Abstract
Surface properties like hydrophobicity, aggregation ability, adhesion to mucosal surfaces and epithelial cells and transit time are key features for the characterization of probiotic strains. In this study, we used two Lactobacillus paracasei subsp. paracasei strains (BGNJ1-64 and BGSJ2-8) strains which were previously described with very strong aggregation capacity. The aggregation promoting factor (AggLb) expressed in these strains showed high level of binding to collagen and fibronectin, components of extracellular matrix. The working hypothesis was that strains able to aggregate have an advantage to resist in intestinal tract. So, we assessed whether these strains and their derivatives (without aggLb gene) are able to bind or not to intestinal components and we compared the transit time of each strains in mice. In that purpose parental strains (BGNJ1-64 and BGSJ2-8) and their aggregation negative derivatives (BGNJ1-641 and BGSJ2-83) were marked with double antibiotic resistance in order to be tracked in in vivo experiments in mice. Comparative analysis of binding ability of WT and aggregation negative strains to different human intestinal cell lines and mucin revealed no significant difference among them, excluding involvement of AggLb in interaction with surface of intestinal cells and mucin. In vivo experiments showed that surviving and transit time of marked strains in mice did not drastically depend on the presence of the AggLb aggregation factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactobacilli are used in the food industry for the production of an array of fermented products. Most lactobacilli present in human feces are clearly allochthonous members derived from fermented food, the oral cavity or proximal parts of the gastrointestinal tract (GIT) (Walter 2008). Given the role of lactobacilli in metabolic, nutritional, physiological and immunological processes in the human body, interest in the promotion of human health and prevention or treatment of several diseases by bacteria of the genus Lactobacillus has recently increased (Dar et al. 2018; Esmaeili et al. 2018; Gerritsen et al. 2011; Rajoka et al. 2018; Messaoudi et al. 2013).
Cell surface proteins of microbes are in primary contact with gut environment, adapting to stress conditions and involved in cell protection and surface recognition (Vinusha et al. 2018). Aggregation factors are important surface components (Lozo et al. 2007; Kojic et al. 2011; Miljkovic et al. 2015). Aggregation factors belong to the family of proteins called Snow-flake Forming Collagen Binding Aggregation Factors (SFCBAF) and represent surface high molecular weight molecules, rich in threonine and lysine and are free of cysteine in all the aggregation factors described so far (Miljkovic et al. 2018). Little is known about specific biological role of aggregation factors in the environment of gut. Probiotic lactobacilli strains, Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibited strong auto-aggregation and co-aggregation phenotypes in the presence of Candida glabrata, which indicate that these lactobacilli strains may exert their probiotic effects through the formation of aggregates and, thus the consequent prevention of colonization by C. glabrata (Chew et al. 2015). One of the proposed mechanisms that could increase the potential of bacteria to survive and persist longer in the GIT is their ability to aggregate (Muthu Selvam et al. 2016; Waśko et al. 2014; García-Cayuela et al. 2014). In addition, adhesion to intestinal cells seems mediated by aggregation factors providing successful colonization and leading to exclusion of pathogens and/or immunomodulation (McNaught and MacFie 2001; Kravtsov et al. 2008; Veljović et al. 2017). However, adhesion to epithelial cells or mucus layer does not necessarily give a selective advantage in the gut (Radziwill-Bienkowska et al. 2017; Turpin et al. 2013) and a long transit time in the gut may not be sufficient to maximize the beneficial effects of a given strain (Miquel et al. 2015).
We previously showed that aggregation factors from Lactobacillus paracasei subsp. paracasei BGNJ1-64 and BGSJ2-8 possess the crucial role in cell aggregation, increase hydrophobicity and contribute to the specific binding of strains to immobilized collagen and fibronectin (Miljkovic et al. 2015, 2016). Since AggLb plays an important role in the interaction between bacteria and binding to the different surfaces, simultaneously changing physico-chemical characteristics on the surface, a main focus of this study was to determine the contribution of AggLb aggregation factor in interaction with intestinal mucosa.
