Abstract
Polyketides and peptides obtained from actinobacteria are important therapeutic compounds which include front line antibiotics and anticancer drugs. Many screening programs are directed towards isolation of bioactive compounds from these organisms but the chances of finding novel antimicrobial leads among common actinobacteria are fast dwindling. As a result, the focus has shifted to the members of less exploited genera of rare actinobacteria. Three isolates, MMS8, MMS16 and KCR3 found to be potent polyketide and peptide producers were identified by 16S rRNA gene sequencing and their sequences deposited in the GenBank under the accession numbers MG407702, MG372012 and MG430204 respectively. MMS8 identified as Micromonospora auratinigra, yielded one potent compound determined to be chloroanthraquinone with an minimum inhibitory concentration (MIC) of 8 µg/ml against Bacillus subtilis and an IC50 value of 10 µg/ml and 4 μg/ml against HeLa and IMR cell lines respectively. This is the first report of the production of chloroanthraquinone by M. auratinigra. MMS16, identified as a member of the family Micromonosporaceae, yielded a potent compound MMS16B analyzed to be a novel bafilomycin analogue. The MIC of the compound was found to be 7 μg/ml against B.subtilis and IC50 value against HeLa and IMR was observed to be 9 μg/ml and 14 μg/ml respectively. MMS16B was also found to exhibit anti-quorum sensing (AQS) activity at sublethal concentrations. KCR3 identified as Kocuria kristinae yielded a novel antimicrobial peptide with antibacterial, antifungal and AQS activity. To the best of our knowledge, no antimicrobial activity has ever been reported from K. kristinae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinobacteria are aerobic, gram-positive, filamentous, soil dwelling bacteria (Anderson and Wellington 2001) with exceptional metabolic diversity and are a rich source of several useful bioactive natural products, such as polyketides and peptides (Bundale et al. 2018a; Zhao et al. 2018). Polyketides, which contain repeating (–CH2–CO–) groups, represent 20% of pharmaceutical drugs in the market (Tiwari and Gupta 2012).
Based on the diversity in structure and function, polyketides can be divided into three classes. The type I polyketides include macrolides like erythromycin, azithromycin and rapamycin and polyenes like amphotericin B and nystatin. The type II polyketides are aromatic polyketides such as tetracycline, doxorubicin, daunorubicin, rhodomycin, actinorhodin etc. The type III polyketides include chalcones and stilbenes in plants and polyhydroxy phenols in bacteria (Shen 2003).
Antimicrobial peptides (AMPs) are a well-known group of therapeutic agents, including tyrocidin, gramicidins, cyclosporine, polymyxins, daptomycin and surfactin. Non-ribosomal peptides, synthesized by non-ribosomal peptide synthetases are known to exhibit a wide range of biological activities including, antiviral, antiprotozoal, hypocholesterolemic, antifungal, siderophore, antimicrobial, antitumor, antioxidant, anti-hypertensive and immunomodulatory activities (Hamedi et al. 2015; Rajanbabu and Chen 2011).
Most of these antibiotics in clinical use today have been developed from compounds isolated from actinobacteria, Streptomyces being the dominant genus (Barka et al. 2016). However, the recent search for the novel compounds from Streptomyces species has often led to the rediscovery of known compounds. Hence, the focus of screening programs has shifted to bioactive compounds from non-Streptomyces group also referred as rare actinobacteria (Bundale et al. 2018b). At present more than 50 rare actinobacterial taxa are reported to produce 2500 bioactive compounds (Kurtböke 2012). Thus, it is crucial that new groups of rare actinobacteria be pursued as sources of novel pharmaceutically active metabolites. Amongst the novel metabolites, anti-quorum-sensing (AQS) agents, which can curb infection without a killing action, are gaining importance. Bacterial cell–cell communication, dubbed quorum sensing, is intricately related to virulence. An associated phenomenon is the bacterial swarming which allows the spread of disease and virulence. With this as a background and the rising incidence of resistance to extant antibiotics, a search for AQS agents as new molecules to treat infection has become logical and gathered momentum (Nashikkar et al. 2011; Kurtböke 2012). Although antimicrobial properties of actinobacteria have been extensively studied, less is known about AQS activities of rare actinobacteria which may be a rich source of active compounds that can act against bacterial quorum sensing systems.
