Abstract
Advancement in proteome analytical techniques and the development of protein databases have been helping to understand the physiology and subtle molecular mechanisms behind biofilm formation in bacteria. This review is to highlight how the evolving proteomic approaches have revealed fundamental molecular processes underlying the formation and regulation of bacterial biofilms. Based on the survey of research reports available on differential expression of proteins in biofilms of bacterial from wide range of environments, four important cellular processes viz. metabolism, motility, transport and stress response that contribute to formation of bacterial biofilms are discussed. This review might answer how proteins related to these cellular processes contribute significantly in stabilizing biofilms of different bacteria in diverse environmental conditions.
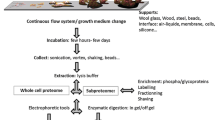
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteomic studies have contributed in better understanding of bacterial biofilms. Biofilm proteomics refers to the comparative identification of the entire set of proteins expressed by the bacteria in the biofilm matrix under various conditions (Khemiri et al. 2016). Profiling and identifying the proteins in bacterial proteome at various stages of biofilm formation and environmental conditions has complemented genomic approaches which are based on DNA sequencing. Networking of data obtained from these studies along with the information obtained from transcriptomics and metabolomics, the approach to profile proteins expressed by multi-species in a biofilm community (metaproteomics) has emerged. This has helped in studying the inter-species interactions in microbial communities (Lassek et al. 2015; Herschend et al. 2017). Freiberg et al. (2016) compared and correlated the RNA and proteome profile of Streptococcus biofilms. Using metaproteomics, the protein profiles of uropathogens such as Pseudomonas aeruginosa, Morganella morganii and Bacteroides sp. that establish biofilms in human catheter have also been explored besides studying the interactions of these pathogens with the human immune system (Lassek et al. 2015). Recently, Chignell et al. (2018), used label-free metaproteomic method to characterize distinct proteins from different stages of biofilm formation in microbial fuel cells. Although metaproteomic approach is still at its infancy, Wang et al. (2016) reviewed its current status in various environments such as soil, aquatic sediments (marine, freshwater, wastewater) and activated sludges. Together, all these studies have contributed enormously to our knowledge on existent but hitherto unknown genes and proteins involved in bacterial biofilm formation and the underlying physiological processes and their regulation. Khemiri et al. (2016) reviewed application of proteomics in studying bacterial biofilms and the review focused on various proteomic tools; contributions of proteomics in developing the biofilm model; differentially expressed proteins during various stages such as attachment, maturation and detachment of biofilms; and the important proteins involved in signal transduction. The major functions of proteins governing bacterial biofilms in almost every environment that have been apparently studied through proteomics can be categorized into proteins that are related to metabolism, motility, transport and stress response.. Hence, this review focuses on the aforesaid functions to provide a deeper perspective on application of proteomics that has deciphered important proteins as possible future targets to combat formation of biofilms. We also highlight the bottlenecks of proteomics and emphasize ‘proteogenomics’ (direct Mass Spectrometry identification of peptides by-passing electrophoresis) as better approach for gap-filling in a 2DE maps by analyzing low abundant proteins to have a better understanding of bacterial biofilms.
Technical advancements in analyzing bacterial biofilm proteome
Bacterial proteome analyses originated with conventional electrophoretic techniques like sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) succeeded by two dimensional polyacrylamide gel electrophoresis (2-DE). The latter was further improved by the introduction of dye labeling methods, complemented with mass spectrometric (MS) analysis. Currently, higher sensitivity proteomic techniques that make use of isotope tagging are used which includes isotope coded affinity tag (ICAT) and isobaric tag for relative and absolute quantitation (iTRAQ), although they are very expensive due to limited labeling efficiency. Use of tandem mass tags (TMTs) and iTRAQ quantitative proteomics approach to study the microbial community biofilms of acid mine drainage (AMD) has been reported (Mosier et al. 2015). Bioorthogonal noncanonical amino acid tagging (BONCAT), is an advanced proteome labeling technique which provides very high sensitivity and relies on pulse labeling azidohomoalanine and helps in identifying the de novo synthesized proteome of translationally active cells (Landgraf et al. 2015). Later, the approach was used to label specific cells (antibiotic tolerant) in the regions of biofilm microcolonies followed by enrichment of low abundant proteins (Babin et al. 2017). Using BONCAT along with co-immunoprecipitation and MS technicuqes, SutA protein has been identified which upregulated post transcriptionally and was expressed during log phase, contributing to the slow growth and survival of P. aeruginosa (Babin et al. 2015). Nevertheless, for more than a decade, studies on biofilm proteome have predominantly reported 2-DE technique with the variants of MS as major proteomic analytical approaches.
2-DE techniques coupled with MS based methods is the major choice to study bacterial proteomes as it helps to explicitly profile the abundant proteins in bacterial biofilms. For this, the general basic proteome extraction procedure includes cell lysis by sonication or lysis buffer or TCA/Acetone method or phenol followed by precipitation step and solubilization in buffer containing urea, thiourea, Triton-X 100, CHAPS and DTT.. 2-DE allows separation of hundreds to thousands of proteins in a single gel. Besides cellular proteins, 2-DE is also the preferred method for analysis of organelle or sub-cellular proteome (Table 1) including the secreted proteins (secretome). Chew et al. (2012) performed 2-DE to investigate cytoplasmic proteome of Fusobacterium nucleatum biofilm in alkaline induced conditions. Islam et al. (2014) used 2-DE to isolate the membrane proteins of Staphylococcus aureus biofilm grown in fluid shear conditions. The protein spots obtained in 2-DE gels are identified, characterized and quantified to estimate the protein expression levels. Another advancement to the conventional 2-DE is 2D fluorescence differential gel electrophoresis (DIGE), which is performed using labeling dyes, thus minimizing the errors produced in conventional 2-DE methods (Rathsam et al. 2005; Mikkelsen et al. 2007). Rathsam et al. (2005) used Cye Dye labeling of cellular proteins for DIGE, in which they used Cy3, Cy5 and Cy2 DIGE fluorescent dyes for each type of protein sample. The differential labeling approach enabled separation of three different samples on the same 2D gel to analyze proteome of biofilm formed by Streptococcus mutans. Likewise, Mikkelsen et al. (2007) also used DIGE proteomic approach to investigate the interrelationship between colonies, biofilms, and planktonic cells of Pseudomonas aeruginosa.
