Abstract
Non-thermal plasma (NTP), generated at atmospheric pressure by DC cometary discharge with a metallic grid, and antibiotics (gentamicin—GTM, ceftazidime—CFZ and polymyxin B—PMB), either alone or in combination, were used to eradicate the mature biofilm of Pseudomonas aeruginosa formed on Ti-6Al-4V alloy. Our aim was to find the conditions for NTP pre-treatment capable of enhancing the action of the antibiotics and thus reducing their effective concentrations. The NTP treatment increased the efficacy of relatively low concentrations of antibiotics. Generally, the highest effect was achieved with GTM, which was able to suppress the metabolic activity of pre-formed P. aeruginosa biofilms in the concentration range of 4–9 mg/L by up to 99%. In addition, an apparent decrease of biofilm-covered area was confirmed after combined NTP treatment and GTM action by SYTO®13 staining using fluorescence microscopy. Scanning electron microscopy confirmed a complete eradication of P. aeruginosa ATCC 15442 mature biofilm from Ti-6Al-4V alloy when using 0.25 h NTP treatment and subsequent treatment by 8.5 mg/L GTM. Therefore, NTP may be used as a suitable antibiofilm agent in combination with antibiotics for the treatment of biofilm-associated infections caused by this pathogen.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of Pseudomonas aeruginosa to form biofilm on human tissues or abiotic materials used in medicine is a serious problem in patient care. Infections associated with the biofilm formation are predominantly related to the hospitalization of patients (Mulcahy et al. 2014). These hospital-acquired or nosocomial infections may be of exogenous or endogenous (i.e. hematogenous) origin (Sendi et al. 2011). Exogenous infections may occur during the surgery or in the early postoperative phase in the case of wound healing disorders. The hematogenous way is associated with the transmission of infection through the bloodstream (Zimmerli and Moser 2012; Zimmerli and Sendi 2011). In addition to the colonization of indwelling devices (venous or urinary catheters), P. aeruginosa can also form biofilm on orthopedic implants (prosthetic joints) often made of titanium alloy Ti-6Al-4V (Cole et al. 2014; Geetha et al. 2009; Pandey et al. 2000; Trautner and Darouiche 2004). P. aeruginosa represents 8% of all isolates causing infections associated with the biofilm formation on implants (Campoccia et al. 2006) and patients with prosthetic joints are at life-long risk of developing a hematogenous infection. Once the infection is established, it might be very difficult to treat with high probability of relapse (Sendi et al. 2011).
The P. aeruginosa biofilm formation is a complex process that is driven by changes in environmental conditions (e.g., nutrient availability) and by bacterial signaling mediated via quorum sensing (QS) molecules (Davey and O´toole 2000; Dufour et al. 2010). The involvement of QS molecules in the formation of P. aeruginosa biofilm and increased resistance of biofilms to biocides have been described (Davies et al. 1998). According to the study, P. aeruginosa mutant unable to synthesize the major QS molecules (N-acyl-homoserine lactones, AHL) has radically altered biofilm architecture. AHL are involved not only in the biosynthesis of exopolysaccharides, contained in the biofilm matrix (Gilbert et al. 2009), but also rhamnolipids which are important for the biofilm architecture and biofilm dispersion (Boles et al. 2005; Davey et al. 2003). They are also involved in the production of other virulence factors that facilitate bacterial colonization, enable efficient host attack and overcome its defense system (van Delden 2004).
For the treatment of P. aeruginosa infections, the oldest but still widely used antibiotics in clinical practice are aminoglycosides, e.g., gentamicin (GTM) (Ren et al. 2019). The mechanism of bacterial resistance to these antibiotics is facilitated via a decrease of their intracellular concentration, i.e., disabling their transport through the cytoplasmic membrane, or via enzymatic inactivation (Ciofu and Tolker-Nielsen 2019). Ceftazidime (CFZ) is the most active cephalosporin available against P. aeruginosa: it is effective at lower concentrations than aminoglycosides, but resistance may also develop (as is typical for other β-lactam antibiotics) (Richards and Brogden 1985). As the last drug of choice in the treatment of infections caused by resistant gram-negative bacteria, polymyxin B (PMB) is still applied despite its nephrotoxicity (Hermsen et al. 2003; Zavascki et al. 2007).
Due to the lack of novel antibiotics, new antimicrobial approaches based on the combination of agents that have not only antibacterial but also antibiofilm and hence anti-virulence effects are sought. One of the possibilities is the use of non-thermal plasma (NTP) which appears to be unable to induce resistance in bacteria even in biofilm form (Alkawareek et al. 2012). In general, NTP is generated by supplying energy to a neutral gas, resulting in the formation of high energy electrons and photons. These give rise to reactive ions, radicals and molecules after collision with neutral atoms and molecules (Conrads and Schmidt 2000). Reactive oxygen (e.g., ozone, superoxide, hydroxyl radicals, singlet oxygen and atomic oxygen) and nitrogen species (e.g., nitric oxide, nitrogen dioxide, nitrite and nitrates) are generated when using air as the neutral gas. These reactive species can cause oxidative stress and peroxidation of membrane lipids, damage bacterial DNA, cause irreversible changes in the native structure of enzymes or membrane permeability. As already mentioned, this multiple-target mechanism reduces the probability of resistance development against this antibiofilm agent (Alkawareek et al. 2012).
