Abstract
This study evaluated the effect of three sulfate salt-based culture media on the reprecipitation of sulfur under the action of two types of bacterial inoculum, a pure strain of Acidithiobacillus ferrooxidans (ATCC 23270) and a consortium of this strain and Acidithiobacillus thiooxidans (ATCC 15494), in a biodesulfurization process for coal (particle size < 0.25 mm) from the ‘La Guacamaya’ mine (Puerto Libertador, Córdoba, Colombia). All of the experiments were periodically monitored, with measurements taken of pH, cell concentration, iron concentration, and pyrite oxidation. Additionally, mineralogical analyses were conducted on the initial and final coal samples, through scanning electron microscopy with an energy-dispersive X-ray spectrometer. The results showed that sulfate reprecipitation occurred primarily, and nearly entirely, during the first 3 days of the process. While all the treatments obtained high levels of mineral oxidation, the reprecipitation processes decreased in media with low concentrations of sulfate, leading to the higher final removal of inorganic sulfur. The bioassays revealed that after 15 days, the maximum pyrite oxidation (86%) and inorganic sulfur removal (53%) was obtained with the treatments using the Kos and McCready culture media. The bacteria evaluated were found to have a great ability to adapt to very simple culture media with minimal nutrient concentrations, and even with some nutrients absent (as in the case of magnesium).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The microorganisms that participate in mineral bioleaching processes are of great interest due to their metabolic diversity and great capacity to adapt to highly hostile environments. However, in order to complete metabolic pathways, proper growth, cell material biosynthesis, and energy production and maintenance, they require a sufficient availability and supply of primary materials and nutrients, appropriate environmental conditions, and adequate control of toxic substances, among other factors (Prescott et al. 2004; Rossi 1990). Silverman and Lundgren (1959) initially proposed the 9 K medium, but it was observed that the excessive concentration of certain nutrients promoted the formation of basic ferric iron hydroxysulfates like jarosite. These generate adverse effects on the process kinetics, since—among other problems—upon forming they precipitate on the surface of minerals, affecting the proper solubilization and recovery of the metal of interest and seriously impacting the efficiency of the process (Kaksonen et al. 2014a, b; Nurmi et al. 2010). Furthermore, this type of material can block pumps, tubes and valves in bioreactors, generating problems at an industrial level (Gómez and Cantero 2005). Additionally, the reprecipitation of hydrated iron sulfates in biodesulfurization processes causes part of the sulfur that has already been leached to remain associated to the coal as a different form of sulfur, decreasing the efficiency of the desulfurization process as such. It is thus thought that more efficient biodesulfurization can be achieved by seeking to understand the iron precipitation phenomena present in the leaching solutions, in order to identify appropriate strategies for managing and controlling the parameters that most influence the proper progression of this type of process (Casas 2000).
The formation of these precipitates depends primarily on the pH, the ionic composition, and the concentration of different compounds in the culture medium (Gómez and Cantero 2005). Additionally, it is believed that high concentrations of cations, ferric ions, and sulfate promote jarosite formation (Gramp et al. 2008; Cunha et al. 2008). Jarosite formation can be caused by an excessive concentration of certain nutrients in the culture media. To decrease these difficulties, Tuovinen and Kelly (1972) proposed the T&K medium, which has lower concentrations of these reagents (Rossi 1990). Other culture media have been developed with low concentrations of phosphate and sulfate; these have been employed in the leaching of uranium minerals (McCready et al. 1986) and different types of coal (Kos et al. 1983; Rossi 1990).
The goal of this study is to show the evaluation of ferric iron sulfate precipitated in coal biodesulfurization. Three culture media were evaluated, holding a different concentration of sulfate salt to assess its influence on this kind of processes.
Materials and methods
Coal sample, physical–chemical and mineralogical analysis
The coal used in this study was extracted from the ‘La Guacamaya’ mine, located in the municipality of Puerto Libertador in the Colombian department of Córdoba. The coal sample was processed until it reached a particle size or diameter that passed through 60 mesh (< 0.25 mm).
The proximate analyses were conducted on air-dried basis (Total sulfur—ASTM D4239; Residual moisture—ASTM D3173; Ashes—ASTM D3174; Volatile material—ASTM D3175; Fixed carbon—ASTM D3172; Superior calorific power—ASTM D5865) and analysis of different forms of sulfur present in the coal (ASTM D 2492) (ASTM 2007a, b; ASTM 2008; ASTM 2011a, b, c, d).
To determine the composition and the presence of minerals in the coal sample, an analysis of Fourier Transformed Infrared Spectroscopy (FTIR) was performed using the spectrometer.
Shimadzu FTIR 8400 S. The sample was crushed up to 200 mesh particle-size and the analyses were carried out in transmittance mode, 24 scans, resolution of 2.0 cm−1, apodization Happ Genzel, in a scan range between 400 cm−1 and 4000 cm−1. The spectra obtained was analysed using Shimadzu IR Solution 1.30 software. For the initial coal sample, the Essential FTIR v3.10.041 software was additionally used, which allowed to find the second derivative of the sample spectrum and identify overlapping peaks.
Culture media
Three culture media reported on the literature were evaluated; they were composed of basal sulfate salts, the T&K medium (Tuovinen and Kelly 1972), commonly used for this type of microorganisms, the Kos medium (Kos et al. 1983) and McCready medium (McCready et al. 1986). The composition of these media is shown in Table 1.