Material and methods
Bacterial strains and growth conditions
The strains and their derivatives used in this study are listed in Table 1. Lactobacillus strains were grown in De Man-Rogosa-Sharpe (MRS) (Merck GmbH, Darmstadt, Germany) medium at 30 °C. Agar plates were prepared by addition of agar (1.5% w/v) to MRS broth. For growth of the labelled strains (BGNJ1-64/SmrSpcrAgg+, BGNJ1-64/SmrSpcrAgg−, BGSJ2-8/SmrSpcrAgg+ and BGSJ2-8/SmrSpcrAgg−) the following antibiotic concentrations were used: streptomycin (Sm) 1000 μg/ml and spectinomycin (Spc) 500 μg/ml (MRS Sm/Spc). All strains were stored in growth medium containing 15% glycerol at − 80 °C.
Adhesion test to Caco2, HT29 and HT29-MTX cell lines
The colonocyte-like cell lines: Caco2 (from passages 47 to 51), HT29 (from passages 62 to 65) and its mucus-secreting derivative HT29-MTX (from passages 50 to 53) were used to determine the adhesion ability of the lactobacilli strains. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, Switzerland) supplemented with heat-inactivated fetal bovine serum (FBS) (20% for Caco2; 10% for HT-29 and HT29-MTX cells) and 1% l-glutamine at 37 °C with 10% of CO2. Intestinal cells were seeded at a concentration of 8 × 106 for Caco-2 and 1 × 107 for HT29 and HT29-MTX cells/well into 24-well plates and cultivated until confluent differentiated monolayers were obtained. Twenty-four hours before bacterial co-culture the culture medium was changed to a medium with 5% heat-inactivated FBS and 1% glutamine.
Overnight cultures of lactobacilli were washed twice with Dulbecco's PBS solution (Sigma-Aldrich) and resuspended in the corresponding cell line media without antibiotics at a concentration of about 108 CFU/ml. Adhesion experiments were carried out for 2 h at 37 °C, 5% CO2 and, afterwards, wells were gently washed (three times with 300 μl of PBS) to release unattached bacteria. The viable adherent bacteria were scraped with 200 μl of 0.05% (v/v) Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA). Dilutions of samples, before and after adhesion, were made in PBS solution and bacteria counts were performed on MRS Sm/Spc agar plates. Adhesion was expressed as the percentage of adhered bacteria with respect to number of added bacteria. Experiments were carried out in two replicated plates and in each plate three wells were used per sample.
Immunomodulation
HT-29 cells were cultured in the same manner by the protocol described above. On co-culture day, bacteria were added at a multiplicity of infection (MOI) of 1:40 in 50 μl DMEM, in a total volume of 500 μl. Cells were stimulated simultaneously with recombinant human TNF-α (5 ng/ml, Peprotech, USA) for 6 h at 37 °C in 5% CO2. After co-incubation, cell supernatants were collected and frozen at − 80 °C until further analysis of IL-8 concentrations by ELISA (Biolegend, USA) (Kechaou et al. 2013).
Binding to mucin
Type III mucin from porcine stomach (PGM) (Sigma-Aldrich, St. Louis, MO, USA) was suspended/solubilized in PBS (10 mg/ml). This solution was left 90 min at 4 °C and 100 µl of mixture was immobilized into polystyrene microtiter plate wells (Thermo Fisher Scientific, Nunc A/S, Waltham, MA, USA), which were then incubated 1 h at 37 °C followed by overnight incubation at 4 °C. Bacterial overnight cultures were centrifuged (5000×g for 10 min) and washed twice with PBS, and the absorbance (A600nm) was adjusted to 0.1 ± 0.01 to standardize the number of bacteria (approximately 1.5 × 108 CFU/ml). After immobilization, wells were washed twice with 200 µl PBS and then bacterial cultures were added (100 µl) and incubated 2 h at 37 °C. Non-adherent bacterial cells were removed by carefully washing the wells three times with 200 μl of PBS. The viable adherent bacteria were scraped with 0.05% (v/v) Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA). Dilutions of samples, before and after adhesion, were made in PBS solution and bacteria counts were performed on MRS Sm/Spc agar plates. Adhesion was expressed as the percentage of adhered bacteria with respect to number of added bacteria. Three independent experiments were carried out in triplicates.
In vivo approach
This study was carried out by using the antibiotic (Sm and Spc) labelled derivatives of two wild type strains (with the strong aggregation ability: BGNJ1-64 and BGSJ2-8) and their derivatives without aggregation ability (BGNJ1-641 and BGSJ2-83).
