Abstract
Anaerobic digestion is an effective process for the treatment of organic solid waste and wastewater and the production of biogas, which is a clean energy source. The carbon dioxide in the biogas can be converted into methane using hydrogen generated from water electrolysis through an approach referred to as power-to-gas. Recently, hydrogen has been added to digesters as an in-situ or ex-situ biogas upgrade to reduce the levels of carbon dioxide. Biogas production systems consist of microbial complexes with highly organized microorganisms in different niches, which can either produce or consume hydrogen. However, the produced endogenous hydrogen should be constantly consumed to maintain a low hydrogen partial pressure. This review addresses the biochemical processes of anaerobic digestion and hydrogen-related microorganisms, including fermentative acid-producing bacteria, syntrophic organic acid degrading bacteria, syntrophic acetate-oxidizing bacteria, homoacetogens, hydrogenotrophic methanogens, and newly reported hydrogen-dependent methylotrophic methanogens. This study also investigates (1) the role of endogenous hydrogen as an intermediate metabolite and of interspecies electron transfer in anaerobic digestion, (2) effects of exogenous hydrogen addition on microbial community structure and metabolic processes, and (3) recent developments regarding in-situ and ex-situ biogas upgrading systems via hydrogen addition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD) is a promising technology for the treatment of various organic wastes and production of renewable energy such as biogas. However, after removal of water, hydrogen sulfide, ammonia, and other trace impurities, the raw biogas with 40–50% CO2 (by volume) has a relatively low calorific value (approximately 20–25 MJ/m3), which cannot compete with that of natural gas (Angelidaki et al. 2018). The off-gas content of CH4 should be higher than 95% (v/v) for it to be introduced into the natural gas grid. The addition of H2 to convert excessive CO2 into CH4 has been proposed as a prospective biogas upgrade strategy. This H2 can be generated from water electrolysis, which can be driven by wind or solar power. The electrical power is transformed into a gaseous substance that can be stored and consumed through the existing natural gas grid, thereby combining two renewable energy sources (electricity and biogas) into biomethane (Luo et al. 2012).
In interspecies electron transport processes, H2 is essential as an external electron donor (Felchner-Zwirello et al. 2013), and the seventh order of methanogens has been reported to be hydrogen-dependent (Lang et al. 2015). Hydrogen is used not only for methanogenesis, but also for cell growth of hydrogenotrophic archaea (Lecker et al. 2017); therefore, the H2:CO2 ratio used for in-situ and ex-situ biological biogas upgrading is typically set to more than 4:1. The biogas upgrading process by H2 addition is mediated by complex microbial interactions (Bassani et al. 2015; Luo and Angelidaki 2012) (Fig. 1). In this context, only dissolved H2 is available to microbes. Therefore, the insoluble essence of H2 demands specific operational parameters, such as gas recirculation, specific reactor configuration, gas diffusion devices, and intense stirring (Guiot et al. 2011), to enhance the gas transfer coefficient (KLa). However, excessive dissolved H2 may inhibit acetogenic reactions and induce the accumulation of volatile fatty acids (VFAs), thereby severely disturbing the balance between VFAs producing and consuming microbes. The endogenous H2 is produced by acidogenic or acetogenic bacteria and consumed by either homoacetogenic bacteria or hydrogenotrophic methanogens. This ensures a low H2 partial pressure in the reactor, which enables proton reduction and energy preservation (Giovannini et al. 2016). Excessive H2 levels (> 10 Pa) can inhibit hydrolytic and fermentative microbial activity in dry AD processes (Liu and Whitman 2008). However, the adverse effect of H2 addition can be reverted as hydrogenotrophic bacteria populations proliferate, especially when extra CO2 is added to the reactor (Cazier et al. 2019). Furthermore, a strengthened hydrogen utilization ability would in turn promote the formation of a close syntrophic association between fermentative bacteria and methanogens. Moreover, fermentative temperature is also critical to determine the KLa value and to modulate the dominant biological pathways for the consumption of dissolved H2 (Zhu et al. 2019a). Many researchers have concluded that the addition of H2 to thermophilic anaerobic reactors is beneficial to hydrogenotrophic methanogens. Nevertheless, the aceticlastic methanogens commonly account for the majority of the archaea in mesophilic reactors (Chen et al. 2020; Xu et al. 2020).