Microorganisms
The microorganisms selected for the study were the commercial bacteria Acidithiobacillus ferrooxidans (ATCC 23270) and Acidithiobacillus thiooxidans (ATCC 15494), sourced from the American Type Culture Collection (ATCC) and supplied by the Biomineralogy and Biohydrometallurgy Laboratory (LBB) of the Universidad Nacional de Colombia at Medellín. The two types of inoculum used were previously adapted to the presence of coal and the different culture media, through progressive stages. The tests were performed with an inoculum of the pure strain 10% v/v of A. ferrooxidans at a concentration of 7.5 × 108 and the consortium composed of 5% v/v of A. thiooxidans at a concentration of 7.8 × 108 and 5% v/v of A. ferrooxidans at 7.5 × 108.
Coal biodesulfurization experiments
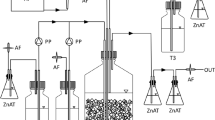
The coal biodesulfurization experiments were conducted in 500 mL Erlenmeyer flasks with an effective volume of 200 mL, 10% v/v of inoculum, 10% w/v of coal pulp, an optimal growth temperature of 30 °C ± 1 °C, initial pH of 1.6 ± 0.03, and an agitation velocity of 180 rpm, during a 15-day process. 10% w/v of coal pulp was selected, based on previous research carried out by Caicedo et al. (2011) and Cardona and Márquez (2009). Besides, this research was conducted to use these results in a pilot-scale process and at full scale. The variables evaluated were: (a) Microorganisms: pure strain and consortium; (b) Culture media: Kos, McCready, and T&K. Assays were performed in duplicate, counted with abiotic controls for each evaluated culture medium, characterized by having no presence of microorganisms. Each process was periodically monitored by measuring pH, cell concentration with Neubauer chamber, iron concentration in solution and reprecipitated iron, using the standard method 3500-Fe B (Eaton et al. 2005; APHA 1992). The Fe3+ concentration was determined by measuring the ferrous ions (Fe2+) and total iron and the concentration of pyrite in the coal, using the standard method ASTM D 2492. A 3 × 2 factorial design was used and the data were processed with R statistical software. A 3 × 2 factorial design was used, and data processing was done through the statistical software R version 3.0.3. Through the R software, the analysis of variance (ANOVA) and comparison of means with the Tukey test were carried out, and the level of significance selected was 0.05 for all analyzes. Statistical analyses apply to ranges of the variables evaluated and are valid on the tests conducted in the research. At the end of the process, scanning electron microscopy (SEM) was performed with a solid-state detector EDX (Energy Dispersive X-ray Spectrometer). In order to characterise the coal sample, it was used the SEM, JEOL JSM 5910 LV, and the EDX Oxford model 7324 detector, which allowed to do microchemical analysis. The conditions used were a 20 kV voltage, on a length of 10 mm, a 50 spot size, BEC signal, and high vacuum.
Results
Sulfur forms and proximate analysis of coal
Table 2 shows the primary characteristics and properties of the type of coal used, found through proximate analysis of total sulfur and different forms of sulfur.
It can be observed that pyritic sulfur was the most abundant form, corresponding to approximately 50% of the total sulfur present in the coal.
Fourier Transformed Infrared Spectroscopy (FTIR)
Figure 1 shows the FTIR spectrum for the initial coal sample. The organic fraction of coal is represented by the bands at 750 cm−1, 810 cm−1, 1250 cm−1, 1372 cm−1, 1436 cm−1, 1600 cm−1, 2850 cm−1, 2920 cm−1, where aromatic bonds CH, ether and aromatic esters, aliphatic chains of CH3 and CH2, aromatic nuclei C=C and carbonyl groups C=O, mainly (Longfei et al. 2020; Bhupendra and Barun 2019; Qiu et al. 2011; Sonibare et al. 2010; Munkhtsetseg et al. 2007; Saikia et al. 2007).
Bands corresponding to Si–O vibrations were observed indicating the presence of quartz at 471 cm−1, 536 cm−1, 693 cm−1, 760 cm−1, 864 cm−1, 1163 cm−1 and clay minerals such as kaolinite and illite at 471 cm−1, 536 cm−1, 760 cm−1, 864 cm−1, 1032 cm−1 (Qiu et al. 2011; Sonibare et al. 2010; Saikia et al. 2007; Suraj et al. 1997; Oinuma and Kodama 1967). A peak between 3600 and 3800 cm−1 corresponds to the OH functional group, associated with clay minerals such as kaolinite (Sonibare et al. 2010; Saikia et al. 2007). The coal sample contains calcium sulfate (gypsum) at 670 cm−1 (Schiavon 2007). The presence of OH groups associated with jarosite in the initial coal sample at 510 cm−1, 630 cm−1, 1090 cm−1, 1170 cm−1 were also verified (Baruah et al. 2003). Pyrite was observed at the end of the spectrum, in the 420 cm−1 band (Qiu et al. 2011; Saikia et al. 2007).
With the second derivative of the spectrum, some bands that could have overlapped in the FTIR analysis were identified, and it was confirmed the presence of some minerals in the initial coal sample. Bands corresponding to clay minerals such as kaolinite and illite (3800–3600 cm−1, 1100 cm−1, 1008 cm−1, 912 cm−1, 800 cm−1–700 cm−1, 529 cm−1) were identified (this analysis is not presented in this article).
Biodesulfurization process
Figure 2 shows the behavior of pH over time for the different treatments. In general, the tendency of this parameter in all of the assays (biotic and abiotic) was to increase during the first 3 days of the process, and later remain nearly constant in the treatments in which the pure strain was used.
