Abstract
Two edible mushrooms Calocybe indica and Pleurotus sajor-caju were chosen as parent strains in this study to approach the concept of hybridization through the protoplast fusion technique. Protoplast fusion in presence of polyethylene glycol (PEG) was conducted between the parent strains and by further double selection screening method, six somatic hybrid lines were developed. Those fruit bodies of the hybrid lines showed phenotypic resemblance with Pleurotus sajor-caju when grown on paddy straw under favorable conditions. The hybridity of the newly developed somatic hybrid strains was established by barrage reaction, morphological traits, fruitbody parameter and, inter single sequence repeat (ISSR) profiling. One-way analysis of variance (ANOVA) was used for the analysis of phenotypic data of hybrid lines and parents. Five ISSR primers were used to generate 51 amplified DNA fragments ranged between 250 and 3000 bp in size in six hybrids and two parents with 90.19% polymorphism. Some of the hybrids contain some non-parental bands which indicate that recombination might happen in the hybrid genome hence confirming the hybridity of newly developed strains. The dendrogram was created using the Average Linkage (Between Groups) method based on ISSR profiling and genetic distance between parent-hybrids and hybrid-hybrid was analyzed by Jaccard’s proximity matrix. A definite improvement in nutritional properties and biological activity was observed in the study. Due to ease in their cultivation, it can play a significant role in the rural economic development.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mushrooms are considered as one of the most significant food items in our diet because of their phenomenal biological properties and pharmacological characteristics (Barros et al. 2008) (Breene 1990). Mushroom cultivation can be practiced all over the world including India throughout the year, as the process is economic and environmental friendly. In developing countries like India, diet primarily includes cereals (wheat, rice and maize) but are deficient in proteins. The aforementioned mushrooms are rich in proteins and vitamins (Heleno et al. 2010), in addition to having a high content of fibres and essential amino acids and an optimum carbohydrate and low fat content, which make them low calorie diet. For this reason, these mushrooms can be used as an effective food supplement to reduce the prevalence of malnutrition in the country.
New edible mushroom strains are produced through para-sexual mating as the incompatibility barrier restricts sexual mating in inter-generic or inter-order. Various approaches including cross breeding and transgenic breeding have been carried out to increase the yield and to improve the strains of mushrooms as well.
Protoplast fusion was first demonstrated in 1993 for inter-generic and intra-specific hybridization of mushroom strains (Peberdy and Fox 1993). The Protoplast fusion method has been able to overcome the natural barriers of vegetative incompatibility successfully to establish heterokaryosis in fungi (Kim et al. 1997) (Yoo et al. 2002). The fusion has been carried out interspecifically (Eyini et al. 2006), intergenerically (Chakraborty and Sikdar 2008, 2010). Different inter-specific, intra-specific fusions between edible mushrooms were attempted through the protoplast fusion. Fusion between Pleurotus florida + Volvariella volvacea and Pleurotus florida + Calocybe indica (Chakraborty and Sikdar , 2008, 2010) successfully produced somatic hybrid and basidiocarp as well. The fruiting bodies of Pleurotus species are popularly known as oyster mushrooms and are liked very much for their nice texture and delicious flavour. The above features have made it gain importance and at present, it has emerged as the second most important mushroom in terms of production in the world.
Calocybe indica, commonly known as milky mushrooms are very attractive, milky white sporophore with large sized fruiting bodies. These have a delicious flavour and are popular among consumers. These are capable of growing at temperatures above 30 °C, so they are suitable for cultivation in summer as well, a condition in which other varieties cannot grow. A variety of this mushroom, APK2 was commercialized from Tamil Nadu Agricultural University.
Our research group is interested to develop new somatic hybrid lines of edible mushrooms with high bio-efficiency, increased shelf life, shorter cropping period and high temperature tolerance through inter-generic protoplast fusion technique between Calocybe indica, also known as milky mushroom, and Pleurotus sajor-caju, an oyster mushroom. Analysis and comparison of the nutritional contents like proteins, carbohydrates, fats, ash of parent mushrooms, and fusant lines have been done. Both the parent mushrooms possess good nutritive value. These are cultivated in the tropical climates and successfully grown on paddy straw at temperature ranging from 22 °C to 35 °C, 65.5–80% humidity. Moreover, their longer shelf-life and lucrative market value have attracted the attention of both consumers and prospective growers.
Materials and methods
Enzyme
Two types of enzymes required for tissue digestion and protoplast isolation were Cellulase Onozuka R10 (2%) (Yakult) and Lysing enzyme (2%) from Trichoderma harzianum (Sigma) with osmotic stabilizer 0.7 M mannitol and 0.2% CaCl2.H2O. Enzymes were mixed at pH 5.5. For all experiments, 2 ml of enzyme mixture was used for 0.5 gm of tissue.
Strains and culture media
Mother culture of parental strain Pleurotus sajor-caju, was obtained from National Research center for Mushroom, Solan, Himachal Pradesh, India and Calocybe indica var APK2 was obtained from Tamil Nadu Agriculture University, Coimbatore, India. These two parent strains were cultured on PDA (i.e. Potato Dextrose Agar, pH- 6.2) solid media and mycelia were maintained on MYG (Maltose 10grm/1ltr, Yeast extract 4grm/1ltr, Glucose 10grm/1ltr, pH-6.2) liquid media for 5–6 days at 24 ± 2 °C for P.sajor-caju and for 10–15 days at 28 ± 2 °C for APK2 prior to protoplast isolation.