The ability of NTP to increase the permeability of an otherwise low permeable membrane of P. aeruginosa, a significant pathogen belonging to the Enterococcus faecium, Klebsiella pneumoniae, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. (ESKAPE) group, is highly important (Jimenez et al. 2012; Kvam et al. 2012). In an early work, Abramzon et al. (2006) described the exposure of Chromobacterium violaceum biofilm to plasma produced by Atomflo 250 reactor. The antibiofilm activity of NTP and its ability to eradicate the biofilm were later studied in many other works (e.g., Alkawareek et al. 2012, 2014; Vaňková et al. 2018; Zelaya et al. 2012) and summarized by Julák et al. (2018). Ziuzina et al. (2015) described the potential of NTP to inactivate other virulence factors. These effects (antibiofilm and anti-virulence) of NTP may be related to the attenuation of the QS systems via degradation of AHL (Flynn et al. 2016). This was examined also in our previous study dealing with the attenuation of AHL-dependent QS systems of P. aeruginosa (Paldrychová et al. 2019).
The combination of NTP with another antimicrobial agent against biofilm cells has been studied only in a handful of publications (Du et al. 2013; Guo et al. 2018; Gupta et al. 2017; Klebes et al. 2015; Koban et al. 2013; Sun et al. 2012; Julák et al. 2020). Koban et al. (2013) dealt with the synergistic effects of NTP with disinfecting agents against dental biofilms (i.e., Streptococcus mutants). Du et al. (2013) focused on the investigation of NTP and antiseptic chlorhexidine (CHX) action on Enterococcus faecalis biofilms. Similarly, the sensitivity of another gram-positive coccus Staphylococcus aureus to gas plasma pre-treatment and a wide range of antibiotics treatment was evaluated by Guo et al. (2018). Sun et al. (2012) focused their research on enhancing the action of antifungal drugs against Candida biofilms by NTP treatment. Klebes et al. (2015) described the efficacy of tissue-tolerable plasma and a modern conventional liquid antiseptic (octenidine dihydrochloride with 2-phenoxyethanol) on chronic wound infections in humans. Finally, Gupta et al. (2017) evaluated the effect of NTP in combination with CHX on P. aeruginosa biofilms on titanium surface.
To the best of our knowledge, our study is the first attempt to use NTP treatment to enhance the antibiotics action towards P. aeruginosa mature biofilm. We determined the effective exposure times of NTP treatment needed for enhancing the efficacy of antibiotics (GTM, CTZ and PMB) in reducing the metabolic activity of mature P. aeruginosa biofilm cells and for decreasing the area of Ti-6Al-4V alloy (the material widely used in clinical practice for joint implant manufacture) covered by biofilm produced by different strains of this bacterium. In addition, we evaluated the ability of such combinations to attenuate AHL-dependent QS systems for better understanding their effect on P. aeruginosa biofilm.
Materials and methods
Pseudomonas aeruginosa strains, culture conditions and mature biofilm formation
Two of the strains (P. aeruginosa DBM 3081 and P. aeruginosa DBM 3777) were kindly provided by the Department of Biochemistry and Microbiology (DBM), University of Chemistry and Technology, Prague. Another two strains (P. aeruginosa ATCC 10145 and P. aeruginosa ATCC 15442) were purchased from the American Type Culture Collection (ATCC). The inoculum of all strains of P. aeruginosa were cultured in LB medium (24 h, 37 °C, shaking at 100 rpm) prior use. An optical density of the prepared bacterial suspension was adjusted to OD600nm = 0.600 ± 0.010. According to the procedure given in detail in Vaňková et al. (2018), 3 mL of this suspension were added to a sterile polypropylene cultivation vessel (height 7 cm, diameter 3 cm, volume 40 mL; p-LAB, Czech Republic) containing one circular titanium alloy Ti-6Al-4V carrier of 1.5 cm in diameter (Prospon, Czech Republic). Mature biofilm was formed for 24 h, at 37 °C and orbital shaking at 100 rpm.
Determination of NTP exposure times for mature biofilm treatment
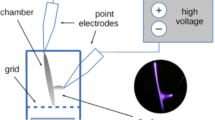
The basic results for NTP effect on P. aeruginosa strains biofilm when using alone were published in our previous study Paldrychová et al. (2019). In present study we tested the combination of NTP treatment with subsequent antibiotic treatment. To find the appropriate conditions for the combination of both antibiofilm agents, the following procedure was used: Mature biofilm-colonized titanium alloy Ti-6Al-4V carriers were washed three times with phosphate buffer saline (PBS) to remove the non-adherent cells. The biofilms on carriers were then exposed to NTP generated by DC cometary discharge with a metallic grid (Fig. 1), previously described in Scholtz et al. (2013) and used in our previous studies (Vaňková et al. 2018; Paldrychová et al. 2019; Julák et al. 2020). It consisted of two needle electrodes connected to a 5 kV power supply (UNI-T UT 513). The electrodes were arranged at an angle of 30°, their tips were 5 mm apart and the tip of the positive electrode was shifted 1 mm above the negative one. The open air discharge burns at a current of 50–70 µA. The electrically insulated metallic grid was inserted between the discharge and the exposed object, improving the inactivation efficiency and the size of the treated area. The grid consisted of stainless steel wire 0.1 mm in diameter forming a net with a mesh size of 1 mm. This arrangement is shown schematically in Fig. 1. The chemical composition is similar to the pulseless glow and curved transition spark discharges described in Khun et al. (2018), in which the excited molecular nitrogen N2, the N2+ ion, and OH· radicals are dominant. The exposure times used were 0.25, 0.5 and 1 h. The carriers were subsequently placed into the cultivation vessels containing 3 mL of LB medium and the effect of NTP treatment on the mature biofilms on carriers alone was evaluated by (3–4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after their cultivation for 24 h at 37 °C and shaking at 100 rpm. Each experiment was performed in triplicates in at least three independent repetitions and the data were averaged. The exposure time which reduced the metabolic activity of biofilm cells by approximately 50% was used for further experiments focused on the effect of the combination of two antibiofilm agents (NTP and antibiotics) on eradication. These exposure times were 1 h for P. aeruginosa DBM 3081, 0.5 h for P. aeruginosa DBM 3777 and P. aeruginosa ATCC 10145, and 0.25 h for P. aeruginosa ATCC 15442.