Construction of derivatives
Lactobacilli strains used for in vivo study were selected for double antibiotic resistance: streptomycin (Sm) 1000 μg/ml and spectinomycin (Spc) 500 μg/ml to distinguish them from indigenous microbiota. This selection was performed as follows: (i) 108 colony forming units (CFU) from an overnight culture of BGNJ1-64 and BGSJ2-8 (in parallel for both strains) were plates on MRS agar supplemented with Sm (1000 μg/ml). Spontaneous Smr mutants, named BGNJ1-64/SmrAgg+ and BGSJ2-8/SmrAgg+ (Table 1) were visible after 2 or 3 days of incubation at 30 °C; (ii) 108 CFU from an overnight culture of BGNJ1-64/SmrAgg+ and BGSJ2-8/SmrAgg+ were plated on MRS agar supplemented with Sm (1000 μg/ml) and Spc (500 μg/ml). Spontaneous mutants were visible after 2 or 3 days of incubation at 30 °C. The obtained derivatives, BGNJ1-64/SmrSpcrAgg+ and BGSJ2-8/SmrSpcrAgg+ (Table 1) were used for further experiments; (iii) to obtain derivatives of BGNJ1-64/SmrSpcrAgg+ and BGSJ2-8/SmrSpcrAgg+, which had lost the ability to aggregate, plasmid curing experiments were performed as described previously (Kojic et al. 2005). Loss of the aggLb gene was confirmed by PCR according to the protocol specified by Miljkovic et al. (2015). Selected aggregation deficient derivatives were named BGNJ1-64/SmrSpcrAgg− and BGSJ2-8/SmrSpcrAgg− (Table 1) and used for in vivo experiments. Clonality of selected derivatives was confirmed by pulse-field gel electrophoresis (PFGE) as described previously by Kojic et al. (2006).
In vivo experiment
Conventional C57Bl/6 mice (males, 6 weeks of age; Janvier Labs, Le Genest Saint Isle, France) were maintained under normal husbandry conditions in the animal facilities of the INRA IERP Unit (Jouy-en-Josas, France). A total of 25 mice were housed in 5 cages (5 mice per cage) and fed with autoclaved food and water ad libitum. All animal experiments began after 1-week acclimatization and next 3 days a dose of 1.4 mg/day of clindamycin was administered subcutaneously. One day later, 109 CFU of each strain in 0.1 ml of 0.9% saline solution were administered by gavage using a feeding tube. Stool samples were collected before antibiotic treatment (day 0), after antibiotic treatment (day 3; immediately before gavage) and after bacterial inoculation (days 4, 5, 6 and 8). Indigenous/ total lactobacilli were monitored by plating on MRS plates, while the inoculated strains were monitored by plating onto MRS supplemented with Sm (1000 μg/ml) and Spc (500 μg/ml) (necessary for elimination of Smr and Spcr microbiota present in faeces and permissive for the BGNJ1-64/SmSpcAgg+, BGNJ1-64/SmSpcAgg−, BGSJ2-8/SmSpcAgg+ and BGSJ2-8/SmSpcAgg− strains). Comparison of kinetics of Lactobacillus derivatives was performed at each time point to assess difference by Mann–Whitney test (Graph Pad prism).
Scanning electron microscopy analyses
Bacterial suspensions of selected strains with aggregation factors expressed on the cell surface (BGSJ2-8) and without aggregation ability (BGSJ2-83) immersed in a fixative solution (2.5% glutaraldehyde in 0.2 M sodium cacodylate buffer, pH 7.4) were deposited on sterile cover-glasses discs (Marienfeld, VWR, France) and stored 1 h at room temperature and overnight at 4 °C. The fixative solution was removed, and samples were rinsed three times for 10 min in the sodium cacodylate solution (pH 7.4). The samples underwent progressive dehydration by soaking in gradually increased concentrations of ethanol (50 to 100%) before critical-point drying under CO2. Samples were mounted on aluminium stubs (10 mm diameter) with carbon adhesive discs (Agar Scientific, Oxford Instruments SAS, GOMETZ-LA-VILLE, France) and sputter coated with platinum (Polaron SC7640, Elexience, Verrières-le-buisson, France) for 200 s at 10 mA. Samples were visualized by field emission gun scanning electron microscopy. They were viewed as secondary electron images (2 kV) with a Hitachi S4500 instrument (Elexience, Verrières-le-buisson, France). Scanning Electron Microscopy analyses were performed at the Microscopy and Imaging Platform MIMA2 (INRA, Jouy-en-Josas, France).
