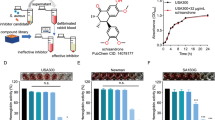
Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a multidrug-resistant pathogen that poses a significant risk to global health today. In S. aureus, α-hemolysin is an important virulence factor as it contributes to the capacity of the bacteria to infect the host. Here, we showed that biochanin A (bioA), an isoflavone present in red clover, cabbage and alfalfa, effectively inhibited hemolytic activity at a dose as low as 32 μg/mL. Further, western blot and RT-qPCR data showed that bioA reduced the production and expression of MRSA hemolysin in a dose-dependent manner. In addition, when different concentrations of bioA were added to a coculture system of A549 cells and S. aureus, it could significantly decrease cell injury. Importantly, the in vivo study showed that bioA could protect mice from pneumonia caused by a lethal dose of MRSA, as evidenced by improving their survival and reducing the number of bacterial colonies in lung tissues, the secretion of hemolysin into alveolar lavage fluid and the degree of pulmonary edema. In conclusion, biochanin A protected the host from MRSA infection by inhibiting the expression of the hemolysin of MRSA, which may provide experimental evidence for its development to a potential anti-MRSA drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus (S. aureus) is a seriously dangerous human and animal pathogen that cause endocarditis, skin and soft tissue infections, bacteremia and pneumonia (Kyaw et al. 2015; McColl et al. 1998; Ni et al. 2018). The difficulty of treating S. aureus infection is due to the emergence of antibiotic-resistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), which is resistant to most antibiotics currently used (Fridman et al. 2014; Levin-Reisman et al. 2017; Mechler et al. 2015). The World Health Organization (WHO) identified MRSA as a high-priority pathogen (Shrivastava et al. 2018). To cope with the increasingly problematic emergence of antibiotic resistance and the postantibiotic era (Dickey et al. 2017), novel strategies against MRSA are urgently needed.
Pathogens express a variety of virulence factors, which affect the host by destroying the relationship between the host immune system and pathogens. In addition, inhibiting the virulence of pathogens will not place selection pressure on the host, which dramatically reduces the probability of drug resistance. Therefore, screening of antivirulence inhibitors is an ideal strategy to control multidrug resistant pathogens (Casadevall and Pirofski 1999).
S. aureus can produce a large number of cytotoxic molecules, including four hemolysins (α, β, δ and γ). Among them, alpha-hemolysin (Hla) is a pore-forming exotoxin that has been widely studied. Hla, a 33 kDa polypeptide, is secreted by 95% of S. aureus (2019; Grumann et al. 2014). One of the remarkable characteristics of hemolysin is its ability to dissolve red blood cells. Hla has been classified as a phenol soluble regulatory protein (PSM), and its hemolytic activity is not dependent on any receptor (Fritz et al. 2013; Kolata et al. 2011). As a water-soluble monomer, it can integrate into the cell membrane to form a transmembrane oligomer β-barrel heptamer channel, leading to host cell lysis or death (Dinges et al. 2000; Wu et al. 2019b). Previous studies have shown that the Hla mutant of S. aureus has significantly reduced virulence compared with the wild-type strain (Tran et al. 2020). Hla has hemolytic, cytotoxic, dermonecrotic, and other lethal properties and is considered an essential virulence target against S. aureus infection.
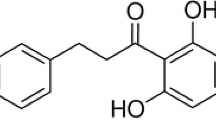
Natural products from plants and microorganisms are the main sources of antivirulence inhibitors because of their extensive physiological activities and environmental friendliness. A number of natural products have been reported to protect the host against S. aureus by inhibiting the production (Jiang et al. 2016; Teng et al. 2017) and self-assembly of Hla (Dong et al. 2013; Marathe et al. 2012). Biochanin A (4′-methoxy-5,7-dihydroxy isoflavone, bioA) (Fig. 1A) is a bioactive isoflavone found in diverse plants (Sarkar et al. 2006). It has been proven to produce marked anti-inflammatory (Kole et al. 2011), antioxidant (Derangula et al. 2020) and antitumor effects (Jain et al. 2015). In addition, bioA has been reported to inhibit the efflux pump of MRSA (Dan et al. 2014) and can be used as an adjuvant in combination with ciprofloxacin against clinical isolates of S. aureus (Liu et al. 2011), while its effect on S. aureus-related virulence factors has not been reported. In our study, a concentration of bioA at 32 μg/mL almost completely inhibited hemolysin activity. In addition, bioA also significantly inhibited the hemolytic activity of MRSA clinical isolates. Subsequently, the protective effect of bioA on S. aureus pulmonary infection was confirmed by establishing a mice pneumonia model. In conclusion, our results showed that bioA effectively inhibit the virulence of S. aureus Hla in vivo and in vitro, indicating it should be further explored as a drug against MRSA infection.