Determination of antibiotics concentrations for mature biofilm treatment
To evaluate the effect of antibiotics (GTM, CFZ, PMB) on mature biofilm alone, biofilms on carriers were washed as described above and placed into the cultivation vessels containing 3 mL of LB medium with the addition of GTM (Sigma-Aldrich, Czech Republic), CTZ (Alfa Aesar, Germany) or PMB (Sigma-Aldrich, Czech Republic) in a final concentration range 0–100 mg/L. After the cultivation of mature biofilm in the presence of antibiotics for next 24 h at 37 °C (100 rpm), the antibiotic action was evaluated by MTT assay. Each experiment was performed in triplicates in at least three independent repetitions and the data were averaged. The concentration which reduced the metabolic activity of biofilm cells by approximately 50% was used in further experiments exploring the effect of the combination of NTP and antibiotics on the biofilm eradication. The antibiotics concentrations were for P. aeruginosa DBM 3081: 4 mg/L of GTM, 0.10 mg/L of CTZ and 3.5 mg/L of PMB; for P. aeruginosa DBM 3777: 9 mg/L of GTM, 1 mg/L of CTZ and 15 mg/L of PMB; for P. aeruginosa ATCC 10145: 6.5 mg/L of GTM, 0.5 mg/L of CTZ and 8.5 mg/L of PMB; and for P. aeruginosa ATCC 15442: 8.5 mg/L of GTM, 0.25 mg/L of CTZ and 7.5 mg/L of PMB.
NTP treatment and subsequent antibiotic treatment of mature biofilm
Titanium alloy Ti-6Al-4V carriers colonized by mature biofilm were washed as described above. The biofilm on carriers was exposed to NTP for the time based on the experiments focused on the NTP treatment alone. The carriers were subsequently placed into cultivation vessels containing 3 mL of LB medium with the addition of antibiotics (GTM, CTZ or PMB) at concentrations determined in the experiments focused on the antibiotic treatment alone. Each experiment was performed in three replicates in at least three independent repetitions and the data were averaged. Fluorescence microscopy with staining by SYTO® 13 dye, and scanning electron microscopy (SEM) for selected combinations were used to confirm the results obtained by the MTT assay and crystal violet staining. In addition, we tested the ability of selected combinations to attenuate AHL-dependent QS systems (determined as β-galactosidase activity).
MTT assay
The MTT assay was used to quantify the effect of both antibiofilm agents alone and their combination on eradication of mature biofilm (expressed as metabolic activity of biofilm cells). The assay is based on yellow colored MTT being metabolized by viable biofilm cells to purple crystals of formazan, which is then quantified spectrophotometrically as given in detail in Vaňková et al. (2018). In brief, 50 µL of MTT (Across Organics, Belgium; 1 g/L) and 60 µL of D-glucose (Penta, Czech Republic; 57.4 g/L) dissolved in PBS were added to washed biofilm. The biofilm was incubated with the reagents for 1–3 h (depending on the strain used) at 37 °C and 150 rpm. After incubation, 100 µL of formazan dissolving solution was added to the biofilm in each well and the mixture was incubated for another 30 min at 37 °C and 230 rpm. The formazan dissolving solution consisted of 40% v/v dimethylformamide (Carl Roth, Germany) dissolved in 2% acetic acid (Penta, Czech Republic) and 16% w/v SDS (Carl Roth, Germany). A 100-µL aliquot from each sample was transferred to another 96-well microtiter plate and measured spectrophotometrically at 570 nm by Infinite Pro 200i Reader (Tecan, Switzerland).
Statistical analysis
The distant values of data obtained by MTT assay were omitted according to Dixon’s Q test. Arithmetic means and standard deviations falling within the interval 0.2 and 28% were calculated for each concentration tested by the assay in relative percentages (control samples were 100%). The significance of the data was evaluated using one-way analysis of variance (ANOVA) with significance level p < 0.05.
Crystal violet staining
In addition to the MTT assay, the effect of NTP treatment and subsequent antibiotic treatment on the total biofilm biomass was evaluated by crystal violet staining. This dye is bound to negatively charged molecules contained in the biofilm matrix (Peeters et al. 2008). The principle and the procedure of this method are given in detail in Paldrychová et al. (2019). Briefly, washed biofilm was stained with 200 µL of 0.1% filtered solution of crystal violet (Carl Roth, Germany) in distilled water at room temperature for 20 min. The excess dye was poured away, and biofilm was washed twice with saline. The dye bound in biofilm biomass was extracted to 200 µL of 96% ethanol (Penta, Czech Republic) at room temperature for 10 min. A 100-µL aliquot of each sample was transferred to another 96-well microtiter plate and measured spectrophotometrically at 580 nm on Infinite Pro 200i Reader (Tecan, Switzerland).