Based on the abovementioned facts, this review provides a comprehensive overview on the role of endogenous and exogenous H2 in the microbiology of biomethane production systems. This study focuses on the various H2-assisted biogas upgrading technologies, and it presents incentives to further develop the biogas upgrading process.
Hydrogen as an interspecies electron transfer mediator
The AD process begins with a sophisticated interspecies electron transfer network driven by the interaction between syntrophic bacteria and methanogens. In this scenario, H2 acts as a diffusive electron mediator in the production of methane, for which methanogens utilize H2-donated protons to reduce CO2 (Shen et al. 2016). A bulk of genera belonging to Anaerolineae has been identified, and it can form syntrophic partnerships with methanogens (Nobu et al. 2016a). The stagnation of interspecies hydrogen transfer can lead to the accumulation of VFAs, particularly propionate and n-butyrate, which require the syntrophic partnership of microorganisms to scavenge the generated H2 and thereby maintain a low H2 partial pressure. When H2 is used for in-situ biogas upgrading through biological processes, continuous mixing is generally adopted to enhance the H2 gas–liquid mass transfer. However, this technique may also enlarge the interspecies distance, which can result in unfavorable conditions for propionate degradation (Shen et al. 2016). Formate is another interspecies electron flow buffer, and it can perform better than H2 when the interspecies distance is long, according to Fick’s diffusion law. In previous study, it has been reported that extra sodium formate can further enhance the methane production of in-situ biogas upgrading reactors to which H2 is already added (Zhu et al. 2019b).
Direct interspecies electron transfer (DIET) can supplement the role of electron donors via electrically conductive pili or outer surface c-type cytochromes, which provide a faster electron transfer rate (> 8.5-fold, e−1cp−1 s−1) than that of non-additive traditional AD fermentation (Park et al. 2018; Xu et al. 2019). The ability to transfer electrons during DIET is required for bacteria such as Desulfomicrobium, Desulfovirga, Geobacter, Streptococcus, and Thermovirga to serve as electron donors. Moreover, the abundance of several bacteria including Geobacter, Smithella, and Syntrophorhabdus that can transfer electrons either through indirect electron carrier (hydrogen or formate) or DIET was also improved after hydrogen was introduced to the anaerobic digester (Xu et al. 2020). This result indicates that the addition of H2 may favor DIET processing in AD.
Effects of hydrogen on microbial community structure and metabolic process
During AD process, complex organic matter must first be degraded into VFAs or alcohols by fermentative bacteria such as those belonging to the Firmicutes and Thermotogae phyla. These intermediates produced by fermentative bacteria should be further converted by acetogenic bacteria into methane precursors, including endogenous H2, acetate, and formate (Schuchmann and Müller 2014). Most reported acetogens belong to the Clostridium and Acetobacterium genera (e.g., Acetobacterium woodii, Clostridium thermaceticum). The introduction of exogenous H2 suppresses acetogens and syntrophic acetate oxidizers such as Syntrophaceticus schinkii and Thermacetogenium phaeum, which depend on the energy from the acetate oxidation to produce H2 and CO2 (Demirel and Scherer 2008).
Methanogenesis is the last and most critical step for biomethane production. It is vital to clearly distinguish the various pathways and the metabolic functional characteristics of methanogens related to H2. Only the Methanosarcina genus and Methanosaetaceae family are responsible for acetoclastic methanogenesis. The Methanosarcina genus can feed on H2, CO2, acetate, methyl alcohol, and methylamine. In contrast, Methanosaetaceae have a higher affinity for acetate even at concentrations below 1 mM, as they cannot live on any other substrate (Smith and Ingramsmith 2007; Vrieze et al. 2012). Another two major methanogenesis pathways have been identified, namely the hydrogenotrophic and methylotrophic pathways, which can convert hydrogen or methyl-C1 compounds into methane. Methylotrophic methanogens can be further classified into hydrogen-dependent and without specific functional genes, in which the latter performs restricted methanogenesis via methylated thiol reduction (Nobu et al. 2016b). In the absence of hydrogen, methanol can be used as the sole fermentative substrate for methane production by strict methyl-dependent methanogens such as Methanolobus and Methanococcoides, which can oxidize 1 mol of methyl groups to obtain enough reducing activity for methane production from 3 mol of methanol. The hydrogen-rich conditions of the anaerobic system may favor Methanosphaera-dominated methylotrophic methanogenesis, whereby the reducing power arises from the H2 catalytic reaction mediated by the membrane-bound methyl viologen hydrogenase. Approximately 30% of the methane is produced by hydrogenotrophic methanogenesis with hydrogen as a proton donor coupled with various CO2 substrates. Alcohols can also be reduced for methane production with the assist of coenzyme F420, and methanogens such as Methanothermobacter thermoautotrophicus and Methanosarcina barkeri can yield methane when CO is provided as the sole carbon and energy source (H2O also participates in this reaction).