Images containing almost the same number of bacteria (precisely 1470 were measured) were used at the same magnification for each strain. The length of bacteria was measured using the imaging software Fiji.
Results
Characterisation of adhesion between bacteria and human intestinal cell lines
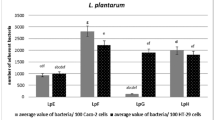
To estimate the role of the aggregation factor AggLr in the adhesion ability of aggregating strains, we performed comparative analysis of strains BGSJ2-8 and BGNJ1-64 and the corresponding non-aggregating derivatives BGSJ2-83 and BGNJ1-641 (Agg−) using Caco2, HT29 and HT29-MTX cell lines that express different molecules on their cell surface with the latter characterized by mucus production.
Results of BGNJ1-64, BGNJ1-641 and BGSJ2-8 binding to the intestinal cell lines showed a low (~ 3%) binding ratio. No difference in binding ability was observed between BGNJ1-64 and BGNJ1-641 with the three cell lines. On the contrary, the binding ratio of strain BGSJ2-83 was slightly higher between twofold and fourfold (Fig. 1). Literature data reported that other probiotic strains showed similar binding ability to Caco2 cells (Tuomola and Salminen 1998; Bogovic-Matijasevic et al. 2003). The results suggest that the binding ability of the non-aggregating BGSJ2-83-derivative correlate with mucin production, but it is not in relation with the presence of AggLb protein, even on the contrary.
We also determined the contribution of aggregation in the modulation of IL-8 secretion by HT29 cells stimulated with TNF-α. Differences/variations in IL-8 production between analysed strains BGNJ1-64 and BGNJ1-641 as well as BGSJ2-8 and BGSJ2-83 were not observed. This result indicates that the presence of AggLb on the bacterial cell surface does not contribute to the IL-8 production/immunomodulatory effect.
Adhesion to mucin
We next analyzed the role of lactobacilli aggregation factor in interaction of the bacterial cells to mucin - the most prevalent ECM component, in comparative binding test of aggregating (BGSJ2-8 and BGNJ1-64) and non-aggregating (BGSJ2-83 and BGNJ1-641) strains. All strains showed very low level of binding to mucin, although non-aggregating derivative BGSJ2-83 showed a higher degree of binding to mucin compared to the other strains (Fig. 2). This result is in line with the higher binding of BGSJ2-83 to HT29-MTX cell line producing mucus. Based on results obtained in adhesion assay, we can conclude that the AggLb aggregation factor is not involved in the interaction with mucin; cells expressing AggLb do not exhibit increased binding, on the contrary in the BGSJ2-83 strain, a slight increase of binding was observed in the absence of AggLb.
In vivo experiments
To evaluate the colonization potential of aggregating and non-aggregating strains, each strain was inoculated to antibiotic-treated mice (Fig. 3A). The level of total lactobacilli was not different between the four groups of mice (Fig. 3B). The results of the enumeration of the inoculated strains on selective media showed that the number of tested strains decreased over time, reaching level between 103 cfu/g of faeces and below the detection level (102 cfu/g of faeces) eighth days after inoculation (Fig. 3C). A comparison of carriage at each time points was performed and revealed no significant differences between aggregating and non-aggregating strains, with the exception of BGNJ1-64 strain at day 4 and at day 5 (p = 0.0079). With four out of five mice still colonized, BGNJ1-8/SmrSpcrAgg− derivative showed the highest persistence in comparison to other strains. To a lesser extent, 3 out of 5 mice were still colonized with BGNJ1-64/SmrSpcrAgg−. These results support the longer transit time and/or colonization potential of the non-aggregating strains, indicating that the AggLb aggregation factor does not contribute to the increase in retention of lactobacilli in the gastrointestinal tract.
In vivo approach. A Schematic representation of in vivo experiment; B results of enumeration of endogenous/total lactobacilli in feces and C survive/retention of ingested tested lactobacilli strains in GIT of mice during period of 8 days. Error bars present standard deviation obtained by enumeration of lactobacilli in feces of five identically treated animals. For details see Materials and methods. Statistical analysis was based on Mann Whitney test, ** for p value = 0.0079 at D4 and D5 between mice inoculated with BGNJ1-64 Agg + and with BGNJ1-64 Agg−
Cells of aggregating positive BGSJ2-8 strain are longer
The size and morphology of cells possessing the ability to aggregate was compared with those that loss the aggregation ability. Using scanning electron microscopy the lengths of the bacterial cells of BGSJ2-8 strain (expressing aggregation ability) and its derivative BGSJ2-83 (without ability to aggregate) were compared. On the same magnification, at first glance, it was noticed that the aggregating cells were longer and more compact (Fig. 4A). After measurement of 1470 cells for both strains, it was found that cells of aggregating strain BGSJ2-8 were significantly longer compared to cells of non-aggregating derivative BGSJ2-83 (Fig. 4B, C). The presence of the AggLb aggregation factor on the cell surface in some way influences the cell size and probably their behaviour.