Protoplast preparation
The mycelial tissues of the two parental mushroom strains (Pleurotus sajor–caju and Calocybe indica) were cultured in liquid MYG medium at pH 6.2 for tissue preparation. Mycelial tissues of both strains were collected from the liquid media and washed with double distilled water twice and were further washed with osmotic stabilizer solution (0.6 M Mannitol with 2% CaCl2.2H2O) at pH 5.5 thrice. Protoplasts were separated from the parental tissue by enzymatic digestion. Here for Calocybe indica, we have followed the protoplast isolation steps stated earlier (Chakraborty and Sikdar 2010). The steps for protoplast isolation and purification from P. sajor-caju had been standardized by using a combination of 2% Lysing enzyme (from Trichoderma harzianum, Sigma) and 2% Cellulose Onazuka R10 (Yakult) enzyme. Fresh mycelial tissues (5–7 days old) were collected from P. sajor-caju and washed with double distilled water and osmoticum (0.6 M Mannitol), followed by chopping of the tissue and suspending into enzyme mixture for 6–8 h at 24 ± 2 °C. Densities of protoplasts were further determined with a hemocytometer. For purification, liberated protoplasts were sieved through sterile cotton, followed by centrifugation at 3,000 rpm for 5 min to collect the pellet. The pelleted protoplasts were further washed thrice with osmotic stabilizer solution by suspending and pelleting through centrifugation. Finally, purified protoplast pellets were suspended in 200 µl stabilizer solution.
Fluorescein diacetate (FDA) staining
FDA staining is a staining procedure to check the viability of the protoplast. It proceeds with mixing of 5 mg FDA with 1 ml acetone kept in dark, followed by mixing of equal volume of protoplast with FDA mixture and then again kept in dark for 5 min. Viable protoplasts are observed under microscope (Widholm 1972).
Protoplast fusion and hybrid Selection Strategy
For the selection of hybrids, a double selection method (Chakraborty and Sikdar 2008) has been used with the salt tolerance property as an important criterion. Pleurotus sajor-caju and Calocybe indica were grown in different concentrations of NaCl (0.1 – 1.0 M) containing MYG medium to test the salt tolerance level in order to use this character for hybrid selection (Chakraborty and Sikdar 2010). In this method it was found that P. sajor-caju could tolerate up to 0.7 mM NaCl, whereas C. indica could tolerate 0.5 mM NaCl, this data was further used for hybrid selection strategy. Parental protoplasts of P. sajor-caju were treated with 10 mM iodoacetamide (IOA) in the dark condition for 5 min before protoplast fusion to inactivate its cytoplasmic gene function, then were fused with normal C. indica protoplasts. Hybrid strains were selected from media (MYG) having concentration 0.7 mM NaCl containing inactivated protoplasts of P. sajor-caju (cytoplasmic gene function was inactivated) and C. indica (sensitive to high salt concentration). Thus, due to complementation of parental genome or nucleo-cytoplasmic interaction only hybrids could develop.
Protoplast fusion was carried out (Anne and Peberdy 1976) with some modifications (Mallick and Sikdar 2014) in the presence of polyethylene glycol (PEG, MW-3350 30%, 0.5 mM CaCl2, and 0.05 M glycine–NaOH at pH 7.5). Combination of 50 µl inactivated P. sajor-caju (IOA treated) protoplasts and 50 µl of normal C. indica var APK2 protoplasts, each with the concentration of 1 × 107 protoplast /ml were taken in a centrifuge tube, then were mixed well by shaking the centrifuge tube at every 5 min interval, then kept at 24 °C for 10–20 min (Anne and Peberdy 1976; Chakraborty and Sikdar 2008, 2010). Minimum volume was taken and inspected under the compound microscope to observe the fusion. After the fusion procedure, the final suspension (same volume of parental protoplast and PEG solution) was diluted 6 times with MYGNa medium (Malt extract −10 g/l, Yeast extract—4 g/l, Glucose—10 g /l + 0.7 mM NaCl) at pH 6.2. Aliquot of 100 µl of fused product was plated with 2 ml of the above mentioned culture medium. P. sajor-caju (normal viable protoplasts as positive control and IOA treated protoplasts as negative control) and C. indica (normal viable protoplast at 0.5 mM concentration in MYG media as positive control and same as in 0.7 mM MYG media as negative control) were also plated in a similar way at pH 6.2. All the cultures were maintained at 24 ± 2 °C, till the formation of macro- colony. The presumptive somatic hybrid colonies were further transferred to PDA medium for further growth.
Spawn and fruit-body production
200 g of each Sorghum grain packets were sterilized for spawn production. Now the selected hybrid mycelia which were grown on the PDA media were inoculated in sterile packets and the packets were kept at 24 ± 2 °C till growth. Matured straws were finely chopped and used as a substrate for fruit body production. Polypropylene packets were used for the production of the fruit body of the mushroom.100 g matured spawn per 0.75 kg substrate was inoculated with an alternate layer of substrate in a cylindrical bag for cultivation to make three to four layers of sterilized substrate. Mushroom bags were made porous for primordial initiation with a regular sprinkling of water after mycelia showed complete colonization. Temperature at 24 ± 2 °C along with high humidity was maintained by misting the room at regular intervals (Mallick and Sikdar 2014).
Barrage reaction
In this process, the selected hybrid lines along with their individual parent are inoculated in the solid PDA media at 2 cm distance from each other. Each plate containing a single hybrid line along with both parents were incubated at 24 ± 2 °C for 7 to 10 days. This technique is known as the “dual technique”. In this experiment, a thick barrage line was observed between the contact zone of two different strains (Adams and Roth 1967).
