Abstract
Rare earth elements (REE) have great demand for sustainable energy and the high-end technology sector. The high similarity of REE owing to the nature of their electronic configurations increases the difficulty and costs of the development of chemical processes for their separation and recovery. In this way, the development of green technologies is highly relevant for replacing conventional unit operations of extractive metallurgy, viz. precipitation, liquid–liquid and solid–liquid extraction, and ion-exchange. Biosorption is a physicochemical and metabolically-independent biological process based on a variety of mechanisms including absorption, adsorption, ion-exchange, surface complexation and precipitation that represents a biotechnological cost-effective innovative way for the recovery of REE from aqueous solutions. This mini-review provides an overview and current scenario of biosorption technologies existing to recover REE, seeking to address the possibilities of using a green technology approach for wastewater treatment, as well as for the recovery of these high valued elements in the REE production chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rare earth elements
The rare earth elements (REEs) are listed in the periodic table located in the lanthanide series (Z = 57 to 71), including yttrium (Z = 39) and scandium (Z = 21), which have very similar chemical and physical properties. On the basis of their atomic weight, REEs are divided into light REE (LREEs)—lanthanum through gadolinium (Z = 57 to 64); and heavy REE (HREEs)—terbium through lutetium (Z = 65 to 71). The high similarity comes from the nature of its electronic configurations that result in a highly stable 3 + oxidation state (Richard and Fan 2018; Owens et al. 2019).
The numerous applications of REEs are due to their spectroscopic and magnetic properties and involve different areas of knowledge. The LREEs have been applied to the manufacture of permanent magnets, phosphors (i.e., substances that emit luminescence), magnetic resonance imaging, battery alloys, fluid catalytic cracking, ceramics, polishing powders, catalysts, and metallurgy excluding batteries. In turn, the HREEs are used in permanent magnets, ceramics, phosphors, portable X-ray devices, and as glass additives (Chararlampides et al. 2015; Suli et al. 2017; Giese 2018; Balaram 2019).
The high market value of REEs is due to the high cost and the extreme difficulty of separating these elements in high purity form (Silva et al. 2018). These elements have been extracted from primary ores in limited regions of the world by energy-intensive processes. Extraction of REEs from bastnasita ((La,Ce)FCO3), monazite ((Ce,La,Y,Th)PO4) and xenotime (YPO4) have been performed using physical processes through particle size separation, flotation and magnetic separation. Classic methods of hydrometallurgy have been used for the separation, preconcentration or removal of trace REEs from various matrices. After the beneficiation steps, REEs are then separated by chemical processes involving hydrometallurgical methods based on liquid–liquid (solvents) or solid–liquid (ion-exchange resins) extraction (Suli et al. 2017).
Recent reports classify REEs as Critical Raw Materials because they are not available in most geographical locations. China provides 95% of the world’s supply of REEs; on the other hand, developed countries whose manufacturing or technology base depends upon imported REEs have commenced seeking alternative secondary sources. Since these countries import REEs and manufacture a number of electrical and electronic equipment, these countries also discard sizeable quantities of e-waste materials that can be in “urban mines” rich in precious metals and REEs (Xavier et al. 2019). Consequently, urban mining of REEs is emerging as a sustainable alternative to primary ore mining (Wang et al. 2017).
Recovery of REEs from secondary sources is also possible, but remains a technological and economic challenge. Impediments on recovering strategic minerals from both primary ores and e-waste are identified with traditional processes, such as pyrometallurgical and hydrometallurgical systems, which are fast and efficient, but cause environmental contamination and are often not economically viable (Das and Das 2013). Although the strategies mentioned above are efficient, innovative work is being conducted to improve the extraction of REEs from different sources, as well as to improve selective separation of each element in the REEs series (Yesiller et al. 2013).
Biosorption as green technology processes in recovering REEs
Biosorption is considered an economical, simple and environmentally-friendly process that has been studied as an alternative to hydrometallurgy for the preconcentration, or as a separation of high-demand and high-valued metals from ores and waste solutions. Over the past decade, a number of significant contributions to the adsorption of REEs using biosorbents have been made (Das and Das 2013; Gupta et al. 2019). In this sense, the use of biosorbents is promising because it also presents as a viable cost-effective industrial process at the expense of the environmental impact of similar technologies (Volesky 2007).
Biosorption is a term that describes “the removal of metal ions, radioisotopes or REE elements by their passive binding to active, or dead, biomass materials in aqueous solutions”. In this process, the ion-biomass interaction is based on the chemical properties of biosorbent cell coatings and not on their biological activity (Gadd 2008; Beni and Esmaeili 2020).
The use of biosorption within an industrial process is encouraged by the lower cost of operation as well as biosorbent material. Other advantages include the high efficiency in the removal of metal ions in solutions containing low metal concentrations, the easy regeneration of the biosorbent for recycling and reuse, and the recovery potential of the biosorbed elements, as well as the minimization of residues and rapid adsorption kinetics (Olukanni et al. 2014; Gupta et al. 2019).