The aggregation factor on cell surface influences the length of bacterial cells. A Representative images of BGSJ2-83 (Agg−) and BGSJ2-8 (Agg+) strains acquired by scanning electron microscope; B the number of cells of different size measured within each group -1470 cells; C graphical presentation of the length of the cells and frequency
Discussion
Lactobacilli play an important role in the life and health of humans and animals. While some resident lactobacilli permanently inhabit distinct mucosal cavities, others are provided by food and may transiently occupy the gastrointestinal tract. Lactobacilli have also been detected in substantial amounts in the different tracts although the actual numbers are subject to huge variations depending on individuals and their health status (George et al. 2018). Aggregation factors expressed on the surface of some strains of lactic acid bacteria present important surface component that could be involved in interaction with bacterial cells of other bacterial species and host. The goal of this study was to evaluate the role of AggLb aggregation factor (belonging to SFCBAF type) in interaction with human intestinal cell lines, matrix components such as the mucin, as well as the contribution to retention of lactobacilli in the gastrointestinal tract. The results of this study show that AggLb is not involved in any of the tested functions. It is interesting that one nonselective phenotype encoded by plasmid-located gene can persist in bacterial population (Miljkovic et al. 2015). Aggregation is very characteristic phenotype of bacterial population that change surface characteristic and behaviour. Only bacterial cells that express AggLb aggregate together indicating that interaction between cells occurs rather between AggLb domains than between AggLb and other cell surface components (Miljkovic et al. 2016). The presence of aggregation molecule of high molecular mass on cell surface could influence the expression of other phenotypes like proteinase activity most probably by reducing the availability of the enzyme substrate interaction and modifying the surface hydrophobicity (Lozo et al. 2007; Miljkovic et al. 2015). Similarly, binding of BGSJ2-83 Agg−strain to mucin and human intestinal cell lines could be explained by a better access of other bacterial cell wall components for binding to mucin or other components of host cells. Furthermore, AggLb does not significantly influence transit time of lactobacilli through the gastrointestinal tract of mice. Retention time of 8 days after administration is usual for probiotic strains administered at once (Van Zyl et al. 2018), and was little prolonged for Agg negative derivatives. Faster shedding of Agg plus strains could be explained by a more efficient removal of bacterial aggregates than individual bacterial cells by intestinal peristalsis rather than by a reduced interaction with epithelial cells. (Lukic et al. 2012, 2014). In addition, we observed that AggLb expressing cells are significantly longer than their Agg- minus derivatives. Interestingly, increased cell size and overall surface would protect from phagocytosis and facilitate enhanced attachment to host surfaces as well as would increase fitness and survive stationary phase (Yang et al. 2016), but in contrast Agg-minus derivatives could benefit from being longer in GIT.
Conclusion
To summarise this study indicates that AggLb aggregation factor is not involved in interaction with mucin and human intestinal cell lines. In vivo experiments strongly support conclusions obtained by in vitro confirming no involvement of AggLb in transit time of lactobacilli in gastrointestinal tract.
