Abstract
In this study, experiments were conducted to isolate, characterize, and evaluate rice rhizosphere bacteria for their arsenic (As) tolerance ability and zinc (Zn) solubilization potential in culture media and soil. Among 20 bacterial isolates recovered, six were found to solubilize inorganic Zn salt(s) efficiently under in vitro culture conditions. 16S rRNA gene sequence-based phylogenetic analysis indicated the affiliation of efficient Zn solubilizing bacteria (ZSB) to Burkholderia vietnamiensis and Burkholderia seminalis. Zinc solubilizing efficiency (ZSE) of the bacteria varied with the concentrations and types of Zn salts used in the experiments. Increasing trend in ZSE of the bacteria was noticed when the percentage of ZnO increased from 0.1 to 0.5 but the same decreased at 1.0%. Increased Zn solubilization was noticed when bacteria were incubated with lower concentration of Zn3(PO4)2 and ZnCO3. In general, Zn solubilization increased with increasing incubation time in lower volume medium, while some isolates failed to solubilize one or more tested Zn salts. However, enriched concentrated cells of the ZSB in glucose amended medium with 0.5% ZnO showed an increasing trend of Zn solubilization with time and were able to solubilize more than 300 mg/L Zn. This increased rate of Zn release by the ZSB was attributed to marked decline in pH that might be due to the enhanced gluconic acid production from glucose. As evident from the decreased ZSE of the bacteria in the presence of As(V) in particular, it seems arsenic imparts a negative effect on Zn solubilization. The ZSB were also able to increase the rate of Zn release in soil. A microcosm-based soil incubation study amending the enriched bacteria and 0.5% ZnO in soil showed an elevated level of both water-soluble and available Zn compared to un-inoculated control. During Zn solubilization in microcosms, viable cells in terms of colony-forming unit (CFU) declined by the same order of magnitude both in the presence and absence of ZnO that might be due to the nutrients limiting condition aroused during the incubation period rather than Zn toxicity. The bacteria in this study also exhibited plant growth promoting traits, such as growth in nitrogen-free medium, production of indole acetic acid (IAA), and solubilization of potassium and phosphate. Our findings suggested that Burkholderia spp. could be the potential candidates for enhancing Zn dissolution in the soil that might reduce the rate of inorganic Zn fertilization in agricultural soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn), an essential element is required in minute quantities for the growth and nutrition of plants and animals. It regulates the structure and functions of various proteins and enzymes in biological systems (Andreini et al. 2009). Plants get zinc from the soil, and animals obtained this primarily from zinc laden plant parts when consumed as food. Thus, a limited supply of available Zn in soil and thereby reduce accumulation in food crops creating Zn deficiency in both plants and animals (Dinesh et al. 2018). Globally, zinc deficiency in agricultural soils is becoming a vital factor that has resulted in reduced productivity of crops with impaired nutritional quality (Hotz and Brown 2004). Consumption of Zn deficient rice causing critical health problems in a large number of people in the countries where rice stands as a principal commodity and serves as a staple food (Wu et al. 2019).
Total Zn content in normal soils has been estimated that varied from 10 to 300 mg kg−1 (Sharma et al. 2013). The total Zn content in various Indian soils ranged between 30 and 75 mg kg−1 (Katyal and Sharma 1991). But literature show very low level of available Zn (4.0–270.0 μg L−1) in soils compared to the mean total Zn (64.0 mg kg−1) (Alloway 2009). Values in the report indicate that the critical limit of Zn in soil is low (< 1.0 mg kg−1); medium (1.0–2.5 mg kg−1) and high (> 2.5 mg kg−1) (Kanwar 1973). Ratan and Shukla (1984) set the critical limits of Zn deficiency and toxicity for rice soil which are 0.39, and 12 mg/kg with DTPA, and 0.78 and 12 mg kg−1 with HC1 (0.05 N), respectively. In agricultural soil, Zn is present in sufficient quantity; however, a significant fraction is unavailable to plant. During the cultivation of rice under flooded condition, the applied fertilizer as zinc sulfate readily forms insoluble complexes and remain unavailable for plant uptake (Zhang et al. 2017; Khanghahi et al. 2018). Also, the exogenous application of chemical fertilizers creates a huge environmental burden (Dinesh et al. 2018). Agricultural practice needs to be carried out in a sustainable way to overcome emergent adverse situations. Past agricultural practices mainly focused on increasing the crop yield without paying much attention to the decreasing mineral concentration in soil. Therefore, an increase in mineral content in the soil as well as in the staple food crops thought to be the promising approach towards the improvement of public health against malnutrition in Zn deficient areas (Shakeel et al. 2015; Kamran et al. 2017; Khanghahi et al. 2018).
The ability of conversion of insoluble zinc into plant accessible soluble form is a plant growth-promoting (PGP) trait exhibited by many bacteria (Kamran et al. 2017; Othman et al. 2017). Also, Zn biofortification of food crops with the help of ZSB is a promising strategy for the eradication of Zn deficiency. Thus, several ZSB are characterized from various soil environments to provide the plant with the soluble form of Zn (Sharma et al. 2012; Gandhi and Muralidharan 2016). Reports based on in vitro study show that the members of the genera Azotobacter, Azospirillum, Acinetobacter, Bacillus, Burkholderia, Gluconacetobacter, Pseudomonas, and Thiobacillus have potential to solubilize Zn (Saravanan et al. 2007; Bapiri et al. 2012; Vidyashree et al. 2016). These microorganisms have proven their potential to improve crop quality using their PGP traits, like nutrient solubilization, nitrogen fixation, and exopolysaccharides and siderophores production (Ahmed et al. 2011). Thus, in recent years, significant attention has been paid to bacteria with PGP traits to replace the burden of chemical fertilizers and also to understand their dynamics, diversity, and beneficial as well as cooperative roles towards agriculture (Gray and Smith 2005; Figueiredo et al. 2008). Studies also conducted to mobilize Zn into various crop plants using ZSB such as in soybean, maize, wheat, green gram, turmeric, etc. (Sharma et al. 2012; Ramesh et al. 2014; Shaikh and Saraf 2017; Mumtaz et al. 2017; Dinesh et al. 2018). Although rice is a staple food worldwide and has a better possibility to eradicate Zn deficiency, only a few reports are available on the role of ZSB in mobilizing Zn in rice plants (Shakeel et al. 2015; Krithika and Balachandar 2016; Othman et al. 2017; Gontia-Mishra et al. 2017).
