
According to our results, to obtain a thermotolerant biomass capable of overcoming heat stress during spray drying, the optimal culture conditions would be pH 5.5 and collection of biomass in exponential growth phase (high cell count and survival to heat shock). Although the survival to heat shock did not show significant differences at free pH and pH 5.5, in this last condition a higher biomass in exponential phase was obtained. These results highlighted the relevance of optimizing the technological conditions, since the results are strain-dependent.
Different survival strategies have been described in LAB to survive stress, such as intracellular accumulation of polyP (Alcántara et al. 2014; Aprea et al. 2005; Archibald and Fridovich 1982; Archibald and Duong 1984; Velly et al. 2014). In bacteria, polyP accumulation is dependent on the phosphate concentration of the culture medium and the growth conditions (Correa Deza 2017; Alcántara et al. 2014; Huang et al. 2016; Schurig-Briccio et al. 2009a, b). In a previous work, the effect of the phosphate concentration of the culture medium on the polyP accumulation in Lr-CRL1505 was already demonstrated (Correa Deza et al. 2017). In this work, the culture pH and growth phase also had a significant effect on the intracellular polyP content of this strain; high levels of polyP were obtained in the exponential phase at both free pH and pH 5.5. To our knowledge, this is the first study on polyP production by probiotic lactobacilli in the exponential phase. Schurig-Briccio et al. (2009a) reported that Escherichia coli grown in media with > 37 mM phosphate maintained a high polyP level in the late stationary phase compared with the exponential one, which could account for changes in gene expression and enzyme activities that enhance stationary-phase fitness.
The relationship between polyP accumulation by Lr-CRL1505 and heat tolerance was also analyzed. High polyP levels coincided with the highest heat shock survival during the exponential phase at both free pH and pH 5.5. In contrast, the lowest polyP content and lowest heat shock survival were determined at pH 6. Many biological functions are attributed to polyP as an energy and phosphate reservoir, metal chelator (Mn2 + and Ca2 +), physiological adjustment during growth and against stress, e.g. starvation, heat, oxidative, acid and osmotic stress (Alcántara et al. 2014; Kornberg 1999; Rao and Kornberg 1999). The mechanisms by which polyP participants are involved in these stress processes are still unclear.
The importance of polyP research (not only for its relation to thermotolerance) is supported by recent publications that have corroborated the beneficial effect of polyP in the prevention of SARS-CoV-2 infection (Ferrucci et al. 2021; Schepler et al. 2021). These results highlight the importance of the technological perspective to broaden the field of application in various types of commercial products. The highest level (17,830 AU) of intracellular polyP was obtained for Lr-CRL1505 in the exponential phase at free pH in this study. For this reason, these growth conditions would be optimal for obtaining intracellular extracts that can be used to formulate a postbiotic product.
In conclusion, our results show the appropriate culture conditions for the production of the probiotic Lr-CRL1505 according to its possible applications, either as a probiotic or as a postbiotic product. In the first case, the best culture conditions are pH 5.5, and harvesting during the exponential phase (high cell count, percentage of living cells and heat shock survival) to obtain a heat tolerant-biomass capable of overcoming heat stress during spray drying. In the second case, free pH, and harvesting in the exponential phase are the optimal conditions that allow obtaining intracellular extracts with a high level of polyP that can be used to formulate a postbiotic product.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Ai Z, Lv X, Huang S, Liu G, Sun X, Chen H et al (2017) The effect of controlled and uncontrolled pH cultures on the growth of Lactobacillus delbrueckii subsp. Bulgaricus LWT 77:269–275
Alcántara C, Blasco A, Zúñiga M, Monedero V (2014) Accumulation of polyphosphate in Lactobacillus spp. and its involvement in stress resistance. Appl Environ Microbiol 80:1650–1659
Aprea G, Mullan W, Mullan A, Murru N, Tozzi M, Cortesi M (2005) Isolation of polyphosphate-accumulating lactic acid bacteria from natural whey starters. Milchwissenschaft 60:256–258
Archibald FS, Duong MN (1984) Manganese acquisition by Lactobacillus plantarum. J Bacteriol 158:1–8