References
Bogovic-Matijasevic B, Narat M, Zoric M (2003) Adhesion of L. gasseri strains on Caco-2 cells. Food Technol Biotechnol 41(1):83–88
Chew SY, Cheah YK, Seow HF, Sandai D, Than L (2015) Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J Appl Microbiol 118(5):1180–1190. https://doi.org/10.1111/jam.12772
Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, Sharma V, Srivastava RK (2018) Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep 8:46–56. https://doi.org/10.1016/j.bonr.2018.02.001
Esmaeili SA, Mahmoudi M, Rezaieyazdi Z, Sahebari M, Tabasi N, Sahebkar A, Rastin M (2018) Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J Cell Biochem 119(9):7865–7872. https://doi.org/10.1002/jcb.27203
García-Cayuela T, Korany AM, Bustos I, Gómez de Cadiñanos LP, Requena T et al (2014) Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res Int 57:44–50. https://doi.org/10.1016/j.foodres.2014.01.010
George F, Daniel C, Thomas M, Singer E, Guilbaud A, Tessier FJ, Revol-Junelles AM, Borges F, Foligné B (2018) Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: a multifaceted functional health perspective (eCollection 2018, Review). Front Microbiol 9:2899. https://doi.org/10.3389/fmicb.2018.02899
Gerritsen J, Smidt H, Rijkers GT, de Vos WM (2011) Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 6(3):209–240. https://doi.org/10.1007/s12263-011-0229-7
Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, Le Goffic R, Courau S, Molimard P, Chatel JM, Langella P, Bermudez-Humaran LG (2013) Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol 79(5):1491–1499. https://doi.org/10.1128/AEM.03075-12
Kojic M, Strahinic I, Topisirovic L (2005) Proteinase PI and lactococcin A genes are located on the largest plasmid in Lactococcus lactis subsp. lactis bv. diacetylactis S50. Can J Microbiol 51:305–314. https://doi.org/10.1139/w05-009
Kojic M, Strahinic I, Fira D, Jovcic B, Topisirovic L (2006) Plasmid content and bacteriocin production by five strains of Lactococcus lactis isolated from semi-hard homemade cheese. Can J Microbiol 52:1110–1120. https://doi.org/10.1139/w06-072
Kojic M, Jovcic B, Strahinic I, Begovic J, Lozo J, Veljovic K, Topisirovic L (2011) Cloning and expression of novel lactococcal aggregation factor from Lactococcus lactis subsp. lactis BGKP1. BMC Microbiol 11:265. https://doi.org/10.1111/jam.12772
Kravtsov EG, Yermolayev AV, Anokhina IV, Yashina NV, Chesnokova VL, Dalin MV (2008) Adhesion characteristics of Lactobacillus is a criterion of the probiotic choice. Bull Exp Biol Med 145:232–234
Lozo J, Jovcic B, Kojic M, Dalgalarrondo M, Chobert JM, Haertlé T, Topisirovic L (2007) Molecular characterization of a novel bacteriocin and an unusually large aggregation factor of Lactobacillus paracasei subsp. paracasei BGSJ2-8, a natural isolate from homemade cheese. Curr Microbiol 55:266–271. https://doi.org/10.1007/s00284-007-0159-1
Lukic J, Strahinić I, Jovčić B, Filipić B, Topisirović L, Kojić M, Begović J (2012) Different roles for lactococcal aggregation factor and mucin binding protein in adhesion to gastrointestinal mucosa. Appl Environ Microbiol 78(22):7993–8000. https://doi.org/10.1128/AEM.02141-12
Lukic J, Strahinic I, Milenkovic M, Nikolic M, Tolinacki M, Kojic M, Begovic J (2014) Aggregation factor as an inhibitor of bacterial binding to gut mucosa. Microb Ecol 68(3):633–644. https://doi.org/10.1007/s00248-014-0426-1
McNaught C, MacFie J (2001) Probiotics in clinical practice: a critical review of the evidence. Nutr Res 21:343–353
Messaoudi S, Manai M, Kergourlay G, Prévost H, Connil N, Chobert JM, Dousset X (2013) Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol 36(2):296–304. https://doi.org/10.1016/j.fm.2013.05.010
Miljkovic M, Strahinic I, Tolinacki M, Zivkovic M, Kojic S, Golic N, Kojic M (2015) AggLb is the largest cell-aggregation factor from Lactobacillus paracasei subsp. paracasei BGNJ1–64, functions in collagen adhesion, and pathogen exclusion in vitro. PLoS ONE 10:e0126387. https://doi.org/10.1371/journal.pone.0126387.