In addition to serving as a preconcentration tool, recovery and selective separation of REEs, biosorption is an eco-friendly approach to eliminate REEs from waste streams as a priority concern due to their accumulation and exertion of toxic effects on living systems. Physicochemical methods such as coagulation, membrane separation and solvent extraction have historically carried out the recovery of heavy metals from aqueous solutions; however, some of the traditional processes are expensive and inefficient at low metal concentrations and also generate unwanted secondary residues (Hisada and Kawase 2018).
Presently, biosorption remains exclusive for the removal of heavy metals or textile dyes from the industrial wastewaters; however, the progressive approach of developing an efficient and selective biosorption process at a viable economic cost has extended the recovery and separation of REEs. Recent published studies by Ponou et al. (2014), Kucuker et al. (2017), Piazza et al. (2017) and Ramasamy et al. (2018) demonstrated respectively, the possibility of biosorption for industrial applications to recover REEs from clay minerals, hard-disk drive magnets, e-waste, and acid mine drainage. Further investigations are anticipated to comprehend the selectivity of biosorption of REEs for their recovery from solutions containing multiple elements (Gupta et al. 2019).
Mechanism of REE biosorption
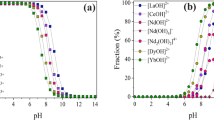
Overall, the uptake mechanism of REE is not an easy issue to tackle. Because of their capacity for complexation by different types of ligands, these metals possess rich solution chemistry. It has been reported that in the presence of OH ligands, a variety of lanthanide species might form, which including Ln(OH)2+, e.g., in addition to polymeric species (Wood 1990). Unless the concentration of hydroxide is high, 3 + is the dominant state of oxidation of REEs in aqueous solution. This high state of oxidation makes REEs behave as strong acids that can strongly bind to groups of oxides and metal hydr(oxy) REE bonds are expected to primarily have an electrostatic rather than a covalent character in such complexes. Furthermore, it was suggested that REEs are taken up on oxide surfaces on heteronuclear surface sites. Such reactions can produce a variety of surface species depending on the conditions of operation, surface nature, and chemical REE form. In this scenario, it is suggested to include two charged and two neutral sites per adsorbed metal (Yesiller et al. 2013).
In addition, biosorbents have been found to be highly specific to certain elements of particular interest and often have a high capacity for adsorption. Biosorption of REE elements can be considered mostly as an ion-exchange process based on the functional groups present on the surface of organisms. Substances present on microbial cells, such as polysaccharides, glycoproteins and lipids can act as functional groups in the uptake of REEs through binding sites such as carboxyl, amino, sulfhydryl, phosphate and hydroxyl groups, among others as shown in Fig. 1.
Different types of algal biomass (red, brown and green) have cellulose, carrageen, and alginate as their constituents. These polysaccharides have functionalities such as –COOH, –OH, –NH2, and –SH, and are responsible for selectively binding metal ions (Cheng et al. 2019). Fungi are eukaryotic organisms that have chitin (a carbohydrate biopolymer composed of N-acetylglucosamine repeat units) as a constituent of their cell wall. The fungal cell wall is filled with functionalities such as amine, imidazole, phosphate, sulfate, thiol and hydroxyl (Beni and Esmaeili 2020).
The total adsorption cycle is entirely dictated by the distribution of active sites on a biosorbent surface. Since adsorption is a surface process, a biosorbent with a very large surface area favours metal ion adsorption, as it provides a large area of contact for the interaction of metal ions (Beni and Esmaeili 2020). The biosorbent used in the biosorption process must be metabolically inactive. This characteristic of the material is important because it allows the recovery of REEs and enables their reuse. The advantage of using inactivated biomass as biosorbents is that living cells have growth limitations, mainly due to the toxicity of the cells by the REEs in solution, in addition to the environmental conditions of the biosorption process, such as the pH of the medium, which tends to be acidic to ensure the solubility of REE ionic species (Andrès et al. 2003).
In algae, specifically, the cell wall matrix is composed of a variety of polysaccharides and proteins, some of which contain anionic functional groups, e.g., carboxylic, sulfate and phosphate groups for biosorption (Klimmek et al. 2001). Since lanthanides are considered strong acids (Brookins 1989), these ions will preferentially bind to strong bases containing oxygen as the electron-donating atom. This means that REEs bind weakly to weak bases, such as those that have S or P as ion donors. Among the ligands on the algal cell surface, the dominant ligand that contains at least one donor oxygen atom are the carboxylic and hydroxyl ionic groups (Birungi and Chirwa 2014; Diniz and Volesky 2005).
Diniz and Voleski (2005) demonstrated that the Ca2+ ions exchange for La3+, Eu3+ and Yb3+ binding in Sargassum polycystum, and occurs in the ratio of 1:1. The cationic exchange was also observed by Vijayaraghavan et al. (2010) using energy-dispersive X-ray spectroscopy (ED-XRF) analysis, and demonstrated that Ca2+ peaks on the cell surface of brown algae, Turbinaria conoides, were reduced when new La3+, Ce3+, Yb3+ and Eu3+ peaks biosorbed were present.