Proteins after being resolved by 2-DE are subjected to MS for their accurate identifications. The principle of MS lies in ionizing the chemical entities followed by their separation based on mass-to-charge ratio (m/z), which is the innate physical property of a particular protein. The proteins are required to undergo enzymatic digestion which is mostly done using trypsin, cleaving the proteins into peptides at the C- terminal of lysine and arginine. The enzymatically digested protein fragments/peptides are used as standards for other peptide samples to execute MS, the results of which are used for database search to identify the proteins of interest. Variations in MS includes soft and non-destructive ionization techniques such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF-MS) and electrospray ionization-quadrupole ion trap MS (ESI-Q-IT-MS) that has made analysis of proteins and peptides feasible. However, the drawback with these techniques is the lack of strict matrix guidelines. MALDI-ToF-MS was used by Sauer et al. (2004) to identify the differentially expressed proteins of nutrient induced P. aeruginosa biofilm proteome separated in 2D gel and MASCOT software for database search. They digested proteins with trypsin to obtain peptides which were used as standards and performed MALDI-TOF-MS to ionize the peptide fragments. Giaouris et al. (2013) also used the same technique of in-gel trypsin digestion followed by MALDI-TOF-MS peptide ionization, to identify differentially expressed protein pattern of planktonic and biofilm cells of Salmonella enterica. In addition to MALDI-TOF-MS, nano liquid chromatography mass spectrometry (nano LC–MS/MS) that includes nanoscale liquid chromatography followed by tandem mass spectrometry, has also been reported (Table 1) for protein identification of mutant E. coli biofilm (Collet et al. 2008). Quantification of proteins can also be performed using labeled approach in which samples are “mass-tagged” with stable heavy isotopes of oxygen, nitrogen, hydrogen or carbon, which implies incorporation of these isotopic elements into proteins or peptides. An example of labeled approach of MS is proteolytic labeling in which samples are labeled with 18O at carboxy terminal during enzymatic digestion of proteins (DeSouza and Siu 2013). Abidin et al. (2012) performed differential proteome analysis of polymicrobial biofilms in which whole cell lysate was analyzed by SDS-PAGE. The separated proteins were quantified by LC-MALDI TOF/TOF MS after in-gel proteolytic H216O/H218O labeling. Besides MALDI-TOF MS, MALDI-TOF(/TOF), LC-MS/MS, NanoLC-MS/MS, ESI-MS/MS have also been reported (Chew et al. 2012; Islam et al. 2014; Lourenco et al. 2013). For accurate protein identification of alkaline induced F. nucleatum biofilm, Chew et al. (2012) used LC-ESI Ion trap MS/MS in addition to MALDI-MS, the spectra of which could not be obtained by MALDI-MS and detected protein spectra peaks using Bruker’s Data Analysis software (version 3.4). Furthermore, a new approach of immune-proteomics has also emerged that has helped in studying immune-reactive proteins of the pathogenic bacterial biofilms. In other words, host-pathogen interactions can be explored using this approach that conjugates 2DE and MS along with immuno-blotting technique, prior to in-gel protein digestion. This approach has also been reported to identify the immunogenic proteins of pathogenic Staphylococcus epidermis biofilm related with dormancy, which affects immune response in humans (Carvalhais et al. 2015). Similarly, immuno-proteomics was used to identify nine unique immunogenic proteins out of which five were specific to Streptococcus suis (a respiratory tract pathogen) biofilm cells and four proteins were found in biofilm as well as planktonic cells (Wang et al. 2012). The proteins identified by immuno-proteomic approaches might be potential candidates for drug target applicable for medical prognosis and treatment of diseases. Thus, various proteome analytical tools and their combinations have been used by many researchers which have contributed to the accumulated information on bacterial biofilm proteins. Nevertheless, each technique suffers from its own limitation in providing comprehensive data and information about proteins involved in this complex biological process (for detailed review, refer Khemiri et al. 2016). Also to understand protein- protein interaction network of P. aeruginosa biofilm and finding out important hub proteins, system biology approach including tools such as Cytoscape and STRING has been applied (Anupama et al. 2018).
Functional proteomics of bacterial biofilms
Many of the genes and their protein products involved in major cellular processes such as metabolism, transport, motility, stress response, virulence, cell division, signal transduction, detoxification, quorum sensing, translation, cell adhesion, are also involved in the biofilm formation (Basic et al. 2017; Mewborn et al. 2017; Park et al. 2014; Sethupathy et al. 2016). Expression of proteins related to these physiological processes changes according to the physical growth conditions such as high temperature, nutrient condition, physical shear stress, wide fluctuation in pH of the environment, damaging oxidative environment or presence of metallic ions in the surroundings. These proteins are either upregulated or downregulated depending on the need, in order to attain maximum adaptability for the bacteria to survive in the form of biofilm even in the adverse conditions (Fig. 1). Sauer et al. (2002) reported a 35% decline in the protein expression pattern of P. aeruginosa biofilm during transition from mature biofilm to planktonic stage. Likewise, several reports are available that have compared proteome of biofilm in planktonic and sessile conditions in bacteria such as Desulphovibrio vulgaris, Xylella fastidiosa, Streptococcus suis, Listeria monocytogenes, S. enterica serovar Enteritidis PT4, E. coli and Neisseria meningitidis (Giaouris et al. 2013; Collet et al. 2008; Lourenco et al. 2013; Wang et al. 2012; Van Alen et al. 2010; Silva et al. 2011; Clark et al. 2012). On the other hand, few reports are available on the comparative proteome analysis between bacterial biofilms grown under normal conditions and stress environments. Table 1 summarizes the work on biofilm of different bacteria in diverse environmental conditions with their differential protein expression profiles. Under unfavorable conditions, alteration of protein expression exhibits either up-regulation or down-regulation of those proteins which are normally expressed moderately, while expression of many proteins remains unaltered as well. The annotation of proteins/genes identified with altered expression demonstrates about their functional attributes in biofilm formation. Anupama et al. (2017) have reported designing of universal primers based on conserved functional domain of proteins of P. aeruginosa PAO1 strain and the orthologs of coding genes, for detection of biofilm genes in any environment. Although many cellular functions are involved in biofilm formation and regulation, metabolism, motility, transport and stress response have been found to be the most common and chiefly associated functions under variety of stress conditions. In the next sections, biofilm related proteins are discussed based on their differential expression levels and in the aforesaid categorization.
