Abstract
The fungus Rhizoctonia solani is one of the causal agents of numerous diseases that affect crop growth and yield. The aim of this present investigation was to identify a biocontrol agent that acts against R. solani and to determine the agent’s protective effect through phytohormones and antioxidant regulation in experimentally infected Chinese cabbage plants. Four rhizospheric soil bacterial isolates GR53, GR169, GR786, and GR320 were tested for their antagonistic activity against R. solani. Among these isolates, GR53 significantly suppressed fungal growth. GR53 was identified as Bacillus amyloliquefaciens subsp. plantarum by phylogenetic analysis of the 16S rDNA sequence. The biocontrol activity of B. amyloliquefaciens subsp. plantarum GR53 was tested in Chinese cabbage plants under controlled conditions. Results showed that R. solani inhibited plant growth (length, width, fresh and dry weight of leaves) by reducing chlorophyll and total phenolic content, as well as by increasing the levels of salicylic acid, jasmonic acid, abscisic acid, and DPPH scavenging activity. By regulating the levels of these compounds, the co-inoculation of B. amyloliquefaciens subsp. plantarum GR53 heightened induced systemic resistance in infected Chinese cabbage, effectively mitigating R. solani-induced damaging effects and improving plant growth. The results obtained from this study suggest that B. amyloliquefaciens subsp. plantarum GR53 is an effective biocontrol agent to prevent the damage caused by R. solani in Chinese cabbage plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese cabbage belongs to the Brassicaceae family and is a major ingredient in kimchi, which is a traditional fermented dish in the Republic of Korea. Over 30,000 ha of land are used to grow Chinese cabbage and 90 % of the domestic production is used for manufacturing kimchi (Lee et al. 2014). Hence, diseases that affect this crop severely hamper the profitable manufacture of cabbage. Of particular consequence is the damping-off in cabbage seedlings caused by the fungus Rhizoctonia solani, which is a major challenge to manage and control (Shiau et al. 1999). More generally, the fungus can persist as sclerotia for several years in the soil and causes diseases in a wide range of their host plants (Gonzalez-Garcia et al. 2006). Soil fumigation is the current strategy used to manage crop diseases, but it also negatively affects beneficial soil microorganisms that assist crop growth (Solanki et al. 2012). Additionally, the application of agrochemicals to plants or soil has been a risk to sustainable agriculture due to resulting contamination of soil, water, and air (Correa et al. 2009). The absence of targeted plant disease control approaches and the growing demand for naturally developed food has inspired the study on biological control (Chowdhury et al. 2013), or the use of microorganisms to eradicate plant pathogens. This method is more environmentally friendly and could be an alternative to chemical fungicides, bactericides, and pesticides (Choudhary 2011; Compant et al. 2005; Radhakrishnan et al. 2013). In the specific case of R. solani, the fungus Trichoderma atroviride and the rhizobacteria Trichoderma harzianum, Pseudomonas fluorescens, Bacillus subtilis, and Bacillus pumilus have been identified as active biocontrol microbes that can contain the spread of the fungal pathogen (Asaka and Shoda 1996; Hadar et al. 1979; Huang et al. 2012; Nagarajkumar et al. 2004; Reithner et al. 2007).
Several reports have revealed the possible use of rhizobacteria such as Bacillus, Pseudomonas and Burkholderia species can able to prevent the phytopathogen effects in crop plants. Specifically, Bacillus species occur ubiquitously in soil and produce a vast array of biologically active metabolites, such as indole acetic acid and gibberellin, in addition to solubilizing the organic phosphate that helps to increase plant growth (Kang et al. 2014). Pavlo et al. (2011) studied the role of microorganisms on plant growth and inhibition of disease causing pathogens; they demonstrated that the stimulation of rapid plant growth results in a failure to develop disease resistance. Previous studies suggest that Bacillus spp. exhibit their antagonistic activity against pathogens through synthesizing antimicrobial peptides, secretion of lytic enzymes, competition for nutrient and space, and importantly, induced systemic resistance, including the increase of pathogenesis-related proteins in plants (Ongena and Jacques 2008; Osman et al. 2011; Sharma et al. 2009). Bacillus amyloliquefaciens secretes antibiotics such as zwittermicin-A, kanosamine, iturin, fengycin, and lipopeptides from surfactins, which are effective against an extensive range of pathogens (He et al. 1994; Stabb et al. 1994). Due to its antibacterial and antifungal activities, B. amyloliquefaciens became commercially developed as an agricultural biomaterial. In fact, B. amyloliquefaciens mediated suppression rates of Phytophthora-blight of peppers and Fusarium-wilt of tomatoes were found to be higher than those of popular chemical fungicides (Chung and Kim 2005). Ji et al. (2013) demonstrated that B. amyloliquefaciens CNU114001 exhibited broad spectrum inhibitory activity against 12 phytopathogenic fungi such as Alternaria panax, Botrytis cinerea, Colletotrichum acutatum, Colletotrichum orbiculare, Corynespora cassiicola, Fusarium oxysporum, Phytophthora capsici, Penicillium digitatum, R. solani, Stemphylium lycopersici, Pyricularia grisea, and Sclerotinia sclerotiorum. Bacterial antibiotics control fungal growth by inhibiting the synthesis of fungal sterols and nucleic acids, as well as changing cell membrane permeability and destroying the fungal cell wall (Michael and Nannette 2003).