Meanwhile, for the assays containing the consortium, after the third day the pH progressively decreased until the twelfth day, when it finally became stable until the end of the process. The abiotic controls increased the pH at the beginning of the process; it later stabilized until the end of the process.
Figure 3 shows the behavior of the cell concentration over time. In all of the treatments, a lag phase was observed at the beginning of the process.
For the assays with the pure strain, this was followed by exponential growth until the ninth day with the Kos and McCready assays and until the twelfth day with the T&K assay, after which point stability was reached for all treatments. Meanwhile, in the treatments containing the consortium, the exponential phase continued until the end of the process.
For the pure strain, the maximum cell concentrations obtained were: 3.1 × 107 cells/mL in the suspensions with T&K, 2.5 × 107 cells/mL with Kos, and 2.0 × 107 cells/mL with McCready. Meanwhile, the maximum cell concentrations reached for the consortium were: 5.0 × 107 cells/mL in the suspensions with Kos, 3.7 × 107 cells/mL with McCready, and 3.0 × 107 cells/mL with T&K.
Figure 4 presents the percentage of solubilized iron over time. In all the treatments, iron solubilization was observed from the beginning of the process. This increased until the twelfth day, after which it had a slight decreasing trend for the pure strain in the assays using Kos and McCready and an increasing trend for the assay with T&K until the end of the process. Meanwhile, for the consortium, this parameter stabilized until the fifteenth day.
Finally, for the pure strain, the iron concentrations observed in solution were 34%, 35% and 38% when the T&K, Kos and McCready media were used, respectively. For the consortium, higher solubilization was observed from the start of the process in the assays with Kos and McCready, and the highest percentage of iron in solution was thus observed in these suspensions (40% Fe), followed by the T&K assay, which obtained 36% Fe in solution.
All of the abiotic controls exhibited iron solubilization at the beginning of the process (day 3), while the controls with the McCready and Kos media showed a slight increasing trend until the end of the process, obtaining final Fe solubilization of 12%. Meanwhile, the control assay using T&K presented a considerable decrease after initial solubilization, which remained constant until the end of the process.
Figure 5 shows the percentage of reprecipitated iron in the mineral over time. In overall, the initial phase in all of the treatments, a considerable reprecipitation occurred, followed by the second phase of stabilized reprecipitation, and finally by a precipitate solubilization phase.
Finally, in the suspensions with the pure strain, the highest reprecipitation percentage was observed when the Kos medium was used (35%), followed by the T&K (34%) and McCready (31%) treatments. With the consortium, lower reprecipitation percentages were obtained, which were similar for all treatments. These were 25% with Kos and 28% with McCready and T&K.
Figure 6 shows the concentration of ferric ions vs. time. In all of the tests, an upward trend in the increase in ferric ions was observed starting at the beginning of the process. This continued until the end of the process for the T&K assay, while growth stabilized after day 12 with the assays using the Kos and McCready media. Meanwhile, the abiotic controls presented a low ionic concentration in solution, which remained nearly constant throughout the process.
Table 3 shows the analysis of different forms of sulfur from the treated coal. In quantitative terms, a clear decrease in pyritic sulfur content can be observed for all of the treatments, with the bioassay composed of the Kos medium and any type of inoculum obtaining the highest removal. Meanwhile, the concentration of sulfur originating from the sulfates was higher than the content present in the initial coal sample, and was highest in the suspensions using the T&K culture medium. Inorganic sulfur depends on the concentration of pyritic sulfur and on the sulfur originating from the sulfates in the solution, therefore, it is considered the highest sulfur removal occurred in the treatments using the Kos and McCready culture media.
In the presence of the consortium it could be guaranteed with greater certainty that high percentages of pyrite oxidation would be obtained with any of the culture media evaluated.
The highest percentage of inorganic sulfur removed for the pure strain occurs in the bioassay with the McCready culture medium, and when a consortium of acidophilic bacteria is used, the suspension with the Kos medium is the most suitable for removing sulfur.
The results of the ANOVA analyzes was not supported, however, statistically, testing showed that there were no significant differences among the different types of culture medium (p value of 0.12), or inoculum (p value of 0.24) used. However, the Tukey test found that the above mentioned statistical results were due to the high similarity that existed between the bioassays with the Kos and McCready media (p value of 0.96), while the results found with the T&K medium were not equally satisfactory (T&K and McCready: p value of 0.19; T&K and Kos: p value of 0.14). This is the reason is due to the highest percentages of inorganic sulfur removal were obtained with the treatments containing the Kos and McCready media.
Characterization of treated coal samples
Figure 7 shows the micrographs corresponding to the initial coal sample. Using this technique, pyrite was found to appear in different forms. The highest proportion appeared as small microcrystals with sizes of around 1 µm, which had mainly cubical shapes and were grouped in an apparently random manner (Fig. 7a) or in a framboidal structure. Euhedral pyrite crystals (Fig. 7b) that were larger than the microcrystals (maximum of 40 µm) were also observed in lesser proportion.
Figure 8 shows the SEM micrographs taken of the samples treated with the different culture media, at the end of the process. This analysis showed that pyrite dissolution occurred near the micro- cracks in the coal grains and that the pyrite framboids transformed jarosite into pyrite pseudomorphs. Furthermore, nearly complete corrosion of the euhedral pyrite crystals was observed.