Excessive dissolved H2 in liquid (either endogenous or exogenous) increases the hydrogen partial pressure in the AD reactor, and this overload of hydrogen partial pressure can hinder VFAs degradation. In AD, at least two pathways are involved in the decrease of H2 partial pressure. The first one is mediated by hydrogenotrophic methanogenic microbes, where CO2 is directly converted to CH4 while consuming H2 as an electron source. This reaction is highly thermodynamically favorable, as shown in Eq. (1). The second pathway indirectly contributes to CH4 production via H2 utilization. Homoacetogenic bacteria and acetoclastic methanogens use hydrogen to convert CO2 into acetate via the Wood-Ljungdahl pathway, after which the acetate is further consumed to generate CH4 (Angelidaki et al. 2018). This indirect pathway is also an exergonic process with two energy gain steps that compensate each other [Eq. (2, 3)].
The seventh order of methanogens (Methanomassiliicoccales), which are obligately dependent on molecular H2 to oxidize their growth substrate of methylamines to CO2, was recently discovered (Lang et al. 2015). Because the H2 concentration is essential for the metabolic processes of some bacteria, H2 addition can disturb the endogenous hydrogen production and consumption balance in AD systems, thus placing strong selective pressure on the microbial community and favoring the proliferation of both hydrogenotrophic methanogens and homoacetogenic bacteria. In turn, as H2 consumption increases, a closer syntrophic association may occur between fermentative bacteria and methanogens. Moreover, H2 addition can improve ATP (adenosine-triphosphate) concentration in AD systems, which suggests that part of the added H2 is utilized for microbial growth rather than for methanogenesis, as shown in Eq. (4) (Dupnock and Deshusses 2019).
In-situ and ex-situ biogas upgrade via hydrogen addition
Among various biogas upgrading strategies, the addition of exogenous H2 to AD reactors has been demonstrated to be an efficient way to upgrade biogas and utilize CO2. For biomethane production, in-situ and ex-situ methods can be used. In addition, hybrid systems combine in-situ and ex-situ pathways to further increase the biogas upgrading efficiency (Fig. 2). In ex-situ biogas upgrade method, exogenous CO2 can be used, and the biogas upgrading efficiency is significantly improved compared to that of the in-situ technique. However, the enrichment of hydrogenotrophic methanogens usually requires a long reaction time. In contrast, the simplified AD process in ex-situ reactors will inevitably decrease the degradation capacity of organic waste. The hybrid system can overcome the limitation of pH increase that usually occurs in the in-situ system, and it requires a relatively small separate reactor such as the one in the ex-situ system.
The most distinct difference between in-situ and ex-situ biogas upgrading reactors is that hydrogenotrophic methanogenesis is selected in a simplified biological system for the ex-situ approach. In the in-situ approach, the injected H2 is combined with the CO2 produced in the reactor, thereby producing CH4 through the activity of autochthonous microbes. Moreover, hydrogenotrophic methanogens are anthropogenically enriched in the AD reactor, while H2 and CO2 are externally supplied to produce CH4 (Rittmann et al. 2015). Previous study on in-situ biogas upgrading demonstrate that pH increase is the main technical challenge for both mesophilic and thermophilic conditions, especially when the pH increases to more than 8.5 (Luo and Angelidaki 2013a). Therefore, pH should be constantly monitored and controlled during the entire AD process for a methane recovery of approximately 99% to be achieved. To address this issue, co-digestion is an effective method that can be used to maintain an optimal pH range during the biogas upgrading process. H2 addition can also lead to problems linked to VFA/alcohol oxidation, which must be carefully considered as high H2 partial pressure (> 10 Pa) is not thermodynamically feasible in the AD process (Siriwongrungson et al. 2007). H2-linked AD inhibition can be reverted, as hydrogenotrophic bacteria proliferates in response to long-term H2 exposure. Nevertheless, ex-situ biogas upgrading has been proposed by several studies to address the drawbacks of the in-situ approach. Compared to in-situ biogas upgrading systems, the ex-situ method has the following advantages (Bassani et al. 2017; Kougias et al. 2017): (1) pure or enriched hydrogenotrophic methanogens can enhance the biogas upgrading rate without generating negative effects on the conventional AD process, (2) the biochemical processes involved are easier to manage, as only hydrogenotrophic methanogenesis occurs, without the need for an initial organic substrate degradation step, and (3) it uses CO2 gas, thus effectively controlling CO2 emissions.