Proteins involved in cellular metabolism
Cellular metabolism involves synthesis and degradation of biological compounds such as carbohydrates, amino acids, proteins, fatty acids, lipids and nucleic acids. The basic cellular metabolic processes such as glycolysis, TCA cycle, protein synthesis and degradation, fatty acid synthesis and degradation etc play pivotal role in governing bacterial biofilm related processes. Change in cellular metabolism according to the changes in environmental conditions and impact on biofilm growth is the commonest phenomenon reported. In addition, carbohydrate and nitrogen metabolism were found to be associated with biofilm formation. In nitrogen source limiting conditions during biofilm formation, the gene nrfA which codes for cytochrome c nitrite reductase (c552) in D. vulgaris was found to be overexpressed (Clark et al. 2012). Interestingly, established stage of biofilms exhibit decrease in metabolic activities as compared to the initial stages of biofilm formation. This phenomenon was demonstrated by Rathsam et al. (2005) in S. mutans. The basic metabolic functions were downregulated when S. mutans growth rate was reduced in mature biofilm. Studies on fluid shear stress induced in S. aureus biofilm (Islam et al. 2014) concluded that about 30% of the proteins identified were involved in carbohydrate metabolism and 20% in protein metabolism. Nine out of thirteen proteins which were underexpressed due to shear stress (1000 s−1), were linked with glycolysis/TCA cycle and importantly glyceraldehydes-3-phosphate dehydrogenase significantly showed reduced expression with such high shear. Any change from wild to mutant type bacteria also alters the expression of metabolism associated proteins in the biofilms. In Enterococcus faecalis, random mutant types AK-F6 and AK-E12 were made and the major difference in glycolytic protein ATP-dependent 6-phosphofructokinase, translation elongation factor G, translation terminator-ribosome recycling factor and ribosomal subunit interface proteins with respect to biofilm formation were observed (Qayyum et al. 2016).
Proteome analysis of Marinobacter hydrocarbonoclastics SP17 biofilm at hexadecane-water interface revealed that enzymes for CO2 release in TCA cycle were downregulated, whereas malate synthase that converts glyoxylate into malate, was upregulated (Vaysse et al. 2009). Also, genes involved in fatty acid synthesis (fabA, fabB and fabF) were found to be downregulated. Fatty acid biosynthesis proteins FabB, FabD, FabG, AccA, and AccD have also been found to increase in Aeromonas hydrophila when its fitness was screened using cocktail of antibiotics, chlortetracycline and triclosan, an antimicrobial fatty acid biosynthesis inhibitor. Moreover, as compared to this antibiotics cocktail, the inhibitors of fatty acid biosynthesis were proved to show more antimicrobial ability against Aeromonas hydrophila biofilms (Li et al. 2016). With regard to the metal stress such as Cu, Zn and Cd on Pseudomonas fluorescens BA3SM1 biofilm, it was found that many metal binding proteins such as ATP-phosphoribosyltransferase, O-acetylserine (thiol)-lyase, aldehyde dehydrogenase and probably saccharopine dehydrogenase and serine protein kinase PrkA were synthesized in response to increase in bacterial metabolic processes (Poirier et al. 2013). The same research group found the effect of CdSe NPs (cadmium selenium nanoparticles), but the process of bacterial aggregation, cell envelope modification, extracellular CdSe NP sequestration, defense against stress and cadmium export were found to be enhanced (Poirier et al. 2016).
Motility related proteins
Motility may be an important factor for biofilm dispersion (Moorthy and Watnick 2004), since biofilms are formed by the sessile communities of bacteria attached to a solid surface. The initial adherence of bacteria requires the involvement of flagella, pili for adhesion as reported in S. enterica, Vibrio cholerae, P. aeruginosa and E. coli (Sauer et al. 2002). P. aeruginosa exhibits loss of flagella and formation of pili while forming biofilms (O’Toole and Kolter 1998; Rashid and Kornberg 2000). As a major release mechanism, flagellum-driven motility has been accounted to enable bacterial escape from the biofilm matrix (Dow et al. 2003). In plant growth promoting bacteria, to colonize plant roots and form biofilms, flagella and chemotaxis related proteins are triggered. In Bacillus amyloliquefaciens SQR9, which is a root colonizing plant growth promoting bacteria, the motility related flagellar proteins such as FlhA, FlgE, FliL and FliY, McpB for chemotaxis and SwrC for swarming motility were found to be downregulated after their colonization onto cucumber plant root (Qiu et al. 2014).
A study identified c-di-GMP as a key regulator and an internal messenger mediating transition between sessile and motile forms in almost all the biofilm forming Gram negative bacteria (Karatan and Watnick 2009). Level of c-di-GMP is directly related to biofilm formation because it helps in enhancing exo-polysaccharide production, motility and flagellar activity, by binding with PilZ domain of different proteins which are responsible for alginate production in bacteria (Hengge 2009).