Sample Collection and morphological analysis
All hybridized mushroom samples including their parent samples Pleurotous sajor-caju, and Calocybe indica were cultivated and collected from Madhyamgram Experimental Farm (MEF) of Bose Institute, West Bengal. Slide culture method has been utilized to measure the mycelial growth of all the hybrids and parental strains. In this method, the mycelial culture is inoculated with a drop of PDA on a slide and kept for 7–10 days at a temperature of 24 ± 2 ºC. The mycelial length was then measured with a scale. Morphological characters like colony diameter, colony color, stipe length, cap diameter, and total yield was recorded, followed by observation under the microscope to record microscopic characters like presence or absence of clamp connection and hyphal width. After collection, the fruit body of mushroom samples were immediately lyophilized by lyophilizer (IIC Instrumentation Corporation, Kolkata).
Genomic DNA extraction and ISSR reaction
Genomic DNA of parent and hybrid strains was extracted by using the modified CTAB (N-cetyl-NNN-trimethyl ammonium bromide) method (Dellaporta et al. 1983). PCR was performed using the extracted DNA for ISSR (Inter single sequence repeat) amplification with slight modifications (Bornet and Branchard 2001). ISSR amplifications were performed in 25 µl reaction mixture made with 75 ng genomic DNA, 1 × Taq polymerase buffer with KCl (Fermentas), 25 mM MgCl2, 2 mM dNTP mixture (all 4 nucleotide triphosphates, Fermentas), 20 µl ISSR primers (from Operon Technologies Inc., USA) and 5 U/µl Taq DNA polymerase (Fermentas) in a DNA Thermal Cycler (Applied Bio-systems 2027). The condition for the ISSR-PCR reaction was: Initially denaturation for 5 min at 94 °C. 40 cycles consisting of 1 min denaturation at 94 °C, followed by primer annealing at a temperature ranging from 37 °C to 61 °C for 1 min, then 3 min extension at 72 °C and a successive final extension at 72 °C for 10 min.
Gel analysis
The PCR amplified genome specific ISSR fragments were separated on a 1.5% agarose gel, which is pre-stained with ethidium bromide solution using 1 × TAE buffer. The gels were run for 2 h at 80 V and the ISSR products were observed under UV Transilluminator. 1 kb plus DNA (MBI, Fermentas) was used as the standard molecular weight marker for determination of the size of amplified fragments.
Polymorphic information content (PIC) value
In genetics, the polymorphism information content (PIC) value is used for quantitative measurement of informativeness of a genetic marker used in linkage analysis.
This value refers to the relative value of each marker with respect to the amount of polymorphism exhibited. According to (Weir, n.d.), it is measured by the formula:
PIC = [1 −R P2i)],
where ‘i’ refers to the total number of alleles detected from ISSR marker,
‘Pi’ is the frequency of the ‘i’ th plus allele in the set of the APS genotype.
Nutrient Content
The nutritional constituents (protein, fat, carbohydrate, and ash) of mushroom samples were analyzed (Cunniff 1995). Crude protein content was examined by the Kjeldahl method (Sweeney and Rexroad 1987). Known amounts of powdered samples were extracted by using Soxhlet apparatus and the crude fat was determined. The ash content was assessed by incineration at 600 ± 15 °C for 5 to 6 h.
Total carbohydrate content was estimated by difference: 100−(g moisture + g protein + g fat + g ash).
Total Energy was calculated according to the following equation: Energy (kcal) = 4 × g protein + g carbohydrate) + 9 x (g lipid).
Statistical analysis
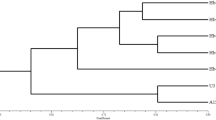
All the morphological and nutritional variations of hybrid and parent mushrooms were analyzed and evaluated using Graph pad prism 5 by One way ANOVA analysis and Turkey Post hoc tests, followed by the comparison of mean, standard deviation and level of significance (p < 0.05). DNA profiling (ISSR data) was done by using IBM SPSS software, version 19. ISSR bands were scored for each primer as the present (1) and absent (0). Then those scored bands from ISSR profile were used to construct a dendrogram with the help of IBM SPSS software, version 19 and genetic distance was analyzed by Jaccard’s proximity matrix (Jaccard, 1901) using the Unweighted Pair-Group method with Mathematic Averages (UPGMA) algorithm.
Results
Protoplast regeneration
Mycelia at their initial phase have a simple cell wall structure because they are in an exponential growth phase. Hence at this moment, the cell wall degrades easily in the presence of enzyme mixture (Djajanegara and Masduki 2013). Parental strains P. sajor-caju and C. indica liberated 8.5 × 106 and 3.1 × 106 protoplast /g tissue respectively (Fig. 1a,b) in the presence of combined cell wall degrading enzyme mixture (2% Cellulase Onozuka R-10 and 2% Lysing enzyme) in 0.6 mM mannitol with 0.2% CaCl2 at pH 5.5, with the incubation period of 6–8 h for P. sajor-caju and 12–15 h for C. indica. After isolation of protoplasts, some protoplasts were regenerated in the liquid MYG + 0.7 mM NaCl medium. Non damaged protoplasts were regenerated after 12–15 h, but some of the protoplasts lacked the ability to regenerate due to damage of nuclei or protoplasts at the time of enzymolysis or isolation procedure. The viable protoplasts were observed after Fluorescein Diacetate (FDA) treatment (Fig. 1d) and viability was calculated as 86.25 ± 2.32% in P. sajor-caju and 70.12 ± 1.43% in C. indica (Figs. 2, 3). The regeneration rate of P. sajor-caju and C. indica was found 31.50 ± 7.12% and 26.10 ± 2.30% respectively (Table 1).