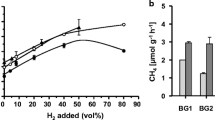
The key obstacle for in-situ and ex-situ biogas upgrading via hydrogen addition is the limited gas–liquid mass transfer rate, previous study suggested a scenario in which the specific transport coefficient of H2 (KLaH2) must reach 21 h−1 to meet the biomethane standard according to the modified AD model No. 1. However, this value is far over the typical KLaH2 value of approximately 9 h−1 in traditional anaerobic digesters (Bensmann et al. 2014). Therefore, several specific operational parameters including reactor type (Kougias et al. 2017), gas recirculation rate (Guiot et al. 2011), gas diffusion device, and stirring intensity (Luo and Angelidaki 2013b; Luo et al. 2014) should be improved to compensate for said limitation. The reactor type determines the basic elements for the anaerobic bioengineering. Recent studies have demonstrated that upflow column, trick-bed, and bubble column reactors can increase H2 and CO2 conversion efficiency by more than 98% (Bassani et al. 2017; Dupnock and Deshusses 2019; Rachbauer et al. 2016). Additionally, Luo and Angelidaki (2013b) determined that installing hollow-fiber membrane biofilms in biogas upgrading reactors can enhance the dissolved H2 rate from 930 to 1760 mL/(L day) along with a 96.1% methane yield. Moreover, a mild gas recirculation and the addition of packing materials (Raschig rings and alumina ceramic sponge) have been adopted to enhance the CH4 yield from 58 to 82% in an in-situ thermophilic granular upflow anaerobic sludge blanket (UASB) reactor (Bassani et al. 2017). Larger pore size diffusion devices for H2 and CO2 injection were also demonstrated to have better kinetics and output-gas quality. The dissolved H2 ratio was enhanced by increasing the mixing speeds or changing the mixing pattern from intermittent to continuous in the stirred tank reactor.
The in-situ and ex-situ biological upgrading technologies are compared in Table 1. The most efficient H2 conversion efficiency of approximately 100% was achieved in a bubble column reactor when the ex-situ biogas upgrading system was adopted. Furthermore, the enrichment of hydrogenotrophic methanogen cultures in ex-situ biogas upgrading systems typically requires substantial time, because an acclimation stage is needed for the microbes to acquire the ability to efficiently ferment the exogenous H2 and CO2 gases. For example, a CH4 content exceeding 96% was reported in an immobilized hydrogenotrophic bacteria culture after 8 months (Rachbauer et al. 2016). However, the homoacetogenesis may gradually increase and consume 11 to 43% of the dissolved H2 after a long-term acclimation period, especially when pretreated inocula is repeatedly used for cultivation (Saady 2013). Moreover, the enrichment of hydrogenotrophic microbes by in-situ H2 addition would compete with acetoclastic methanogens, and this may severely disturb the inherent micro-ecological balance based on the acetate metabolism. In contrast, the ex-situ microbiological biogas upgrading system has the advantage of applying individual hydrogenotrophic methanogenesis bioprocesses, thereby being more suitable for industrial applications.
The hybrid system combines the in-situ and ex-situ pathways. Partially upgraded biogas produced from an in-situ bioreactor is subsequently injected into an ex-situ reactor, which is currently under development for further improvement of the biomethane production efficiency. A major issue for all the aforementioned power-to-gas technologies in industrial application is the intermittency caused by the intermittent production of the renewable energy (wind or solar power) used for the water electrolysis (Ren et al. 2017). In this context, the biological reactions should not be stopped, because microbes may be affected by changes in hydrogen input. Therefore, further research to avoid this negative interruption of H2 supply is needed (Frank et al. 2018).