Biosensor assay for AHL detection
The detection of AHL was performed with Agrobacterium tumefaciens NTL4 (pZLR4) ATCC BAA 2240 as a biosensor. This detection was performed in samples derived from the cultivation of mature biofilm of all P. aeruginosa strains formed on titanium alloy Ti-6Al-4V carriers (as described above) in the presence of the antibiotic. The antibiotics displaying the strongest effect enhanced by NTP treatment, as well as their combination were used. The biosensor contains a plasmid that responds to the presence of total AHL level with the production of β-d-galactosidase. Its enzymatic activity can be determined using colorimetric assay with X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Omega Bio-tek, USA). This assay was carried out according to the procedure described in detail in Paldrychová et al. (2019). In short, AHL-containing P. aeruginosa supernatants surrounding its biofilm (2 μL) were transferred into a 96-well microtiter plate (Gama group, Czech Republic). Cultured A. tumefaciens NTL4 (pZLR4) cell suspension adjusted to OD600nm = 0.250 ± 0.020 (50 μL) was added into each well and incubated for 16–18 h at 30 °C and 100 rpm. An aliquot of 50 μL of lysis buffer was then added for 90 min at 25 °C and 150 rpm to release β-d-galactosidase and activate it by the addition of X-gal solution (50 μL) for 1 h at 25 °C and 150 rpm. The resulting blue product was determined spectrophotometrically at 660 nm on Infinite Pro 200i Reader (Tecan, Switzerland). Each experiment was performed in six replicates.
Fluorescence microscopy with SYTO® 13 staining
To investigate the entire biofilm structure affected by NTP treatment and subsequent antibiotic treatment, the mature biofilm of all P. aeruginosa strains tested (formed on titanium alloy Ti-6Al-4V carriers as described above) was influenced by the antibiotic whose action was the most strongly enhanced by NTP treatment and the samples were stained with SYTO® 13 dye and visualized using fluorescence microscope Eclipse E400 (Nikon, Japan). The effect of antibiotic alone and its effect after NTP treatment were compared. SYTO® 13 binds to the nucleic acids and to the extracellular DNA of the cells (Ullal et al. 2010). The visualization of untreated biofilm and biofilm treated by one or both antibiofilm agents were performed in the same way as in our previous study Paldrychová et al. (2019).
Scanning electron microscopy (SEM)
For detailed visualization of biofilm structure, the mature biofilm of type strain P. aeruginosa ATCC 15442 formed on titanium alloy Ti-6Al-4V carriers was observed using SEM as described in detail by Volejníková et al. (2019). The image of the untreated surface of Ti-6Al-4V alloy was depicted in our previous study Vaňková et al. 2020. The effect of NTP treatment alone and the sole treatment by the antibiotic whose effect was the most strongly enhanced by NTP treatment, as well as their successive application on mature biofilm was observed with Nova NanoSEM 450 (Fei, USA) electron microscope. Samples were gently washed with sterile distilled water and let completely dry out by laminar flow and subsequently in a desiccator under vacuum at least five days before visualization. The images of the dried samples were taken under low vacuum by LVD detector at magnification of ×2500, spot size 5 and dwell time 20 µs, scale up 30 µm.
Results
Effect of NTP on P. aeruginosa mature biofilm
The ability of NTP to affect P. aeruginosa mature biofilm formed on the surface of titanium alloy Ti-6Al-4V alone was demonstrated by the MTT assay. The results are summarized in Table 1, where the lowest exposure times causing an approximately 50% eradication of mature biofilm (as compared to the control 100%) are highlighted in bold. The lowest NTP exposure time of 0.25 h was needed for P. aeruginosa ATCC 15442. The longer exposure times of 0.5 h were determined for P. aeruginosa ATCC 10145 and P. aeruginosa DBM 3777, the longest exposure (1 h) being determined for P. aeruginosa DBM 3081. Therefore, individual P. aeruginosa strains differ in their sensitivity to NTP treatment. All results were significant according to ANOVA (p < 0.05).
Effect of GTM, CTZ and PMB antibiotics alone and combined with NTP pre-treatment on mature P. aeruginosa biofilm
The concentrations of antibiotics used for assessing the combined effect of NTP treatment with subsequent antibiotic treatment on P. aeruginosa mature biofilm were those causing approximately 50% decrease in metabolic activity of biofilm cells. An example of selecting a suitable concentration of the antibiotic is given for GTM in Supplementary Material (Table A1). With the tested P. aeruginosa strains, these concentrations of GTM ranged from 4 to 9 mg/L, for CTZ from 0.1 to 1 mg/L and for PMB from 3.5 to 15 mg/L.
The NTP treatment used to enhance antibiotic action against P. aeruginosa mature biofilm was based on the determined NTP exposure times and concentrations of antibiotics reducing the metabolic activity of biofilm cells approximately by 50% when acting alone (Fig. 2).
Effect of gentamicin (GTM), ceftazidime (CTZ) and polymyxin B (PMB) alone or with non-thermal plasma (NTP) pre-treatment of Pseudomonas aeruginosa biofilm. a GTM + NTP, metabolic activity; b GTM + NTP total biofilm biomass; c CTZ + NTP, metabolic activity; d CTZ + NTP total biofilm biomass; e PMB + NTP, metabolic activity; f PMB + NTP, total biofilm biomass. Results are given in relative percentages; control (untreated by any antibiofilm agent) represents 100%
The combination of NTP treatment with GTM (4–9 mg/L) action (Fig. 2a, b) resulted in significant decrease in metabolic activity of mature biofilm cells as well as total biofilm biomass in all strains (p < 0.05) except P. aeruginosa DBM 3081. The NTP treatment with subsequent GTM action (6.5 mg/L) caused a significant (93%) decrease in the metabolic activity of P. aeruginosa ATCC 10145 biofilm cells (p < 0.01) (Fig. 2a) and the decrement of the total biofilm biomass by 87% (Fig. 2b). The metabolic activity of P. aeruginosa DBM 3777 and P. aeruginosa ATCC 15442 biofilm cells was significantly (99%) reduced after NTP treatment with subsequent GTM action (at 9 and 8.5 mg/L, respectively) with p < 0.00001 (Fig. 2a). In addition, the total biomass of mature P. aeruginosa ATCC 15442 biofilm was completely eradicated by such combination of antibiofilm agents (Fig. 2b), indicating a great promise of this combined action.