Given these facts, the main objective of this study was to isolate an array of ZSB from paddy soil rich in arsenic (As) content and characterize them for their Zn solubilization potential in culture media and soil. Arsenic is the potential contaminant of agricultural land that entered the rice field during the irrigation of arsenic laden groundwater. Since there is a chance of reducing the zinc solubilization activity in the presence of arsenic, we isolated ZSB that could tolerate the toxicity of As and continued their performance. As the Zn solubilization attribute of bacteria in soil is an unusual characteristic, we also tested the ability of the bacteria to mobilize Zn in soil (Dinesh et al. 2018). Beside, bacteria were further tested for their multi-tasking PGP abilities that included solubilization of phosphorus and potassium, and production of IAA.
Materials and methods
Soil sampling and analysis
Soil samples were collected from rice root rhizosphere from rice fields (23° 16′ 48.54″ N; 88° 23′ 6.7416″ E) of Nadia district, West Bengal where deep irrigation was practiced. A preliminary survey was performed for As rich rice field by collecting soil samples randomly and analyzing by atomic absorption spectrophotometer (AAS) (ThermoFisher Scientific ICE 3300). From the potential As rich rice field, rice plants in the late tillering stage were uprooted and soil adhering to the root surface was collected aseptically. The collected soils were then immediately placed in sterile polythene bags and taken to the laboratory for analysis and isolation of ZSB and stored at 4 °C for further study.
The pH, as well as conductivity of the soil samples, was measured using standard procedure (Hendershot et al. 2007). Available soil organic carbon was estimated by the Walkley–Black chromic acid wet oxidation method and available phosphorus by the Bray method. Concentrations of different anions like (F−, Cl−, Br−, NO3−, PO43− and SO42−) were measured using ion chromatography (Thermo Scientific Dionex Aquion) and the available metals like Nickel, Zinc, Copper, Chromium, Iron, and Arsenic were measured in mg/kg using AAS.
Isolation, screening and characterization of the isolates
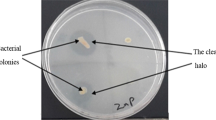
Enrichment technique was used to isolate the bacteria from soil samples. Aliquots of soil sample (4 g) was inoculated in nitrogen-free sterile medium (100 ml) with the following composition (g/L): (K2HPO4: 0.8; KH2PO4: 0.8; MgSO4.7H2O: 0.2; MnSO4.4H2O: 0.002; NaCl: 0.1; Na2MoO4.2H2O: 0.002; Na-Vanadate: 0.002; FeCl3: 0.01; Mannitol: 15; CaCl2.2H2O: 0.02; Sucrose: 20) in Erlenmayer flask (Chakraborty et al. 2017). Following incubation at 30 °C on an orbital shaker for 7 days, enriched culture was transferred (1%, v/v) into the same fresh medium and incubated further in the same condition. The latter well-grown enrichment culture was serially diluted in mineral salt medium (MSM) (g/L): (Dextrose: 10.0; (NH4)2SO4: 1.0; KCl: 0.2; K2HPO4: 0.1; MgSO4: 0.2; pH: 7.0) and streaked on the surface of the same MSM agar plate and incubated at 30 °C. Colonies that appeared on the MSM agar surface were randomly picked and purified by repeated streaking onto the same medium surface. The ZSB were screened by streaking the pure culture on the MSM agar plate supplemented with 0.1% of the pre-sterile insoluble Zn salts (ZnO, Zn3(PO4)2 and ZnCO3), separately and incubated at 30 °C for 7 days (Gontia-Mishra et al. 2017). Pure cultures that exhibited clearing zone around the colony were taken for further characterization and preserved at –20 °C with 15% glycerol following growth in MSM broth.
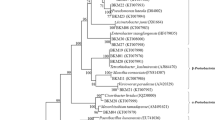
Beside phenotypic characterization, pure cultures were phylogenetically characterized by 16S rRNA gene analysis. Genomic DNA was extracted from each well-grown pure culture using the Purelink genomic DNA isolation kit (Invitrogen). Extracted genomic DNA was used in a polymerase chain reaction (PCR) for amplification of 16S rRNA gene using 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) primers following the PCR reaction and thermal cycle regime as described by Chakraborty and Islam 2018. Cloned PCR products in the pGEM-T vector were sequenced commercially. Obtained 16S rRNA gene sequences were compared with sequences in GenBank database using BLAST. Phylogenetic analysis was performed with MEGA 4.0 software by neighbor-joining method incorporating Jukes-Cantor distance correction. Bootstrap analysis was performed with 1000 replication and > 50% bootstrap values are indicated at the nodes. Obtained sequences were submitted in the GenBank database with the accession number MN263845-MN263850.
Determination of Zn solubilizing efficiency of the bacteria
Zn solubilization efficiency of the bacteria was tested on MSM agar supplemented separately with different concentrations (0.1%, 0.5%, and 1%) of the Zn salts (ZnO, ZnCO3 and Zn3(PO4)2) (Fasim et al. 2002).
Required quantities of each Zn salt, separately wrapped in aluminium foil, were sterilized and mixed with molten sterilized MSM agar just before the pouring of the plates. Zinc solubilization efficiency in the presence of arsenic was also tested by using ZnO (0.3%) amended MSM agar plate supplemented with either As(III) (1.0 mM) or As(V) (5 mM). Well grown liquid culture of the bacteria in MSM were spot inoculated by drop-casting on the surface of the MSM agar plate and incubated at 30 °C in dark to observe zone of clearing around the colonies. After 7 days of incubation, both diameters of the cleared zone and the respective colony were recorded. Zinc solubilization efficiency was calculated as ZSE% = (diameter of the clearing zone/diameter of the colony) × 100 (Gonita-Mishra et al. 2017).