In addition, from the microchemical analysis (made in the place where the white arrow indicates, Fig. 8a), the formation of jarosite (K+) and natrojarosite (Na+) is evident. A percentage of aluminum was also found in this sampling area, indicating it can precipitate from the clay minerals.
Discussion
Coal biodesulfurization experiments
As noted previously, the results regarding the behavior of pH over time for the pure strain and the consortium showed that all of the culture media presented a significant increase in this parameter during the initial days of the process. Since this initial increase was also seen in the abiotic controls, this conduct does not seem to be predominantly due to proton consumption caused by biological oxidation of ferrous ions, as has been found in other studies from the literature (Caicedo et al. 2011). Instead, this increase was caused by the consumption of acid, which led to the dissolution of some minerals (Kiani et al. 2014; Gómez et al. 1999; Tillet and Myerson 1987), such as those present in clays, including kaolinite and illite. These were found through mineralogical analysis with XRD, FTIR and SEM/EDS (Ye et al. 2018), and could have a greater contribution. Minerals from clays are characterized by having hydroxyl or oxygen groups exposed on their edges and surfaces, due to the breaking of links and to the general loss of the regularity of the crystalline structure (López 2012). This permits them to adsorb protons at an acidic pH, contributing to the initial increase in the pH of the solution (Manafi 2002; Sarcheshmehpour et al. 2009). Chen et al. (2013) showed that in acidic solutions, clays prefer to adsorb H+ ions over other types of metallic ions, leaving the heavy metals in solution. Additionally, in these conditions, clays could dissolve and consume acid, as proposed by Fitzpatrick and Shand (2008).
Additionally, A. thiooxidans bacteria was found to be important as a generator of sulfuric acid (Kamimura et al. 2005), since after the initial increase in pH in the suspensions with the pure strain, insufficient protons were generated to acidify the suspensions, while in the treatments with the consortium, a decrease in pH was observed during the process (Fig. 2).
Meanwhile, in the abiotic controls, a small percentage of iron was observed in solution, which likely originated from the dissolution of non-pyritic iron present in the initial coal sample. This solubilization was not originated from the pyrite, because this is not an acid-soluble mineral and the impossibility of regenerating Fe3+ ions in these abiotic suspensions provide elements to indicate that pyrite is only dissolved when ferrous ion-oxidizing bacteria exist in the environment (Schippers 2007; Rohwerder and Sand 2007; Olson 1991). In addition, the pyrite is part of the group of acid-insoluble metal sulphides and to dissolve it basically requires oxidation processes as a fact (Rohwerder and Sand 2007); and that under acidic conditions in the absence of bacteria and without considerable amounts of iron ions (III), the leaching processes of pyrite are extremely deficient (Schippers and Sand 1999). The fact that solubilization remained around 10% throughout the process in the controls for the Kos and McCready assays, while precipitation problems were generated after initial solubilization in the control for the T&K assay, provides evidence of the chemical solubility problem that likely occurred in the culture media with high sulfate concentrations. This indicates that the concentration of sulfates in the T&K culture medium was more easily able to induce precipitate formation.
The occurrence of iron precipitation during the initial days of the process indicates that this precipitation was likely due to the initial increase in pH and/or to the effect of the coal on the process (Kiani et al. 2014). This is supported by the fact that some researchers believe that precipitation is strongly influenced by factors like temperature, mineral age, pH and ionic composition (Kaksonen et al. 2014a, b; Wang and Zhou 2012; Caicedo and Márquez 2013; Nurmi et al. 2010; Liao et al. 2009; Cardona and Márquez 2009; Wang et al. 2006).
It is generally accepted that jarosite formation only occurs at a pH higher than 2.0 and that culture media with a pH lower than 1.8 can limit the precipitation of this iron hydroxysulfate (Gómez and Cantero 2005; Carranza and García 1990), while the solubility of ferric ions decreases at a pH higher than 2.5 (Nurmi et al. 2010). However, some research studies have found that jarosite can form at a highly acidic pH, even lower than 1.0 (Gramp et al. 2008; Dutrizac 1980; Babcan 1971), and in environments rich in sulfate (Kaksonen et al. 2014a, b). Additionally, other studies have found jarosite precipitation to occur around pyrite and coal starting at the beginning of the biodesulfurization process (Cardona and Márquez 2009).
Kaksonen et al. (2014a, b) used empirical models to determine the oxidation and precipitation velocity of iron, based on the initial pH of the leaching solutions (1.0 to 2.2). They found that the oxidation of ferrous ions and the precipitation of ferric ions occurred as a function of the initial pH of the solution and that this precipitation increased as acidity decreased. This indicates that an increase in pH at the beginning of the process, caused by minerals present in the coal (clays), could have contributed to the precipitation process in all of the culture media evaluated.
Although the T&K treatment appeared to have the greatest tendency toward precipitation, jarosite generation was observed in all of the bioassays. The final reprecipitation of iron in the present study was around 38% in all treatments. This is similar to what has been obtained in other studies, such as that of Nurmi et al. (2010), who observed precipitation between 30 and 40% in a process of biological oxidation of low-grade sulfide.
Meanwhile, high pyrite solubilization was obtained during this process (86% in the Kos bioassays), while earlier studies carried out with the same research group using Colombian coal obtained oxidation of 68% (Caicedo and Márquez 2010) and 96% (Cardona and Márquez 2009). However, the latter of these studies was conducted with a particle size of < 75 µm, which is a much finer grain than the one used in the present study (250 µm). This contributed to improving the bioleaching process at the expense of a larger contact surface area. The results are also satisfactory when compared with other studies such as that of Jorjani et al. (2007), who obtained 91.58% oxidation in 11 days with a pulp percentage of 5% (w/w), using a particle size of 180 µm. Meanwhile, He et al. (2012) were able to remove 47% of pyritic sulfur in 30 days in an Erlenmeyer using Acidithiobacillus caldus.