Materials and methods
Bacterial strains, reagents and growth conditions
S. aureus Newman and S. aureus BAA-1717 (USA 300) were purchased from American Type Culture Collection (ATCC, Manassas, V A, United States). Clinically isolated MRSA strain SA28 was obtained within the past 3 years and was identified by 16S RNA and quality control. BioA was obtained from the Rui Fensi Biological Company (Chengdu, China). BioA was dissolved in dimethyl sulfoxide (DMSO, Sigma, United States) to obtain a master stock (20 mg/mL) for standby application. Other chemical reagents were provided by Sangon Biotech (Shanghai, China). S. aureus was grown in trypticase soy broth (TSB, Hopebio, Qingdao, China) in an incubator with shaking at 220 rpm at 37 °C.
Susceptibility testing
The minimum inhibitory concentration (MIC) was determined by the microdilution method in 96-well plates according to the NCCLS guidelines as described previously. BioA was serially diluted twofold in 100 μL of cation-adjusted medium (CAMHB) at concentrations ranging from 2 to 512 µg/mL. Then, S. aureus USA300 (105 CFU/mL) was added to a 96-well plate. After incubation at 37 °C for 16 h, the MIC value was determined by reading the absorbance at 600 nm with a microplate reader.
Growth assay
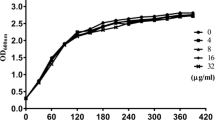
For the growth curve assay, S. aureus USA300 was cultivated at 37 °C to an OD value of 0.3 at 600 nm in TSB. Following the addition of bioA at concentration of 64 μg/mL, the bacteria were further cultured at 37 °C with constant shaking for 24 h. S. aureus USA300 cultures without added bioA were used as a control group, and S. aureus USA300 cultures supplemented with 1% DMSO were used only to verify whether DMSO affects bacterial growth. Subsequently, the OD600 value was measured every hour for 24 h, and the curve of the time versus the absorbance was drawn using the statistical program GraphPad Prism version 8.0.
Hemolysis assay
S. aureus Newman, S. aureus USA300 and MRSA strain SA28 were grown in TSB in the absence or presence of bioA to the postexponential growth phase, respectively. Bacterial supernatants were collected by centrifugation, and a hemolysis assay of the culture supernatants was conducted based on our previously described method using rabbit red blood cells (Qiu et al. 2010). Briefly, 100 μL supernatant was added to 1 mL phosphate buffered saline (PBS) buffer, followed by the addition of 25 μL defibrinated rabbit blood (Sbjbio, Nanjing, China). It was gently mixed and then incubated at 37 ℃ for 1 h. Low-speed centrifugation (4000×g, room temperature, 10 min) was used to collect any intact blood cells. The hemolytic activity of the culture supernatant was determined by measuring the absorbance at 543 nm. In addition, PBS was used as a negative control group. The assays were performed in triplicate.
RT-qPCR
A total of 2 mL of S. aureus USA300 grown with different concentrations of bioA (0 to 32 μg/mL) was incubated at 37 °C with shaking at 220 rpm for 16 h. S. aureus USA300 grown without bioA was treated in the same manner and used as a negative control. Subsequently, each sample was centrifuged at 10,000×g for 10 min to collect the bacteria. Total RNA was extracted using a Beyozol Total RNA Extraction Kit (Biotechnology, Shanghai, China) according to the manufacturer's instructions. Then, 1 µg of the purified RNA was used to generate cDNA using a PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). The Hla gene and regulatory gene RNAIII were determined by RT-qPCR using an ABI 7900HT Real-time PCR system. Table 1 shows the oligonucleotide primers, and the RNA transcript levels were calculated using the 2−ΔΔCT method. Each reaction was performed at least in triplicate.