Quantitative assay of Zn-solubilization in the liquid medium
Quantitative Zn solubilization ability of the bacteria was performed in liquid MSM in presence of (0.1%) each insoluble Zn compounds ZnO, Zn3(PO4)2 and ZnCO3. In 10 ml MSM equal amount of cells (~ 108) of the bacteria were used in each treatment. Similarly, the same amount of each Zn salts was also incubated in the absence of bacteria as a control. All the treatments and controls in triplicates were incubated at 30 °C in shaking condition (150 rpm). Destructive sampling was performed, and in each time point, two milliliteres of each culture were taken for determination of pH and analysis of soluble Zn by AAS following centrifugation at 13,000 × g for 10 min at room temperature and filtration by 0.22 μm filter paper.
Zinc solubilization with enriched culture and influence of glucose
Solubilization of ZnO was studied in more detail in presence and absence of glucose with the addition of enriched bacteria at the onset of experiment. Bacteria were first enriched in MSM in absence of ZnO. Well grown cells were harvested by centrifugation and concentrated in MSM. For each bacterium, the experiment was conducted in 100 ml Erlenmeyer flasks containing 45 ml of sterile MSM in two sets, with and without glucose. Sterile ZnO (0.1%) was added to each flask and subsequently inoculated with concentrated cells (~ 108 CFU/ml). Uninoculated Erlenmeyer flasks containing sterile MSM amended with ZnO but with and without glucose were taken as the controls. For all bacteria, triplicate experiments were conducted and incubated at 30 °C in shaking condition (150 rpm) for 18 days. 2-ml aliquots of each treatment were sampled from the Erlenmeyer flasks at various time intervals, and following centrifugation and filtration solubilized Zn, pH and organic acids were measured in the supernatant. Organic acid content was determined by Dionex, Aquion ion chromatography system (Thermo Fisher Scientific) equipped with an IonPac™ AS23 column (4 × 250 mm), an AERS™ 500 carbonate suppressor, and a conductivity detector.
Microcosm study
Zinc solubilization ability of the bacteria in soil was tested by setting up laboratory microcosms. Details of soil used in the microcosm and the sampling procedure are available in the previous report by Chakraborty et al. 2017. An aliquot of soil sample (100 g) was taken into each 250 ml screw-capped glass jar and various microcosm sets were prepared as follows, (i) microcosms with only soil, (ii) microcosms with soil + ZnO (0.5%), (iii) microcosms with soil + added bacteria, and (iv) microcosms with soil + ZnO (0.5%) + added bacteria. All the glass jars containing soil were sterilized before the addition of ZnO and bacterial cultures. Separately sterilized ZnO was mixed with the respective treatments. Each of the bacterial isolates was enriched in Luria Bertani broth; cells were aseptically harvested by centrifugation at 10,000×g for 5 min, washed twice in sterile saline (0.9%) to remove any leftover medium and used in microcosm. Microcosms were inoculated with similar numbers (~ 108–109 cells/g soil) of each cell, separately. An equal amount of sterile water was added to each treatment to flood the soil and mixed well. The triplicate set of each treatment was incubated for two months at 30 °C. Aliquots of soil were withdrawn at different time intervals (15 days, 1 month, and 2 months) and analyzed for water and DTPA extractable Zn and viable cells in terms of colony-forming units (CFU). For CFU count, part of the microcosm soil sample (0.25 g) was resuspended in 5 ml sterile saline, serially diluted and applied onto the LB agar plates and incubated at 30 °C. Available Zn was extracted from 1.0 g air-dried soil sample using 2 ml DTPA extractant according to Katyal and Sharma 1991. For water-extractable Zn, 1.0 g soil sample was resuspended in 2 ml water and shaken for 2 h. Following centrifugation at 13,000×g for 10 min, the supernatant of both water and DTPA extracts were taken and Zn was estimated by AAS.
Determination of pH
The pH of the culture supernatant and the soil sample from each treatment before and after incubation was monitored. Aliquots of bacterial culture were centrifuged and the pH of the supernatant was determined by using the glass electrode. Soil pH was measured with the standard CaCl2 method (Hendershot et al. 2007). An aliquot (10 g) of air-dried soil sample was resuspended in 20 ml freshly prepared 0.01 M CaCl2. The suspension was stirred occasionally for 30 min then the entire setup was left undisturbed for at least 1 h to make the content out distinctly into two layers. Without disturbing the bottom sediment, pH probe was immersed carefully into the clear supernatant at top and pH was recorded.
Zinc tolerance study
Tolerance towards Zn by each of the isolates was performed in MSM agar amended with different concentrations of ZnCl2 according to Bhakat et al. 2019. Cultures were streaked on the MSM agar surface in Petri plates and incubated at 30 °C for a week and observed for the appearance of colonies.
Determination of arsenic tolerance by the isolates
Arsenic tolerance ability of the bacteria was studied in liquid MSM. Bacteria were allowed to grow in MSM in presence of different concentrations of arsenite (0.5–2.0 mM) and arsenate (5.0–50.0 mM) of sodium salts and incubated at 30 °C under shaking condition for 7 days according to Bhakat et al. 2019. The presence or absence of the bacterial growth was monitored every 24 h by measuring absorbance at 660 nm on a spectrophotometer and cross-checked by spot inoculation on LB agar medium for the viable cell. The result was expressed as the minimum inhibitory concentration (MIC), the least concentration of the As that completely inhibit the bacterial growth.
Determination of plant-growth promoting (PGP) traits
Phosphate (P) and potassium (K) solubilization
Phosphate and K solubilization were tested by spot inoculating the well grown (24 h) culture of each isolate on Pikovskaya's agar medium and Aleksandrov medium, respectively (Pikovskaya 1948; Hu et al. 2006). The plates were incubated at 30 °C for 7 days and were checked for the appearance of the hallow zone around the colony. The SE of P and K, in percentage, were calculated as (diameter of solubilization/diameter of the colony) × 100%.