Miljkovic M, Bertani I, Fira D, Jovcic B, Novovic K, Venturi V, Kojic M (2016) Shortening of the Lactobacillus paracasei subsp. paracasei BGNJ1–64 AggLb protein switches its activity from auto-aggregation to biofilm formation. Front Microbiol 7:1422. https://doi.org/10.3389/fmicb.2016.01422
Miljkovic M, Marinkovic P, Novovic K, Jovcic B, Terzic-Vidojevic A, Kojic M (2018) AggLr, a novel aggregation factor in Lactococcus raffinolactis BGTRK10-1: its role in surface adhesion. Biofouling 34(6):685–698. https://doi.org/10.1080/08927014.2018.1481956
Miquel S, Beaumont M, Martín R, Langella P, Braesco V, Thomas M (2015) A proposed framework for an appropriate evaluation scheme for microorganisms as novel foods with a health claim in Europe. Microb Cell Fact 14:48. https://doi.org/10.1186/s12934-015-0229-1
Selvam RM, Vinothini G, Thaiyammal SP, Latha S, Chinnathambi A, Dhanasekaran D, Padmanabhan P, Alharbi SA, Archunan G (2016) The cell aggregating propensity of probiotic actinobacterial isolates: isolation and characterization of the aggregation inducing peptide pheromone. Biofouling 32(1):71–79. https://doi.org/10.1080/08927014.2015.1122759
Radziwill-Bienkowska JM, Robert V, Drabot K, Chain F, Cherbuy C, Langella P, Thomas M, Bardowski JK, Mercier-Bonin M, Kowalczyk M (2017) Contribution of plasmid-encoded peptidase S8 (PrtP) to adhesion and transit in the gut of Lactococcus lactis IBB477 strain. Appl Microbiol Biotechnol 101:5709–5721. https://doi.org/10.1007/s00253-017-8334-1
Rajoka MS, Zhao H, Lu Y, Lian Z, Li N, Hussain N, Shao D, Jin M, Li Q, Shi J (2018) Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct 9(5):2705–2715. https://doi.org/10.1039/c8fo00547h
Tuomola EM, Salminen SJ (1998) Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol 41(1):45–51. https://doi.org/10.1016/S0168-1605(98)00033-6
Turpin W, Humblot C, Noordine M-L, Wrzosek L, Tomas J, Mayeur C, Chetbuy C, Guyot JP, Thomas M (2013) Behavior of lactobacilli isolated from fermented slurry (ben-saalga) in gnotobiotic rats. PLoS ONE 8(4):e57711. https://doi.org/10.1371/journal.pone.0057711
Van Zyl WF, Deane SM, Dicks LMT (2018) In vivo bioluminescence imaging of the spatial and temporal colonization of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA in the intestinal tract of mice. BMC Microbiol 18(1):171. https://doi.org/10.1186/s12866-018-1315-4
Veljović K, Popović N, Miljković M, Tolinački M, Terzić-Vidojević A, Kojić M (2017) Novel aggregation promoting factor AggE contributes to the probiotic properties of Enterococcus faecium BGGO9-28. Front Microbiol 8:1843. https://doi.org/10.3389/fmicb.2017.01843
Vinusha KS, Deepika K, Johnson TS, Agrawal GK, Rakwal R (2018) Proteomic studies on lactic acid bacteria: a review. Biochem Biophys Rep 14:140–148. https://doi.org/10.1016/j.bbrep.2018.04.009
Walter J (2008) Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74(16):4985–4996. https://doi.org/10.1128/AEM.00753-08
Waśko A, Polak-Berecka M, Paduch R, Jóźwiak K (2014) The effect of moonlighting proteins on the adhesion and aggregation ability of Lactobacillus helveticus. Anaerobe 30:161–168. https://doi.org/10.1016/j.anaerobe.2014.10.002
Yang DC, Blair KM, Salama NR (2016) Staying in shape: the impact of cell shape on bacterial survival in diverse environments. Microbiol Mol Biol Rev 80:187–203. https://doi.org/10.1128/MMBR.00031-15
Acknowledgements
This work was co-supported by The Ministry of Education, Science and Technological Development of the Republic of Serbia, Republic of Serbia (Grant No. 173019 and Grant for Co-financing of Postdoctoral Training of Researchers in 2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Mice experiments were carried out in accordance with the European guidelines for the care and use of laboratory animals (Directive 2010/63/UE). The studies received ethical approval from the local ethics committee (COMETHEA), authorized by the Ministry of Education of Higher Education and Research for the period 2015–2018 (APAFIS # 00680.01). The animal facility was accredited by the Direction des Services Vétérinaires (reference A78-187).
Rights and permissions
About this article
Cite this article
Miljkovic, M., Thomas, M., Serror, P. et al. Binding activity to intestinal cells and transient colonization in mice of two Lactobacillus paracasei subsp. paracasei strains with high aggregation potential. World J Microbiol Biotechnol 35, 85 (2019). https://doi.org/10.1007/s11274-019-2663-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2663-4