Despite this, while the usage of Bacillus strains to improve plant growth has been documented widely, only a limited number of studies were conducted to understand their role in suppressing R. solani-induced damage in plants (Solanki et al. 2012). Moreover, in spite of these few reports regarding the improved suppression of R. solani by B. amyloliquefaciens, no data are available on the hormonal and antioxidant changes in the host plant. An increase of endogenous salicylic acid in plants is involved in signal transfer during pathogen infection and activates the pathogenesis-associated gene expression (Buonaurio et al. 2002). The purposes of the current study were twofold: first, to identify a potent biocontrol rhizobacteria against R. solani from available strains collected in the field, and second, to evaluate their mitigation effects by analyzing salicylic acid, jasmonic acid, abscisic acid, and antioxidant activity in experimentally infected Chinese cabbage.
Materials and methods
Isolation of bacterial strains from rhizosphere soil
Rhizosphere soil samples were collected from various agricultural field of Chungcheongbuk-do, Danyang, Republic of Korea. One gram of soil sample was added to 50 mL of sterilized saline solution. Resultant suspensions were successively diluted (10−4) and 0.1 mL aliquots were spread on plates containing tryptic soy/agar (TSA; Merck Co., Germany) medium. The plates were incubated for 48 h at 30 °C. Bacterial colonies were separated by their morphology, pigmentation, and growth rate.
In vitro antifungal activity of bacterial strains against R. solani
The effects of bacterial strains GR53, GR169, GR786, and GR320 against R. solani were assayed by dual culture method, following Radhakrishnan et al. (2013). Rhizoctonia solani culture was obtained from the National Institute of Agricultural Science and Technology (Suwon, Korea). GR53, GR169, GR786, and GR320 bacterial strains were applied to R. solani grown on plates containing potato dextrose agar. Plates were then incubated for 10 days at 28 ± 2 °C. Subsequently, R. solani mycelial growth was measured, and the inhibition of mycelial growth was calculated by measuring the clear zone between the fungus and bacterial isolates.
Identification and phylogenetic analysis of bacterial isolate GR53
An isolated antagonistic bacterial strain GR53 was identified by a partial 16S ribosomal DNA (rDNA) sequence. Chromosomal DNA was isolated and extracted by following the methods (Adachi et al. 1996). Primers 27F (5′-AGAGTTTGATC(AC)TGGCTCAG-3′) and 1492R (5′-CGG (CT) TACCTTGTTACGACTT-3′) were used for PCR amplification of the 16S rDNA. We then used BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) to determine the nucleotide sequence homology of this bacterial isolate. Closely related nucleotide sequences were aligned using ClustalW and MEGA (version 5.0), and the neighbor-joining tree was generated using same software. Bootstrap analysis (1000 replications) was performed to assess the stability of tree nodes and branches.
Bacterial and fungal treatments and plant growth condition
Chinese cabbage seeds were surface sterilized with 1 % Tween 80 solution and 2 % perchloric acid, then washed thoroughly with sterile distilled water: first, for 5 min in a shaking incubator (120 rpm), followed by rinsing three times. The seeds were aseptically cultured in magenta boxes (10.0 × 7.0 × 7.0 cm) containing 50 mL of MS basal medium (Murashige and Skoog 1962) supplemented with 0.8 % agar at 25 °C; untreated seed cultures served as controls. The bacterial strain GR53 was cultured in LB media for 3 days at 30 °C in a shaking incubator (200 rpm). The pathogenic fungus R. solani was inoculated in potato dextrose broth media and cultivated in a shaking incubator with 200 rpm at 25 °C for 5 days. One hundred micro-litter of bacterial culture GR53 was applied on the surface of MS agar media after the emergence of the first leaf from Chinese cabbage seeds. 3 days later, 200 micro-litter of R. solani culture was inoculated to GR53-treated and untreated plants. The length, width, fresh and dry weight of leaf, chlorophyll, salicylic acid, jasmonic acid, abscisic acid, total polyphenol, and DPPH scavenging activity were recorded in Chinese cabbage plants at 10 days post R. solani treatment.