The current study involves the purification and characterization of the bioactive polyketides and peptides from the three isolates selected from our previous study and their evaluation for antimicrobial, anticancer and AQS properties. These isolates were identified by 16S rRNA gene sequencing as Micromonospora auratinigra, Family Micromonosporaceae and Kocuria kristinae and their sequences deposited in the GenBank under the accession numbers MG407702, MG372012 and MG430204 respectively (Bundale et al. 2018b).
Materials and methods
Chemicals and media
All chemicals and solvents were of analytical grade and purchased from Merck, Germany and culture media from Hi-media, Mumbai, India.
Test organisms and animal cell lines
The target strains used for screening antimicrobial activity were procured from Microbial Type Culture Collection (MTCC), IMTECH, Chandigarh, India and were: Bacillus subtilis MTCC 441, Escherichia coli MTCC 443, Proteus mirabilis 425, Serratia marcescens MTCC 86 and Candida albicans MTCC 227. HeLA and IMR cell lines were purchased from National Centre for Cell Science (NCCS), Pune, India.
Methods
Production of polyketides and peptides
The selected potent isolates were grown in potato dextrose broth and incubated in a rotary shaker incubator (REMI CIS-24 BL) at 130 rpm at 28 °C. 1 ml aliquots were withdrawn after every 24 h for a period of 12 days to optimize incubation period for maximum bioactive metabolite production. The cell free supernatant was concentrated fivefold in a vacuum concentrator and 50 µl was used to determine antimicrobial bioactivity against test organisms. The diameters of zones of inhibition were noted and correlated to the concentration of the bioactive compound in the cell free supernatant (Bundale et al. 2015).
Extraction of the bioactive compounds
For polyketides
Crude antimicrobial compound was recovered from the mycelium as well as culture filtrate of both bioactive isolates by solvent extraction with ethyl acetate (1:1 v/v). The solvent was evaporated to dryness in a vacuum concentrator to obtain the crude cell and broth extracts which were stored at – 20 °C until further use (Bundale et al. 2018a).
For peptides
The cell free broth was cooled overnight and acetone precipitation/ammonium sulphate precipitation was carried out. The protein precipitate was separated by centrifugation. Ammonium sulphate precipitate was resuspended in phosphate buffer and was subjected to dialysis using a dilute buffer. The dialysed peptide was used for bioactivity studies. The solvent in the acetone precipitate was allowed to evaporate completely overnight at 4 °C. This precipitate was resuspended in phosphate buffer and used for bioactivity studies. The presence of protein in the precipitate was confirmed by biuret and ninhydrin tests.
Purification of the bioactive compounds
For polyketides
The dried crude extract was dissolved in ethyl acetate and 100–500 µl was loaded over the silica gel column. A stepwise gradient of chloroform/methanol was applied and the fractions thus separated were collected.
Preparative thin layer chromatography (TLC) with silica gel plate 60 F254 was used for the partial purification of antimicrobial products. The crude extracts were spotted and developed in different solvent systems. The solvent systems used were chloroform:petroleum ether:methanol (10:10:3), chloroform:acetone:methanol (75:15:10), chloroform:methanol (8:2), petroleum ether:chloroform:methanol (7:2:1), chloroform:methanol (9:1), benzene:acetone:methanol (100:10:1). The developed plates were air dried and the separated bands were detected by observations of the color of the bands. The TLC was repeated several times and the mean Rf of the bands was calculated. The fractions were physically separated from each other by scraping the bands from the plates, extracting with methanol, concentrating the extracts and again subjecting each concentrate to TLC using the same solvent system, thereby confirming the purity of each fraction (Bundale et al. 2018a; Johdo et al. 1991; Kim et al. 1996).