On the other hand, it was observed that when the Kos and McCready culture media were used in the treatments with the pure strain, low bacterial concentrations were obtained. It is likely that the nutrient concentration in the Kos and McCready culture media was not sufficient for the bacteria or that this concentration was decreased by the jarosite, which precipitated with some of the culture media nutrients upon forming (Kiani et al. 2014). Microorganisms therefore need a balanced mixture of nutrients, and if any of these is missing or limited, microbial growth decreases, independent of the concentration of other primary materials in the culture media (Rossi 1990). Additionally, in the Kos assay, the absence of magnesium in this culture medium could have contributed to the generation of low pure bacterial cell concentrations, since maximum microorganism growth is thought to depend—among many factors—on an optimal concentration of magnesium in the medium, particularly in a simple culture medium (Lodge and Hinshelwood 1939). Although it has been found that the magnesium requirement of gram-negative bacteria is 10 times lower than that of gram-positive bacteria (Webb 1949), this element appears to be essential for the synthesis of cellular material and plays an important role in cell division (Webb 1949).
Meanwhile, in contrast to what was observed with the pure strain, in the assays containing the consortium, the highest bacterial concentrations were obtained in the treatments using the Kos and McCready media. Additionally, higher bacterial densities were obtained when the consortium was used instead of the pure strain. This can be attributed to the sulfur that was added to these cultures as a source of energy for A. thiooxidans, since it has been found that the addition of sulfur induces new proteins in A. ferrooxidans cells when these grow with an additional iron source (Ohmura et al. 1996). Another factor that could have generated a higher cell concentration when using a consortium is that phosphate stimulates the growth and oxidation of iron on bacteria growing in the presence of sulfur (Harahuc et al. 2000). This stimulating effect of phosphate was not seen in the bioassays with the pure strain, because it has been found that in the absence of sulfur, this element can have the effect of inhibiting oxidation and bacterial growth (Harahuc et al. 2000; Ramírez et al. 2004).
Rossi (1990) assert that high microorganism growth velocities can ensure good mineral oxidation and solubilization velocities. However, based on the results of this study, it can be presumed, as other researchers have determined, that the bacterial concentration does not affect pyrite oxidation (at least not in the ranges found here) when this is greater than 106 cells/mL (Gleisner et al. 2006; Olson 1991). This can be concluded because the lowest bacterial concentration was obtained with the assays using the Kos and McCready media, and particularly using the pure strain, but throughout the process, these assays presented the highest concentrations of ferric ions in solution, contributing to the mineral solubilization process (Gleisner et al. 2006; Crundwell 2001). A high concentration of ferric ions in suspensions accelerates sulfide degradation (Ye et al. 2018), since it contributes to increasing the quantity of these ions at the cell-mineral interface or extracellular polymeric membrane (EPS), thus additionally promoting the adherence of bacteria to the mineral and accelerating the dissolution process (Yu et al. 2017; Aguirre 2012; Gómez and Cantero 2005; Kinzler et al. 2003; Sand et al. 1999).
The fact that high pyrite oxidation values were obtained despite the fact that high bacterial concentrations were not observed in all the treatments with the Kos and McCready culture media could indicate that the degree of oxidation does not depend on the quantity of cells present in the suspension. It could depend, rather, on other factors such as maintaining a high Fe3+/Fe2+ ratio in the medium. In other words, chemical attack (indirect mechanism) has a greater impact on this type of process. It can certainly be said that the pyrite solubilization velocity does not depend on a high bacterial population existing in the suspension, but rather on this population presenting significant ion-regenerating activity.
The bioleaching process effectively decreased the content of inorganic sulfur present in the initial coal sample. Biodesulfurization percentages ranging from 37 to 53% were obtained for the inorganic forms of coal. Similar research studies have obtained 50% removal of total sulfur over the course of 30 days, using an initial 5% (w/w) of pulp and 15% (v/v) of inoculum (Kiani et al. 2014). Ye et al. (2018) achieved a maximum total sulfur removal of 45.8% after 36 days of treatment in coals from Mongolia, China, using exotic microorganisms and 10% w/v coal pulp density.
It is important to highlight the great ability of the bacteria evaluated to adapt to very simple culture media, with minimal nutrient concentrations and even with some nutrients absent (as in the case of magnesium). The results show that in a coal biodesulfurization process, significant mineral oxidation can be obtained with any of the treatments evaluated; however, media with high concentrations of sulfates promote reprecipitation processes and do not obtain the same level of sulfur removal as media with low sulfate concentrations, due to the fact that jarosite formation promotes high concentrations of Fe3+ and SO 2− ions (Gramp et al. 2008; McCready et al. 1986).
In light of the positive results obtained with the assays using the Kos and McCready media, these can be considered a good option that would help reduce costs in processes on an industrial scale.