Chemical modification of biomass is generally intended to increase biosorption capacity and affinity for an ion of interest. In general, modification procedures include pretreatment, enhancement of the binding site, modification of binding sites and polymerization (Vijayaraghavan and Balasubramanian 2015). Acid pretreatment is one of the most common methods for cleaning biomass, but it is also possible to perform alkaline treatment, ethanol and acetone (Zhang et al. 2010; Rehman et al. 2013; Giese and Jordão 2019). In order to inactivate the biomass to be used as biosorbent, drying, heating and freezing processes are routinely used—the lyophilization process does not compromise the biomass adsorption capacity. Maintaining the integrity of the cell wall lining during the inactivation process is essential to preserve the biosorptive characteristics of biomass (Ribeiro et al. 2010).
Factors affecting REE biosorption
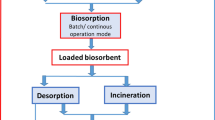
Application of biosorbents for the preconcentration of REEs is greatly justified as the process is environmentally benign, simple and can be economic (Gupta et al. 2019). Figure 2 summarizes the biosorption process technology for REE ions. A huge range of biosorbents have been used for the adsorption and recuperation of REEs, and biosorption of REEs was governed by certain parameters, viz., contact time, pH, biosorbent dosage, temperature, ionic strength; and must be optimized using statistic designed methods for maximizing absorption effectiveness, and recovery.
In most cases, metal binding occurs by electrostatic interaction, surface complexation, ion transfer, and precipitation that can occur individually or in combination. Biomass (living or non-living cells), biomaterial shapes, chemical properties of metal solutions, environmental conditions, such as pH, influences the biosorption of REEs (Gadd 2008; Gupta et al. 2019).
The solution pH is an important factor regulating the biosorption process, which affects the speciation of REEs in solution through hydrolysis, complexation and redox reactions (Coimbra et al. 2017; Heidelmann et al. 2017; Hisada and Kawase 2018). The temperature also influences metal ion biosorption, and during the sorption process, several studies have suggested different views on the temperature effects.
An increase in the concentration of biosorbent dose usually increases the amount of REE adsorbed once the number of binding sites is rising due to the increased surface area of the biosorbent. On the other hand, the amount of biosorbent adsorbed ion per unit weight decreases as the biosorbent dose increases, which may be due to the complex interaction of several variables (Gadd 2008; Das and Das 2013; Gupta et al. 2019). Finally, pore distribution controls the internal diffusion of metal ions contributing to intracellular metal accumulation in biosorbents. REE' adsorption in a biosorbent depends on the size and volume of the pore. The pore entry of REE ions is highly feasible when the pore size is comparatively larger than the size of the hydrated REE ion. This is also the pore width, these REE ions it can handle, which ultimately leads to higher adsorption efficiency (Beni and Esmaeili 2020).
Microbial biosorbents for recovery of REEs
Due to their high adsorption capacity and easy availability, algal biomass has been used for the adsorption of heavy metal ions and other toxic pollutants from industrial wastewater. In the case of lanthanides, the concentration of REEs adsorbed on macroalgae such as seaweed can reach 102–106-fold above that found in seawater (Sakamoto et al. 2008). REEs can be found at 1.3 μg per seaweed (Xiao-Jun et al. 1998) which is considered a high concentration when compared to the concentration found in the environment, between 10–3 and 10−1 μg/L (Sahoo et al. 2012; Liang et al. 2014; Richards and Mullins 2013).
Among the algae studied, the group most used in REE biosorption studies has been the brown marine macroalgae, mainly of the Sargassacea family. These algal species contain high concentrations of alginate that comprize abundant carboxylic groups capable of capturing cations metallic species present in solution (Davis et al. 2003). Additionally, in laboratory studies for La3+ biosorption, the freshwater green microalgae C. reinhardtii was able to recover 1.03 mmol/g within 5 h (Birungi and Chirwa 2014), which was very close to 1.11 mmol/g in 6 h for the brown marine Turbinaria conoides (Vijayaraghavan et al. 2010). The same can be observed for Nd3+, where studies have shown that microalgae Ankistrodesmus gracilis and Monoraphidium sp. biosorbed 0.98 and 0.94 mmol/g, respectively (Palmieri et al. 2000), while the best result found for brown macroalgae Sargassum spp. was 0.70 mmol/g in 40 min (Oliveira and Garcia 2009).
Bacteria are widely studied for their potential as biosorbents because they have a high surface area in relation to their small size, which facilitates the process of adsorption of metal ions present in solution (Vijayaraghavan and Yun 2008). Among the bacterial strains studied as biosorbents for REEs, are those belonging to the genera Bacillus (Coimbra et al. 2017, 2019; Giese and Jordão 2019), Streptomyces (Tsuruta 2006), Pseudomonas (Andrès et al. 2000; Texier et al. 2002), Myxococcus (Merroun et al. 2003) and Agrobacterium (Xu et al. 2011).