For peptides
The protein precipitate was purified by preparative TLC using the solvent system butanol:acetic acid:water (3:1:1) (Dharmaraj 2011). The developed plate was air dried and the band was visualized by spraying ninhydrin and the Rf was noted. The TLC was repeated in parallel and the band calculated by Rf was scraped and later extracted in the same solvent system. The purified extract was concentrated and again subjected to TLC using the same solvent system to confirm its purity. The peptide thus purified was sent for LC–MS analysis to confirm its purity and to assess its molecular weight.
Spectral studies
The UV–vis absorption spectra (190–1100 nm) of the purified fractions were determined to identify the chromophores present in the metabolites by using a double beam bio-spectrophotometer (BL-198, Elico Ltd.) (Silverstein et al. 2014). Furthermore, Fourier transform infra red (FT-IR) spectrum of each active extract was obtained (as KBr discs) between 400 and 4000 cm−1 on Perkin Elmer 2000 FT-IR spectrophotometer and plotted as intensity versus wave number (Augustine et al. 2005). 1H NMR spectra of the purified bioactive compounds was measured using a Bruker AMX 300 Coupling constants (J) in Hz. The mass spectra were obtained on a Bruker micro TOF-Q II 10,330 between 50 and 3000 m/z.
Bioactivity studies
Antimicrobial activity
The antimicrobial activity of the pure compounds was assessed by the agar well diffusion method using Mueller–Hinton agar for the antibacterial and potato dextrose agar for anti fungal assays.
15 μl of 1 mg/ml stock were used for the tests. The diameter of the inhibition zones was determined after 24 h of incubation at 37 °C for bacteria and 28 °C for C. albicans. The minimum inhibitory concentrations (MICs) of the bioactive compounds were determined via a microdilution method using sterile 24-well plates with tetracycline as a standard (Arthington-Skaggs et al. 2002).
MTT-based cytotoxicity assay
The cytotoxicity of bioactive fractions on established cell lines like HeLa and IMR was determined in vitro by the MTT based cytotoxicity assay (Mosmann 1983; Begde et al. 2011). The adherent cells were exposed to a concentration gradient of 1–40 μg/ml of the purified compounds.
Anti-quorum sensing activity
Pigment quenching assay Quorum sensing inhibition (QSI) by the bioactive compounds was determined by studying pigment quenching using agar well diffusion method with S. marcescens as the indicator organism. A positive QSI result was indicated by a lack of pigmentation of the indicator strain around the vicinity of the well. Negative results were indicated by no pigmentation inhibition (Kanagasabhapathy et al. 2009; Bundale et al. 2018b).
Swarming motility assay To study the effect of bioactive compounds from rare actinobacteria on the swarming motility of P. mirabilis, 5 µl of an overnight culture was centrally inoculated on swarm agar plates containing various concentrations of the compounds. Two control plates were also set up for each study; one containing no additives and termed positive control and the other containing 20% DMSO and termed solvent control. All the plates were incubated at 37 °C for 20 h. Thereafter, the diameter of the swarm zone was measured and compared to the control (Nashikkar et al. 2011).
Statistical analysis
The MIC values were expressed as average of four independent replicates ± SD and IC50 values in the MTT based cytotoxicity assay as an average of eight replicates ± SD. Student’s t-test was performed using SYSTAT Software (Systat Software, Inc., Chicago, IL, USA). P value ≤ 0.05 was considered significant unless otherwise mentioned.
Results
Three rare actinobacterial strains, MMS8, MMS16 and KCR3, which exhibited the ability to produce bioactive polyketides and peptides on the basis of pre-screening results were selected for this study.
Production and extraction of bioactive compounds
The bioactive compound production by the three isolates was monitored over a period of 12 days and was found to start only after 48 h for all the three isolates. It reached a maximum (24 mm) for KCR3 on the 4th day, and remained almost stable till the 12th day. A similar pattern was observed for MMS8 where, maximum bioactive metabolite production was observed on the sixth day (16 mm). But for MMS16, maximum production reached on 6th day (19 mm) and then showed a sharp decline from day 10 (Fig. 1).