Characterization of treated coal samples
The intense corrosion that was observed in the pyrite grains based on the SEM micrographs of the treated coal (Fig. 8) was primarily associated with the presence of fractures and cracks inside the coal grains and with the presence of framboidal sulfide structures. This may be because the fractures gave rise to an oxidative process, which facilitated the diffusion processes and the contact of the mineral with different ions and microorganisms involved in dissolution (Gleisner et al. 2006; Hone et al. 1987). It is also likely that due to a certain ‘roughness’ present in the cracks, the microorganisms were more easily able to colonize these surfaces, since this provided them with some protection from the shear forces and the hydrodynamic stress that is produced in bacteria by contact with mineral particles (Donlan 2002). Meanwhile, the framboidal structures, which are highly porous because they are composed of small microcrystals and because of the size of these microcrystals (many times smaller than 1 µm), provide increases in the surface area, facilitating the transfer of mass and the bacterial and chemical attack of ferric iron (Donlan 2002).
It has been observed that metallic sulfide dissolution does not occur at random. Studies of images taken by atomic-force microscopy (AFM) have shown that microorganisms have an 80% higher possibility of attacking the sites that present imperfections on the surface of the mineral, since different forces of attraction that likely originate from the metallic ions and the sulfur are released more easily through the cracks (Kinzler et al. 2003; Rodriguez-Leiva and Tributsch 1988).
The euhedral form of the pyrite eroded from the edges toward the inside of the grain during the biodesulfurization process, thus losing its geometry of well-defined faces. Clear corrosion furrows were observed, compared with the shapes found in the initial coal sample. There was also evidence of the accumulation or generation of precipitates on the edges and surface of the dissolved pyrite grains, associated with sulfide bioleaching, as has been seen in other studies (Kiani et al. 2014; Sampson et al. 2000); this indicated its reprecipitation.
Conclusions
The increase in pH at the beginning of the process caused by the minerals present in the coal (clays) contributed to the precipitation process during the initial days of the biodesulfurization process.
Pyrite oxidation did not depend only on a high cell concentration existing in the suspension, but rather, on that population presenting significant oxidizing activity.
The nutrient concentration in the culture media and the minerals contained in the coal are determining factors in the effectiveness of the bioleaching process, since they significantly impact sulfate reprecipitation.
The occurrence of iron precipitation during the initial days of the process indicates that this precipitation was likely due to the initial increase in pH and/or the nutrient concentration in the culture media and the effect of the coal on the process.
The bioleaching process contributed to decreasing inorganic sulfur content by up to 53% in the treatments with the McCready culture medium.
Fractures and cracks in the coal grains and the porosity that was present in the framboidal pyrite structures were important, since the larger surface area of these structures likely facilitated oxidation.
Recommendations
The use of media with low sulfate concentrations minimizes reprecipitation, which not only provides benefits at a process level, but also could contribute to minimizing costs at an industrial scale.
Additionally, washing the coal with water at the beginning and controlling pH during the first 3 days of the process could contribute to diminishing the effect of the clays and reducing initial precipitation.
References
Aguirre M (2012) Extracellular polymeric substances (EPS) production in Sulfobacillus thermosulfidooxidans and its relevance on attachment to metal sulfides. Master’s thesis. Universidad Nacional de Colombia.
APHA (1992) American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 18th ed. American Public Health Association, Washington, DC
ASTM D2492 (2007a) Standard test method for forms of sulfur in coal. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D2492-02R07
ASTM D3172 (2007b) Standard practice for proximate analysis of coal and coke. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D3172-07A
ASTM D3173 (2008) Standard test method for moisture in the analysis sample of coal and coke. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D3173-03R08
ASTM D3174 (2011a) Standard test method for ash in the analysis sample of coal and coke from coal. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D3174-11
ASTM D3175 (2011b) Standard test method for volatile matter in the analysis sample of coal and coke. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D3175-11
ASTM D4239 (2011c) Standard test method for sulfur in the analysis sample of coal and coke using high temperature tube furnace combustion. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D4239-11
ASTM D5865 (2011d) Standard test method for gross calorific value of coal and coke. ASTM International, West Conshohocken, PA. https://www.astm.org. https://doi.org/10.1520/D5865-11a
Babcan J (1971) Synthesis of jarosite KFe3(SO4)2(OH)6. Goel Zb 22 (2): 299–304
Baruah M, Kotoky P, Borah G (2003) Distribution and nature of organic/mineral bound elements in Assam coals, India. Fuel 82:1783–1791
Bhupendra S, Barun K (2019) Desulfurization of high sulfur Indian coal by oil agglomeration using Linseed oil. Powder Technol 342:690–697
Caicedo G, Márquez M (2010) Mecanismo de selección de consorcios bacterianos compatibles con A. ferrooxidans y A. thiooxidans en procesos de biodesulfurización de carbón. Rev Fac Ing Univ Antioquia 52:88–94
Caicedo G, Márquez M (2013) Effect of chloride salts on biodesulfurization process of a colombian coal. Rev Fac Ing Univ Antioquia 68:115–123
Caicedo G, Márquez M, Moreno C (2011) Influencia de la concentración de hierro y pH iniciales en un proceso de biodesulfurización de carbón – ensayos a nivel de laboratorio. Rev Colomb Biotechnol 13(2):199–209
Cardona I, Márquez M (2009) Biodesulfurization of two Colombian coals with native microorganisms. Fuel Process Technol 90:1099–1106
Carranza F, García M (1990) Kinetic comparison of support materials in the bacteria ferrous iron oxidation in a packed-bed column. Biorecovery 2:15–27
Casas JM (2000) Modelacion cinetica de la precipitacion de hierro como jarosita en soluciones lixiviantes utilizando la bacteria Thiobacillus ferrooxidans. XIV Chilean congress of chemical engineering.