Indole acetic acid (IAA) production
To check the production of IAA, the bacteria were allowed to grow in the presence and absence of (0.5 mg/ml) L-tryptophan in Luria Bertani medium following the method of Sawar and Kremer (1995). Simultaneously un-inoculated control sets were also prepared. Following incubation, at 30 °C under shaking condition for a week cultures were centrifuged at 8000×g for 10 min. Then 2 ml of the supernatant was taken and to it 4 ml of freshly prepared Salkowski’s reagent (50 ml of 35% perchloric acid and 1 ml 0.5 M FeCl3 solution) was added with the further addition of 25 µl of orthophosphoric acid and was mixed thoroughly. The mixture was incubated in dark at room temperature for 25 min for the development of pink colour which indicates the presence of IAA.
Other PGP traits
A preliminary screening test for the ability of the bacteria to grow in nitrogen deprived condition was tested according to Goswami et al. 2015 by allowing the bacteria to grow in nitrogen-free medium ((g/L), Sucrose: 10; K2HPO4: 0.6; MgSO4: 0.20; NaCl: 0.2; K2SO4: 0.1; CaCO3: 2.0; pH 6.8) supplemented with 0.5% bromothymol blue. Nirogen-fixing bacteria increase the pH of the medium which change the medium colour from green to blue. Siderophore production by the bacteria was tested on CAS-agar according to Louden et al. (2011). King’s B medium and chrome azurol S (CAS-HDTMA) solution were separately prepared and sterilized. 100 ml of CAS-HDTMA solution was added into 900 ml sterilized King’s B medium drop wise and gently mixed with molten agar. Bacteria were spot inoculated on the prepared CAS agar plates and incubated at 30 °C for 3–4 days to allow the formation of orange-pink colour around the colonies.
Statistical analysis
All statistical calculation was performed with OriginPro 8.0. One-way ANOVA analysis was performed at the p = 0.05 level to find out the significant differences between the means. Unless otherwise mentioned, we used triplicate data to estimate statistical significance.
Results
Isolation of potential ZSB
From soil sample, microorganisms were successfully enriched in nitrogen free medium. From this enriched culture total twenty colonies were recovered through spread plate technique using MSM agar. Among the colonies that exhibited zone of clearance around them on insoluble Zn salt-amended MSM-agar plate were taken for further study (Supplementary Fig. S1). Six isolates, that showed different extent of Zn solubilization were purified, charcterized and named as EIKU12, EIKU13, EIKU14, EIKU15, EIKU16 and EIKU17. The physicochemical properties of the collected soil, from which the bacteria were isolated, are presented in supplementary Table S1.
Phenotypic and molecular characterization
Gram staining followed by light microscopy showed that the isolated bacteria were gram-negative, short rods and arranged in single or pairs. 16S rRNA gene sequencing followed by similarity search in the NCBI nucleotide database indicated the relatedness of the six ZSB to class β-proteobacteria. Phylogenetic analysis incorporating sequence of the same gene of type strains from the GenBank database indicated all the six isolates were affiliated with Burkholderia spp. All the isolates showed more than 99% 16S RNA gene sequence identity with Burkholderia vietnamiensis LMG 10,929 (CP009632.1) reported from rice rhizosphere and Burkholderia seminalis R-2419 (Fig. 1).
Zn-solubilization in culture media and change of pH
The percent SE of different Zn-salts by the ZSB was calculated and is presented in the Fig. 2. Except EIKU15, which did not show Zn solubilization in 0.5% ZnCO3, all the isolates registered more than 110% ZSE in all 0.1% and 0.5% insoluble Zn-salt amended plates. Isolate EIKU13 showed highest SE (377%) at 0.5% ZnO and effectively (227%) solubilized 0.1% Zn3(PO4)2. EIKU17 showed the highest Zn solubilization (356%) with 0.1% ZnO. EIKU15 performed well both in 0.1% and 0.5% ZnO. Three isolates EIKU13, EIKU14 and EIKU17 showed more than 200% ZSE at 0.1% Zn3(PO4)2, while EIKU12 at 0.1% ZnCO3. The trend of Zn solubilization by the isolates in presence ZnCO3 and Zn3(PO4)2 was decreased with the increasing concentration of the Zn-salts from 0.1 to 1%. But in presence of ZnO, the same was found to increase from 0.1 to 0.5% but decrease at 1.0% ZnO.
ZSE of the bacteria was also tested in presence of arsenic on 0.3% ZnO amended MSM agar. Compared to control, in presence of either 1 mM As(III) or 5 mM As(V), ZSE decreased for all the bacteria (Fig. 3). More decreased ZSE was noticed in As(V) amended plate compared to As(III) amended plate.
The ability of the ZSB to solubilize Zn was quantitatively determined while growing in liquid media and the data is presented in Fig. 4. Zn solubilization study was performed with 0.1% insoluble Zn salts because all the isolates showed more than 100% ZSE with this concentration. In liquid culture all the test bacteria were found to solubilize ZnCO3. Isolate EIKU12, EIKU13, and EIKU14 solubilized all the tested Zn salts. EIKU15 and EIKU17 failed to solubilize ZnO, while EIKU16 and EIKU17 were unable to solubilize Zn3(PO4)2. Amount of soluble Zn was found to increase with increasing incubation time among all bacteria in presence of respective Zn salts. Different bacteria exhibited different levels of Zn solubilization capability with different Zn salts. Zinc solubilization in liquid culture was also found to be accompanied by change in pH of the culture medium (Table 1). In general, lowering of pH was observed during solubilization of tested Zn-salts. The major drop in pH of culture medium was found during solubilization of ZnCO3 while maximum drop in pH was recorded for the isolate EIKU12, EIKU14 and EIKU16. Very little fall in medium pH was recorded during solubilization of ZnO. Pearson product moment correlation analysis between amount of solubilized Zn and medium pH showed the correlation coefficient value of –0.745, 0.936 and –0.926 with the p value of 0.089, 0.006 and 0.008 while solubilizing ZnO, Zn3(PO4)2 and ZnCO3, respectively. Significant negative correlation (p < 0.05) was only observed with the solubilization of ZnCO3. This analysis indicates that the pH of culture medium was only inversely proportional to the amount of soluble Zn while solubilizating ZnCO3 but not with ZnO and Zn3(PO4)2.