Although the selected effective concentrations of CTZ (0.1–1 mg/L) were generally lower than those of GTM, some biofilms (P. aeruginosa DBM 3777 and P. aeruginosa ATCC 15442) were not fully eradicated even at 250 mg/L CTZ (data not shown). In combination with NTP treatment, 1 mg/L CTZ reduced the metabolic activity of P. aeruginosa DBM 3777 biofilm cells by 96% (p < 0.05) (Fig. 2c) and eradicated the total biofilm biomass of P. aeruginosa DBM 3777 by 99% (Fig. 2d). In the case of P. aeruginosa ATCC 10145 and P. aeruginosa ATCC 15442 (6.5 mg/L or 8.5 mg/L of CTZ, respectively), the NTP treatment resulted in 83% and 84% decrease of total biofilm biomass, respectively.
The selected concentrations of PMB were the highest of the tested antibiotics (3.5–15 mg/L), P. aeruginosa DBM 3777 mature biofilm cells being the least sensitive to its action (Fig. 2e, f). The NTP treatment plus subsequent PMB action (15 mg/L) reduced the metabolic activity of P. aeruginosa DBM 3777 biofilm cells by 85%, with p < 0.05 (Fig. 2e) and caused an almost complete (98%) eradication of mature biofilm (Fig. 2f). Overall, the NTP treatment with subsequent PMB action resulted in more than 70% reduction of metabolic activity of P. aeruginosa mature biofilm cells and was found very effective in enhancing the antibiotic action.
Generally, the greatest effect of the combination of both antibiofilm agents on the P. aeruginosa total biofilm biomass reduction or eradication was observed for NTP treatment and GTM action rather than for CTZ or PMB (Fig. 2b, d, f).
Effect of NTP pre-treatment and subsequent GTM treatment on AHL relative level
Based on the above results, we studied the effect of GTM and its combination with NTP treatment on the AHL relative level. With GTM alone, the AHL relative level was decreased by 53–60% in P. aeruginosa DBM 3081, P. aeruginosa DBM 3777 and P. aeruginosa ATCC 10145 (Fig. 3a–c). The only insensitive strain was P. aeruginosa ATCC 15442, which was not affected (Fig. 3d). We assumed that this AHL decrease was related to the decrease of optical density of suspension cells surrounding P. aeruginosa biofilm. The NTP treatment with subsequent GTM action in sensitive strains resulted in a 59–90% decrease of AHL relative level (Fig. 3a–c), being the most effective in P. aeruginosa DBM 3777 and ATCC 10145, similarly to the decrease of optical density of their suspension cells. Although the effect of GTM on AHL relative level in P. aeruginosa ATCC 15442 was less enhanced by NTP pre-treatment, the optical density of its suspension cells was completely abolished (Fig. 3d). In fact, we do not yet have a satisfactory answer to this phenomenon.
Effect of gentamicin (GTM) alone and with non-thermal plasma (NTP) pre-treatment on β-galactosidase activity (black columns) and optical density (white columns) of suspension cells surrounding Pseudomonas aeruginosa biofilm-covered Ti-6Al-4V carriers. aP. aeruginosa DBM 3081, bP. aeruginosa DBM 3777, cP. aeruginosa ATCC 10145, dP. aeruginosa ATCC 15442. NTP treatment exposure time was 1 h for P. aeruginosa DBM 3081, 0.5 h for P. aeruginosa DBM 3777 and P. aeruginosa ATCC 10,145 and 0.25 h for P. aeruginosa ATCC 15,442. Antibiotic concentrations were for P. aeruginosa DBM 3081: 4 mg/L of GTM, 0.10 mg/L of CTZ and 3.5 mg/L of PMB; for P. aeruginosa DBM 3777: 9 mg/L of GTM, 1 mg/L of CTZ and 15 mg/L of PMB; for P. aeruginosa ATCC 10145: 6.5 mg/L of GTM, 0.5 mg/L of CTZ and 8.5 mg/L of PMB; and for P. aeruginosa ATCC 15442: 8.5 mg/L of GTM, 0.25 mg/L of CTZ and 7.5 mg/L of PMB. Results are given in relative percentages; control (untreated by any antibiofilm agent) represents 100%
Fluorescence microscopic visualization of P. aeruginosa mature biofilm exposed to NTP pre-treatment and subsequent GTM treatment
To confirm the results obtained by MTT assay, the untreated mature biofilm of P. aeruginosa and biofilm treated with GTM alone and with the combination of NTP treatment with subsequent GTM treatment were visualized using fluorescence microscope (Fig. 4). All strains of P. aeruginosa formed coherent mature biofilm on the surface of Ti-6Al-4V alloy carriers when untreated (Fig. 4a–d). The thickest complex biofilm structure was apparent in the case of P. aeruginosa ATCC 15442 (Fig. 4d), whereas P. aeruginosa DBM 3777 appeared to be the least potent biofilm producer (Fig. 4b). The treatment of P. aeruginosa formed mature biofilm with GTM alone resulted in a slight reduction in biofilm-covered surface (Fig. 4e–h). The mature biofilms of all P. aeruginosa strains tested were evenly eliminated on the entire surface (the uniform decrement of green signal) as shown in Fig. 4e–h. In the case of P. aeruginosa ATCC 15442, the strongest colonizer of Ti-6Al-4V alloy carriers, the NTP treatment with subsequent addition of GTM resulted in an almost complete eradication of the cells from the surface of the carrier.