Dispersion of biofilms is also stimulated by availability of required nutrients. However, excess of nutrients cause biofilm repression as well. Observations of Sauer et al. (2004) on nutrient induced dispersion of P. aeruginosa from its biofilm matrix helps us to make a closer step in understanding this process. It was found that the genes related to flagella development (fliC) were highly expressed and genes responsible for type IV pilus formation (pilA) showed decrease in expression in sessile conditions, when the bacteria were suddenly supplied with carbon source. The genes fliC and pilA, expressed oppositely in planktonic condition revealing the significant role of flagella in surface attachment. Also, up-regulation of type IV pili protein and down-regulation of flagella protein for the development on of biofilm were observed in an investigation on biofilm proteome of Pseudomonas putida (Sauer et al. 2002). Type IV pili assemblage protein PilF was also found to be upregulated in P. aeruginosa when it was subjected to colistin antibiotic treatment and when pilF mutant was complemented with pUCP18 vector containing pilF gene, the colistin antibiotic tolerance was regained (Chua et al. 2015). The carbon source either induces or suppresses biofilm growth as reported in Bacillus subtilis, S. mutans and E. coli with the similar conclusions (Stanley et al. 2003). The strains of E. coli BL21AI resistant to proline-rich antimicrobial peptides (PrAMPs) could not express Type I fimbriae related proteins indicating that these strains cannot form pili and thus are unable to form biofilm (Schmidt et al. 2016). It has been shown that a few specific RNase enzymes (RNase II, RNase R and PNPase) have a major role in affecting mobility of E. coli (Pobre and Arraiano 2015), since these enzymes modified the expression of flagellum assembling genes and deletion of these genes had an impact on motility and consequently on biofilm formation.
Biofilm formation as a result of de-escalating motility is also a kind of defense mechanism that bacteria express in the presence of heavy metals or any pollution in the environment. Effect of metal stress on motility of P. fluorescens and P. brassicacearum was studied and the down-regulation of proteins involved in synthesis of flagella indicates slowing down of the bacterial motility and enhancement of biofilm formation (Poirier et al. 2013).
Transporter proteins
Proteome analysis of planktonic and biofilm bacteria has revealed the role of proteins responsible for the transport of molecules through the cells. Most of the transporter proteins reside in the membrane as primary active transporters and secondary transporters serving as channels and carriers (Gelfand and Rodionov 2008). Expression of various transporters (ABC transporters) are altered with the change in the external environments, as reported in B. subtilis, S. aureus, Thermotoga maritime, E. coli, S. mutans and P. aeruginosa (Sauer and Camper 2001; Schembri et al. 2003; Pysz et al. 2004; Shemesh et al. 2007). Rathsam et al. (2005) investigated the planktonic and the biofilm mode of growth of S. mutans, and identified eight ATP-Binding Cassette (ABC) transporter proteins, four of them were related to sugar uptake. In the maltose uptake system, an ATP-binding protein was related to multiple sugar binding transport systems and was found to be 4.3 fold downregulated in the biofilm mode of growth, as compared to the planktonic mode. Studies on D. vulgaris biofilm with respect to planktonic cells also showed enhanced expression of 4 ABC transporter genes and 2 amino acid ABC transporter proteins (Clark et al. 2012). Other groups of bacterial transport proteins such as substrate and electron transport proteins are even extremely sensitive to any change in pH of the environment. When the pH was changed from 7.4 to 8.2, 30% of the proteins expressed by F. nucleatum belonged to substrate transport protein group and 31% of them were found to be proteins involved in electron transport (Chew et al. 2012). Furthermore, the intracellular concentration of 16 transporter proteins were significantly altered along with tripartite ATP-independent periplasmic transporter (TRAP transporter) and ABC transporter binding protein inside bacterial cell, with the increase of alkalinity of environment. This phenomenon could be implicated to energy conservation process in bacterial biofilms. There is considerably high significance of involvement of transport function not only in single bacterial species, rather in polymicrobial biofilms as well. In a study on periodontitis, which is caused by the consortia of bacteria involving Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia (Abiko et al. 2010), bacteria were observed to acquire a synergic strategy in order to survive better. This synergism among bacteria is the consequence of transport activity and cellular metabolism that were related to iron uptake, nutrient sharing and glycine regulation (Abidin et al. 2012). The important role of phosphate and thiosulphate ABC transporters (which form a large group of proteins) in sharing of nutrients by bacteria through cell envelope has been studied. These proteins were over-produced in M. hydrocarbonoclasticus SP17 biofilm when it was grown at the interface between hexadecane and water (Vaysse et al. 2009). Similarly, dipeptide permease (DppA) is a protein involved in transport of dipeptides across the cell membrane and found to be upregulated in E. coli when it was subjected to glucose limitation growth condition (Wick et al. 2001). The same protein was found to be upregulated by tenfold in S. enterica serovar Typhimurium during its growth on silicone rubber tubing surface as substratum, as compared to planktonic cells (Hamilton et al. 2009). DppA along with other nutrient transportors such as Fur, SufC and proteins involved in complementary degradation process such as GcvT, GpmA and RibB were induced during biofilm formation by S. enterica serovar Enteritidis PT4 on stainless steel surface substratum (Giaouris et al. 2013). All these findings clearly indicate differential expression of these transport proteins in different conditions of bacterial growth, revealing their importance in biofilm formation and stability.
Stress responsive proteins
One of the major functional aspects of bacterial biofilms in which our understating has improved as a result of cumulative proteomic research is the stress response. Biofilm formation is a strategy owned by bacterial community that gives them greater stability to survive in wide range of environments. This extra ability of bacteria to adapt successfully even in the adverse environment is attributed to the stress response, and this is achieved through expression of certain proteins that help in adaptation.
Pham et al. (2010) observed up-regulation of oxidative stress related proteins (up to 10- to 20-fold) which provide resistance and protection from oxidative stress during biofilm formation by Tannerella forsythia in subgingivalis plaque. Later, Silva et al. (2011) suggested bacterial biofilm cells tend to constitutively express stress proteins even without any adverse conditions as observed in X. fastidiosa. Nutrient starvation stress was studied in Helicobacter pylori, which causes gastritis and peptic ulcers in human (Shao et al. 2013). Two chaperone proteins GroES and GroEL and β-subunit of RNA polymerase were found to highly express under serum starvation. Also, two oxidative stress related proteins, alkyl hydroperoxide reductase and thioredoxin were over-expressed in the biofilm of H. pylori.