Zinc solubilization mechanism and influence of glucose
To understand the probable mechanism of Zn solubilization by the bacteria and the influence of glucose therein if any, ZnO solubilization was investigated in MSM in the presence and absence of glucose. As some of the isolates (EIKU15 and EIKU17) failed to solubilize ZnO when it added at the onset of culture, cells were first enriched with MSM in absence of ZnO, harvested, resuspended in MSM and exposed to ZnO. In seven days EIKU12, EIKU13, EIKU14 and EIKU16 solubilized more than 300 mg/L Zn in glucose amended medium (Fig. 5). Except with EIKU12, in presence of glucose, Zn solubilization was gradually increased up to 18 days of incubation for other isolates and maximum solubilization (443 mg/L) was noticed with EIKU14. All isolates showed very low Zn solubilization (< 30 mg/L) in absence of glucose. The recorded pH during solubilization of ZnO in absence of glucose was almost invariant; however, in presence of glucose, pH of medium declined compared to un-inoculated control. Except EIKU12, for all bacteria notable decrease of pH was observed around 3–7 h of incubation. The decline in pH of culture supernatant was validated by measuring the organic acids (gluconic acid, malonic acid, succinic acid and oxalic acid) produced by the bacteria (Table 2). Gluconic acid was found to maximally produced which ranged from 638.3 to 1625.7 mg/L. Malonic acid and succinic acid ranged from 1.2 to 16.4 mg/L and 8.0–121.8 mg/L, respectively. Among the tested bacteria, EIKU13 produced maximum amount of gluconic acid. No significant correlation was found between organic acid production and the amount of solubilized ZnO or pH of the medium in presence of glucose.
Zinc solubilization in soil-based microcosm
Zinc solubilization potential of the bacteria was also performed in soil by incubating the bacteria in soil with the amendments of insoluble ZnO. Different fraction of Zn in the used soil is presented in supplementary Table S2. Water soluble and available Zn in soil was estimated in mg Zn/kg soil and presented in Fig. 6. Zn solubilization by the bacteria in soil was monitored in three successive periods, 15, 30 and 60 days. All the six isolates solubilized ZnO at varying degree in 60 days incubation. In all microcosms, maximum water soluble Zn was observed in 15 days of incubation which decreased with the incubation time (Fig. 6a). However, increased level of both water soluble and DTPA extractable Zn was found in bacteria amended microcosms than those were not amended with bacteria. In all time periods, microcosms with soil and added bacteria (EIKU15, EIKU16 and EIKU17) showed more water soluble Zn than microcosms with only soil. Similarly, bacteria amended microcosms containing soil and added ZnO showed increased water soluble and DTPA extractable Zn than microcosms with soil and ZnO but without bacteria. Noticeably, more than 4.5 and 650 mg Zn/kg soil of water soluble and DTPA extractable Zn, respectively, were observed in ZnO amended soil at 15 days of incubation which were gradually decreased while extending the incubation time. But in presence of bacteria increasing trends of DTPA extractable Zn was observed while incubating the ZnO amended soil (Fig. 6b). This ranged from 700 to 900 mg Zn/kg soil and higher Zn solubilization was registered by EIKU14. In different time period, viable bacterial populations in the microcosms were also enumerated in terms of CFU/g soil (Table 3). In 15 days incubation nearly one order and in two months incubation nearly 2–3 order decreased in bacterial population in the microcosms was observed. Relatively similar level of decline of bacterial population was also noticed in ZnO amended microcosms.
Zn tolerance by the isolates
Maximum Metal Tolerance (MTC) was studied for the bacteria in presence of ZnCl2 and is presented in the Table 1. All the studied Burkholderia spp. showed higher tolerance to ZnCl2. Except EIKU14 that showed lower metal tolerance (7.3 mM), all the other Burkholderia spp. showed higher tolerance (22 mM) to Zn.
Aresnic tolerance by the isolates
Tolerance towards different salts of arsenic [As(III) and As(V)] was studied and expressed as MIC, and presented in Table 1. All the six Burkholderia spp. were found to tolerate > 1.0 mM of As (III) where EIKU15 and EIKU17 showed lower tolerance. Isolates EIKU12, EIKU13, EIKU14 and EIKU16 showed 2 mM of arsenite tolerance. The tolerance level of As(V) was found to be more than 50 mM for all of the six isolates.
Plant growth promoting traits of the bacteria
All the isolated bacteria were tested for PGP traits like P and K solubilization, IAA production and the result is presented in Table 1. All the six Burholderia spp. were able to produce clear zones around their colonies on Pikovskaya's and Aleksandrov agar media which indicated solubilization of insoluble P and K salt by the bacteria, respectively. Except EIKU13, all the isolates showed more than 200% P SE. All the isolates registered more than 175% K SE. Isolates EIKU15 and EIKU17 showed maximum P SE, whereas isolate EIKU14 registered maximum K solubilization efficiency. All the isolates showed weak ability of IAA production. None of the isolates were found to produce siderophore in the tested condition. Although, all the bacteria were found to grow in nitrogen free medium and they turned the BTB colour from green to yellow indicates production of acidic substances.
Discussion
Every food crops will be affected if grown in Zn deficient soil but our major concern is with the rice soil as rice is the major staple food worldwide. The translocation of Zn into rice plants depends on the available Zn in soil. Zinc solubilizing rhizospheric bacteria might play a pivotal role in increasing the available Zn by mobilizing insoluble Zn in rice soil. Our focus was to convert the insoluble Zn to the rice plant accessible form in soil by augmenting the ZSB. A number of bacteria were initially isolated through enrichment process using nitrogen free medium but few of them solubilized zinc but at different extent in in vitro and in vivo to the soil. The isolated potential ZSB were affiliated with aerobic gram negative rod shaped bacteria Burkholderia vietnamiensis and Burkholderia seminalis. It has been assumed that member of the genus Burkholderia may pose a risk to human health, and specially the member of the group Burkholderia cepacia complex are well known as plant pathogen. However, recent investigations have proposed existence of phylogenetically distinct Burkholderia cluster that include many plant-beneficial environmental Burkholderia species able to promote plant growth and to fix nitrogen (Eberl and Vandamme 2016). Member of this cluster are rarely isolated from patients and do not impart risk to human health (Coenye et al. 2002; Gerrits et al. 2005; Deris et al. 2010; Eberl and Vandamme 2016). Moreover, the taxonomic position of a bacterial strain is not always a right indicator of its pathogenic potential. Isolated bacteria in this study showed closer relationship with Burkholderia vietnamiensis which is reported to increase rice grain yield and used as biocontrol agent (Gillis et al. 1995; Govindarajan et al. 2008). B. seminalis is reported to inhabit several environments including water, soil, plants and human and might involve in plant growth promotion and biocontrol of plant pathogen (Araújo et al. 2017). Thus, Burkholderia spp., in this study, might be able to play significant role in agriculture.