Archibald FS, Fridovich I (1982) Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys 215:589–596
Aschar-Sobbi R, Abramov AY, Diao C, Kargacin ME, Kargacin GJ, French RJ et al (2008) High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J Fluoresc 18:859–866
Chiba E, Tomosada Y, Vizoso-Pinto MG, Salva S, Takahashi T, Tsukida K et al (2013) Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice against respiratory syncytial virus infection. Int Immunopharmacol 17:373–382
Chitarra LG, Breeuwer P, Abee T, Bulk RW (2006) The use of fluorescent probes to assess viability of the plant pathogenic bacterium Clavibacter michiganensis subsp. michiganensis by flow cytometry. Fitopatol Bras 31:349–356
Correa Deza MA, Grillo-Puertas M, Salva S, Rapisarda VA, Gerez CL, Font de Valdez G (2017) Inorganic salts and intracellular polyphosphate inclusions play a role in the thermotolerance of the immunobiotic Lactobacillus rhamnosus CRL 1505. PLoS ONE 12:e0179242
Correa Deza MA, Martos GI, Nuñez M, Fiori M, Gerez CL, Font G (2018) Artisanal tanneries: potential application of inoculants formulated with lactic acid bacteria. J Basic Microbiol 58:296–301
Correa Deza MA, Rodríguez de Olmos A, Suárez NE, Font de Valdez GF, Salva S, Gerez CL (2021a) Inorganic polyphosphate from the immunobiotic Lactobacillus rhamnosus CRL1505 prevents inflammatory response in the respiratory tract. Saudi J Biol Sci 28:5684–5692
Correa Deza MA, Díaz Vergara L, Salva S, Montenegro M, Font de Valdez G, Gerez CL (2021b) Inorganic additive improves the survival of the probiotic Lacticaseibacillus rhamnosus CRL1505 during spray drying, rehydration, and storage. Curr Microbiol 78:3863–3871
Daniali M, Nikfar S, Abdollahi M (2020) Antibiotic resistance propagation through probiotics. Expert Opin 16:1207–1215
Delgado S, Sánchez B, Margolles A, Ruas-Madiedo P, Ruiz L (2020) Molecules produced by probiotics and intestinal microorganisms with immunomodulatory activity. Nutrients 12:391
Ferrucci V, Kong DY, Asadzadeh F, Marrone L, Boccia A, Siciliano R et al (2021) Long-chain polyphosphates impair SARS-CoV-2 infection and replication. Sci Signal 14:eabe5040
Grillo-Puertas M, Villegas JM, Rintoul MR, Rapisarda VA (2012) Polyphosphate degradation in stationary phase triggers biofilm formation via LuxS quorum sensing system in Escherichia coli. PLoS ONE 7:e50368
Haugland R (1992) Handbook of fluorescent probes and research chemicals fluorescent dyes for assessing vital cell functions. Elsevier, London, pp 172–180
Herrera M, Salva S, Villena J, Barbieri N, Marranzino G, Alvarez S (2014) Dietary supplementation with lactobacilli improves emergency granulopoiesis in protein-malnourished mice and enhances respiratory innate immune response. PLoS ONE 9:e90227
Huang S, Rabah H, Jardin J, Briard-Bion V, Parayre S, Maillard MB et al (2016) Hyperconcentrated sweet whey, a new culture medium that enhances Propionibacterium freudenreichii stress tolerance. Appl Environ Microbiol 82:4641–4651
Janning B, In’t Veld P (1994) Susceptibility of bacterial strains to desiccation: a simple method to test their stability in microbiological reference materials. Anal Chim Acta 286:469–476
Kim WS, Perl L, Park JH, Tandianus JE, Dunn NW (2001) Assessment of stress response of the probiotic Lactobacillus acidophilus. Curr Microbiol 43:346–350
Kitazawa H, Villena J (2014) Modulation of respiratory TLR3-anti-viral response by probiotic microorganisms: lessons learned from Lactobacillus rhamnosus CRL1505. Front Immunol 5:201
Kornberg A (1999) Inorganic polyphosphate: a molecule of many functions. Inorganic polyphosphates. Springer, London, pp 01–18
Lloyd D, Hayes AJ (1995) Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett 133:1–7
McDonald L, Fleming H, Hassan H (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56:2120–2124
Parente E, Ciocia F, Ricciardi A, Zotta T, Felis GE, Torriani S (2010) Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: a multivariate screening study. Int J Food Microbiol 144:270–279
Perdana J, Bereschenko L, Fox MB, Kuperus JH, Kleerebezem M, Boom RM et al (2013) Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: comparing single droplet drying to spray and freeze drying. Food Res Int 54:1351–1359
Polak-Berecka M, Waśko A, Kordowska-Wiater M, Targoński Z, Kubik-Komar A (2011) Application of response surface methodology to enhancement of biomass production by Lactobacillus rhamnosus E/N. Braz J Microbiol 42:1485–1494
Raibaud P, Galpin J, Ducluzeau R, Mocquot F, Oliver G (1973) The “Lactobacillus” genus in the digestive tract of the rat. II. Characteristics of heterofermentative strains isolated from “holo-“ and “gnotoxenic” rats (author’s transl). Ann Microbiol 124:223–235