The polyketide complexes extracted from MMS8 and MMS16 were purified using silica gel adsorption column chromatography followed by preparative TLC. The purified fractions were named MMS8A, MMS8B, MMS8C and MMS8D for the isolate MMS8. A similar numbering scheme was used for MMS16 too. The AMP from KCR3 was purified by acetone precipitation followed by preparative TLC. The solvent system used, colour, and Rf’s of the fractions obtained from each organism are given in the Table 1.
Characterization of the polyketides and peptides produced by rare actinobacterial isolates
This section describes the characterization of the most potent bioactive compounds produced by the respective organisms.
Identification of bioactive compound from MMS8
The UV–vis spectra of MMS8B, which was the most potent compound showed the peaks at 247, 303, 379 and 410 (Fig. 2a). The IR (KBr) spectra of MMS8B showed prominent peaks at 3326, 2946, 2833, 1651, 1447, 1418, 1113, 1020, 666 (cm−1) (Fig. 2b). The 1H NMR spectra showed chemical shifts at δ 9.99 (aldehyde), δ 7.06–δ 7.57 (3 aromatic protons), δ 2.36 (C attached to Cl), δ 1.15–δ 1.39 (alkyl, methylene), δ 0.9 (methyl), δ 0.02–δ 0.096 (Fig. 2c). The mass spectrum of the compound showed a peak at m/z 242. The obtained molecular ion peak showed a further fragmentation to give a base peak at m/z 214 (Fig. 2d).
Identification of bioactive compound from MMS16
MMS16B, the most potent compound was found to be soluble in acetone, methanol and chloroform and showed a sharp yellow band with an Rf of 0.48 in chloroform:methanol (9:1). The UV–vis spectra of MMS16B showed the peaks at 255, 280, 318, 342, 417 nm (Fig. 3a). The IR (KBr) spectra of MMS16B showed prominent peaks at 3344, 2943, 2833, 1650, 1438, 1131, 1028 (cm−1) (Fig. 3b). The chemical shifts shown by the proton NMR were at δ 7.07–δ 7.57 (3 aromatic protons), δ 2.19–δ 2.29 (C attached to N), δ 1.28–δ 1.57 (alkyl, methine, methylene), δ 0.9 (methyl), δ 0.02–δ 0.096 (Fig. 3c). The molecular weight was determined by mass spectra and by high resolution of the molecular ions to be m/z 811(Fig. 3d).
Identification of AMP from KCR3
The peptide nature of the bioactive compound, KCR3A obtained from KCR3 has been established in a previous study by us. The purified compound from KCR3 resolved as one single band with an Rf of 0.52 in the solvent system butanol:acetic acid:water (3:1:1) which turned purple on spraying with ninhydrin. The UV–vis spectra showed prominent peaks at 232 and 372 nm (Fig. 4a). The IR (KBr) spectra showed prominent peaks at 2281, 1595, 1488, 1153, 1069, 1039 and 962 (cm−1) (Fig. 4b). High resolution LC–MS of the KCR3A yielded highest molecular mass of 1097 with a range of several fragmentation peaks (m/z 579, 637, 695, 753, 811, 869, 927, 985) (Fig. 4c, Supplementary Fig. 1).
Table 2 summarizes the physicochemical properties of the purified compounds obtained from all the three isolates.
Bioactivity studies
Antimicrobial activity
MMS8A and MMS8C did not exhibit any activity against B. subtilis but MMS8B and MMS8D were active. None of the compounds of MMS8 was active against E. coli. All purified compounds of MMS8 were active against C. albicans. MIC of MMS8B against B. subtilis was found to be 8 µg/ml. The purified compounds from isolate MMS16 were found to be active against B. subtilis with zones of inhibition in the range of 14–17 mm. These compounds were not active against E. coli. The compounds were also antifungal exhibiting zones of inhibition against C. albicans. The MIC of MMS16B, the most potent compound, against B. subtilis was found to be 7 µg/ml. The purified peptide from KCR3 was potent against all test organisms (refer Table 1).