Pretreatment of bacterial cells with NaCl, HCl or NaOH solutions may be necessary for the active cell surface sites to have sufficient negative charge density to favor interaction between the cells. For Bacillus subtilis, for example, pretreatment with 1 M NaOH favored the La3+ sorption process, which was equal to 100% within 40 min with 100 µM La3+ solution at pH 3.0. With cells pretreated with 1 M HCl, the maximum sorption was only 34% in 60 min (Giese and Jordão 2019). On the other hand, both alkaline and acid pretreatments did not promote an increase of La3+ and Ce3+ biosorption by Agrobacterium sp. HN1 (Xu et al. 2011).
Takahashi et al. (2005) observed that the distribution coefficient (Kd) between the B. subtilis cell wall and the REE solution tends to increase proportionally to the cellular concentration (at a fixed pH value), as well as proportionally to pH values (in a fixed cell concentration). The dependence of Kd values on pH solution indicated that phosphate groups participated in the interactions between REE and biomass at higher pH values (Bonificio and Clarke 2016).
Higher rates of metal percentage removal in diluted solutions have been observed in the literature for some fungal and yeast strains. Muraleedharan et al. (1994) evaluated the use of pulverized biomass (particle size 600–1200 μm) from the fruiting body of Ganoderma lucidum, a wood-decomposing basidiomyceteous fungus, on REE biosorption. The authors simulated a synthetic effluent from the monazite process consisting of Th4+, RCl3, Zn2+, Cd2+, F− and PO43− ions, and used G. lucidum biomass packed in a fixed-bed reactor for the treatment of this effluent. The use of biosorbent decreased the concentration of REE (< 0.15 mg/L) and thorium (< 0.1 mg/L) to lower levels.
Recently, Giese et al. 2019 described the La3+ and Sm3+ biosorption by Botryosphaeria rhodina, a fungus used in the industrial production of β-glucan. The maximum uptake capacity of La3+ was observed at low amounts of La ions in solution, decreasing from 100 to 25% when the initial lanthanide concentration increased from 15 to 100 mg/L. The scaled-up production of β-glucan by this fungus results in the production of large amounts of mycelial biomass as an industrial by-product that represents a potentially cost-effective biosorbent for REEs (Giese et al. 2019), and heavy metals (Muñoz et al. 2019).
Vlachou et al. (2009) evaluated the biosorption capacity of Kluyveromyces marxianus, Candida colliculosa and Debaryomyces hansenii, and compared them to Saccharomyces cerevisiae. These yeast species are present as waste streams originating from breweries, wineries, and bakeries, and can be used as low-cost biosorbents. The authors described the biosorption behavior of Nd3+ by adsorption isotherms at pH 1.5 with the initial concentration of Nd3+ between 10 and 200 mg/L, where the qmax value was from 10 to 12 mg/g, and a Kf value of 0.9–1.2. The analysis of the mathematical model indicated the existence of two types of Nd3+ ion binding sites that were common to the four yeast strains evaluated. The same behavior was also observed for the bacterium, Mycobacterium smegmatis, cells for REE ions La3+, Eu3+, and Yb3+ biosorption (Andrès et al. 1993).
Two strains of S. cerevisiae, one wild type the other a mutant (rim20Δ), were evaluated for the capacity of biosorption of La3+. Di Caprio et al. (2016) observed that for both strains, ion-biomass interactions occurred through bonding to carboxylic, amino and phosphate groups on the yeast surface. Both processes reached equilibrium between 10 and 20 min, with a qmax of 70 mg/g at pH 4.0 for the wild type strain, and 80 mg/g at pH 6.0 for the mutant strain.
Table 1 summarizes some data from the literature on the biosorption of REEs by various microbial species. Further studies are needed to understand the behavior of biosorption using microbial biosorbents in real wastewater and leachate solutions, where selective adsorption of REEs from an element matrix develops the technology towards large-scale application of the method of biosorption. Although REEs partitioning from multi-element solutions has been achieved by tailoring chelating ligands to the biosorbent surface, selectivity among REEs still needs future work (Gupta et al. 2019).
Final considerations
Currently, the biggest challenge in the REE industry is to separate and retrieve the elements to obtain pure REE compounds, as a result of the high chemical similarity between the group elements. Biosorption offers an economically-feasible technology for efficient removal and recovery of REEs from diluted leachates combining biotechnology with extractive hydrometallurgy, as a potential alternative for REE concentration through interactions between REEs and certain active sites present in the biosorbent.
In general, studies involving biosorption of REEs by algae, bacteria, fungi and yeasts are still at an early stage, with few species of organisms exploited, and not all REEs have been studied. The use of packed-bed column technology with immobilized biomass is an economically and operationally viable alternative that could be employed in REE biosorption processes, and feasibility studies of this type of bioprocess should be conducted. The use of fixed or fluidized bed reactors is preferred because of the easier recovery of the treated effluent. For this purpose, successful bacterial immobilization on different matrices is required.