Western blot
To evaluate the effect of bioA on α-toxin expression, bioA (0 to 32 μg/mL) was added to the S. aureus USA300 cultures and incubated at 37 °C until the OD600 reached 2.5. The α-toxin protein in the supernatant of the bacterial culture was isolated by 12% SDS-PAGE and then transferred to a polyvinylidene fluoride (PVDF) membrane by a transblot semidry system. The nonspecific binding sites of the PVDF membrane were blocked by incubating in 5% bovine serum albumin (BSA) for 2 h at room temperature. The membranes were incubated with rabbit anti-α-toxin polyclonal antibody (diluted 1:8000) (Sigma-Aldrich) for 1 h. After washing with PBST three times, the membranes were incubated with HRP-labeled goat anti-rabbit IgG (Bioworld, China) diluted 1:10,000 in PBST + 1% BSA at 37 °C for 1 h. The blots were visualized using an enhanced chemiluminescence (ECL) detection system (GE Healthcare, UK), and the bands were quantified using ImageQuant TL software (GE Healthcare).
Live/dead and cytotoxicity assay
A549 cells (adenocarcinomic human alveolar basal epithelial cells) were seeded in 96-well plates (Nest, United States) at a density of 4 × 105 cells/well and then incubated at 37 °C in a 5% CO2 incubator for 24 h. After that, the medium was removed gently and the dish was washed with sterile PBS to remove the nonadherent cells. Afterward, 100 µL of S. aureus USA300 culture and Dulbecco’s modified Eagle’s medium (DMEM, 400 µL) containing various concentrations of bioA (0 to 32 µg/mL) were added to the cells. After incubation at 37 °C for 6 h, the medium was collected for LDH assays by an LDH Cytotoxicity Detection kit (Beyotime, Shanghai, China). The amount of released LDH was measured using a microplate reader (Thermo Fisher, USA) at an absorbance of 490 nm.
For the live/dead assay, the dye was added to the cells and incubated in the dark for 30 min. The A549 cells were stained red/green to evaluate cell viability, and the fluorescence was measured from images obtained using fluorescence microscopy (Olympus, Japan).
Murine model of pneumonia
Seven-week-old female C57BL/6J mice (~ 22 g) were used to establish a pneumonia model as previously described (Brown et al. 2009; Labandeira-Rey et al. 2007). For the survival experiments, a group of 10 mice was infected with a lethal dose of 2 × 108 CFUs (in 30 µL of PBS) of S. aureus culture via the intranasal route. After infection for one hour, the mice were dosed with bioA (50 mg/kg of body weight) via the intraperitoneal route at intervals of 12 h. Similarly, mice were injected with sterile PBS containing 0.5% DMSO as the negative control. The survival of the animals was monitored every 12 h for 96 h postchallenge to calculate their survival rate.
The bacterial count in the lung tissue and pathological correlates of pneumonia were also evaluated, and for this, the mice were infected with 30 µL (1 × 108 CFUs) of S. aureus culture. After infection for two days, the mice were euthanized, and the lungs were collected, weighed, and homogenized. After that, the lung tissue homogenate was serially diluted and plated on TSA agar plates and incubated at 37 ℃ until isolated colonies appeared to be counted.
The level of Hla in the alveolar lavage fluid of mice in each group was determined by ELISA. For histopathological analysis, the lung tissues of mice in each group were aseptically removed and fixed in 10% formalin. After conventional hematoxylin and eosin (H&E) staining, the lung tissue sections were observed and analyzed by light microscopy. The W/D ratio was used to reveal the degree of tissue edema and it was recorded in each case. The wet lung weight was determined 1 min after removal of the surface moisture. The dry lung weight was measured after dehydration at 80 ℃ (Xia et al. 2016).