Conclusion
Biological biogas upgrading by external H2 addition is a promising technology that can provide a novel alternative for the integration of electricity storage and bio-natural gas production. However, conventional AD is a complex reaction between microorganisms, and the endogenous H2 concentration is essential for the equilibrium of biochemical reactions. The injection of exogenous H2 into bioreactors increases the hydrogen partial pressure and disturbs the balance between microbes. However, only the hydrogenotrophic methanogenesis is essential to convert CO2 and added H2 into CH4. Moreover, hydrogenotrophic methanogens have higher competence compared to aceticlastic methanogens under thermophilic digestion conditions. Therefore, the thermophilic ex-situ biogas upgrading system is recommended for industrial application.
References
Angelidaki I, Treu L, Tsapekos P, Luo G, Campanaro S, Wenzel H, Kougias PG (2018) Biogas upgrading and utilization: current status and perspectives. Biotechnol Adv 36(2):452–466
Bassani I, Kougias PG, Treu L, Angelidaki I (2015) Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ Sci Technol 49(20):12585–12593
Bassani I, Kougias PG, Treu L, Porté H, Campanaro S, Angelidaki I (2017) Optimization of hydrogen dispersion in thermophilic up-flow reactors for ex situ biogas upgrading. Bioresour Technol 234:310–319
Bensmann A, Hanke-Rauschenbach R, Heyer R, Kohrs F, Benndorf D, Reichl U, Sundmacher K (2014) Biological methanation of hydrogen within biogas plants: a model-based feasibility study. Appl Energy 134:413–425
Burkhardt M, Busch G (2013) Methanation of hydrogen and carbon dioxide. Appl Energy 111:74–79
Cazier EA, Trably E, Steyer J-P, Escudie R (2019) Reversibility of hydrolysis inhibition at high hydrogen partial pressure in dry anaerobic digestion processes fed with wheat straw and inoculated with anaerobic granular sludge. Waste Manage 85:498–505
Chen H, Hao S, Chen Z, Sompong O, Fan J, Clark J, Luo G, Zhang S (2020) Mesophilic and thermophilic anaerobic digestion of aqueous phase generated from hydrothermal liquefaction of cornstalk: Molecular and metabolic insights. Water Res 168:115199
Demirel B, Scherer P (2008) The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev Environ Sci Bio/Technol 7(2):173–190
Dupnock TL, Deshusses MA (2019) Detailed investigations of dissolved hydrogen and hydrogen mass transfer in a biotrickling filter for upgrading biogas. Bioresour Technol 290:121780
Felchner-Zwirello M, Winter J, Gallert C (2013) Interspecies distances between propionic acid degraders and methanogens in syntrophic consortia for optimal hydrogen transfer. Appl Microbiol Biotechnol 97(20):9193–9205
Frank E, Gorre J, Ruoss F, Friedl MJ (2018) Calculation and analysis of efficiencies and annual performances of power-to-gas systems. Appl Energy 218:217–231
Giovannini G, Donoso-Bravo A, Jeison D, Chamy R, Ruíz-Filippi G, Vande Wouwer A (2016) A review of the role of hydrogen in past and current modelling approaches to anaerobic digestion processes. Int J Hydrog Energy 41(39):17713–17722
Guiot SR, Cimpoia R, Carayon G (2011) Potential of wastewater-treating anaerobic granules for biomethanation of synthesis gas. Environ Sci Technol 45(5):2006–2012
Kim S, Choi K, Chung J (2013) Reduction in carbon dioxide and production of methane by biological reaction in the electronics industry. Int J Hydrog Energy 38(8):3488–3496
Kougias PG, Treu L, Benavente DP, Boe K, Campanaro S, Angelidaki I (2017) Ex-situ biogas upgrading and enhancement in different reactor systems. Bioresour Technol 225:429–437
Lang K, Schuldes J, Klingl A, Poehlein A, Daniel R, Brune A (2015) New mode of energy metabolism in the seventh order of methanogens as revealed by comparative genome analysis of “candidatus methanoplasma termitum”. Appl Environ Microb 81(4):1338–1352
Lecker B, Illi L, Lemmer A, Oechsner H (2017) Biological hydrogen methanation—a review. Bioresour Technol 245:1220–1228
Liu Y, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the Methanogenic archaea. Ann N Y Acad Sci 1125(1):171–189
Luo G, Angelidaki I (2012) Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol Bioeng 109(11):2729–2736