Production of hybrid strains through protoplast fusion technique between Pleurotus sajor-caju and Calocybe indica var APK2. a, b Isolated protoplasts from Pleurotus sajor-caju and Calocybe indica respectively. c Fused protoplasts after PEG treatment. d FDA stained viable protoplasts of Pleurotus sajor-caju showing green fluorescence (marked by arrow). e Formation of somatic hybrid colonies through protoplast fusion technique. f A plate showing barrage reaction (triple culture of Pleurotus sajor-caju, Calocybe indica and one hybrid line. g Plates showing somatic hybrid culture (APS-1, APS-2, APS-3, APS-4, APS-5 and APS-6). h, i Pictures showing white colored basidiocarp of APS-5 & APS-4 respectively. j. Fruit bodies of first flush of APS-4. Size bar 80 µm in a, b & d, 10 µm in c, 3 cm in g, 25 mm in h & i, 5 mn in j
Assessment of morphological traits
Six hybrid lines and parents were compared based on morphological characters like hyphal growth rate, colony diameter, cell width, and colony morphology, presence or absence of clamp connection and barrage reaction. Barrage reaction was seen as a thick brownish barrage at their contact zone on PDA medium when the reaction was performed between parent (P. sajor-caju and C. indica) and hybrid lines (APS-1, APS-2, APS-3, APS-4, APS-5, APS-6). It was observed that Clamp connection was present in all hybridized mushroom strains. Morphological characterization was done for these putative hybrid lines and results showed significant variations between hybrids and parents. Hybrid mycelia grew rapidly than parents in PDA media. Morphologically all hybrids showed similarity with parent P. sajor-caju, except APS-1, which exhibits completely different color and mycelial nature. Initially APS-1 appeared white in color, but eventually, it turned into off white color. Mycelial growth diameter, and colony diameter (based on the slide culture on day 4 and day 7 respectively), hyphal cell width and colony morphology are represented in tabular form (Table 2). Here we observed that all the selected hybrid lines can grow well at 24 ± 2 °C. Colony growth (in 7 days) of the hybrid lines which were grown on solid media, diameter ranged from 3.48 ± 1.21 cm (APS-1) to 8.97 ± 1.62 cm (APS-4), whereas the diameter of Colony (in 7 days) of P. sajor-caju was 3.23 ± 0.35 cm and C. indica was 2.15 ± 0.56 cm. Out of six, diameter of APS-1 (3.48 ± 1.21 cm), APS-2 (4.52 ± 0.68 cm), and APS-6 (4.24 ± 0.63 cm) are very close to the parent P.sajor-caju (i.e. 3.23 ± 0.35 cm) while for APS-4 (8.97 ± 1.62 cm) it was higher than that of other fusant products as well as parents. Remaining two hybrid lines APS-3 and APS -5 showed satisfactory results. Hyphal cell width of fusant lines also varied from parent cultures.
The minimum cell width of the hybrid line was 16.02 ± 0.82 µm (APS-3) but the parent strain C. indica was found to show lowest cell width i.e. 5.02 ± 0.14 µm among all and the maximum cell width was 46.61 ± 0.68 µm (APS-4) compared to both parents and other hybrids.
Production of spawn and fruit body
We used Sorghum grains for the production of spawn packets. The spawn maturation time varied from hybrid to hybrid. The hybrid cultures APS- 4 required only 5–7 days for spawn maturation, whereas APS-1 took the maximum time of 10–15 days. On the other hand, we observed that the hybrid APS -5 required 10–12 days like the parent P. sajor-caju while another parent C. indica took about 15–20 days for production of the same. The spawn maturation time for APS-2, and APS-3 was 7–10 days, hence APS-2, APS-3 and APS-4 can be considered better to produce spawn than other hybrid lines at the temperature 24 ± 2 °C.
The strip length of the hybrid lines varied from 3.66 ± 1.012 cm (APS-5) to 6.71 ± 0.473 cm (APS-4). Maximum pileus diameter was 4.22 ± 0.93 cm (APS-6) while the minimum diameter was 2.43 ± 0.50 cm (APS-1). Here we observed that the yield of some of the hybrid lines (weight of three flushes in g/0.75 kg dry substrate) was remarkably higher than parents, whereas some of the hybrid lines yielded less amount compared to the parents. Hybrid lines APS-4, APS-5 and APS-3 yielded the highest crop in three flushes 865.33 ± 66.64 g, 737.80 ± 66.60 g and 533.33 ± 69.00 g respectively, while the yield of parent P. sajor-caju, and C. indica was 692.66 ± 53.71 and 822.50 ± 54.44 respectively, which were lower than APS-4, APS-5 and APS-3. The minimum yield among the hybrid line was shown by APS-2, i.e. 285.66 ± 37.44 g.
In the present study, we found that P. sajor-caju showed about 88.17% biological efficiency (BE). We harvested another parent C. indica on paddy straw with a 1 cm casing layer, where we found a better yield of 108.48%. In the present study, it was observed that the BE of APS-4 and APS-5 was undoubtedly high i.e. 117.68% and 97.95% respectively which are higher than the remaining four hybrid cultures including their parents, whereas BE of APS-2 was the lowest (35.91%) among them. Although APS-1, APS-3 and APS-6 exhibited satisfying results. Comparing all the morphological characteristics and yield, APS-4 showed best results among all hybrid lines and the parents. (All the above data are shown in table S1.)
ISSR profile analysis
To conform hybridity of somatic hybrid lines (APS) of P. sajor−caju and C. indica, ISSR primers, have been used. Among ten ISSR primers five were selected. These five primers exhibited a clear and reproducible banding pattern. Table 3 displays details of ISSR repeat sequence, no. of scored bands, the size range of bands, and PIC values of five hybrid strains. In this analysis, we found 51 amplified fragments in six hybrids and two parents with 90.19% polymorphism. The amplified fragments ranged from 250 to 3000 bp in size. Varying genomic sequences against ISSR primer repeat sequences in individual and parent strains led to different band numbers in each ISSR primer.