Chen Y, He Y, Ye W, Sui W, Xiao M (2013) Effect of shaking time, ionic strength, temperature and pH value on desorption of Cr(III) adsorbed onto GMZ bentonite. Transactions of Nonferrous Metals Society of China, Elsevier
Crundwell FK (2001) How do bacteria interact with minerals? In: Ciminelli VST, Garcia O Jr (eds) International biohydrometallurgy symposium. Elsevier, Amsterdam, pp 149–157
Cunha ML, Gahan CS, Menad N, Sandström A (2008) Possibilities to use oxidic byproducts for precipitation of Fe/As from leaching solution for subsequent base metal recovery. Miner Eng 21:38–47
Donlan R (2002) Biofilms: microbial life on surfaces. Emerging Infect Dis 8:881–890
Dutrizac JE (1980) The physical chemistry of iron precipitation in the zinc industry. In: Cigan JM, Mackey TS, Okeefe TJ (Eds) Lead–zinc–tin 80. AIME, New York, pp 532–564
Eaton AD, Clesceri LS, Rice EW, Greenberg AE, Franson MAH (2005) 3500-Fe. Standard methods for the examination of water and wastewater. 21st ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Fitzpatrick RW, Shand P (2008) Inland acid sulfate soils: overview and conceptual models. In: Fitzpatrick R, Shand P (eds) Inland acid sulfate soil systems across Australia. Perth, Australia
Gleisner M, Herbert RB, Frogner P (2006) Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen. Chem Geol 225:16–29
Gómez J, Cantero D (2005) Biooxidación del ion ferroso. Fundamentos y perspectivas de las tecnologías Biomineras 2:25–41
Gómez E, Ballester A, González F, Blázquez ML (1999) Leaching capacity of a new extremely thermophilic microorganism, Sulfolobus rivotincti. Hydrometallurgy 52:349–366
Gramp J, Jones F, Bigham J, Tuovinen O (2008) Monovalent cation concentrations determine the types of Fe(III) hydroxysulfate precipitates formed in bioleach solutions. Hydrometallurgy 94:29–33
Harahuc L, Lizama H, Suzuki I (2000) Selective inhibition of the oxidation of ferrous iron or sulfur in Thiobacillus ferrooxidans. Appl Environ Microbiol 1031–1037.
He H, Hong FF, Tao XX, Li L, Ma CY, Zhao YD (2012) Biodesulfurization of coal with Acidithiobacillus caldus and analysis of the interfacial interaction between cells and pyrite. Fuel Process Technol 101:73–77
Hone HJ, Beyer M, Ebner HG, Klein J, Juntgen H (1987) Microbial desulphurization of coal-development and application of a slurry reactor. Chem Eng Technol 10:173–176
Jorjani E, Chehreh S, Mesroghli Sh (2007) Prediction of microbial desulfurization of coal using artificial neural networks. Miner Eng 20:1285–1292
Kaksonen AH, Morris C, Rea S, Li J, Wylie J (2014a) Biohydrometallurgical iron oxidation and precipitation: part I-effect of pH on process performance. Hydrometallurgy 147:255–263
Kaksonen AH, Morris C, Rea S, Li J, Usher KM (2014b) Biohydrometallurgical iron oxidation and precipitation: part II: jarosite precipitate characterisation and acid recovery by conversion to hematite. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2014.04.015
Kamimura K, Higashino E, Kanao T, Sugio T (2005) Effects of inhibitors and NaCl on the oxidation of reduced inorganic sulfur compounds by a marine acidophilic, sulfur-oxidizing bacterium A. thiooxidans. Extremophiles 9:45–51
Kiani MH, Ahmadi A, Zilouei H (2014) Biological removal of sulphur and ash from fine-grained high pyritic sulphur coals using a mixed culture of mesophilic microorganisms. Fuel 13:89–95
Kinzler K, Gehrke T, Telegdi J, Sand W (2003) Bioleaching: a result of interfacial process caused by extracellular polymeric substances (EPS). Hydrometallurgy 71:83–88
Kos CH, Bijleveld W, Grotenhuis T, Box P, Kuenen JG, Poorter RPE (1983) Composition of mineral salts medium for microbial desulfurization of coal. In: Rossi G, Torma AE (eds) Recent progress in biohydrometallurgy. Canada Center for Mineral and Energy Technology, Ottawa
Liao Y, Zhou L, Bai S, Liang J, Wang S (2009) Occurrence of biogenic schwertmannite in sludge bioleaching environments and its adverse effect on solubilization of sludge-borne metals. Appl Geochem 24:1739–1746
Lodge R, Hinshelwood C (1939) Physicochemical aspects of bacterial growth. Part V. Influence of magnesium on the lag phase in the growth of Bact. Zactis aerogenes in synthetic media containing phosphate. J. Chem. Soc. 1943:213–219
Longfei T, Songjiang C, Dongjiao G, Xiangnan Z, Huan H, Xiuxiang T (2020) Effect of removal organic sulfur from coal macromolecular on the properties of high organic sulfur coal. Fuel 259:116264
López AE (2012) Estudio experimental de permeabilidad de materiales depositados en pilas de lixiviación. Universidad de Chile. Master’s thesis.
Manafi Z (2002) Column bioleaching of agglomerated low-grade copper ore by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Islamic Azad University of Jahrom, Iran. Master’s thesis.