Anticancer activity
In vitro antitumor activity of the bioactive compounds was judged by MTT based cytotoxicity assay against established cancer cell lines, HeLa and IMR. The IC50 of MMS8B determined from the graph was ~ 10 μg/ml for HeLa and ~ 4 μg/ml for IMR and that of MMS16B was observed to be ~ 9 μg/ml for HeLa and ~ 14 μg/ml for IMR (Fig. 5a, b).
a MTT based cytotoxicity assay of MMS8B on HeLa and IMR, IC50 against HeLa was found to be 10 μg and 4 μg respectively and b MTT based cytotoxicity assay of MMS16B on HeLa and IMR, IC50 against HeLa was found to be 9 μg and 14 μg respectively. Each point represents mean of three independent observations ± SD
Anti-QS activity
All purified compounds from both the polyketide producers exhibited AQS activity. The most potent compounds, MMS8B and MMS16B showed pigment quenching zones of 10 mm and 12 mm respectively against S. marcescens. KCR3A showed extremely high AQS activity against S. marcescens with a turbid zone of inhibition of 32 mm (Fig. 6) However, nisin used as a standard, failed to show AQS even at concentration of 10 mg/ml. MMS16B also showed antiswarming activity against P. mirabilis at a concentration of 0.3 mg/ml and 0.5 mg/ml with 70–80% reduction in swarm zones as can be seen in Fig. 7.
Anti swarming activity of MMS16B against Proteus mirabilis: a positive control, b solvent control, c swarm zone inhibition by compound MMS16B at 0.3 mg/ml concentration (50% reduction in swarm zone) and d swarm zone inhibition by compound MMS16B at 0.5 mg/ml concentration (80% reduction in swarm zone)
Discussion
The current study was undertaken with the aim of obtaining novel polyketides and peptides from rare actinobacteria isolated from soil. These were purified and their antimicrobial, anticancer and anti-QS activities were assessed followed by their structure elucidation.
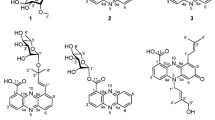
Compound MMS8B isolated from M. auratinigra, was obtained as an orange yellow compound with an Rf of 0.45 in the solvent system chloroform:methanol (9:1). The UV–vis spectrum indicates the compound to belong to anthraquinones showing bands in the wavelength range 220–350 nm and one absorption band at longer wavelengths, close to 400 nm (Osman et al. 2014). IR spectra and NMR indicate the compound MMS8B to be chloroanthraquinone, with the presence of a peak at 660 cm−1 (C–Cl) in IR spectrum and chemical shift at δ 2.37 (C attached to Cl) in 1H NMR. The structure of the compound was further confirmed by the fragmentation pattern of the molecule in the mass spectrum showing a sharp peak at 242 and fragmentation peak at 214 as reported in Pubchem. The compound was thus identified as chloroanthraquinone, reported to have a molecular mass of m/z 242 in published data.
Anthraquinone derivatives other than chloroanthraquinones have been previously reported from Micromonospora rhodorangea (Xue et al. 2009) and Micromonospora lupini (Igarashi et al. 2007). However, to the best of our knowledge, this is the first report of chloroanthraquinone being isolated from M. auratinigra which is a relatively less studied species of this widely reported genus with only one report wherein, Talukdar et al. have reported the bioactive compound, 2-methylheptylisonicotinate, similar to isoniazids from this organism (Talukdar et al. 2016).
MMS8B was found to have antimicrobial, antifungal and anticancer activity. A similar range of activity was also reported in 7-chloroemodin, a novel chloroanthraquinone isolated from lichen (Rosso et al. 2003). Moreover, 2-methylheptylisonicotinate isolated from M. auratinigra has been reported to have very high values of MIC of 40 μg/ml against B. subtilis (Talukdar et al. 2016). As compared to it, chloroanthraquinone, isolated from this strain of M. auratinigra has been found to have a much lower MIC of 8 µg/ml. There are practically no reports on anticancer activities of chloroanthraquinone. However our compound was found to be potent against both the tested cell lines.