Visualization of Pseudomonas aeruginosa biofilm on Ti-6Al-4V carriers treated with gentamicin (GTM) alone or combined with non-thermal plasma (NTP) pre-treatment using flurescent microscope. Untreated biofilm of P. aeruginosa DBM 3081 (a), P. aeruginosa DBM 3777 (b), P. aeruginosa ATCC 10145 (c) and P. aeruginosa ATCC 15442 (d); biofilm treated with GTM: P. aeruginosa DBM 3081 (e), P. aeruginosa DBM 3777 (f), P. aeruginosa ATCC 10145 (g) and P. aeruginosa ATCC 15442 (h); biofilm exposed to NTP and subsequent addition of GTM: P. aeruginosa DBM 3081 (i), P. aeruginosa DBM 3777 (j), P. aeruginosa ATCC 10145 (k) and P. aeruginosa ATCC 15442 (l). Scale bar = 50 µm
SEM visualization of P. aeruginosa ATCC 15442 mature biofilm exposed to NTP pre-treatment and subsequent GTM treatment
Detailed images of P. aeruginosa ATCC 15442 mature biofilm formed on Ti-6Al-4V alloy carriers and then treated by NTP alone or with subsequent GTM action were obtained using SEM (Fig. 5). The untreated mature biofilm of P. aeruginosa ATCC 15442 was very robust and the entire surface of otherwise highly porous carrier was completely covered by the biofilm (Fig. 5a). In the case of NTP treatment of mature biofilm alone (Fig. 5b), the biofilm still covered the whole carrier surface, although its complexity was disrupted as the pores of Ti-6Al-4V alloy were apparent (darker areas under cells in the picture), indicating a slight decrement in biofilm-covered area. GTM treatment alone resulted in a greater decrease in the biofilm-covered carrier area (Fig. 5c). The biofilm was eradicated from the carrier surface (light areas in the image) but the cells remained adhered to the pores of the alloy (dark areas in the image). The NTP treatment and subsequent addition of GTM to mature biofilm of P. aeruginosa ATCC 15442 resulted in a complete eradication of the biofilm from the carrier (Fig. 5d); this indicates a great potential of NTP exposure combined with antibiotic prophylaxis for the treatment of biofilm-related infections.
Visualization of Pseudomonas aeruginosa ATCC 15442 biofilm (a) on Ti-6Al-4V carriers treated with non-thermal plasma (NTP) (b) and gentamicin (GTM) (c) alone or with combined NTP pre-treatment and subsequent GTM treatment (d) using scanning electron microscope. NTP treatment exposure time was 0.25 h. Concentration of GTM was 8.5 mg/L. Scale bar = 30 µm, magnification: ×2500, detector: LVD, dwell time: 20 µs, spot size: 5
Discussion
Due to the emergence of antimicrobial resistance (particularly in biofilm cells) to conventional antibiotics that leads to an increase in their effective doses to excessive and toxic concentrations, novel approaches are being sought to treat biofilm-associated infections. One of the most serious pathogens is P. aeruginosa, a member of the ESKAPE group, whose complete biofilm eradication is extremely difficult (Mulani et al. 2019). For this purpose, we used the NTP treatment of mature P. aeruginosa biofilm to enhance the action of antibiotics and reduce their effective concentrations. The conventional antibiotics (GTM, CTZ, PMB) that we tested are routinely used in clinical practice, but the biofilm resistance towards them and severe side effects at high dosage have been described (Ciofu and Tolker-Nielsen 2019; Du et al. 2010; Zavascki et al. 2007).
The effect of NTP on P. aeruginosa biofilm has been investigated in several studies over the last two decades (Alkawareek et al. 2012; Matthes et al. 2013; Paldrychová et al. 2019; Soler-Arango et al. 2019; Triandafillu et al. 2003; Ziuzina et al. 2015). In general, it has been shown that NTP is an effective antibiofilm agent capable of complete reduction of metabolic activity of biofilm cells or their culturability but not fully eradicating them. Alkawareek et al. (2012) described a rapid decline in the number of surviving cells after NTP exposure. Their confocal laser scanning images depicted the biofilm cells with disrupted cell membranes but approximately the same biofilm biomass remained and the biofilm on their polystyrene carriers was thus not eradicated. Soler-Arango et al. (2019) demonstrated the same findings using SEM. Similarly, our previous study revealed a significant NTP activity in reducing total biofilm biomass of some P. aeruginosa strains, but the biofilms were not completely eradicated even after long exposure times, as proved also by fluorescence microscopy (Paldrychová et al. 2019). Therefore, the use of NTP for biofilm weakening appeared to be an appropriate alternative and we assumed that it should be better used as treatment to enhance subsequent antibiotic action.
Gupta et al. (2017) described the possibility of direct NTP treatment generated by plasma jet in combination with biocide CHX to sterilize titanium surface covered by P. aeruginosa PAO1 biofilm. The ability of NTP and CHX to sterilize titanium surface was evaluated by counting colony forming units and XTT metabolic assay after biofilm harvesting from titanium coupons using sonication. These methods showed a complete sterilization of this surface. However, SEM images depicted numerous residues of P. aeruginosa biofilm after combined treatment by NTP and CHX (used at a very high concentration of 10 g/L). Similarly, confocal laser scanning microscopy showed the remaining biofilm biomass after NTP plus CHX treatment. In the case of further cultivation, these persistent cells can re-develop into the mature biofilm.