Data analysis
Independent samples were statistically analyzed using Student’s t test, and P < 0.05 was considered significant. The data are presented as the mean ± SD, and all statistical analyses in this study were conducted using the statistical program GraphPad Prism version 8.0. The number of independent replicates for in vitro experiments is three or four replicates and ten replicates for in vivo experiments.
Results
BioA does not affect the growth of S. aureus
The MIC of bioA against S. aureus USA300 was determined by the microdilution method. The MIC value was 512 μg/mL, which proved that it has no antibacterial activity. In addition, the growth curve experiment further revealed that there was no effect on the growth of S. aureus USA300 when cocultured with bioA at a concentration of 64 μg/mL (Fig. 1B). Thus, bioA can be considered a candidate anti-S. aureus virulence inhibitor because it does not have direct anti-S. aureus activity.
Effects of bioA on hemolysis of S. aureus USA300, Newman and MRSA clinical isolates
The effects of bioA (0 to 32 μg/mL) on the hemolytic activity of S. aureus USA300, Newman and MRSA clinical isolates were determined. As shown in Fig. 2A–C, when the medium supernatant of S. aureus USA300, Newman and SA28 was co-incubated with rabbit blood cells for 1 h, blood cell rupture and obvious hemolysis were observed. However, no hemolysis was observed when the supernatant of these strains was pretreated with bioA at 32 μg/mL, and the hemolytic inhibitory activities of S. aureus USA300, Newman and MRSA clinical isolates were 3.73%, 4.58% and 4.36%, respectively (P < 0.001).
BioA downregulates the transcription of Hla and RNAIII
To further explore the inhibitory mechanism of bioA on hemolysis of S. aureus, we evaluated the effect of bioA on the transcription level of Hla and its upstream regulatory gene RNAIII. As shown in Fig. 3A, when different concentrations of bioA were added to S. aureus, the transcriptional levels of Hla and RNAIII were decreased in a dose-dependent manner. These results suggested that bioA inhibited hemolytic activity by downregulating Hla and RNAIII transcription.
Determination of bioA affected the expression of Hla and the transcription of the related regulatory gene. A The effects of different concentrations of bioA on the transcription of Hla and RNAIII in S. aureus USA300 (n = 3). B Western blot analysis of Hla expression by S. aureus USA300 treatment with bioA (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001 were calculated using one-way ANOVA
BioA decreases the secretion of Hla
According to the RT-qPCR results, we further evaluated whether bioA could inhibit the expression of Hla, and western blotting was performed. As shown in Fig. 3B, compared to the WT group (untreated S. aureus), the addition of different concentrations of bioA (0, 8, 16 or 32 µg/mL) inhibited the expression of Hla in a dose-dependent manner. This implied that bioA could effectively inhibit the activity of Hla by inhibiting the expression of Hla.
BioA prevents Hla‑mediated A549 cell injury
After S. aureus infection of A549 cells for 6 h, the survival state of the A549 cells was observed by live/dead cell staining. As shown in Fig. 4A, A549 cells without S. aureus showed green fluorescence under the microscope, which proved that there were few injured cells. When S. aureus was incubated with A549 cells, many cells were stained red, indicating that S. aureus cause the death of A549 cells (Fig. 4B). Remarkably, the addition of various concentrations of bioA (8–32 μg/mL) to the system provided significant protection against A549 injury and death, as indicated by a significant reduction in red fluorescence (Fig. 4C–E).
Effects of bioA on A549 cells injury induced by S. aureus Hla. A549 cells were stained with live (green)/dead (red) agent. A A549 cells, B A549 cells were infected by S. aureus USA300. C–E A549 cells were infected by USA300 with bioA (8, 16 or 32 µg/mL). F The effect of bioA on S. aureus USA300 induced A549 cells cytotoxicity by detecting the release of LDH (n = 3). *P < 0.05, ***P < 0.001 compared with the untreated group
Subsequently, S. aureus-induced cytotoxicity was further determined by detecting the release of LDH. Consistent with the results of the live and dead fluorescent assay, the bioA treatment group inhibited A549 cell death induced by S. aureus in a dose-dependent manner. BioA has a significant protective effect against A549 cell injury induced by S. aureus.