Isolate MMS16, identified as novel member of Family Micromonosporaceae sp., yielded a yellow coloured compound, MMS16B. The UV–vis spectra indicate that it may belong to bafilomycin group of macrolide antibiotics exhibiting maximum absorptions at 242, 248 and 280 nm. Bafilomycins B and C show in addition, shoulders between 340 and 360 nm (Werner et al. 1984). The additional shoulder at 343 in our compound, indicates that it may be bafilomycin B. Further the Rf also matches to that reported for setamycin which is a type of bafilomycin B1 (Omura et al. 1981). The IR spectra too was very similar to that reported for setamycin by Omura et al. (1981), showing peaks in the range of 3300–3500 cm−1 and around 1650 cm−1 as reported for bafilomycins (Werener et al. 1981). However, the peak due to an ester group at 1710–1730 cm−1 and at 1220 cm−1, reported for bafilomycin B1, was missing.
High resolution LC–MS of the compound yielded molecular weights of 811 which matches the published data for bafilomycin B1. The mass showed the same fragmentation peaks (m/z 568, 525, 399, 368, 338, 211, 169, 137, 113, 109) as reported by Otoguro for setamycin (bafilomycin B1). Also the appearance of characteristic fragment peak at around m/z 211 in our compound is assignable to flavensomycinoic acid which has been involved in the molecules of bafilomycin B1 (Otoguro et al. 1988). The chemical shift values shown by the 1H NMR of MMS16B were very similar to that of bafilomycins. Thus Rf, UV spectra, and mass spectra of MMS16B is almost identical to setamycin (bafilomycin B1) but IR spectra is similar to bafilomycin A. This indicates that MMS16B might be slightly different from the known bafilomycins.
MIC of MMS16B (7 µg/ml) was found to be much lower than the reported value of 25 µg/ml for setamycin against B. subtilis (Omura et al. 1981; Otoguro et al. 1988). As MMS16B did not show any appreciable activity against E. coli and P. mirabilis but exhibited pigment quenching against S. marcescens, it was explored further for its AQS properties. MMS16B showed antiswarming activity against P. mirabilis at a concentration of 0.3 mg/ml and 0.5 mg/ml with 70–80% reduction in swarm zones. To the best of our knowledge there are no previous reports on the AQS properties of any analogue of bafilomycins.
The IC50 value against cancer cell lines HeLa and IMR is comparable to that of reported values of bafilomycin B1; 5.88 nM and 14.37 nM for other cell lines like leukemia and melanoma (Laakso et al. 2003). These studies on the antimicrobial, AQS and anti cancer properties of MMS16B, which may be a bafilomycin analogue, make it a good candidate for being studied as an AQS and anticancer drug.
KCR3 identified as K. kristinae, was found to produce an AMP. The IR spectra of the AMP obtained from KCR3 shows the characteristic IR bands of peptide linkage (2281-C≡N stretch, 1595-C = O amide region, 1488-NH bending and CN stretching, 1153-C–OH stretch, C–O–C stretch, 962-C–H out of plane bending) (Fabian and Mäntele 2006). However, the peak of 2500 cm−1 indicative of S–H stretch, was lacking in KCR3A, confirming the absence of thiol group in this AMP. The mass spectra indicated that the component with m/z 753 was present at the highest concentration and is likely to be the active compound. Our peptide showed a much broader bioactivity range unlike the AMP’s produced by other species of Kocuria such as kocurin produced by Kocuria palustris and variacin produced by Kocuria varians. KCR3A showed activity against both gram positive as well as gram negative bacteria and was also found to be antifungal in nature. Further, it was found to have very high AQS activity at sublethal concentrations as judged by the pigment quenching capability using S. marcescens as the test organism. Kocurin is a thiazolyl peptide, reportedly showing in vitro activity against gram-positive bacteria (Martín et al. 2013). Similarly, variacin, a lantipeptide has also been reported to be active against gram positive bacteria with no antifungal activity (Pridmore et al. 1996). To the best of our knowledge, K. kristinae has not been previously studied for production of any bioactive compounds.