The development of a bioprocess to make up the REEs production chain promising and innovative is for the mineral sector. Alternative routes and clean technologies for REE extraction and separation are needed to fit current trends seeking better performance of hydrometallurgical facilities. Researchers progressive approach to developing an effective, selective, and stable biosorbent at minimal cost, should also be extended to preconcentration for REE. The move from the laboratory to an industrial scale of the biosorption process is difficult for preconcentration of REEs.
There are two trends for the use of biosorption as an industrial process of REE removal and separation: (a) use of hybrid technologies using active biomass, and (b) efficient biomaterials as biosorbents for reuse and recycling. Industry and the bioeconomy will strengthen investigative biases for the application of tools, such as molecular biotechnology, which can be used in future to construct manipulated microorganisms with higher sorption capacities and specificity for each REE species.
References
Andrès Y, Maccordick JH, Hubert JC (1993) Adsorption of several actinide (thorium, uranium) and lanthanide (lanthanum, europium, ytterbium) ions by Mycobacterium smegmatis. Appl Microbiol Biotechnol 39(3):413–417. https://doi.org/10.1007/BF00192103
Andrès Y, Thouand G, Boualam M, Mergeay M (2000) Factors influencing the biosorption of gadolinium by micro-organisms and its mobilisation from sand. Appl Microbiol Biotechnol 54(2):262–267. https://doi.org/10.1007/s002530000368
Andrès Y, Texier AC, Le Cloirec P (2003) Rare earth elements removal by microbial biosorption: a review. Environ Technol 24(11):1367–1375. https://doi.org/10.1080/09593330309385681
Balaram V (2019) Rare earth elements: a review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci Front 10:1285–1303. https://doi.org/10.1016/j.gsf.2018.12.005
Beni AA, Esmaeili A (2020) Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environ Technol Innov 17:100503. https://doi.org/10.1016/j.eti.2019.100503
Birungi ZS, Chirwa EMN (2013) Phytoremediation of lanthanum using algae from eutrophic freshwater sources. Proc Water Environ Fed 2013:357–366. https://doi.org/10.2175/193864713813667728
Birungi ZS, Chirwa EMN (2014) The kinetics of uptake and recovery of lanthanum using freshwater algae as biosorbents: comparative analysis. Biores Technol 160(1):43–51. https://doi.org/10.1016/j.biortech.2014.01.033
Bonificio WD, Clarke DR (2016) Rare-earth separation using bacteria. Environ Sci Technol Lett 3(4):180–184. https://doi.org/10.1021/acs.estlett.6b00064
Brookins DG (1989) Aqueous geochemistry of rare earth elements. Rev Miner Geochem 21(1):201–225. https://doi.org/10.1016/0009-2541(90)90080-Q
Chararlampides G, Vatalis KI, Apostoplos B, Ploutarch-Nikolas B (2015) Rare earth elements: industrial applications and economic dependency of Europe. Procedia Econ Financ 24:126–135. https://doi.org/10.1016/S2212-5671(15)00630-9
Cheng SY, Show P-L, Lau BF, Chang J-S, Ling TC (2019) New prospects for modified algae in heavy metal adsorption. Trends Biotechnol 37(11):1255–1268. https://doi.org/10.1016/j.tibtech.2019.04.007
Coimbra NV, Nascimento M, Giese EC (2017) Evaluation of the use of bacterial biomass immobilized in biosorption of light and medium rare earth elements. HOLOS 6(33):136–146. https://doi.org/10.15628/holos.2017.6445
Coimbra NV, Gonçalves FS, Nascimento M, Giese EC (2019) Study of adsorption isotherm models on rare earth elements biosorption for separation purposes. Int Schol Sci Res Innov 13:200–203
Das N, Das D (2013) Recovery of rare earth metals through biosorption: an overview. J REE 31(10):933–943. https://doi.org/10.1016/S1002-0721(13)60009-5
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37(18):4311–4330. https://doi.org/10.1016/S0043-1354(03)00293-8
Di Caprio F, Altimari P, Zanni E, Uccelletti D, Toro L, Pagnanelli F (2016) Lanthanum biosorption by different Saccharomyces cerevisiae strains. Chem Eng Trans 49:37–42. https://doi.org/10.3303/CET1649007
Diniz V, Volesky B (2005) Biosorption of La, Eu and Yb using Sargassum biomass. Water Res 39(1):239–247. https://doi.org/10.1016/j.watres.2004.09.009