In our study we demonstrated that NTP pre-treatment can lead to a significant decrease of effective concentrations of antibiotics; especially the GTM action was highly enhanced. This antibiotic was able to reduce the metabolic activity of P. aeruginosa biofilm cells after NTP treatment in the concentration range of 4–9 mg/L and fluorescence microscopy showed notable biofilm eradication rate in all strains tested. The greatest effect of the combination of the two antibiofilm agents was confirmed in the case of P. aeruginosa ATCC 15442 whose mature biofilm was almost completely eradicated (as shown by SYTO® 13 staining of biofilm biomass) after 0.25 h of NTP exposure and subsequent treatment with 8.5 mg/L of GTM. Simultaneously, the SEM images obtained in our work depicted a strong coverage of Ti-6Al-4V alloy carrier by P. aeruginosa ATCC 15442 mature biofilm untreated with any antibiofilm agent. The NTP or GTM treatment alone did not significantly affect the P. aeruginosa mature biofilm but their successive action caused complete eradication (only the typical surface of Ti-6Al-4V alloy without any adhered cells was shown). Hence, in comparison with other studies in this field, we achieved the eradication of P. aeruginosa biofilm biomass. Furthermore, it is important to note that our experimental setup included also the ability of persistent cells to re-develop biofilm after NTP exposure. These results can therefore be considered very important. In addition, the ability of the NTP/GTM combination to attenuate AHL-dependent QS systems of P. aeruginosa was evaluated. In general, the role of QS systems in the microbial world is an important area of research that has an impact on the strategies preventing biofilm formation or eradication (Choudhary and Schmidt-Dannert 2010). Although it was described that NTP is able to decompose commercially available AHL molecules exposed directly in suspension form (Flynn et al. 2016), in our previous study we showed that NTP is not a significant in vitro QS inhibitor in P. aeruginosa strains also tested in the present study (not more than 20% inhibition of QS). However, NTP treatment obviously enhanced the ability of GTM to either inhibit AHL-dependent QS systems or reduce microbial load and stress the bacteria resulting in reduced ALH production of most P. aeruginosa strains used (up to 90% inhibition of QS). It should be noted that the strongest colonizer of Ti-6Al-4V alloy, P. aeruginosa ATCC 15442, whose biofilm was completely eradicated by a combined action of NTP and GTM, was not greatly affected in its AHL production by the NTP/GTM treatment; thus QS is not the primary target for their action.
In conclusion, the use of a combination of NTP and antibiotics, which were primarily designed to inhibit the suspension growth of bacteria, could, by enhancing the effects of antibiotics, prolong their use in clinical practice.
References
Abramzon N, Joaquin JC, Bray J, Brelles-Marińo G (2006) Biofilm destruction by RF high-pressure cold plasma jet. IEEE Trans Plasma Sci 34:1304–1309
Alkawareek MY, Algwari QT, Laverty G et al (2012) Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS ONE 7:e44289
Alkawareek MY, Gorman SP, Graham WG et al (2014) Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int J Antimicrob Agents 43:154–160
Boles BR, Thoendel M, Singh PK (2005) Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57:1210–1223
Campoccia D, Montanaro L, Arciola CR (2006) The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27:2331–2339
Choudhary S, Schmidt-Dannert C (2010) Applications of quorum sensing in biotechnology. Appl Microbiol Biotechnol 86:1267–1279
Ciofu O, Tolker-Nielsen T (2019) Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol 10:913
Cole SJ, Records AR, Orr MW et al (2014) Catheter-associated urinary tract infection by Pseudomonas aeruginosa is mediated by exopolysaccharide-independent biofilms. Infect Immun 82:2048–2058
Conrads H, Schmidt M (2000) Plasma generation and plasma sources. Plasma Sources Sci Technol 9:441
Davey ME, O´toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
Davey ME, Caiazza NC, O'Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185:1027–1036
Davies DG, Parsek MR, Pearson JP et al (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298
Du S, Kuo H, Cheng C et al (2010) Molecular mechanisms of ceftazidime resistance in Pseudomonas aeruginosa isolates from canine and human infections. Vet Med 55:172–182
Du T et al (2013) Effect of modified nonequilibrium plasma with chlorhexidine digluconate against endodontic biofilms in vitro. J Endod 39:1438–1443
Dufour D, Leung V, Lévesque CM (2010) Bacterial biofilm: structure, function, and antimicrobial resistance. Endod Topics 22:2–16
Flynn PB, Busetti A, Wielogorska E et al (2016) Non-thermal plasma exposure rapidly attenuates bacterial AHL-dependent quorum sensing and virulence. Sci Rep 6:26320
Geetha M, Singh AK, Asokamani R et al (2009) Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Prog Mater Sci 54:397–425
Gilbert KB, Kim TH, Gupta R et al (2009) Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol Microbiol 73:1072–1085
Guo L et al (2018) Gas plasma pre-treatment increases antibiotic sensitivity and persister eradication in methicillin-resistant Staphylococcus aureus. Front Microbiol 9:537
Gupta TT, Karki SB, Matson JS et al (2017) Sterilization of biofilm on a titanium surface using a combination of nonthermal plasma and chlorhexidine digluconate. Biomed Res Int 2017:1–11
Hermsen ED, Sullivan CJ, Rotschafer JC (2003) Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect Dis Clin N Am 17:545–562
Jimenez PN, Koch G, Thompson JA et al (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65
Julák J, Scholtz V, Vaňková E (2018) Medically important biofilms and non-thermal plasma. World J Microbiol Biotechnol 34:178
Julák J., Vaňková E., Válková M et al (2020) Combination of non-thermal plasma and subsequent antibiotic treatment for biofilm re-development prevention. Folia Microbiol.