Compounds like KCR3A and MMS16B, which interfere with the QS mechanism of pathogens, may prove to be highly effective in controlling their pathogenicity, especially opportunistic pathogens, that cause disease only when their population becomes quorate, and they are able to express their virulence factors. AQS compounds like these can hence attenuate the virulence of the pathogens without challenging their growth, thereby preventing the emergence of drug resistant strains.
Although screening programs do not always result in the discovery of new compounds, this study yielded two novel polyketides and an AMP which can be taken up in a drug development program wherein they may be chemically modified to further increase their activity. These can be subsequently evaluated in clinical trials rendering valuable chemotherapeutic compounds in future.
References
Anderson AS, Wellington EM (2001) The taxonomy of Streptomyces and related genera. Int J Syst Evol Microbiol 51(3):797–814
Arthington-Skaggs BA, Lee-Yang W, Ciblak MA, Frade JP, Brandt ME, Hajjeh RA, Harrison LH, Sofair AN, Warnock DW, Candidemia Active Surveillance Group (2002) Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother 46(8):2477–2481
Augustine SK, Bhavsar SP, Kapadnis BP (2005) A non-polyene antifungal antibiotic from Streptomyces albidoflavus PU 23. J Biosci 30(2):201–211
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80(1):1–43
Begde D, Bundale S, Mashitha P, Rudra J, Nashikkar N, Upadhyay A (2011) Immunomodulatory efficacy of nisin—a bacterial lantibiotic peptide. J Pept Sci 17(6):438–444
Bundale SB, Begde DN, Nashikkar NA, Kadam TA, Upadhyay AU (2015) Optimization of culture conditions for production of bioactive metabolites by Streptomyces spp. isolated from soil. Adv Microbiol 5(06):441–451
Bundale SB, Begde DN, Pillai D, Gangwani K, Nashikkar NA, Kadam TA, Upadhyay AU (2018a) Novel aromatic polyketides from soil Streptomyces spp.: purification, characterization and bioactivity studies. World J Microbiol Biotechnol 34:1–6
Bundale SB, Singh J, Begde DN, Nashikkar NA, Upadhyay AU (2018b) Culturable rare actinomycetes from Indian forest soils: molecular and physicochemical screening for biosynthetic genes. Iran J Microbiol 10(2):132–142
Dharmaraj S (2011) Antagonistic potential of marine Actinobacteria against fish and shellfish pathogens. Turk J Biol 35(3):303–311
Ding Y, Huang Y, Ruan J, Gao Y (2009) Selective isolation and diversity of acidophilic filamentous actinomycetes from acidic soils. Wei sheng wu xue bao = Acta microbiol Sin 49(6):710–717
Ding D, Chen G, Wang B, Wang Q, Liu D, Peng M, Shi P (2013) Culturable actinomycetes from desert ecosystem in northeast of Qinghai-Tibet Plateau. Ann Microbiol 63(1):259–266
Fabian H, Mäntele W (2006) Infrared spectroscopy of proteins. In: Handbook of vibrational spectroscopy. Wiley Online Library. https://doi.org/10.1002/0470027320.s8201
Hamedi J, Imanparast S, Mohammadipanah F (2015) Molecular, chemical and biological screening of soil actinomycete isolates in seeking bioactive peptide metabolites. Iran J Microbiol 7(1):23
Igarashi Y, Trujillo ME, Martínez-Molina E, Yanase S, Miyanaga S, Obata T, Sakurai H, Saiki I, Fujita T, Furumai T (2007) Antitumor anthraquinones from an endophytic actinomycete Micromonospora lupini sp. nov. Bioorg Med Chem Lett 17(13):3702–3705
Johdo O, Watanabe Y, Ishikura T, Yoshimoto A, Naganawa H, Sawa T, Takeuchi T (1991) Anthracycline metabolites from Streptomyces violaceus A262. J Antibiot 44(10):1121–1129
Kanagasabhapathy M, Yamazaki G, Ishida A, Sasaki H, Nagata S (2009) Presence of quorum-sensing inhibitor-like compounds from bacteria isolated from the brown alga Colpomenia sinuosa. Lett Appl Microbiol 49:573–579