Gadd GM (2008) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84(1):13–28. https://doi.org/10.1002/jctb.1999
Giese EC (2018) Rare earth elements: therapeutic and diagnostic applications in modern medicine. Clin Med Rep 2:1–2. https://doi.org/10.15761/CMR.1000139
Giese EC, Jordão CS (2019) Biosorption of lanthanum and samarium by chemically modified Bacillus subtilis free cells. Appl Water Sci 9:182. https://doi.org/10.1007/s13201-019-1052-3
Giese EC, Barbosa AM, Dekker RFH (2019) Biosorption of lanthanum and samarium by viable and autoclaved mycelium of Botryosphaeria rhodina MAMB-05. Biotechnol Prog 36:e2783. https://doi.org/10.1002/btpr.2783
Guo P, Wang J, Li X, Zhu J, Reinert T, Heitmann J, Butz T (2000) Study of metal bioaccumulation by nuclear microprobe analysis of algae fossils and living algae cells. Nucl Instrum Methods Phys Res 161:801–807. https://doi.org/10.1016/S0168-583X(99)00933-7
Gupta NK, Gupta A, Ramteke P, Sahoo H, Sengupta A (2019) Biosorption-a green method for the preconcentration of rare earth elements (REE) from waste solutions: a review. J Mol Liq 274:148–164. https://doi.org/10.1016/j.molliq.2018.10.134
Heidelmann GP, Roldão TM, Egler SG, Nascimento M, Giese EC (2017) Microalgae biomass use for lanthanides biosorption. HOLOS 6(33):170–179. https://doi.org/10.15628/holos.2017.6436
Heilmann M, Jurkowski W, Buchholz R, Brueck T, Becker AM (2015) Biosorption of neodymium by selected photoautotrophic and heterotrophic species. J Chem Eng Process Technol 6:1–5. https://doi.org/10.4172/2157-7048.1000241
Hisada M, Kawase Y (2018) Recovery of rare-earth metal neodymium from aqueous solutions by poly-γ-glutamic acid and its sodium salt as biosorbents: Effects of solution pH on neodymium recovery mechanisms. J Rare Earths 36(5):528–536. https://doi.org/10.1016/j.jre.2018.01.001
Kano N (2013) Biosorption of lanthanides using select marine biomass. In: Engineering “biomass now - sustainable growth and use”. Intech Open, Rijeka, pp 101–124. https://doi.org/10.5772/51164
Kim JA, Dodbiba G, Tanimura Y, Mitsuhashi K, Fukuda N, Okaya K, Matsuo S, Fujita T (2011) Leaching of rare-earth elements and their adsorption by using blue-green algae. Mater Trans 52:1799–1806. https://doi.org/10.2320/matertrans
Klimmek S, Stan HJ, Wilke A, Bunke G, Buchholz R (2001) Comparative analysis of the biosorption of cadmium, lead, nickel, and zinc by algae. Environ Sci Technol 35(21):4283–4288. https://doi.org/10.1021/es010063x
Korenevsky AA, Sorokin VV, Karavaiko GI (1999) Biosorption of rare earth elements. Process Metall 9:299–306. https://doi.org/10.1016/S1572-4409(99)80119-9
Kücüker MA, Nadal JB, Kuchta K (2016) Comparison between batch and continuous reactor systems for biosorption of neodymium (Nd) using microalgae. Int J Plant Animal Environ Sci 6(3):197–203
Kucuker MA, Wieczorek N, Kuchta K, Copty NK (2017) Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS ONE 12:e0175255. https://doi.org/10.1371/journal.pone.0175255
Liang T, Li K, Wang L (2014) State of rare earth elements in different environmental components in mining areas of China. Environ Monit Assess 186(3):1499–1513. https://doi.org/10.1007/s10661-013-3469-8
Merroun ML, Ben Chekroun K, Arias JM, Gonzalez-Muñoz MT (2003) Lanthanum fixation by Myxococcus xanthus: cellular location and extracellular polysaccharide observation. Chemosphere 52:113–120. https://doi.org/10.1016/S0045-6535(03)00220-0
Muñoz AJ, Ruiz E, Espínola F, Barbosa-Dekker AM, Dekker RFH, Castro E (2019) Assessment of by-product from Botryosphaeria rhodina MAMB-05 as an effective biosorbent of Pb(II). Molecules 24:3306. https://doi.org/10.3390/molecules24183306
Muraleedharan TR, Philip L, Iyengar L, Venkobachar C (1994) Application studies of biosorption for monazite processing industry effluents. Biores Technol 49:179–186. https://doi.org/10.1016/0960-8524(94)90082-5
Oliveira RC, Garcia O Jr (2009) Study of biosorption of rare earth metals (La, Nd, Eu, Gd) by Sargassum sp. biomass in batch systems: physicochemical evaluation of kinetics and adsorption models. Adv Mater Res 71:605–608. https://doi.org/10.4028/www.scientific.net/AMR.71-73.605
Oliveira RC, Jouannin C, Guibal E, Garcia O (2011) Samarium (III) and praseodymium (III) biosorption on Sargassum sp.: batch study. Process Biochem 46:736–744. https://doi.org/10.1016/j.procbio.2010.11.021