Among all five, primer ISSR−7 [(AAAGAG)3 A] amplified the highest no of scorable bands i.e. twelve and minimum amplification was generated by ISSR−11 [(CAC)3 GC] i.e. eight band patterns. The Polymorphism information content value (PIC) was calculated based on ISSR profile. Average PIC value of five ISSR primers was 0.360. We found the highest value as 0.413 in ISSR−2 whereas the lowest value was observed as 0.321 in ISSR−7.
Based on the ISSR profile, the genetic distance between the parent and hybrid as well as hybrid and hybrids were analysed by Jaccard’s proximity matrix (Data are shown in S table no.2). According to the data, it can be seen that the parent P. sajor−caju and the hybrid APS−1 (6.557) has the highest genetic distance which means maximum dissimilarity whereas APS−4 shows the least genetic distance i.e. 4.796. It can also be seen that the parent C. indica var APK2 and APS−4 are genetically most distinct (6.633). We found that APS−4 and APS−6 (4.690) closely resemble each other, while APS−1 and APS−3(6.633) exhibit maximum diversity among the hybrid strains.
Nutritional analysis
In this study, we found that P.sajor-caju contains 3.37 g of carbohydrate and another parent C.indica showed 3.33 g. Now among all the hybrids, APS-5 (4.48 g) expressed the highest carbohydrate content, whereas APS-2 showed the minimum amount (2.96 g) of carbohydrate among all.
In our study, the total fat content of 100 g of dried C.indica is 3.93 g and it closely resembles the data obtained earlier (Sumathy et al. 2015). P.sajor-caju contains 4.37 g fat as per our study. This result was almost similar to the preceding experiments (Shin et al. 2007). The fat content of hybrid strains ranges from 1.13 g in APS-4 to 2.02 g in APS-2 (presented in Table 4). Among the hybrids and parents, P.sajor-caju expressed the highest value i.e. 4.37 g. The rest of the hybrid lines APS-1, APS-3, APS-5, APS-6 exhibited 1.80 g, 1.87 g, 1.76 g and 1.40 g per 100 g of dried sample respectively. In this study, we observed that APS-4 contain the lowest amount of fat while APS-1, APS-3, APS-5 and APS-6 also exhibit lower fat content than parents. Hence, hybrid strains are found to be better than parents in terms of less fat content.
In this study, we measured the protein content of hybrid mushrooms as well the parent strains (C. indica and P. sajor-caju). The data in Table 4 reflects that the protein content of hybrid mushroom samples (APS-1, APS-2, APS-3, APS-4, APS-5, APS-6) are 1.174 g, 1.92 g, 1.80 g, 1.55 g, 2.21 g, and 1.94 g respectively. The parent strain C. indica expressed a protein content of 1.74 g/100 g, although it was reported previously that it was about 2.75 g/100 g of fresh mushroom (Alam et al. 2008). Another parent P. sajor-caju, in our study, reflected a protein value of 3.63 g/100 g. Overall, APS-5 showed the highest protein values among all the hybrids, whereas APS-1 has the minimum protein content. Summarily, we concluded that parent mushroom P. sajor-caju is rich in protein among all strains studied here, but some hybrid lines like APS-2, 3,5 and 6 are also rich in protein close to P. sajor-caju.
Usually, mushrooms are also high in moisture content. Fresh and air dried mushrooms contain mostly 85–90% and 10–12% of moisture respectively (Chang 1980; Danell and Eaker 1992). In the present study, we found that the moisture content of the fresh parent mushrooms P. sajor-caju and C. indica was 89.94% and 87.24% respectively. Hybrid strains comprised almost the same moisture content as their parents (Displayed in Table 4).
Discussion
The isolation and regeneration of protoplasts have been considered as a very useful method in the biotechnological manipulations of higher plants and fungi (Illing et al. 1989). For the last 40 years, isolation of protoplasts has been done from filamentous or mycelial fungi. In this study Lysing enzyme (Sigma) from Trichoderma harzianum and Cellulose Onazuka R10 (Yakult) enzyme mixture have been used to generate a maximum yield from the parent strains P. sajor-caju and C. indica. In a previous study, a similar enzyme mixture was used to obtain maximum yield from strain P. florida i.e. 1.5 × 107 protoplasts/g and L. edodes 1.6 × 106 protoplasts/g (Mallick and Sikdar 2014), whereas in another study it has been observed that Venturia Inaequalis released an utmost number of protoplasts in the presence of a mixture of enzymes containing cellulase, chitinase, pectinase and β-glucuronidase (Lalithakumari et al. 1996). In our experiment, we observed that the protoplast regeneration rate was lower than some Basidiomycetes (Chakraborty and Sikdar 2008, 2010), but much more higher than M. importuna #4 and M. sextelata #6 (He et al. 2020). Polyethylene Glycol 30% (PEG 3350) was used for the fusion process for 5 min. Here the PEG concentration is a very essential factor for protoplast fusion, as higher concentration can damage the protoplasts (Abe et al. 1982), hence in this study maximum tolerable concentration i.e. 30% PEG has been used. Osmotic stabilizer helps to get maximum yield. In the above experiment, we used mannitol as osmotic stabilizer, which played an important role in the protoplast isolation and fusion process. In past experiments, several other osmotic stabilizers such as potassium (Eyini et al. 2006), sucrose (Kim et al. 1997), sorbitol (Porselvi and Vijayakumar, n.d.), etc. also have been used. After a successful fusion procedure, the fused protoplasts were transferred to the selective media (MYGNa). A Double selection strategy (Chakraborty and Sikdar 2008) was used for selection of the hybrids. Salt tolerance and IOA treatment are the two principal factors for hybrid selection. In this study, we found that C. indica var APK2 could tolerate up to 0.5 mM NaCl (Fig. 1j), whereas P. sajor-caju showed tolerance up to 0.7 mM NaCl concentration. Inactivation of the protoplasts of P. sajor-caju was done by using IOA (10 mM). MYG + 0.7 mM NaCl was prepared to be used as selection media. Protoplasts of P. sajor-caju were unable to regenerate due to inactivation of their nucleus by IOA while C. indica var APK2 also did not show regeneration due to a higher concentration of salt (0.7 mM) than its tolerance level, but the fused protoplasts showed regeneration due to the complementation between the two genomes of two different parents (Fig. 1c). Thirteen putative hybrid colonies were obtained from the fusion media (Fig. 1e), then transferred to the solid media (PDA) at pH 6.2 and incubated at 24 ± 2 °C. For further study, out of thirteen hybrid colonies six colonies (Fig. 1g) (APS-1, APS-2, APS-3, APS-4, APS-5, APS-6) were separated and maintained in PDA media. No regeneration from negative control plates of parental strains was found, while positive control plates exhibited normal growth which strongly suggests the success of the double selection strategy.