Khun J, Scholtz V, Hozák P et al (2018) Various DC-driven point-to-plain discharges as non-thermal plasma sources and their bactericidal effects. Plasma Sources Sci Technol 27:065002
Klebes M, Ulrich C, Kluschke F et al (2015) Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J Biophotonics 8:382–391
Koban I, Geisel MH, Holtfreter B et al (2013) Synergistic effects of nonthermal plasma and disinfecting agents against dental biofilms in vitro. ISRN Dent 2013:1–10
Kvam E, Davis B, Mondello F et al (2012) Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob Agents Chemother 56:2028–2036
Matthes R, Koban I, Bender C et al (2013) Antimicrobial efficacy of an atmospheric pressure plasma jet against biofilms of Pseudomonas aeruginosa and Staphylococcus epidermidis. Plasma Processes Polym 10:161–166
Mulani MS, Kamble EE, Kumkar SN et al (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10:539
Mulcahy LR, Isabella VM, Lewis K (2014) Pseudomonas aeruginosa biofilms in disease. Microb Ecol 68:1–12
Paldrychová M, Vaňková E, Scholtz V et al (2019) Effect of non-thermal plasma on AHL-dependent QS systems and biofilm formation in Pseudomonas aeruginosa: difference between non-hospital and clinical isolates. AIP Adv 9:055117
Pandey R, Berendt A, Athanasou N et al (2000) Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. Arch Orth Traum Surg 120:570–574
Peeters E, Nelis HJ, Coenye T (2008) Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165
Ren H, Liu Y, Zhou J, Long Y, Liu C, Xia B, Shi J, Fan Z, Liang Y, Chen S, Xu J, Wang P, Zhang Y, Zhu G, Liu H, Jin Y, Bai F, Cheng Z, Jin S, Wu W (2019) Combination of azithromycin and gentamicin for efficient treatment of Pseudomonas aeruginosa infections. J Infect Dis 220:1667–1678
Richards DM, Brogden R (1985) Ceftazidime. Drugs 29:105–161
Scholtz V, Kvasničková E, Julák J (2013) Microbial inactivation by electric discharge with metallic grid. Acta Phys Pol A 124:62–65
Sendi P, Banderet F, Graber P et al (2011) Clinical comparison between exogenous and haematogenous periprosthetic joint infections caused by Staphylococcus aureus. Clin Microbiol Infect 17:1098–1100
Soler-Arango J, Figoli C, Muraca G et al (2019) The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: a comprehensive model explaining plasma-mediated biofilm eradication. PLoS ONE 14:1–27
Sun Y, Yu S, Sun P et al (2012) Inactivation of Candida biofilms by non-thermal plasma and its enhancement for fungistatic effect of antifungal drugs. PLoS ONE 7:e40629
Trautner BW, Darouiche RO (2004) Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32:177–183
Triandafillu K, Balazs DJ, Aronsson BO et al (2003) Adhesion of Pseudomonas aeruginosa strains to untreated and oxygen-plasma treated poly (vinyl chloride)(PVC) from endotracheal intubation devices. Biomaterials 24:1507–1518
Ullal AJ, Pisetsky DS, Reich CF III (2010) Use of SYTO 13, a fluorescent dye binding nucleic acids, for the detection of microparticles in vitro systems. Cytometry Part A 77:294–301
van Delden C (2004) Virulence factors in Pseudomonas aeruginosa virulence and gene regulation. Springer, Boston, pp 3–45
Vaňková E, Válková M, Kašparová P et al (2018) Prevention of biofilm re-development on Ti-6Al-4V alloy by cometary discharge with a metallic grid. Contrib Plasm Phys 59:166–172
Vaňková E, Kašparová P, Dulíčková N et al (2020) Combined effect of lasioglossin LL-III derivative with azoles against Candida albicans virulence factors: biofilm formation, phospholipases, proteases and hemolytic activity. FEMS Yeast Res 20:foaa020
Volejníková A, Melicherčík P, Nešuta O et al (2019) Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J Med Microbiol 68:961–972
Zavascki AP, Goldani LZ, Li J et al (2007) Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215
Zelaya A, Vandervoort K, Brelles-Mariño G (2012) Battling bacterial biofilms with gas discharge plasma. Plasma for bio-decontamination. medicine and food security. Springer, Dordrecht, pp 135–148
Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65:158–168
Zimmerli W, Sendi P (2011) Pathogenesis of implant-associated infection: the role of the host. Semin Immunopathol 33:295–306
Ziuzina D, Boehm D, Patil S et al (2015) Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS ONE 10:e0138209
Acknowledgements
This work was supported by the “Operational Programme Prague—Competitiveness” (CZ.2.16/3.1.00/24503) and the “National Programme of Sustainability I”—NPU I LO1601 and Charles University research program “Progress Q25”.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MP, PK and ES. The first draft of the manuscript was written by MP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: MP and EV, Introduction: MP, Methodology: MP, Formal analysis and investigation: MP, PK and ES, Writing—original draft preparation: MP and EV, Writing—review and editing: VS, Funding acquisition: OM and JM, Resources: OM, Supervision: JJ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paldrychová, M., Vaňková, E., Kašparová, P. et al. Use of non-thermal plasma pre-treatment to enhance antibiotic action against mature Pseudomonas aeruginosa biofilms. World J Microbiol Biotechnol 36, 108 (2020). https://doi.org/10.1007/s11274-020-02891-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02891-6