BioA alleviates lung injury caused by S. aureus infection
To evaluate the therapeutic activity of bioA in the lung model, mice were challenged with S. aureus (2 × 108 CFUs) followed by treatment with bioA (50 mg/kg). The untreated mice began to die at 12 h after inoculation with S. aureus USA300, and the survival rate was 20% within 72 h. After treatment with 50 mg/kg bioA, the survival rates of the mice were 50% within 72 h (Fig. 5A). Administration of bioA significantly improved the survival rate and prolonged the survival time of the mice (P < 0.01).
The therapeutic and protective effects of bioA on mice. A Effect of bioA treatment on the survival of mice (n = 10) infected with S. aureus USA300 at 12 h intervals for 96 h. B W/D ratio of lung tissue of mice (n = 6) infected with the S. aureus USA300 and treated with bioA (50 mg/kg). C Hla level in alveolar lavage fluid of mice (n = 6) treated or untreated with bioA after infection with S. aureus USA300. D Effect of bioA treatment on bacterial load in lungs of mice (n = 6). E Histopathology of the mouse lung tissue after 48 h of treatment with bioA or DMSO. Lung sections were analyzed by H&E staining. Scale bar, 50 µm
The W/D ratio was further measured to evaluate the effect of bioA on the degree of pulmonary edema. As shown in Fig. 5B when the mice were infected with S. aureus USA300, the pulmonary W/D ratio increased from 4.04 ± 0.18 to 8.62 ± 0.25. After treatment with bioA, the W/D ratio recovered to 6.30 ± 0.27, suggesting that bioA significantly reduce the degree of pulmonary edema (P < 0.01). In addition, the level of Hla in the alveolar lavage fluid was detected by ELISA. As shown in Fig. 5C, compared with the untreated group, the level of Hla in the alveolar lavage fluid decreased significantly after bioA treatment (P < 0.01). Subsequently, we evaluated the colony count in the lung tissue in each group. For the untreated infected mice, the colony count was 9.81 ± 0.11 log10 CFU/g. After treatment with 50 mg/kg bioA, the number of bacteria in the lungs was significantly reduced to 6.54 ± 0.32 log10 CFU/g compared with the untreated group. BioA significantly reduced the number of viable S. aureus USA300 in the lungs of the mice (Fig. 5D).
Histopathology was used to examine the changes in the lung tissue of the mice in each group. The lungs of the untreated infected mice showed serious acute injury, which was characterized by congestion and edema in the interstitial area and a large amount of inflammatory cell infiltration. After treatment with 50 mg/kg bioA, the inflammatory symptoms and inflammatory cell infiltration were significantly reduced (Fig. 5E). In short, these results indicated that bioA protected mice from a lethal S. aureus challenge in vivo.
Discussion
MRSA is a multidrug-resistant pathogen that poses a significant risk to global health today. Antibiotics have always played a leading role in the prevention and therapy of diseases caused by S. aureus. However, the resistance of S. aureus to antibiotics is increasing rapidly, and the development of new antibiotics is difficult, slow, and far behind the pace of development of drug-resistant strains. Innovative anti-infective drugs are urgently needed to overcome drug resistance. Targeting bacterial virulence is an alternative antimicrobial therapy that provides a promising opportunity to inhibit pathogenesis and will not cause direct death to the target bacteria. Many virulence factors have been proven to be potential targets for drug design and therapeutic intervention. Targeted virulence represents a new model to enable clinicians to prevent and treat current multidrug-resistant pathogens (Diep et al. 2016).
The pathogenicity of S. aureus mainly depends on the secretion of a large number of extracellular toxins. In general, infections caused by bacterial toxins are becoming uncontrollable. Hla has been proven to be a significant pathogenic toxin secreted by S. aureus. This small β-barrel pore-forming cytotoxin dissolves erythrocytes and leukocytes but not neutrophils by binding to the protein receptor ADAM10, a disintegrin and metalloproteinase (Valeva et al. 1997; Wilke and Bubeck Wardenburg 2010). Therefore, Hla is an ideal target to fight S. aureus infection.