Giaouris et al. (2013) observed expression of 20 proteins in S. enterica serovar Enteritidis PT4 biofilm grown on stainless steel surface, of which 9 (ArcA, BtuE, Dps, OsmY, SspA, TrxA, YbbN and Yhbo) were categorized into stress response proteins. Dps is a ferritin-like DNA-binding protein that offers protection from oxidative stress. Likewise, Ssp is a stringent starvation protein, a RNA polymerase which has an essential role in response to stress during stationary phase under a nutrient deprived condition (Williams et al. 1994) as well as acid-induced stress in E. coli (Hansen et al. 2005). YhbO is another protein in E. coli, which was also found to be over-expressed in hyperosmotic and acidic stress conditions (Weber et al. 2006).
Presence of metal ions in the environment also leads to oxidative stress by triggering reactive oxygen species (ROS) that further directs cell destruction by damaging DNA and proteins and causing lipid peroxidation (Bertrand and Poirier 2005). Poirier et al. (2013) subjected P. fluorescens BS3SM1 to metal stress such as zinc, cadmium and copper to understand the proteome regulation in response to metal stress. Cell envelope modification and intracellular sequestration of Zn and Cu were reported. Over-synthesis of enzyme, metal efflux, inactivated protein replacement and cell aggregation are the mechanisms reported in relation to Cd resistance. With Cd toxicity, the bacterium displayed up-regulation of aldehyde dehydrogenase and oxidoreductase, but a down-regulation of ferredoxin NADP+ reductase, transcription elongation factor NusA and serine protein kinase PrkA. Whereas, OmpR-like osmolarity response regulator protein enhanced bacterial defense against Cu toxicity.
The proteome of P. aeruginosa biofilm matrix is known to be derived from secreted proteins and proteins from outer membrane vesicles (OMVs) which contribute to the majority of the matrix. OMVs are the globular bilayer entities made of phospholipids, lipopolysaccharides, proteins and DNA released by bacteria in the matrix. OMVs of P. aeruginosa contains KatE and KatA proteins which function as protective agents against oxidative stress caused by hydrogen peroxide and several proteases, thus shielding the bacteria from oxidative phagocytic attacks (Toyofuku et al. 2012). Chaperones are well known for their prominent role in maintaining integrity of proteins by ensuring proper folding and protection from denaturation. Clp and DnaK are the major chaperones found to be highly expressed in Vibrio fischeri biofilms (Chavez-Dozal et al. 2015).
Planktonic versus biofilm cells
Planktonic and biofilms are two growth models of bacteria in which biofilm cells are sessile adhering to the abiotic surface. Planktonic and biofilm cells differ in their cellular physiology that contribute to their differential behavior (Stewart and Franklin 2008). The transition from planktonic to sessile form is the result of cell gene programming due to induction of initiation factors and other cascade proteins (Qayyum et al. 2016). Compared to planktonic state, recombinant protein production potential is higher in biofilm cells. Soares et al. (2018) found that the expression of recombinant protein (enhanced green fluorescent protein, eGFP) by E. coli biofilm cells was ten times more than that of planktonic cells. Differential expression of proteins either by upregulation or downregulation is triggered by transition periods between different growth phases. DEPs vary in their patterns that are mainly categorized under metabolism, amino acid transporters and regulator proteins (Tremoulet et al. 2002). Outer membrane proteins and cytoplasmic proteins which were immunogenic in nature have been found to be over-expressed in biofilm of Aggregatibacter actinomycetemcomitans and Mycoplasma bovis respectively, as compared to their planktonic states (Chen et al. 2018). A study on Pseudoalteromonas lipolytica TC8 suggested that proteins related to sulphur metabolism were downregulated in biofilm cells (Favre et al. 2018). Although many reports have shown differential protein expression of planktonic and biofilm mass in various conditions, specific universal proteins common for bacteria confined to a particular mode is still a research gap that needs to be filled. Moreover, most of the available research papers mention almost similar proteomics strategy to study both the growth models in different stress conditions without discussing much about the future applications and scope of the findings. Hence, more investigations are required generate and interpret huge data sets of differentially expressed proteins for application based objective such as disease treatment. In addition, methods for solubilization and analysis of proteome separately from planktonic and biofilm cells have to evolve.