Oliveira RC, Guibal E, Garcia O (2012) Biosorption and desorption of lanthanum (III) and neodymium (III) in fixed-bed columns with Sargassum sp.: perspectives for separation of rare earth metals. Biotechnol Prog 28:715–722. https://doi.org/10.1002/btpr.1525
Olukanni DO, Agunwamba JC, Ugwu EI (2014) Biosorption of heavy metals in industrial wastewater using microorganisms (Pseudomonas aeruginosa). Am J Sci Ind Res 5(2):81–87. https://doi.org/10.5251/ajsir.2014.5.2.81.87
Owens CL, Nash GR, Hadler K, Fitzpatrick RS, Wall F (2019) Apatite enrichment by rare earth elements: a review of the effects of surface properties. Adv Colloid Interface Sci 265:14–28. https://doi.org/10.1016/j.cis.2019.01.004
Ozaki T, Kimura T, Ohnuki T, Yoshida Z, Francis AJ (2003) Association Mechanisms of europium (III) and curium (III) with Chlorella vulgaris. Environ Toxicol Chem 22:2800–2805. https://doi.org/10.1897/02-481
Palmieri MC, Garcia O (2001) Biosorption of erbium and ytterbium using biomass of microorganisms. Process Metall 11:137–144
Palmieri MC, Garcia O Jr, Melnikov P (2000) Neodymium biosorption from acidic solutions in batch system. Process Biochem 36:441–444. https://doi.org/10.1016/S0032-9592(00)00236-3
Palmieri MC, Volesky B, Garcia O (2001) Biosorption of lanthanum using Sargassum fluitans in batch system. Hydrometallurgy 67:31–36. https://doi.org/10.1016/S0304-386X(02)00133-0
Pan X, Wu W, Chen Z, Rao W, Guan X (2017) Biosorption and extraction of europium by Bacillus thuringiensis strain. Inorg Chem Commun 75:21–24. https://doi.org/10.1016/j.inoche.2016.11.012
Park DM, Reed DW, Yung MC, Eslamimanesh A, Lencka MM, Anderko A, Fujita Y, Riman RE, Navrotsky A, Jiao Y (2016) Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ Sci Technol 50:2735–2742. https://doi.org/10.1021/acs.est.5b06129
Piazza SD, Cecchi G, Cardinale AM, Carbone C, Mariotti MG, Giovine M, Zotti M (2017) Penicillium expansum link strain for a biometallurgical method to recover REEs from WEEE. Waste Manag 60:596–600. https://doi.org/10.1016/j.wasman.2016.07.029
Ponou J, Wang LP, Dodbiba G, Okaya K, Fujita T, Mitsuhashi K, Atarashi T, Satoh G, Noda M (2014) Recovery of rare earth elements from aqueous solution obtained from Vietnamese clay minerals using dried and carbonized parachlorella. J Environ Chem Eng 2:1070–1081. https://doi.org/10.1016/j.jece.2014.04.002
Ramasamy DL, Puhakka V, Iftekhar S, Wojtuś A, Repo E, Hammouda SB, Iakovleva E, Sillanpää M (2018) N- and O-ligand doped mesoporous silica-chitosan hybrid beads for the efficient, sustainable and selective recovery of rare earth elements (REE) from acid mine drainage (AMD): understanding the significance of physical modification and conditioning of the polymer. J Hazard Mater 348:84–91. https://doi.org/10.1016/j.jhazmat.2018.01.030
Rehman R, Anwar J, Mahmud T (2013) Sorptive removal of lead (II) from water using chemically modified mulch of Madhuca longifolia and Polyalthia longifolia as novel biosorbents. J Desal Water Treat 51(13–15):2624–2634. https://doi.org/10.1080/19443994.2012.749200
Ribeiro RF, Magalhães SM, Barbosa FA, Nascentes CC, Campos IC, Moraes DC (2010) Evaluation of the potential of microalgae Microcystis novacekii in the removal of Pb2+ from an aqueous medium. J Hazard Mater 179(1):947–953. https://doi.org/10.1016/j.jhazmat.2010.03.097
Richard AR, Fan M (2018) Rare earth elements: properties and applications to methanol synthesis catalysis via hydrogenation of carbon oxides. J Rare Earths 36(11):1127–1135. https://doi.org/10.1016/j.jre.2018.02.012
Richards RG, Mullins BJ (2013) Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol Model 249(1):59–67. https://doi.org/10.1016/j.ecolmodel.2012.07.004
Sahoo PK, Tripathy S, Equeenuddin SM, Panigrahi MK (2012) Geochemical characteristics of coal mine discharge vis-à-vis behavior of rare earth elements at Jaintia Hills coalfield, northeastern India. J Geochem Explor 112(1):235–243. https://doi.org/10.1016/j.gexplo.2011.09.001
Sallam AM, El-Sayed EM, Amin MM, El-Aassy IE, El-Feky MH, Nada AA, Harpy NM (2014) Biosorption of rare earth elements by two fungal genera from lower carboniferous carbonaceous shales, in southwestern Sinai, Egypt. J Appl Environ Biol Sci 4:146–154