Quantification of salicylic acid (SA)
SA was extracted and quantified using the methods of Enyedi et al. (1992) and Seskar et al. (1998). One hundred milligrams of leaf samples were extracted with 90 and 100 % methanol by centrifuging at 10,000×g. The combined methanol extracts were vacuum-dried and re-suspended in 2.5 mL of 5 % trichloroacetic acid, and the supernatant was partitioned with ethyl acetate/cyclopentane/isopropanol (49.5:49.5:1, v/v). The top organic layer was transferred to a 4 mL vial for drying with nitrogen gas. After drying, it was again suspended in 1 mL of 70 % methanol. HPLC analyses were carried out on a Shimadzu fluorescence detector (Shimadzu RF-10AXL, excitation and emission detected at 305 and 365 nm, respectively), fitted with a C18 reverse-phase HPLC column (HP hypersil ODS). The flow rate was 1.0 mL/min. Salicylic acid content in samples was calculated by authentic standard peak values.
Quantification of abscisic acid (ABA)
ABA contents were extracted from the leaf samples following the procedures of Qi et al. (1998). An extraction solution containing 95 % of isopropanol and 5 % glacial acetic acid was first filtered through filter paper, and then 10 ng ABA was added as internal standard. The filtrate was concentrated by a rotary evaporator. The concentrated residue was dissolved in 1N NaOH solution and then washed with methylene chloride to remove lipophilic materials. Next, the aqueous phase was adjusted to pH 3.5 with the addition of 6N HCl and partitioned with ethyl acetate (EtOAc). EtOAc extract was then evaporated; the residue was dissolved in phosphate buffer (pH 8.0) and run through a polyvinylpolypyrrolidone (PVPP) column. The phosphate buffer mixture was adjusted to pH 3.5 with the addition of 6 N HCl and partitioned with EtOAc. The EtOAc extract was again evaporated; the residue was dissolved in dichloromethane and passed through a silica cartridge (Sep-Pak; Waters Associates, Milford, MA, USA), which was pre-washed with diethyl ether:methanol (3:2, v/v) and dichloromethane. ABA was recovered from the cartridge by elution with diethyl ether:methanol (3:2, v/v). The extracts were dried and methylated by adding diazomethane for GC–MS SIM (6890N network GC system, and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA) analysis. For quantification, the Lab-Base (ThermoQuset, Manchester, UK) data system software was used to monitor the responses to ions of m/e 162 and 190 for Me-ABA and 166 and 194 for Me-[2H6]-ABA.
Quantification of jasmonic acid (JA)
JA from plant leaf samples was extracted according to the method of McCloud and Baldwin (1997). The Me esters of extracts were analyzed by GC–MS SIM (6890N network GC system, and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). For JA determination, the fragment ion at m/z = 83 amu, corresponding to the base peaks of JA and (9,10-2H2)-9,10-dihydro-JA, was monitored. The amount of endogenous JA was calculated from the peak areas of endogenous JA in comparison to the corresponding standards.
Total phenolic content
Total phenolic content was determined by the Folin-Ciocalteu colorimetric method (Amerine and Ough 1980). Leaf tissues were extracted with 80 % MeOH. Fifty microliters of extract was mixed with 1 mL of 2 % Na2CO3 and 50 µL of 1N Folin–Ciocalteu reagent at room temperature for 30 min. The absorbance of total phenol content was measured at 750 nm. Gallic acid was used as the standard to quantify total phenol levels.
DPPH scavenging activity
The free radical scavenging activity of leaf samples was determined using a DPPH assay following an established method (Blois 1958). Leaf samples were extracted with MeOH; the reaction mixture contained 5 mg diphenyl-1-picrylhydrazyl (DPPH) dissolved in 50 mL MeOH. The reaction mixture and MeOH extract (1:1) were incubated at room temperature in the dark for 30 min. The absorbance of samples was detected at 517 nm. The radical scavenging activity was calculated and expressed as percentage by following equation.
Acon—the absorbance of the control reaction; Atest—the absorbance in the presence of the sample of the extracts.
Statistical analysis
The experiments were repeated three times. Sigma Plot Software (10.0) was used to calculate the standard errors and compare the statistical difference of treatments based on Duncan’s multiple range test (DMRT), at a significance level of p ≤ 0.05.
Results
Identification of bacterial strains antagonistic against R. solani
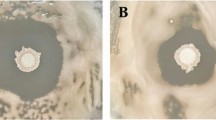
In this study, the four bacterial isolates, GR53, GR169, GR786, and GR320, were tested to identify the strain that effectively prevented the spread of R. solani. Among these, the bacterial strain GR53 (Bacillus amyloliquefaciens) alone inhibited the mycelial growth of R. solani (Supplementary material Fig. 1). The clear zone formation between bacterial and fungal mycelial growth was observed in petri-plates, revealing that bacterial isolate GR53 effectively prevented the spread of R. solani (Fig. 1). The rate of mycelial growth inhibition was checked at different time intervals, and within seven days, R. solani growth was completely halted by the effect of co-inoculation of GR53 (Fig. 2).