Conclusions
Evolution of the robust and sophisticated proteomic techniques complemented by the advancement in bioinformatic tools and softwares has helped us to improve the overall understanding of bacterial biofilm proteomes. It is also to be noted that most of the biofilm proteome analysis were done on the cultivable bacteria. Proteome of bacterial communities in complex environmental samples need to be explored to a deeper detail to understand the influence of bacterial species on each other, as well as the influence of diverse environmental conditions on the biofilm formation. The conventional 2-DE electrophoresis suffers from poor coverage of the proteome and the identification of low abundant proteins still remains a challenge, although fractionation techniques are used. In addition, the MS data are compared with the annotated bacterial protein and genome databases which are majorly computational and some of the protein information is believed to be erroneous. After a bacterial genome is sequenced, automated computation predictions and comparison among the existing genome sequences remains the major choice in genome annotation. Proteogenomic approaches which involve direct MS of the proteins without electrophoretic separation have helped in identifying proteins from new genes as well as from alternate spliced variants. Data from direct proteomics are used to re-annotate protein coding genes on a genome-wide scale. Thus, gene annotations have been corrected for many eukaryotic genes. Kumar et al. (2016) reviewed the proteogenomics based re-annotated bacterial genomes (70+ bacteria) which has helped to redefine the distribution of taxa in their phylogenetic tree. Unique proteins of bacterial biofilms that are expressed at various stages of formation, interaction and diverse environments can be identified using proteogenomics. Metaproteomics and proteogenomics approaches are expected to be the next closer steps to take forward the bacterial biofilm proteome studies.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- SDS-PAGE:
-
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- 2-DE:
-
Two dimensional polyacrylamide gel electrophoresis
- MS:
-
Mass spectrometry
- ICAT:
-
Isotope coded affinity tag
- iTRAC:
-
Isobaric tag for relative and absolute quantitation
- TMTs:
-
Tandem mass tags
- AMD:
-
Acid mine drainage
- 3D, OD:
-
Optical densitometry
- DIGE:
-
Difference gel electrophoresis
- MALDI-ToF-MS:
-
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry
- ESI-Q-IT-MS:
-
Electrospray ionization-quadrupole ion trap mass spectrometry
- nanoLC-MS/MS:
-
Nano liquid chromatography-mass spectrometry
- LC-ESI, TCA:
-
Tricarboxylic acid cycle
- c-di-GMP:
-
Bis-(3′-5′)-cyclic dimeric guanosine monophosphate
- RNA:
-
Ribonucleic acids
- ABC:
-
ATP-binding cassette
- ATP:
-
Adenosine triphosphate
- TRAP:
-
Tripartite ATP-independent periplasmic
- ROS:
-
Reactive oxygen species
- OMVs:
-
Outer membrane vesicles
References
Abidin ZZ, Veith PD, Dashper SG, Zhu Y, Catmull DV, Chen YY, Heryanto DC, Chen D, Pyke JS, Tan K, Mitchell HL, Peynolds EC (2012) Differential proteomic analysis of a polymicrobial biofilm. J Proteome Res 11:4449–4464
Abiko Y, Sato T, Mayanagi G, Takahashi N (2010) Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res 45:389–395
Anupama R, Mukherjee A, Babu S (2017) Gene-centric metegenome analysis reveals diversity of Pseudomonas aeruginosa biofilm gene orthologs in fresh water ecosystem. Genomics 110:89–87
Anupama R, Sajitha LS, Mukherjee A, Babu S (2018) Cross-regulatory network in Pseudomonas aeruginosa biofilm genes and TiO2 anatase induced molecular perturbations in key proteins unraveled by a systems biology approach. Gene 64:289–296
Babin BM, Bergkessel M, Sweredoskie MJ, Moradiane A, Hesse S, Newman DK, Tirrel DA (2015) SutA is a bacterial transcription factor expressed during slow growth in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 103:2833–2838
Babin BM, Atangcho L, van Eldijk MB, Sweredoski MJ, Moradian A, Hess S, Tolker-Nielsen T, Newman DK, Tirrell DA (2017) Selective proteomic analysis of antibiotic-tolerant cellular subpopulations in Pseudomonas aeruginosa biofilms. mBio 8:e01593–e01517
Basic A, Blomqvist M, Dahlén G, Svensäter G (2017) The proteins of Fusobacterium spp. involved in hydrogen sulfide production from l-cysteine. BMC Microbiol 17:61
Bertrand M, Poirier I (2005) Photosynthetic organisms and excess of metals. Photosynthetica 43:345–353
Carvalhais V, Cerveira F, Vilanova M, Cerca N, Vitorino R (2015) An immunoproteomic approach for characterization of dormancy within Staphylococcus epidermidis biofilms. Mol Immunol 65:429–435
Chavez-Dozal A, Gorman C, Nishiguchi MK (2015) Proteomic and metabolomic profiles demonstrate variation among free-living and symbiotic Vibrio fischeri biofilms. BMC Microbiol 215:226
Chen S, Hao H, Zhao P, Ji W, Li M, Liu Y, Chu Y (2018) Differential immunoreactivity to bovine convalescent serum between Mycoplasma bovis biofilms and planktonic cells revealed by comparative immunoproteomic analysis. Front Microbiol 9:379
Chew J, Zilm SP, Fuss JM, Gully NJ (2012) A proteomic investigation of Fusobacterium nucleatum alkaline-induced biofilms. BMC Microbiol 12:189
Chignell JF, De Long SK, Reardon KF (2018) Meta-proteomic analysis of protein expression distinctive to electricity-generating biofilm communities in air-cathode microbial fuel cells. Biotechnol Biofuels 11:121
Chua SL, Yam JKH, Hao P, Adav SS, Salido MM, Liu Y, Givskov M, Sze SK, Nielsen TT, Yang L (2016) Selective labeling and eradication of antibiotic-tolerant bacterial populations in Pseudomonas aeruginosa biofilms. Nat Commun 7:10750. https://doi.org/10.1038/ncomms10750
Clark ME, He Z, Redding AM, Joachimiak MP (2012) Transcriptomic and proteomic analyses of Desulfovibrio vulgaris biofilms: carbon and energy flow contribute to the distinct biofilm growth state. BMC Genom 13:138
Collet A, Cosette P, Beloin C, Ghigo JM, Rihouey C, Lerouge P, Junter GA, Jouenne T (2008) Impact of rpoS deletion on the proteome of Escherichia coli grown planktonically and as biofilm. J Proteome Res 7:4659–4669