Rao N, Kornberg A (1999) Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase. In: Inorganic polyphosphates, pp 183–195
Russell JB (1991) Resistance of Streptococcus bovis to acetic acid at low pH: relationship between intracellular pH and anion accumulation. Appl Environ Microbiol 57:255–259
Salva S, Villena J, Alvarez S (2010) Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: impact on intestinal and respiratory infections. Int J Food Microbiol 141:82–89
Savoie S, Champagne C, Chiasson S, Audet P (2007) Media and process parameters affecting the growth, strain ratios and specific acidifying activities of a mixed lactic starter containing aroma-producing and probiotic strains. J Appl Microbiol 103:163–174
Schepler H, Wang X, Neufurth M, Wang S, Schröder HC, Müller WE (2021) The therapeutic potential of inorganic polyphosphate: a versatile physiological polymer to control coronavirus disease (COVID-19). Theranostics 11:6193
Schurig-Briccio LA, Farías RN, Rintoul MR, Rapisarda VA (2009a) Phosphate-enhanced stationary-phase fitness of Escherichia coli is related to inorganic polyphosphate level. J Bacteriol 191:4478–4481
Schurig-Briccio LA, Farías RN, Rodríguez-Montelongo L, Rintoul MR, Rapisarda VA (2009b) Protection against oxidative stress in Escherichia coli stationary phase by a phosphate concentration-dependent genes expression. Arch Biochem Biophys 483:106–110
Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H et al (2013) Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol 14:01–16
Tonetti FR, Islam MA, Vizoso-Pinto MG, Takahashi H, Kitazawa H, Villena J (2020) Nasal priming with immunobiotic lactobacilli improves the adaptive immune response against influenza virus. Int Immunopharmacol 78:106–115
van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216
Velly H, Fonseca F, Passot S, Delacroix-Buchet A, Bouix M (2014) Cell growth and resistance of Lactococcus lactis subsp. lactis TOMSC161 following freezing, drying and freeze-dried storage are differentially affected by fermentation conditions. J Appl Microbiol 117:729–740
Villena JC, Salva MS, Nuñez MS, Corzo MJ, Tolaba R, Faedda J et al (2012a) Beneficial lactobacilli for improving respiratory defenses: the case of Lactobacillus rhamnosus CRL1505. Lactobacillus: classification, uses and health implications. Nova Science Publishers, London
Villena J, Salva S, Núñez M, Corzo J, Tolaba R, Faedda J et al (2012b) Probiotics for everyone! The novel immunobiotic Lactobacillus rhamnosus CRL1505 and the beginning of Social Probiotic Programs in Argentina. IJBWI 1:189
Villena J, Vizoso-Pinto MG, Kitazawa H (2016) Intestinal innate antiviral immunity and immunobiotics: beneficial effects against rotavirus infection. Front Immunol 7:563
Zelaya H, Tada A, Vizoso-Pinto MG, Salva S, Kanmani P, Agüero G et al (2015) Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation–coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm Res 64:589–602
Zelaya H, Alvarez S, Kitazawa H, Villena J (2016) Respiratory antiviral immunity and immunobiotics: beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front Immunol 7:633
Zheng J, Wittouck S, Salvetti E, Franz CM, Harris H, Mattarelli P et al (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W (2020) Postbiotics—a step beyond pre-and probiotics. Nutrients 12:2189
Zotta T, Ricciardi A, Ciocia F, Rossano R, Parente E (2008) Diversity of stress responses in dairy thermophilic streptococci. Int J Food Microbiol 124:34–42
Acknowledgements
The authors would like to thank Drs. JM Villegas and VA Rapisarda for their collaboration.
Funding
This work was financially supported by Grant Consejo Nacional de Investigaciones Científicas y Técnicas-CONICET (PIP 384) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 0786).
Author information
Authors and Affiliations
Contributions
GCL, FG and CDMA contributed to the study data analysis, conception and design. Material preparation, data collection and data analysis were performed by CDMA, SS, and G-PM. The first draft of the manuscript was written by [CDMA] and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Correa Deza, M.A., Salva, S., Grillo-Puertas, M. et al. Effect of culture parameters on the heat tolerance and inorganic polyphosphate accumulation by Lacticaseibacillus rhamnosus CRL1505, a multifunctional bacterium. World J Microbiol Biotechnol 39, 182 (2023). https://doi.org/10.1007/s11274-023-03625-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03625-0