Phenotypic dominance of P. sajor-caju has been observed over C. indica as the all six hybrid strains show more phenotypic resemblance to the parent P. sajor-caju, however, in the molecular study, the hybridity of the six strains have been confirmed. Previous studies suggest that the phenotypic character of one of the parent strains may be expressed in hybrid strains (Chakraborty and Sikdar 2008, 2010; Mallick and Sikdar 2014). This phenotypic resemblance of the hybrids with P. sajor-caju may be either due to genomic dominance of P. sajor-caju over C. indica or may be due to absence of C. indica chromosomes in the hybrids needed for expression of fruitbody phenotypic character.
A barrage is a demarcation zone that is usually seen at the interface of genetically different fungi. This phenomenon is due to the heterokaryon incompatibility system which helps in non-self recognition and hence prohibits genetically different nuclei to exist in the same cytoplasm during the asexual phase of life cycle (Micali and Smith 2003). Thus nuclear exchange during growth of two mycelia into each other is inhibited by barrage formation (Glass et al. 2000). In our experiment barrage can be seen between mycelia of parents and hybrid strains (Fig. 1f). In addition, clamp connections are also noted in all the hybrid lines. The barrage reaction and clamp connection confirmed that the hybrid lines were genetically diverse from the parent strains.
It was observed that five ISSR primers exhibited dissimilar banding profiles and neither of the hybrid strains represents the sum total of parental banding profiles. The ISSR profile analysis of the hybrids and the parents depicted that hybrids contained some non-parental bands along with parental bands. This indicates recombination might have occurred in the hybrid genome and hence the hybrid nature of the newly developed lines was confirmed. This finding indicates that the hybrid strains were synkaryon formed after protoplast fusion with subsequent chromosome elimination and somatic recombination. The possibility of the hybrids of being heterokaryons of the two parental nuclei can be excluded (Primrose, 1987). Based on the ISSR profiling dendrogram was generated, which partially demonstrates that the hybrid lines were phylogenetically close to the parent P. sajor-caju while C. indica seemed to be genetically distinct from their hybrid progeny.
Substrate selection for the cultivation of mushroom fruit body depends on the availability and cost of the substrate. For growing oyster mushrooms many agricultural, forest and agro-industrial by-products rich in cellulose, lignin and hemicellulose have been proved to be useful. The substrate should be fresh, dry, free from mold infestation and properly stored, as the yield of mushrooms largely depends on these qualities of these substrate. In most of the cases, paddy straw can be used for cultivation of mushrooms, but they can grow profusely in lignocellulose biomass from coconut brunch, leaf, leaf stalk and coir pith (Thomas et al. 1998). Here we used Sorghum grain for the production of spawn packets while paddy straw was used as substrate for the fruit body. A Shorter life cycle and highest weight gain was observed in the hybrid APS-4, whereas in APS-5 and APS-3 similar yield was seen.
We also studied Biological efficiency (BE%), frequently used in mushroom industries to describe the yield potential of mushrooms. The important role of hybridization is to improve the strain quality by increasing BE significantly. Also, the Biological efficiency (BE) of mushroom samples varies significantly with the substrate.
Nutritional analysis
Environmental factors like temperature, humidity, pH, light, etc. also have an impact on the nutritional components of the mushrooms. Carbohydrates in the mushrooms are one of the major components which provide energy. Carbohydrates are present in digestible and nondigestible forms (Vaz et al. 2011). In mushrooms digestible forms like mannitol, glucose and glycogen are present in smaller amount, while non digestible forms of carbohydrates like oligosaccharides and non-starch polysaccharides (such as chitin, β-glucans etc.) are dominant (Cheung 2010). Non-digestible carbohydrates contribute as crude fibers which play an important role in normal functioning of digestive system and also have been proved beneficial in lowering blood glucose and cholesterol level (Wang et al. 2014).
In our experiment we found carbohydrate content as less than 10% of total dry sample in all species including parent strains. We found that P. sajor-caju contains 3.37 g of carbohydrate. This result is close to a previous study (Rashidi et al. 2016). The carbohydrate content of another parent C. indica is almost similar to an,earlier experiment (Alam et al. 2008).
Mushrooms are also rich in proteins, hence mushrooms are said to be “poor man’s protein”. Mushroom proteins exhibit higher protein value than other plant proteins. Protein content of the mushrooms not only depend upon environmental conditions but also on the stages of growing mushrooms and species (Ayaz et al., n.d.). Mushrooms consist of 12–29.3% of protein (Wang et al. 2014), while some of the researchers have claimed to observe higher protein content up to 54–59% (Barros et al. 2008). It was reported earlier that the protein content was about 7.3–7.6 mg/g in C. indica which was grown on a different substrate (Saranya et al. 2011). We observed that the Protein content of the strains we studied ranged from 1.17 to 3.36 g/100 g on a dry weight basis.