DeSouza LV, Siu KWM (2013) Mass spectrometry-based quantification. Clin Biochem 46:421–431
Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100:10995–11000
Favre L, Ortalo-Magne A, Pichereaux C, Gargaros A, Burlet-Schiltz O, Cotelle V, Culioli G (2018) Metabolome and proteome changes between biofilm and planktonic phenotypes of the marine bacterium Pseudoalteromonas lipolytica TC8. Biofouling 34:132–148
Freiberg JA, Le Breton Y, Tran BQ, Scott AJ, Harro JM, Ernst RK, Goo YA, Mongodin EF, Goodlett DR, McIver KS, Shirtliff ME (2016) Global analysis and comparison of the transcriptomes and proteomes of group A Streptococcus biofilms. mSystems 1(6):e00149–e00116
Gelfand MS, Rodionov DA (2008) Comparative genomics and functional annotation of bacterial transporters. Phys Life Rev 5:22–49
Giaouris E, Samoilis G, Chorianopoulos N, Ercolini D, Nychas GJ (2013) Differential protein expression patterns between planktonic and biofilm cells of Salmonella enterica serovar Enteritidis PT4 on stainless steel surface. Int J Food Microbiol 162:105–113
Hamilton S, Bongaerts RJ, Mulholland F, Cochrane B, Porter J, Lucchini S, Lappin-Scott HM, Hinton JCD (2009) The transcriptional programme of Salmonella enterica serovar Typhimurium reveals a key role for tryptophan metabolism in biofilms. BMC Genom 10:599
Hansen AM, Qiu Y, Yeh N, Blattner FR, Durfee T, Jin DJ (2005) SspA is required for acid resistance in stationary phase by downregulation of H-NS in Escherichia coli. Mol Microbiol 56:719–734
Hengge R (2009) Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273
Herschend J, Damholt ZBV, Marquard AM, Svensson B, Sørensen SJ, Hägglund P, Burmølle M (2017) A meta-proteomics approach to study the interspecies interactions affecting microbial biofilm development in a model community. Sci Rep 7(1):16483
Islam N, Kim Y, Ross JM, Marten MR (2014) Proteome analysis of Staphylococcus aureus biofilm cells grown under physiologically relevant fluid shear conditions. Proteome Sci 12:21
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347
Kavanagh P, Botting CH, Jana PS, Leech D, Abram F (2016) Comparative proteomics implicates a role for multiple secretion systems in electrode-respiring Geobacter sulfurreducens biofilms. J Proteome Res 15:4135–4145
Khemiri A, Jouenne T, Cosette P (2016) Proteomics dedicated to biofilmology: what have we learned from a decade of research? Med Microbiol Immunol 205(1):1–19
Kumar D, Mondal AK, Kutum R, Dash D (2016) Proteogenomics of rare taxonomic phyla: a prospective treasure trove of protein coding genes. Proteomics 16:226–240
Landgraf P, Antileo ER, Schuman EM, Dieterich DC (2015) BONCAT: metabolic labeling, click chemistry, and affinity purification of newly synthesized proteomes. Methods Mol Biol 1266:199–215
Lassek C, Burghartz M, Chaves-Moreno D, Otto A, Hentschker C, Fuchs S, Bernhardt J, Jauregui R, Neubauer R, Becher D, Pieper DH, Jahn M, Jahn D, Riedel K (2015) A metaproteomics approach to elucidate host and pathogen protein expression during catheter-associated urinary tract infections (CAUTIs). Mol Cell Proteom 14(4):989–1008
Lee JH, Kim YG, Cho MH, Lee J (2014) ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formationand virulence factor production. Microbiol Res 169:888–896
Li W, Yao Z, Sun L, Hu W, Cao J, Lin W, Lin X (2016) Proteomics analysis reveals a potential antibiotic cocktail therapy strategy for Aeromonas hydrophila infection in biofilm. J Proteome Res 15:1810–1820
Lourenço A, de Las Heras A, Scortti M, JVazquez-Boland J, Frank JF, Britoa L (2013) Comparison of Listeria monocytogenes exoproteomes from biofilm and planktonic state: Lmo2504, a protein associated with biofilms. Appl Environ Microbiol 79:6075–6082
Mewborn L, Benitez JA, Silva AJ (2017) Flagellar motility, extracellular proteases and Vibrio cholerae detachment from abiotic and biotic surfaces. Microb Pathog 113:17–24
Mikkelsen H, Duck Z, Lilley KS, Welch M (2007) Interrelationships between colonies, biofilms and planktonic cells of Pseudomonas aeruginosa. J Bacteriol 189:2411–2416
Moorthy S, Watnick PI (2004) Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol Microbiol 52:573–587
Mosier AC, Li Z, Thomas BC, Hettich RL, Pan C, Banfield JF (2015) Elevated temperature alters proteomic responses of individual organisms within a biofilm community. ISME J 9:180–194
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
Oosthuizen MC, Steyn B, Lindsay DJ, Brözel VS, Cosette P, von Holy A (2001) Novel method for the proteomic investigation of a dairy-associated Bacillus cereus biofilm. Appl Environ Microbiol 194:47–51
Park AJ, Murphy K, Krieger JR, Brewer D, Taylor P, Habash M, Khursigara CM (2014) A temporal examination of the planktonic and biofilm proteome of whole cell Pseudomonas aeruginosa PAO1 using quantitative mass spectrometry. Mol Cell Proteom 13(4):1095–1105
Pérez-Ibarreche M, Mendoza LM, Vignolo G, Fadda S (2017) Proteomic and genetics insights on the response of the bacteriocinogenic Lactobacillus sakei CRL1862 during biofilm formation on stainless steel surface at 10 °C. Int J Food Microbiol 3:258:18–27
Pham TK, Roy S, Noirel J, Douglas I, Wright PC, Stafford GP (2010) A quantitative proteomic analysis of biofilm adaptation by the periodontal pathogen Tannerella forsythia. Proteomics 10:313–341
Pobre V, Arraiano CM (2015) Next generation sequencing analysis reveals that the ribonucleases RNase II, RNase R and PNPase affect bacterial motility and biofilm formation in E. coli. BMC Genom 16:72
Poirier I, Hammannb P, Kuhnb L, Bertrand M (2013) Strategies developed by the marine bacterium Pseudomonas fluorescens BA3SM1 to resist metals: a proteome analysis. Aquat Toxicol 128–129:215–232
Poirier I, Kuhnb L, Demortière A, Mirvaux B, Hammann P, Chicher J, Caplat C, Palluda M, Bertrand M (2016) Ability of the marine bacterium Pseudomonas fluorescens BA3SM1 to counteract the toxicity of CdSe nanoparticles. J Proteom 148:213–227