Luo G, Johansson S, Boe K, Xie L, Zhou Q, Angelidaki I (2012) Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol Bioeng 109(4):1088–1094
Luo G, Angelidaki I (2013a) Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: process performance and microbial insights. Appl Microbiol Biotechnol 97(3):1373–1381
Luo G, Angelidaki I (2013b) Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl Microbiol Biotechnol 97(8):3739–3744
Luo G, Wang W, Angelidaki I (2014) A new degassing membrane coupled upflow anaerobic sludge blanket (UASB) reactor to achieve in-situ biogas upgrading and recovery of dissolved CH4 from the anaerobic effluent. Appl Energy 132:536–542
Nobu MK, Dodsworth JA, Murugapiran SK, Rinke C, Gies EA, Webster G, Schwientek P, Kille P, Parkes RJ, Sass H, Jørgensen BB, Weightman AJ, Liu W-T, Hallam SJ, Tsiamis G, Woyke T, Hedlund BP (2016a) Phylogeny and physiology of candidate phylum ‘Atribacteria’ (OP9/JS1) inferred from cultivation-independent genomics. ISME J 10(2):273–286
Nobu MK, Narihiro T, Kuroda K, Mei R, Liu W-T (2016b) Chasing the elusive euryarchaeota class WSA2: genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J 10(10):2478–2487
Park J-H, Kang H-J, Park K-H, Park H-D (2018) Direct interspecies electron transfer via conductive materials: a perspective for anaerobic digestion applications. Biores Technol 254:300–311
Rachbauer L, Voitl G, Bochmann G, Fuchs W (2016) Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor. Appl Energy 180:483–490
Ren G, Liu J, Wan J, Guo Y, Yu D (2017) Overview of wind power intermittency: impacts, measurements, and mitigation solutions. Appl Energy 204:47–65
Rittmann S, Seifert A, Herwig C (2015) Essential prerequisites for successful bioprocess development of biological CH4 production from CO2 and H2. Crit Rev Biotechnol 35(2):141–151
Saady NMC (2013) Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrog Energy 38(30):13172–13191
Schuchmann K, Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12(12):809–821
Shen L, Zhao Q, Wu X, Li X, Li Q, Wang Y (2016) Interspecies electron transfer in syntrophic methanogenic consortia: from cultures to bioreactors. Renew Sustain Energy Rev 54:1358–1367
Siriwongrungson V, Zeng RJ, Angelidaki I (2007) Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res 41(18):4204–4210
Smith KS, Ingramsmith C (2007) Methanosaeta, the forgotten methanogen? Trends Microbiol 15(4):150–155
Vrieze JD, Hennebel T, Boon N, Verstraete W (2012) Methanosarcina : the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol 112(5):1–9
Xu H, Chang J, Wang H, Liu Y, Zhang X, Liang P, Huang X (2019) Enhancing direct interspecies electron transfer in syntrophic-methanogenic associations with (semi)conductive iron oxides: Effects and mechanisms. Sci Total Environ 695:133876
Xu H, Wang KJ, Zhang XQ, Gong H, Xia Y, Holmes DE (2020) Application of in-situ H2 assisted biogas upgrading in high-rate anaerobic wastewater treatment. Bioresour Technol 299:122598
Zhu X, Chen L, Chen Y, Cao Q, Liu X, Li D (2019a) Differences of methanogenesis between mesophilic and thermophilic in situ biogas-upgrading systems by hydrogen addition. J Ind Microbiol Biot 46(11):1569–1581
Zhu XP, Cao Q, Chen YC, Sun XY, Liu XF, Li D (2019b) Effects of mixing and sodium formate on thermophilic in-situ biogas upgrading by H2 addition. J Clean Prod 216:373–381
Acknowledgements
This research was jointly supported by the National Key R&D Program of China (2019YFD1100603), Chengdu International Science and Technology Cooperation Project (2019-GH02-00024-Hz), West Light Foundation of the Chinese Academy of Sciences (2018XBZG_XBQNXZ_A_004, 2019XBZG_JCTD_ZDSYS_001), Youth Innovation Promotion Association of the Chinese Academy of Sciences (2017423), and Special fund for talented persons of Sichuan provincial Party Committee Organization Department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, X., Zhou, P., Chen, Y. et al. The role of endogenous and exogenous hydrogen in the microbiology of biogas production systems. World J Microbiol Biotechnol 36, 79 (2020). https://doi.org/10.1007/s11274-020-02856-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02856-9