The fat content in mushrooms is very low and hence are preferred in diabetic patients. According to the earlier reports mushrooms contain less than 1% to as high as 15–20% of its dry weight with 2–8% of fat as an average range (Sánchez 2004). Due to low fat content, mushrooms are considered as very beneficial in patients with heart diseases (Gregori et al. 2007). Fat content in different species of mushrooms ranged from 1.1 to 8.3% in a dry weight basis and it represents all classes of lipid compounds including free fatty acids, monoglycerides, diglycerides, triglycerides, sterols, sterol esters and, phospholipids (Miles and Chang 2004). Now crude fat content of hybrid strains ranged between 1.13 and 2.02 g/100 g of dry weight, which is lower than parent strains and hence are favorable for diabetic patients.
Ash is the remaining inorganic residue after the separation of water and organic matter in the presence of oxidising agents and this inorganic residue is considered to be rich in minerals. In the above experiment, we found that the ash content of the studied strains ranges from 0.91 g to 1.25 g/100 g of dry weight. Previous study results were almost similar to this result (Shin et al. 2007; Dundar et al. 2008).
The nutritional content of mushrooms depends upon various factors like atmospheric condition, variation in species, age of the species, including growing region, type of substratum, etc. These all can affect the nutritional composition, accumulation of metal ions and water content (Vetter 1993; Rudawska and Leski 2005). We observed a good nutritional value of hybrid strains cultivated in the farm. These have been found to be rich in proteins, carbohydrates and minerals. In addition, its low-fat content makes it healthy food, hence these can be valuable for diabetics and malnourished people.
Conclusion
Edible mushrooms are known for their high nutritional values due to which they are being preferred nowadays over most of the other food items. In the present study, we used two edible mushrooms to generate six intergeneric hybrid strains. The nutritional values, sensory properties and biological efficiencies of the hybrid mushrooms are diverse from each other and these variations might have happened due to chromosomal alterations or somatic crossing over leading to genetic variations. We used distinct parameters to look over the effectiveness of hybrid mushrooms over their parents. Based on the results of the composition of proximate analysis, it can be concluded that hybrid mushrooms are rich in proteins and have very low-fat content. Thus, these can play a good role in becoming part of a healthy balanced diet especially for diabetics. Due to ease in their cultivation, they can also contribute to the rural economic development. Hence, further nutraceutical and biological evaluation is needed to be done.
Abbreviations
- PEG:
-
Polyethylene glycol
- PDA:
-
Potato Dextrose Agar
- MYG:
-
Maltose +Yeast + Glucose
- IOA:
-
Iodoacetamide
- FDA:
-
Fluorescein diacetate
- BE:
-
Biological efficiency
- ISSR:
-
Inter Single Sequence Repeat
- PIC:
-
Polymorphic information content
- UPGMA:
-
Unweighted pair-group method with mathematic averages
References
Abe M, Umetsu H, Nakai T, Sasage D (1982) Regeneration and fusion of mycelial protoplasts of Tricholoma matsutake. Agric Biol Chem 46:1955–1957
Adams DH, Roth LF (1967) Demarcation lines in paired cultures of Fomes cajanderi as a basis for detecting genetically distinct mycelia. Can J Bot 45:1583–1589
Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee TS (2008) Nutritional analysis of cultivated mushrooms in Bangladesh-Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 36:228–232
Anne J, Peberdy JF (1976) Induced fusion of fungal protoplasts following treatment with polyethylene glycol. Microbiology 92:413–417
Ayaz, F.A., Torun, H., Özel, A., Col, M., Duran, C., Sesli, E., Colak, A., n.d. Nutritional value of some wild edible mushrooms from Black Sea Region (Turkey). Turk J Biochem 9.
Barros L, Venturini BA, Baptista P, Estevinho LM, Ferreira IC (2008) Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. J Agric Food Chem 56:3856–3862
Bornet B, Branchard M (2001) Nonanchored inter simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Mol Biol Report 19:209–215
Breene WM (1990) Nutritional and medicinal value of specialty mushrooms. J Food Prot 53:883–894
Chakraborty U, Sikdar SR (2010) Intergeneric protoplast fusion between Calocybe indica (milky mushroom) and Pleurotus florida aids in the qualitative and quantitative improvement of sporophore of the milky mushroom. World J Microbiol Biotechnol 26:213–225
Chakraborty U, Sikdar SR (2008) Production and characterization of somatic hybrids raised through protoplast fusion between edible mushroom strains Volvariella volvacea and Pleurotus florida. World J Microbiol Biotechnol 24:1481–1492
Chang ST (1980) Mushrooms as human food. Bioscience 30:399–401
Cheung PCK (2010) The nutritional and health benefits of mushrooms. Nutr Bull 35:292–299
Cunniff, P., 1995. Official methods of analysis. Assoc. Off. Anal. Chem. AOAC 16th Ed Arlingt. Va. USA.
Danell E, Eaker D (1992) Amino acid and total protein content of the edible mushroom Cantharellus cibarius (Fries). J Sci Food Agric 60:333–337
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Report 1:19–21
Djajanegara I, Masduki A (2013) Protoplast fusion between white and brown oyster mushrooms. Indones J Agric Sci 11:16–23
Dundar, A., Acay, H., Yildiz, A., 2008. Yield performances and nutritional contents of three oyster mushroom species cultivated on wheat stalk. Afr. J. Biotechnol. 7.