The main characteristic of Hla is its ability to dissolve red blood cells, and rabbit blood cells are especially susceptible to this effect, being 100 times higher than that of other mammalian animal cells (Bernheimer and Schwartz 1963; Bhakdi et al. 1984). Based on this, hemolysin inhibitors were screened from an in-house library containing 300 natural small molecular compounds. Many natural products from microorganisms and plants have been reported as candidate antivirulence agents against S. aureus and have shown specific activity and high safety (Wu et al. 2019a). We identified the natural compound bioA that could block the hemolytic activity of MSSA, MRSA and MRSA clinical isolates against rabbit blood cells. The supernatant of S. aureus treated with bioA (32 μg/mL) almost completely lost hemolytic activity. As a natural flavonoid, bioA has been found in a variety of plants and has a variety of pharmacological functions. Its development cost is much lower than that of antibiotics and vaccines.
To further clarify the mechanism by which bioA inhibits hemolysis, RT-qPCR and western blotting experiments were carried out. BioA decreased the level of Hla transcription by downregulating RNAIII, which was consistent with the western blot results. BioA significantly inhibited hemolytic activity by affecting the expression of Hla. As one of the major toxins secreted by S. aureus, Hla has been proven to affect a variety of human cell types, including epithelial cells, macrophages and endothelial cells (Berube and Bubeck Wardenburg 2013; Powers et al. 2012). In our experiment, we observed that the survival rate of A549 cells was significantly improved after adding bioA. In addition, Hla mutants of S. aureus have been shown to significantly reduce the pathogenicity in a variety of models, such as skin and soft tissue infection and pneumonia (Bubeck Wardenburg et al. 2007). BioA has significant therapeutic effects on S. aureus-induced pneumonia in mice, including improving the survival rate of the mice, prolonging the survival time, reducing the lung colony number, decreasing the D/W ratio of the lung tissue, reducing the level of Hla in alveolar lavage fluid and alleviating lung tissue damage.
Collectively, in the face of the global antibiotic crisis, there is an urgent need for new clinical treatments. BioA has significant anti-Hla activity in vivo and in vitro and can be further developed against S. aureus infection.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bernheimer AW, Schwartz LL (1963) Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol 30:455–468. https://doi.org/10.1099/00221287-30-3-455
Berube BJ, Bubeck Wardenburg J (2013) Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins 5(6):1140–1166. https://doi.org/10.3390/toxins5061140
Bhakdi S, Muhly M, Füssle R (1984) Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun 46(2):318–323. https://doi.org/10.1128/iai.46.2.318-323.1984
Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG (2009) The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect 15(2):156–164. https://doi.org/10.1111/j.1469-0691.2008.02648.x
Bubeck Wardenburg J, Patel RJ, Schneewind O (2007) Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75(2):1040–1044. https://doi.org/10.1128/iai.01313-06
Casadevall A, Pirofski LA (1999) Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 67(8):3703–3713. https://doi.org/10.1128/iai.67.8.3703-3713.1999
Dan Z, Xie K, Wang H, Chen Y, Xie MJAMS (2014) Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Mol Med Rep 54(10):1204–1211
Derangula M, Panati K, Narala VR (2020) Biochanin A ameliorates ovalbumin-induced airway inflammation through peroxisome proliferator-activated receptor-gamma in a mouse model. EMIDDT 21:145
Dickey SW, Cheung GYC, Otto M (2017) Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16(7):457–471. https://doi.org/10.1038/nrd.2017.23