Although Zn is vital trace element, it imparts toxicity at higher concentration, inhibiting iron-sulfur cluster biogenesis in E. coli (Li et al. 2019). A concentration of 2.5 mM or 0.35 mM of ZnSO4 in LB medium or M9 minimal medium, respectively, showed toxicity to E.coli (Brocklehurst and Morby 2000; Lee et al. 2005). Thus it is expected that bacteria should show tolerance to increased soluble fraction of Zn while solubilizing Zn from insoluble salt. In this respect higher tolerance to Zn (7–22 mM) by the isolated Burkholderia spp. is noteworthy. Elevated Zn tolerance of the ZSB is comparable to the standard tolerable limit as reported by Abou-Shanab et al. 2007 and referred as Zn resistant those survived in 1 mM Zn.
Zinc solubilization in soil depends on the continued metabolic potential of the indigenous bacteria which might be influence by the geo-physicochemical property of the soil (Dinesh et al. 2018). In the rice fields, especially in the Bengal Delta, where mostly arsenic laden ground water is used for irrigation, arsenic toxicity might limit the metabolic activity necessary for zinc solubilization (Chakraborty et al. 2017). Therefore, it might be assumed that to perform Zn solubilazation in contaminated sites microorganisms must be resistant to As. In this study, we found that isolated bacteria tolerated elevated level of arsenic, however, they showed decreased Zn solubilization efficiency in presence of As.
One of the plant growth promoting traits exhibited by the bacteria is the ability to transform the mineral nutrients from their insoluble form to plant accessible form (Bashri et al. 2017). From various type of agricultural soil plenty of ZSB bacteria are isolated and characterized, however, the study on ZSB from rice field is scarce (Ramesh et al. 2014; Gandhi and Muralidharan 2016; Kumar et al. 2019). Only a few studies reported the role of Burkholderia sp. in Zn solubilization for rice cultivation (Gonita-Mishra et al. 2017; Dinesh et al. 2018). In this study all the Burkholderia spp. showed variable degree of Zn solubilization as indicated by the variation in solubilization efficiency. This observation is very much collinear with the earlier reports regarding differential solubilization of ZnO, ZnCO3 and Zn3(PO4)2 (Li et al. 2010; Gontia-Mishra et al. 2017).
In general, we observed higher ZSE during solubilization of ZnO (0.5%) which varied among bacteria and differed with the amount of ZnO used. It seems the SE increased when amount of ZnO increased from 0.1 to 0.5% but at 1.0% the same was decreased. With some isolates higher efficiency was noticed with lower concentration of Zn3(PO4)2 and ZnCO3. This observation is partially corroborated with the findings of earlier reports where larger dissolution was with ZnO followed by ZnCO3 and Zn3(PO4)2 (Gonita-Mishra et al. 2017; Dinesh et al. 2018). However, reports on the Zn source used and Zn solublization is contradictory in respect to C source used during the assay (Saravana et al. 2007; Vidyeshree 2016).
Zn solubilization was quantitatively determined in liquid medium as plate assay does not reflect the true Zn solubilization potential (Dinesh et al. 2018). Here we also observed differential pattern of Zn solubilization. Two of the six isolates did not solubilize Zn when assayed with ZnO at the onset of culture with 1% inoculum. Thus, quantitative Zn solubilization also determined with the enriched culture. Enriched culture of the bacteria in presence of glucose demonstrated higher ZnO solubilization which ranged from 300 to 450 (mg Zn/L). Zinc solubilization potential of our bacteria in broth is comparable to that of previous reports (Saravanan et al. 2007; Bapiri et al. 2012; Gontia-Mishra et al. 2017). Medium acidification with organic acid production is thought to be the principal mechanism of Zn solubilization, where pH is inversely changing with the increasing solubilized Zn (Costerousse et al. 2018). However, we only observed significant negative correlation between pH of medium and amount of solubilised Zn while solubilising ZnCO3. Similar to earlier report, that indicates stimulatory effect of glucose on organic acid production, we also observed drop in pH of the medium and increased Zn solubilization by the bacteria in presence of glucose. In this regard production of higher level of gluconic acid by the bacteria in presence of glucose is corroborated well with the previous findings, however, we did not observe significant correlation between amounts of gluconic acid produced and solubilised Zn (Costerousse et al. 2018). Conversely, Li et al. (2010) observed Burkholderia cepacia produce organic acid while solubilizing Zn despite the presence of glucose.
The bacteria that solubilising Zn efficiently in culture medium might not be able to perform well in soil, as Zn solubilization depends on growth conditions. Our microcosm based study revealed that the tested bacteria not only solubilised ZnO in soil but also able to maintain certain level of water soluble and available Zn during the incubation period. Increase in both water and DTPA extractable Zn in absence of any added ZnO indicated that the bacteria when amended in soil solubilised naturally occurring Zn in the soil. The solubilised Zn concentration is comparable to critical limit of 0.05 (M) HCl and DTPA extractable Zn in rice soils that has been established as 1.0 mg/kg and 0.75 mg/kg, respectively, below which a soil is said to be Zn deficient (Ponnamperuma et al. 1981).
Solubilization of different soil minerals and making them available to plants are regarded to be the important traits which are directly associated with PGPR activities (Nahas 1996; Rokhbakhsh-Zamin et al. 2011). The present investigation showed that besides solubilization of Zn, the isolated bacteria have the in vitro PGP potential characteristics like phosphate and potassium solubilization, IAA production. We also noticed the ability of the tested bacteria to grow in nitrogen free medium which indicates their survivability in nitrogen limited environment. Recent reports indicated Burkholderia spp. could fix nitrogen with the association of plants (Estrada-delos Station et al. 2001; Shinjo et al. 2018).