Pysz MA, Conners SB, Montero CI, Shockley KR, Johnson MR, Ward DE, Kelly RA (2004) Transcriptional analysis of biofilm formation processes in the anaerobic, hyperthermophilic bacterium Thermotoga maritim. Appl Environ Microbiol 70:6098–6112
Qayyum S, Sharma D, Bisht D, Khan AU (2016) Protein translation machinery holds a key for transition of planktonic cells to biofilm state in Enterococcus faecalis: a proteomic approach. Biochem Biophys Res Commun 474:652–659
Qiu M, Xu Z, Li X, Li Q, Zhang N, Shen Q, Zhang R (2014) Comparative proteomics analysis of Bacillus amyloliquefaciens SQR9 revealed the key proteins involved in in situ root colonization. J Proteome Res 13:5581–5591
Rashid MH, Kornberg A (2000) Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 97:4885–4890
Rathsam C, Eaton RE, Simpson CL, Browne GV, Valova VA, Harty DWS, Jacques NA (2005) Two-dimensional fluorescence difference gel electrophoretic analysis of Streptococcus mutans biofilms. J Proteome Res 4:2161–2173
Sadeghinejad L, Cvitkovitch DG, Siqueira WL, Santerre JP, Finer Y (2016) Triethylene glycol up-regulates virulence- associated genes and proteins in Streptococcus mutans. PLoS ONE 11(11):e0165760
Sauer K, Camper AK (2001) Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J Bacteriol 183:6579–6589
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biolfilm. J Bacteriol 184:1140–1154
Sauer K, Cullen MC, Rickard AH, Zeef LAH, Davies DG, Gilbert P (2004) Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol 186:7312–7326
Schembri MA, Kjaergaard K, Klemm P (2003) Global gene expression in Escherichia coli biofilms. Mol Microbiol 48:253–267
Schmidt R, Krizsan A, Volke D, Knappe D, Hoffmann R (2016) Identification of new resistance mechanisms in Escherichia Coli against apidaecin 1b using quantitative gel and LC–MS-based proteomics. J Proteome Res 15:2607–2617
Sethupathy S, Prasath KG, Ananthi S, Mahalingam S, Balan SY, Pandian SK (2016) Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production. J Proteom 145:112–126
Shao C, Sun Y, Wang N, Yu H, Zhou Y, Chen C, Jia J (2013) Changes of proteome components of Helicobacter pylori biofilms induced by serum starvation. Mol Med Rep 8:1761–1766
Shemesh M, Tam A, Steinberg D (2007) Differential gene expression profiling of Streptococcus mutan cultured under biofilm and planktonic conditions. Microbiol 153:1307–1317
Silva MS, De Souza AA, Takita MA, Labate CA, Machado MA (2011) Analysis of the biofilm proteome of Xylella fastidiosa. Proteome Sci 9:58
Silva AF, Dos Santos AR, Coelho Trevisan DA, Ribeiro AB, Zanetti Campanerut-Sá PA, Kukolj C, de Souza EM, Cardoso RF, Estivalet Svidzinski TI, de Abreu Filho BA, Junior MM, Graton Mikcha JM (2018) Cinnamaldehyde induces changes in the protein profile of Salmonella Typhimurium biofilm. Res Microbiol 169:33–43
Soares A, Gomes LC, Mergulhao FJ (2018) Comparing the recombinant protein production potential of planktonic and biofilm cells. Microorganisms 6:48. https://doi.org/10.3390/microorganisms6020048
Stanley NR, Britton RA, Grossman AD, Lazazzera BA (2003) Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol 185:1951–1957
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199
Sun L, Chen H, Lin W, Lin X (2017) Quantitative proteomic analysis of Edwardsiella tarda in response to oxytetracycline stress in biofilm. J Proteom 150:141–148
Sze SK, Nielsen TT, Yang L (2016) Selective labelling and eradication of antibiotictolerant bacterial populations in Pseudomonas aeruginosa biofilms. Nat Commun 7:10750
Toyofuku M, Roschitzki B, Riedel K, Eberl L (2012) Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J Proteome Res 11:4906–4915
Tremoulet F, Duche O, Namane A, Martinie B, Labadie JC (2002) Comparison of protein patterns of Listeria monocytogenes grown in biofilm or in planktonic mode by proteome analysis. FEMS Microbiol Lett 210:25–31
Van Alen T, Claus H, Zahedi RP, Groh J, Blazyca H, Lappann M, Sickmann A, Vogel U (2010) Comparative proteomic analysis of biofilm and planktonic cells of Neisseria meningitides. Proteomics 24:4512–4521
Vaysse PJ, Prat L, Mangenot S, Cruveiller S, Goulas P, Grimaud R (2009) Proteomic analysis of Marinobacter hydrocarbonoclasticus SP17 biofilm formation at the alkane-water interface reveals novel proteins and cellular processes involved in hexadecane assimilation. Res Microbiol 160:829–837
Wang Y, Yi L, Wu Z, Shao J, Liu G (2012) Comparative proteomic analysis of Streptococcus suis biofilms and planktonic cells that identified biofilm infection-related immunogenic proteins. PLoS ONE https://doi.org/10.1371/journal.pone.0033371
Wang DZ, Kong LF, Li YY, Xie ZX (2016) Environmental microbial community proteomics: status, challenges and perspectives. Int J Mol Sci 17(8):1275
Weber A, Kögl SA, Jung K (2006) Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J Bacteriol 188:7165–7175
Wick LM, Quadroni M, Egli T (2001) Short- and long-term changes in proteome composition and kinetic properties in a culture of Escherichia coli during transition from glucose-excess to glucose-limited growth conditions in continuous culture and vice versa. Environ Microbiol 3:588–599
Williams MD, Ouyang TX, Flickinger MC (1994) Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coli protein synthesis and survival during growth and prolonged starvation. Mol Microbiol 11:1029–1043
Wood TK, Hong SH, Qun M (2010) Engineering biofilm formation and dispersal. Trends Biotechnol 29:87–94
Acknowledgements
The authors gratefully acknowledge the support rendered by the management of Vellore Institute of Technology, Vellore, India in carrying out their research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Research involving human participants and/or animals
The research work does not involve human participants and/or animals.
Informed consent
The research work does not involve human participants and hence informed consent does not arise.
Rights and permissions
About this article
Cite this article
Rani, A., Babu, S. Environmental proteomic studies: closer step to understand bacterial biofilms. World J Microbiol Biotechnol 34, 120 (2018). https://doi.org/10.1007/s11274-018-2504-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2504-x