Diep BA, Le VTM, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E (2016) Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13(1):16–34. https://doi.org/10.1128/cmr.13.1.16
Dong J, Qiu J, Zhang Y, Lu C, Dai X, Wang J, Li H, Wang X, Tan W, Luo M, Niu X, Deng X (2013) Oroxylin A inhibits hemolysis via hindering the self-assembly of α-hemolysin heptameric transmembrane pore. PLoS Comput Biol 9(1):e1002869. https://doi.org/10.1371/journal.pcbi.1002869
Fridman O, Goldberg A, Ronin I, Shoresh N, Balaban NQ (2014) Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513(7518):418–421. https://doi.org/10.1038/nature13469
Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA (2013) A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56(11):1554–1561. https://doi.org/10.1093/cid/cit123
Grumann D, Nübel U, Bröker BM (2014) Staphylococcus aureus toxins–their functions and genetics. Infect Genet Evol 21:583–592. https://doi.org/10.1016/j.meegid.2013.03.013
Jain A, Lai JC, Bhushan A (2015) Biochanin A inhibits endothelial cell functions and proangiogenic pathways: implications in glioma therapy. Anticancer Drugs 26(3):323–330. https://doi.org/10.1097/cad.0000000000000189
Jiang L, Li H, Wang L, Song Z, Shi L, Li W, Deng X, Wang J (2016) Isorhamnetin attenuates Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. J Microbiol Biotechnol 26(3):596–602. https://doi.org/10.4014/jmb.1507.07091
Kolata J, Bode LG, Holtfreter S, Steil L, Kusch H, Holtfreter B, Albrecht D, Hecker M, Engelmann S, van Belkum A, Völker U, Bröker BM (2011) Distinctive patterns in the human antibody response to Staphylococcus aureus bacteremia in carriers and non-carriers. Proteomics 11(19):3914–3927. https://doi.org/10.1002/pmic.201000760
Kole L, Giri B, Manna SK, Pal B, Ghosh SJEJP (2011) Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur J Pharmacol 653(1–3):8–15
Kyaw MH, Kern DM, Zhou S, Tunceli O, Jafri HS, Falloon J (2015) Healthcare utilization and costs associated with S. aureus and P. aeruginosa pneumonia in the intensive care unit: a retrospective observational cohort study in a US claims database. BMC Health Serv Res 15:241. https://doi.org/10.1186/s12913-015-0917-x
Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Höök M, Etienne J, Vandenesch F, Bowden MG (2007) Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science (new York, NY) 315(5815):1130–1133. https://doi.org/10.1126/science.1137165
Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ (2017) Antibiotic tolerance facilitates the evolution of resistance. Science (new York, NY) 355(6327):826–830. https://doi.org/10.1126/science.aaj2191
Liu G, Liang JC, Wang XL, Li ZH, Wang W, Guo N, Wu XP, Shen FG, Xing MX, Liu LH, Li L, Liu MY, Yu L (2011) In vitro synergy of biochanin A and ciprofloxacin against clinical isolates of Staphylococcus aureus. Molecules (basel, Switzerland) 16(8):6656–6666. https://doi.org/10.3390/molecules16086656
Marathe SA, Sen M, Dasgupta I, Chakravortty D (2012) Differential modulation of intracellular survival of cytosolic and vacuolar pathogens by curcumin. Antimicrob Agents Chemother 56(11):5555–5567. https://doi.org/10.1128/aac.00496-12
McColl K, Murray L, El-Omar E, Dickson A, El-Nujumi A, Wirz A, Kelman A, Penny C, Knill-Jones R, Hilditch T (1998) Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med 339(26):1869–1874. https://doi.org/10.1056/nejm199812243392601
Mechler L, Herbig A, Paprotka K, Fraunholz M, Nieselt K, Bertram R (2015) A novel point mutation promotes growth phase-dependent daptomycin tolerance in Staphylococcus aureus. Antimicrob Agents Chemother 59(9):5366–5376. https://doi.org/10.1128/aac.00643-15
Ni S, Li B, Chen F, Wei H, Mao F, Liu Y, Xu Y, Qiu X, Li X, Liu W, Hu L, Ling D, Wang M, Zheng X, Zhu J, Lan L, Li J (2018) Novel staphyloxanthin inhibitors with improved potency against multidrug resistant Staphylococcus aureus. ACS Med Chem Lett 9(3):233–237. https://doi.org/10.1021/acsmedchemlett.7b00501