Eyini M, Rajkumar K, Balaji P (2006) Isolation, regeneration and PEG-induced fusion of protoplasts of Pleurotus pulmonarius and Pleurotus florida. Mycobiology 34:73–78
Glass NL, Jacobson DJ, Shiu PK (2000) The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu Rev Genet 34:165–186
Gregori A, Švagelj M, Pohleven J (2007) Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol Biotechnol 45:238–249
He P, Yu M, Wang K, Cai Y, Li B, Liu W (2020) Interspecific hybridization between cultivated morels Morchella importuna and Morchella sextelata by PEG-induced double inactivated protoplast fusion. World J Microbiol Biotechnol 36:58
Heleno SA, Barros L, Sousa MJ, Martins A, Ferreira IC (2010) Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem 119:1443–1450
Illing GT, Normansell ID, Peberdy JF (1989) Protoplast isolation and regeneration in Streptomyces clavuligerus. Microbiology 135:2289–2297
Kim C, Choi E-C, Kim B-K (1997) Characteristics of artificial hybrids between Lentinula edodes and Coriolus versicolor. Arch Pharm Res 20:384–386
Lalithakumari, D., Mrinalini, C., Chandra, A.B., Annamalai, P., 1996. Strain improvement by protoplast fusion for enhancement of biocontrol potential integrated with fungicide tolerance in Trichoderma spp./Stammverbesserung durch Fusion der Protoplasten von Trichoderma-Arten zur Steigerung der Leistungsfähigkeit in der biologischen Bekämpfung integriert mit Fungizidtoleranz. Z. Für Pflanzenkrankh. PflanzenschutzJournal Plant Dis. Prot. 206–212.
Mallick P, Sikdar SR (2014) Production and molecular characterization of somatic hybrids between Pleurotusflorida and Lentinula edodes. World J Microbiol Biotechnol 30:2283–2293
Micali CO, Smith ML (2003) On the independence of barrage formation and heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 38:209–219
Miles PG, Chang S-T (2004) Mushrooms: cultivation, nutritional value, medicinal effect, and environmental impact. CRC Press
Peberdy, J.F., Fox, H.M., 1993. Protoplast technology and edible mushrooms, in: Genetics and Breeding of Edible Mushrooms. Overseas Publishers Association NV, pp. 125–155.
Porselvi, A., Vijayakumar, R., n.d. Strain improvement of Pleurotus eous and Pleurotus florida by protoplast fusion.
Primrose, S.B., n.d. Cell and Enzyme Immobilization, Modern Biotechnology, Chapter 7. Blackwell Scientific Publications (Oxford, 1987).
Rashidi M, Nuran A, Yang TA (2016) Nutritional and antioxidant values of oyster mushroom (P. sajor-caju) cultivated on rubber sawdust. Int J Adv Sci Eng Inf Technol 6:161–164
Rudawska M, Leski T (2005) Macro-and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem 92:499–506
Sánchez C (2004) Modern aspects of mushroom culture technology. Appl Microbiol Biotechnol 64:756–762
Saranya V, Madhanraj P, Panneerselvam A (2011) Cultivation, composting, biochemical and molecular characterization of Calocybe indica (C and A). Asian J Pharm Res 1:55–57
Shin CK, Yee CF, Shya LJ, Atong M (2007) Nutritional properties of some edible wild mushrooms in Sabah. J Appl Sci 7:2216–2221
Sumathy R, Kumuthakalavalli R, Krishnamoorthy AS (2015) Proximate vitamin, aminoacid and mineral composition of milky mushroom, Calocybe indica (P&C). Var. Apk2 commonly cultivated in Tamil Nadu. J Nat Prod Plant Resour 5:38–43
Sweeney RA, Rexroad PR (1987) Comparison of LECO FP-228" nitrogen determinator" with AOAC copper catalyst Kjeldahl method for crude protein. J Assoc Off Anal Chem 70:1028–1030
Thomas GV, Prabhu SR, Reeny MZ, Bopaiah BM (1998) Evaluation of lignocellulosic biomass from coconut palm as substrate for cultivation of Pleurotus sajor-caju (Fr.) Singer. World J Microbiol Biotechnol 14:879–882
Vaz JA, Barros L, Martins A, Santos-Buelga C, Vasconcelos MH, Ferreira ICFR (2011) Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem 126:610–616. https://doi.org/10.1016/j.foodchem.2010.11.063
Vetter J (1993) Toxic elements in certain higher fungi. Food Chem 48:207–208
Wang X-M, Zhang J, Wu L-H, Zhao Y-L, Li T, Li J-Q, Wang Y-Z, Liu H-G (2014) A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem 151:279–285
Weir, B.S., n.d. Sinauer Associates, Inc; Sunderland, MA (1996) Data Anal. II Methods Discrete Popul. Genet, DataGoogle Sch
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Yoo, Y.B., Lee, K.H., Kim, B.G., 2002. Characterization of somatic hybrids with compatible and incompatible species by protoplast fusion in genera Pleurotus (Fr.) P. Karst. and Ganoderma P. Karst. by RAPD-PCR analysis. Int. J. Med. Mushrooms 4.
Acknowledgment
We sincerely acknowledge the financial support by Rajiv Gandhi National Fellowship, University Grants Commission (F1-17.1/2013-14/RGNF-2013-14-SC-WES-54351/SA-III/Website). We are also grateful to Bose Institute, Kolkata for instrumental and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, P., Sikdar, S.R. & Samanta, A. Nutritional analysis and molecular characterization of hybrid mushrooms developed through intergeneric protoplast fusion between Pleurotus sajor-caju and Calocybe indica with the purpose to achieve improved strains. World J Microbiol Biotechnol 37, 69 (2021). https://doi.org/10.1007/s11274-021-03032-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03032-3