Conclusion
Among the 20 bacterial isolates obtained six bacteria were found to solubilize Zn efficiently at different conditions. 16S rRNA gene sequencing and phylogenetic analysis indicated the affiliation of all ZSB with the genus Burkholderia although they solubilized different Zn salts at different magnitude. All ZSB were able to grow in nitrogen free medium and showed significant tolerance to Zn and As. ZSE on solid medium was found to increase with ZnO concentration from 0.1 to 0.5% but decreased at 1.0% but in other Zn salts (Zn3(PO4)2 and ZnCO3) higher solubilization was noticed at lower concentration. Some isolates failed to solubilize one or more tested Zn salts in low volume liquid medium. With enriched bacterial cells ZnO solubilization increased with increasing incubation time where glucose was found to positively influence the Zn solubilization. Gluconic acid produced by the bacteria seems to be the major cause of medium acidification and zinc solubilisation. Soil incubation study revealed that all the tested Burkholderia spp. were able to solubilize Zn significantly under soil condition and could maintain the solubilized fraction of Zn above critical level. The tested Burkholderia spp. solubilized potassium and phosphate efficiently and were able to produce indole acetic acid (IAA) in vitro. This study suggests that the Burkholderia spp. could be used in agricultural soil to enhance Zn dissolution.
References
Abou-Shanab RA, vanBerkum P, Angle JS (2007) Heavy metal resistance and genotypic analysis of metal resistance genes in grampositive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 68:360–367
Ahmed AMA, Ahmed G, Magda MH, TawfikIntegrated MM (2011) Effect of organic and biofertilizers on wheat productivity in new reclaimed soils. Res J Agric Biol Sci 7:105–114
Andreini C, Bertini I, Rosato A (2009) Metalloproteomes: a bioinformatic approach. AccChem Res 42:1471–1479
Araújo FD, Araújo WL, Eberlin MN (2017) Potential of Burkholderia seminalis TC342R3 as biocontrol agent against Fusariumoxysporum evaluated by mass spectrometry imaging. J Am Soc Mass Spectrom 28:901. https://doi.org/10.1007/s13361-017-1610-6
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548
Bapiri A, Asgharzadeh A, Mujallali H, Khavazi K, Pazira E (2012) Evaluation of zinc solubilization potential by different strains of fluorescent pseudomonads. J Appl Sci Environ Manag 16:295–298
Bashri G, Patel A, Singh R, Parihar P, Prasad SM (2017) Mineral solubilization by microorganism: mitigating strategy in mineral deficient soil. Microbial Biotechnol. https://doi.org/10.1007/978-981-10-6847-8_12
Bhakat K, Chakraborty A, Islam E (2019) Characterization of arsenic oxidation and uranium bioremediation potential of arsenic resistant bacteria isolated from uranium ore. Environ Sci Pollut R 26:12907–12919. https://doi.org/10.1007/s11356-019-04827-6
Brocklehurst KR, Morby AP (2000) Metal-ion tolerance in Escherichia coli: analysis of transcriptional profiles by gene-array technology. Microbiology 146:2277–2282. https://doi.org/10.1099/00221287-146-9-2277
Chakraborty A, Bhakat K, Islam E (2017) Arsenic contamination in agricultural soil reduces metabolic activity of total and free-living nitrogen-fixing bacteria as revealed by real-time qPCR. Soil Sediment Contam 26:736–748
Chakraborty A, Islam E (2018) Temporal dynamics of total and free-living nitrogen-fixing bacterial community abundance and structure in soil with and without history of arsenic contamination during a rice growing season. Environ Sci Pollut Res 25:4951–4962. https://doi.org/10.1007/s11356-017-0858-5
Coenye T, Goris J, Spilker T et al (2002) Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinuslimosus gen nov., sp. nov. J Clin Microbiol 40(6):2062–2069
Costerousse B, Schönholzer-Mauclaire L, Frossard E, Thonar C (2018) Identification of heterotrophic zinc mobilization processes among bacterial strains isolated from wheat rhizosphere (TriticumaestivumL.). Appl Environ Microbiol 84:01715–01717. https://doi.org/10.1128/AEM.01715-17
Deris ZZ, Van Rostenberghe H, Habsah H et al (2010) First isolation of Burkholderiatropica from a neonatal patient successfully treated with imipenem. Int J Infect Dis 14(1):e73–e74
Dinesh R, Srinivasan V, Hamza S, Sarathambal C, Gowda SA, Ganeshamurthy AN, Gupta SB, Nair VA, Subila KP, Lijina A, Divya VC (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186
Eberl L, Vandamme P (2016) Members of the genus Burkholderia: good and bad guys. F1000Research, 5, F1000 Faculty Rev-1007. https://doi.org/https://doi.org/10.12688/f1000research.8221.1
Estrada-delos Station P, Bustitio-Cristales R, Caballero-Mallado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol 67:279–2798
Fasim F, Ahmed N, Parsons R, Gadd GM (2002) Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett 213:1–6
Figueiredo MV, Burity HA, Martı´nez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182188
Gandhi A, Muralidharan G (2016) Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Eur J Soil Biol 76:1–8
Gerrits GP, Klaassen C, Coenye T et al (2005) Burkholderia fungorum septicemia. Emerg Infect Dis 11(7):1115–1117
Gillis M, Van TV, Bardin R, Goor M, Hebbar P, Willems A, Segers P, Kersters K, Heulin T, Fernandez MP (1995) Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov for N2-fixing isolates from rice in Vietnam. Int J Syst Bacteriol 45:274–289
Gontia-Mishra I, Sapre S, Tiwari S (2017) Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere 3:185–190
Goswami D, Parmar S, Vaghela H, Dhandhukia P, Thakker J (2015) Describing Paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR). Cogent Food Agric 1:1000714
Govindarajan M, Balandreau J, Kwon S-W, Weon H-Y, Lakshminarasimhan C (2008) Effects of the inoculation of Burkholderia vietnamensis and related endophyticdiazotrophic bacteria on grain yield of rice. MicrobEcol 55:21–37
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plantbacterium signaling processes. Soil Biol Biochem 37:395412
Hendershot WH, Lalande H, Duquette M (2007) Soil Reaction and Exchangeable Acidity. In M. R. Carter and E. G. Gregorich, editors. Soil sampling and methods of analysis. Second edition. CRC Press, Taylor & Francis Group, Boca Raton, Florida, USA.