Pavičić M (2019) Retraction note: Can two-way direct communication protocols be considered secure? Nanosc Res Lett 14(1):242. https://doi.org/10.1186/s11671-019-3086-8
Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J (2012) ADAM10 mediates vascular injury induced by Staphylococcus aureus α-hemolysin. J Infect Dis 206(3):352–356. https://doi.org/10.1093/infdis/jis192
Qiu J, Wang D, Hua X, Feng H, Jiang Y, Xia L, Jing D, Lu J, Yu L, Deng X (2010) Subinhibitory concentrations of thymol reduce enterotoxins A and B and α-hemolysin production in Staphylococcus aureus isolates. PLoS ONE 5(3):e9736
Sarkar FH, Adsule S, Padhye S, Kulkarni S, Li Y (2006) The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem 6(4):401–407. https://doi.org/10.2174/138955706776361439
Shrivastava S, Shrivastava PS, Ramasamy JJJMS (2018) World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc 32(1):76
Teng Z, Shi D, Liu H, Shen Z, Zha Y, Li W, Deng X, Wang J (2017) Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression. Appl Microbiol Biotechnol 101(17):6697–6703. https://doi.org/10.1007/s00253-017-8417-z
Tran VG, Venkatasubramaniam A, Adhikari RP, Krishnan S, Wang X, Le VTM, Le HN, Vu TTT, Schneider-Smith E, Aman MJ, Diep BA (2020) Efficacy of active immunization with attenuated α-hemolysin and panton-valentine leukocidin in a rabbit model of Staphylococcus aureus necrotizing pneumonia. J Infect Dis 221(2):267–275. https://doi.org/10.1093/infdis/jiz437
Valeva A, Walev I, Pinkernell M, Walker B, Bayley H, Palmer M, Bhakdi S (1997) Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci USA 94(21):11607–11611. https://doi.org/10.1073/pnas.94.21.11607
Wilke GA, Bubeck Wardenburg J (2010) Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA 107(30):13473–13478. https://doi.org/10.1073/pnas.1001815107
Wu SC, Liu F, Zhu K, Shen JJJA, Chemistry F (2019a) Natural products that target virulence factors in antibiotic-resistant Staphylococcus aureus. J Agric Food Chem 67:13195
Wu SC, Liu F, Zhu K, Shen JZ (2019b) Natural products that target virulence factors in antibiotic-resistant Staphylococcus aureus. J Agric Food Chem 67(48):13195–13211. https://doi.org/10.1021/acs.jafc.9b05595
Xia F, Li X, Wang B, Gong P, Xiao F, Yang M, Zhang L, Song J, Hu L, Cheng M, Sun C, Feng X, Lei L, Ouyang S, Liu ZJ, Li X, Gu J, Han W (2016) Combination therapy of LysGH15 and apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus. Appl Environ Microbiol 82(1):87–94. https://doi.org/10.1128/aem.02581-15
Funding
This work was partly supported by a grant from the Science and Technology Development Plan Project (2019) of Jilin Province Science and Technology Department (20190103080JH), “Xinglin Scholar Project” of Changchun University of Chinese Medicine (2019, Wu song), “Xinglin Scholar Project” of Changchun University of Chinese Medicine (QNKXJ2-2021ZR05) and “Thirteenth Five-Year Plan” of Science and Technology Project of Education Department of Jilin Province (No. JJKH20200906KJ).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by JF, DS, XL, LW, DY and JG. The first draft of the manuscript was written by LW and XW; YZ and HY revised the manuscript; WS, BW conceived and designed the experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest in this work.
Ethical approval
The animal work in this report was approved by the Experimental Animal Ethics Committee of Changchun University of Chinese Medicine, and it complied with the regulations on the use of experimental animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, J., Sun, D., Wang, L. et al. Biochanin A as an α-hemolysin inhibitor for combating methicillin-resistant Staphylococcus aureus infection. World J Microbiol Biotechnol 38, 6 (2022). https://doi.org/10.1007/s11274-021-03182-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03182-4