Sakamoto N, Kano N, Imaizumi H (2008) Determination of rare earth elements, thorium and uranium in seaweed samples on the coast in Niigata Prefecture by inductively coupled plasma mass spectrometry. Appl Geochem 23(10):2955–2960. https://doi.org/10.1016/j.apgeochem.2008.04.011
Silva GA, Petter CO, Albuquerque NR (2018) Factors and competitiveness analysis in rare earth mining, new methodology: case study from Brazil. Heliyon 4(3):e00570. https://doi.org/10.1016/j.heliyon.2018.e00570
Suli L, Ibrahim WH, Aziz B, Deraman MR, Ismail N (2017) A review of rare earth mineral processing technology. Chem Eng Res Bull 19:20–35. https://doi.org/10.3329/cerb.v19i0.33773
Takahashi Y, Chatellier TX, Hattori KH, Kato K, Fortin D (2005) Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats. Chem Geol 219:53–67
Texier AC, Andrès Y, Faur-Brasquet C, Le Cloireç P (1999) Selective biosorption of lanthanide (La, Eu, Yb) ions by Pseudomonas aeruginosa. Environ Sci Technol 33(1):489–495. https://doi.org/10.1021/es9807744
Tsuruta T (2006) Bioaccumulation of uranium and thorium from the solution containing both elements using various microorganisms. J Alloys Compd 408–412:1312–1315. https://doi.org/10.1016/j.jallcom.2005.04.131
Vijayaraghavan K (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. https://doi.org/10.1016/j.biotechadv.2008.02.002
Vijayaraghavan K, Sathishkumar M, Balasubramanian R (2010) Biosorption of lanthanum, cerium, europium, and ytterbium by a brown marine alga, Turbinaria conoidesa. Ind Eng Chem Res 49(9):4405–4411. https://doi.org/10.1021/ie1000373
Vijayaraghavan K, Sathishkumar M, Balasubramanian R (2011) Interaction of rare earth elements with a brown marine alga in multi-component solutions. Desalination 265:54–56. https://doi.org/10.1016/j.desal.2010.07.030
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manag 160:283–296. https://doi.org/10.1016/j.jenvman.2015.06.030
Vijayaraghavan K, Jegan J (2015) Entrapment of brown seaweeds (Turbinaria conoides and Sargassum wightii) in polysulfone matrices for the removal of praseodymium ions from aqueous solutions. J Rare Earths 33(11):1196–1203. https://doi.org/10.1016/S1002-0721(14)60546-9
Vlachou A, Symeopoulos BD, Koutinas AA (2009) A comparative study of neodymium sorption by yeast cells. Radiochim Acta 97:437–441. https://doi.org/10.1524/ract.2009.1632
Volesky B (2007) Biosorption and me. Water Res 41(18):4017–4029. https://doi.org/10.1016/j.watres.2007.05.062
Xavier LH, Duthie AC, Giese EC, Lins FAF (2019) Sustainability and the circular economy: a theoretical approach focused on e-waste urban mining. Res Policy 1:101467. https://doi.org/10.1016/j.resourpol.2019.101467
Xiao-Jun Y, Xiao-Lin H, Biao S, Xiao F, Li-Jun H (1998) Element composition of Sargassum thunbergii. Chin J Oceanol Limnol 16(2):189–192. https://doi.org/10.1007/BF02845187
Xu S, Zhang S, Chen K, Han J, Liu H, Wu K (2011) Biosorption of La3+ and Ce3+ by Agrobacterium sp. HN1. J Rare Earths 29(3):265–270. https://doi.org/10.1016/S1002-0721(10)60443-7
Yesiller SU, Eroğlu AE, Shahwan T (2013) Removal of aqueous rare earth elements (REE) using nano-iron based materials. J Ind Eng Chem 19(3):898–907. https://doi.org/10.1016/j.jiec.2012.11.005
Wang M, Tan Q, Chiang JF, Li J (2017) Recovery of rare and precious metals from urban mines—a review. Front Environ Sci Eng 11(5):1. https://doi.org/10.1007/s11783-017-0963-1
Wood SA (1990) The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem Geol 82:159–186. https://doi.org/10.1016/0009-2541(90)90080-Q
Zhang Y, Liu W, Xu M, Zheng F, Zhao M (2010) Study of the mechanisms of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J Haz Mater 178(1):1085–1093. https://doi.org/10.1016/j.jhazmat.2010.02.051
Acknowledgements
The author gratefully acknowledges the financial support from the National Council for Scientific and Technological Development (CNPq, Brazil) that supported her research program at CETEM, Rio de Janeiro, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giese, E.C. Biosorption as green technology for the recovery and separation of rare earth elements. World J Microbiol Biotechnol 36, 52 (2020). https://doi.org/10.1007/s11274-020-02821-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02821-6