McCready RGL, Wadden D, Marchbank A (1986) Nutrient requirements for the in-place leaching of uranium by Thiobacillus ferrooxidans. Hydrometallurgy 17:61–71
Munkhtsetseg S, Khomich A, Poklonskii N (2007) Infrared absorption spectra of coals with different degrees of coalification. J Appl Spectrosc 74(3):338–343
Nurmi P, Özkaya B, Sasaki K, Kaksonen AH, Riekkola-Vanhanen M, Tuovinen OH, Puhakka JA (2010) Biooxidation and precipitation for iron and sulfate removal from heap bioleaching effluent streams. Hydrometallurgy 101:7–14
Ohmura N, Tsugita K, Koizumi J, Saiki H (1996) Sulfur-binding protein of flagella of Thiobacillus ferrooxidans. J Bacteriol 178:5776–5780
Oinuma K, Kodama H (1967) Use of infrared absorption spectra for identification of clay minerals in sediments. J Tokyo Univ General Educ (Nat Sci) 7:1–23
Olson GJ (1991) Rate of pyrite bioleaching by Thiobacillus ferrooxidans: results of an interlaboratory comparison. Appl Environ Microbiol 57(3):642–644
Prescott ML, Harley JP, Klein DA (2004) Microbiología. McGraw Hill Interamericana, Madrid, p 167
Qiu Y, Zhang Q, Tian Y, Zhang J, Cao J, Xiao T (2011) Composition and structure of Luxing coal with different particle sizes. Petrol Coal 53(1):45–55
Ramírez P, Guiliani N, Valenzuela L, Beard S, Jerez C (2004) Differential protein expression during growth of Acidithiobacillus ferrooxidans on ferrous iron, sulfur compounds, or metal sulfides. Appl Environ Microbiol 70:4491–4498
Rodriguez-Leiva M, Tributsch H (1988) Morphology of bacterial leaching patterns by Thiobacillus ferrooxidans on synthetic pyrite. Arch Microbiol 149:401–405
Rohwerder T, Sand W (2007) Mechanisms and biochemical fundamentals of bacterial metal sulfide oxidation. Microbial Process Metal Sulfides 2:35–58
Rossi G (1990) Biohydrometallurgy. Hamburg, McGraw Hill Book Company
Saikia B, Boruah RK, Gogoi PK (2007) FT-IR and XRD analysis of coal from Makum coalfield of Assam. J Earth Syst Sci 116(6):575–579
Sampson MI, Phillips CV, Ball AS (2000) Investigation of the attachment of Thiobacillus ferrooxidans to mineral sulfides using scanning electron microscopy analysis. Miner Eng 13(6):643–656
Sand et al (1999) Direct versus indirect bioleaching. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment toward the mining of the 21st century Part A. Elsevier, Amsterdam, pp 27–49
Sarcheshmehpour Z, Lakzian A, Fotovat A, Reza BA, Hosain HG, Seyed- Bagheri SA (2009) The effects of clay particles on the efficiency of bioleaching process. Hydrometallurgy 98:33–37
Schiavon N (2007) Kaolinisation of granite in an urban environment. Environ Geol 52:399–407
Schippers A (2007) Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. Microbial Process Metal Sulfides 1:3–34
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol 77(5):642–647
Sonibare O, Haeger T, Foley SF (2010) Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 35:5347–5353
Suraj G, Iyer CSP, Rugmini S, Lalithambika M (1997) The effect of micronization on kaolinites and their sorption behavior. Appl Clay Sci 12:111–130
Tillet D, myerson a (1987) the removal of pyritic sulfur from coal employing Thiobacillus ferrooxidans in a packed column reactor. Biotechnol Bioeng 24:146–150
Tuovinen O, Kelly D (1972) Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ores. Zeitschrift für allgemeine Mikrobiologie 12(4):311–346
Wang M, Zhou L (2012) Simultaneous oxidation and precipitation of iron using jarosite immobilized Acidithiobacillus ferrooxidans and its relevance to acid mine drainage. Hydrometallurgy 125–126:152–156
Wang W, Qin Y, Sang S, Jiang B, Guo Y, Zhu Y, Fu X (2006) Partitioning of minerals and elements during preparation of Taixi coal, China. Fuel 85:57–67
Webb M (1949) The influence of magnesium on cell division. J Gen Microbiol 2(3):275–287
Ye J, Zhang P, Zhang G, Wang S, Nabi M, Zhang Q, Zhang H (2018) Biodesulfurization of high sulfur fat coal with indigenous and exotic microorganisms. J Cleaner Prod 197(1):562–570
Yu ZJ, Yu RL, Liu AJ, Liu J, Zeng WM, Liu XD, Qiu GZ (2017) Effect of pH values on extracellular protein and polysaccharide secretions of Acidithiobacillus ferrooxidans during chalcopyrite bioleaching. Trans Nonferrous Metals Soc China 27(2):406–412
Acknowledgements
The authors would like thank the National University of Colombia, Argos and Administrative Department of Science, Technology and Innovation of Colombia for the supports. In addition, the authors appreciate the assistance from Coals Laboratory and CIMEX of National University of Colombia, Medellín and Centricol Industries for providing the facilities to fulfill the experimental measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte Briceño, P.G., Caicedo Pineda, G.A. & Márquez Godoy, M.A. Early reprecipitation of sulfate salts in coal biodesulfurization processes using acidophilic chemolithotrophic bacteria. World J Microbiol Biotechnol 36, 81 (2020). https://doi.org/10.1007/s11274-020-02855-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02855-w