Kim HS, Hong YS, Kim YH, Yoo OJ, Lee JJ (1996) New anthracycline metabolites produced by the aklavinone 11-hydroxylase gene in Streptomyces galilaeus ATCC 31133. J Antibiot 49(4):355–360
Kurtböke DI (2012) Biodiscovery from rare actinomycetes: an eco-taxonomical perspective. Appl Microbiol Biotechnol 93(5):1843–1852
Laakso JA, Mocek UM, Van Dun J, Wouters W, Janicot M (2003) R176502, a new bafilolide metabolite with potent antiproliferative activity from a novel Micromonospora species. J Antibiot 56(11):909–916
Martín J, da Sousa ST, Crespo G, Palomo S, González I, Tormo JR, de la Cruz M, Anderson M, Hill RT, Vicente F, Genilloud O (2013) Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar drugs 11(2):387–398
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Nashikkar NA, Begde DN, Bundale SB, Pise MV, Rudra JA, Upadhyay AU (2011) Inhibition of swarming motility, biofilm formation and virulence factor expression of urinary pathogens by Euphorbia trigona latex extracts. Int J Pharm Sci Res 2(3):558
Omura S, Otoguro K, Nishikiori T, Oiwa R, Iwai Y (1981) Setamycin, a new antibiotic. J Antibiot 34(10):1253–1256
Osman CP, Weber JF, Ismail NH (2014) UV/visible spectra of a series of natural and synthesised anthraquinones: experimental and quantum chemical approaches. SpringerPlus 3(1):233. https://doi.org/10.1186/2193-1801-3-233
Otoguro K, Nakagawa A, Omura S (1988) Setamycin, a 16-membered macrolide antibiotic identification and nematocidal activity. J Antibiot 41(2):250–252
Pridmore D, Rekhif N, Pittet AC, Suri B, Mollet B (1996) Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: comparison to lacticin 481 of Lactococcus lactis. Appl Environ Microbiol 62(5):1799–1802
Rajanbabu V, Chen JY (2011) Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 32(2):415–420
Rosso ML, Bertoni MD, Adler MT, Maier MS (2003) Anthraquinones from the cultured lichen mycobionts of Teloschistes exilis and Caloplaca erythrantha. Biochem Syst Ecol 31(10):1197–1200
Shen B (2003) Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol 7(2):285–295
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL (2014) Spectrometric identification of organic compounds. Wiley, Hoboken
Talukdar M, Bordoloi M, Dutta PP, Saikia S, Kolita B, Talukdar S, Nath S, Yadav A, Saikia R, Jha DK, Bora TC (2016) Structure elucidation and biological activity of antibacterial compound from Micromonospora auratinigra, a soil Actinomycetes. J Appl Microbiol 121(4):973–987
Tiwari K, Gupta RK (2012) Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol 32(2):108–132
Werner G, Hagenmaier H, Drautz H, Baumgartner A, Zahner H (1984) Metabolic products of microorganisms. 224. J Antibiot 37(2):110–117
Xue CM, Tian L, Lin WH, Deng ZW (2009) Anthraquinone derivatives from Micromonospora rhodorangea. Nat Prod Res 23(6):533–538
Zhao P, Xue Y, Gao W, Li J, Zu X, Fu D, Feng S, Bai X, Zuo Y, Li P (2018) Actinobacteria-derived peptide antibiotics since 2000. Peptides. https://doi.org/10.1016/j.peptides.2018.03.011
Acknowledgements
The authors would like to acknowledge the financial assistance provided by the Department of Science and Technology, Government of India for the present study under the Scheme WOS-A. The authors would also like to thank Central Instrumentation Facility, IISER Bhopal, India for providing NMR and Mass Spectra Analysis and Material Science Department of VNIT, Nagpur, India for FT-IR analysis respectively. We acknowledge our student, Deepak Khushalani for his help in acquiring the spectral data of KCR3A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bundale, S., Singh, J., Begde, D. et al. Rare actinobacteria: a potential source of bioactive polyketides and peptides. World J Microbiol Biotechnol 35, 92 (2019). https://doi.org/10.1007/s11274-019-2668-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2668-z