Hotz C, Brown KH (2004) Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:94–204
Hu XF, Chen J, Guo JF (2006) Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990
Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S (2017) Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front Microbiol 8:2593. https://doi.org/10.3389/fmicb.2017.02593
Kanwar JS (1973) Soil Fertility-Theory and Practice. Publ. Indian Council of Agricultural Research, New Delhi.
Katyal JC, Sharma BD (1991) DTPA extractable and total Zn, Cu, Mn and Fe in Indian soils. Geoderma 49:165–179
Khanghahi MY, Ricciuti P, Allegretta I, Terzano R, Crecchio C (2018) Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res 25:25862–25868
Krithika S, Balachandar D (2016) Expression of zinc transporter genes in rice as influenced by zinc-solubilizing Enterobacter cloacae strain ZSB14. Front Plant Sci 7:446
Kumar A, Dewangan S, Lawate P, Bahadur I, Prajapati S (2019) Zinc-Solubilizing Bacteria: A Boon for Sustainable Agriculture. Microorganisms for Sustainability. Plant Growth Promoting Rhizobacteria for Sustainable Stress Management https://doi/org/https://doi.org/10.1007/978-981-13-6536-2_8
Lee LJ, Barrett JA, Poole RK (2005) Genome-wide transcriptional response of chemostat cultured Escherichia coli to zinc. J Bacteriol 187:1124–1134. https://doi.org/10.1128/JB.187.3.1124-1134.2005
Li J, Ren X, Fan B, Huang Z, Wang W, Zhou H, Lou Z, Ding H, Lyu J, Tan G (2019) Zinc toxicity and iron-sulfur cluster biogenesis in Escherichia coli. Appl Environ Microbiol 85:01967–02018. https://doi.org/10.1128/AEM.01967-18
Li WC, Ye ZH, Wong MH (2010) Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil 326:453–467
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12:51–53
Mumtaz MZ, Ahmad M, Jamil M, Hussain T (2017) Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol Res 202:51–60
Nahas E (1996) Factors determining rock phosphate solubilization by microorganisms isolated from soil. World J Microbiol Biotechnol 12(6):567–572
Othman NMI, Othman R, Saud HM, Wahab PEM (2017) Effects of root colonization by zinc-solubilizing bacteria on rice plant (Oryza sativa MR219) growth. Agric Nat Resour 51:532–537
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 17:362–370
Ponnamperuma FN, Caylon MT, Lantin RS (1981) Dilute hydrochloric acid as an extractant for available zinc, copper and boron in rice soils. Plant Soil 61:291–310
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014) Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in vertisols of central India. Appl Soil Ecol 73:87–96
Rattan RK, Shukla LM (1984) Critical limits of deficiency and toxicity of zinc in paddy in a typic Ustipsamment. Commun Soil Sci Plant Anal 15(9):1041–1050. https://doi.org/10.1080/00103628409367541
Rokhbakhsh-Zamin F, Sachdev D, Kazemi-Pour N, Engineer A, Pardesi KR, Zinjarde S, Dhakephalkar PK, Chopade BA (2011) Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetumglaucum. J Microbiol Biotechnol 21:556–566
Saravanan VS, Madhaiyan M, Thangaraju M (2007) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798
Sawar M, Kremer RJ (1995) Determination of bacterially derived auxins using a microplate method. Lett Appl Microbiol 20:282–285
Shaikh S, Saraf M (2017) Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal Agric Biotechnol 9:120–126
Shakeel M, Rais A, Hassan MN, Hafeez FY (2015) Root associated Bacillus sp improves growth, yield and zinc translocation for Basmati Rice (Oryza sativa) varieties. Front Microbiol 6:1286. https://doi.org/10.3389/fmicb.2015.01286
Sharma SK, Sharma MP, Ramesh A, Joshi OP (2012) Characterization of zinc-solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J Microbiol Biotechnol 22:352–359. https://doi.org/10.4014/jmb.1106.05063
Sharma A, Patni B, Shankhdhar D, Shankhdhar SC (2013) Zinc - an indispensable micronutrient. Physiol Mol Biol Plants 19:11–20. https://doi.org/10.1007/s12298-012-0139-1
Shinjo R, Uesaka K, Ihara K, Sakazaki S, Yano K, Kondo M, Tanaka A (2018) Draft genome sequence of Burkholderia vietnamiensis strain RS1, a nitrogen-fixing endophyte isolated from sweet potato. Microbiol Resour Announc 7:00820–00918. https://doi.org/10.1128/MRA.00820-18
Vidyashree DN, Muthuraju R, Panneerselvam P, Saritha B, Ganeshamurthy AN (2016) Isolation and characterization of zinc solubilizing bacteria from stone quarry dust powder. Int J Agri Sci 8:3078–3081
Wu TY, Gruissem W, Bhullar NK (2019) Targeting intracellular transport combined with efficient uptake and storage significantly increases grain iron and zinc levels in rice. Plant Biotechnol J 17:9–20
Zhang X, Jiang B, Ma Y (2017) Aging of zinc added to soils with a wide range of different properties: factors and modeling. Environ Toxicol Chem 36:2925–2933
Acknowledgements
We thankfully acknowledged the received of Grant from DST-SERB (File No. EMR/2016/003761), Government of India for carrying out this research work. A support from DST-PURSE, University of Kalyani, is also highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhakat, K., Chakraborty, A. & Islam, E. Characterization of zinc solubilization potential of arsenic tolerant Burkholderia spp. isolated from rice rhizospheric soil. World J Microbiol Biotechnol 37, 39 (2021). https://doi.org/10.1007/s11274-021-03003-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03003-8