Phylogenetic analysis was carried out on GR53 after DNA extraction and subsequent sequencing of the 16S rDNA. Sequence of the selected strains was analyzed by BLAST, presenting the highest sequence homology proportion, query coverage, and the lowest E values. Sequences of other genera were also used to determine the actual relationship among participating candidates in the group. BLAST search results revealed that GR53 shares a 100 % sequence homology with B. amyloliquefaciens (Fig. 3). The sequence was submitted to NCBI GenBank (accession no. KJ937782). Based on sequence similarity and phylogenetic analysis, we identified bacterial isolate GR53 as B. amyloliquefaciens.
Biocontrol activity of B. amyloliquefaciens subsp. plantarum GR53 on R. solani infection in Chinese cabbage
The treatment of B. amyloliquefaciens subsp. plantarum GR53 did not cause any significant change in Chinese cabbage growth under normal conditions (Table 1). The disease causing agent R. solani significantly inhibited the length and width of experimentally infected cabbage leaves when compared to uninfected control plants. The co-treatment of B. amyloliquefaciens subsp. plantarum GR53 to infected plants mitigated the damaging effect on leaf growth by enhancing the length and width of leaves. Similarly, Chinese cabbage infected by R. solani had lower fresh and dry leaf weight in comparison to controls. GR53 treatment effectively prevented this damage; Chinese cabbage co-treated with B. amyloliquefaciens experienced an increase in their fresh weight (3.08–3.20 g) and dry weight (0.21–0.26 g). Indeed, photosynthetic pigment chlorophyll content declined in R. solani infected plants, while B. amyloliquefaciens subsp. plantarum GR53 co-treatment enhanced the chlorophyll content in diseased plants. To confirm the mitigation effect of GR53 against R. solani infection, same experiment was conducted on pots containing autoclaved horticultural soil. Fifteen-days-old plants were treated with B. amyloliquefaciens subsp. plantarum GR53 and subsequently R. solani culture was applied after 7 days. The significant increase of plant growth by biocontrol agent on diseased plants was found in 1 month old plants (Supplementary table 1), and the R. solani and B. amyloliquefaciens subsp. plantarum GR53 colonies in roots of Chinese cabbage were observed by SEM analysis (supplementary figure 2).
Hormonal and antioxidants changes in diseased plants during B. amyloliquefaciens subsp. plantarum GR53 interaction
The mitigation effect of B. amyloliquefaciens subsp. plantarum GR53-inoculated Chinese cabbage was assessed by measuring levels of stress hormones (SA, JA, and ABA) and antioxidants (total phenolic content and DPPH scavenging activity). In comparison to controls, SA content increased in both plants treated with R. solani only and co-treated with B. amyloliquefaciens subsp. plantarum GR53 (Fig. 4). The synergistic interaction of disease causing fungi R. solani and biocontrol agent GR53 on cabbage plants resulted in a drastic elevation of SA synthesis. Moreover, our results from measuring JA levels demonstrated the alleviating effects of B. amyloliquefaciens subsp. plantarum GR53 on diseased plants. First, we found that, as expected, R. solani-infected plants had higher levels of JA than healthy control plants. However, although JA synthesis was also enhanced in plants treated only with GR53, co-inoculation of B. amyloliquefaciens subsp. plantarum GR53 with R. solani significantly reduced JA levels in disease-infected plants. In addition, differential expression of ABA synthesis was observed in plants during pathogen-only treatment and bacterial + pathogen co-treatment. The accumulation of ABA was two times higher in R. solani-infected Chinese cabbage plants than in untreated control plants. Bacterial co-treatment using GR53 significantly blocked ABA accumulation in plants and mitigated disease-induced stress.
Influence of B. amyloliquefaciens subsp. plantarum GR53 on salicylic acid, jasmonic acid, and abscisic acid levels in R. solani-infected plants. Bars represent means plus standard error (n = 3). Means followed by the same letter are not significantly different (p < 0.05), as determined by Duncan’s multiple range test
Finally, the microbial interaction also alters the antioxidant levels in affected plants. Chinese cabbage infected by R. solani failed to synthesize phenolic compounds (Fig. 5), while no significant changes to total phenolic levels were observed between control plants and those given a B. amyloliquefaciens subsp. plantarum GR53 treatment. The high production of phenolic compounds in diseased plants during the effect of B. amyloliquefaciens subsp. plantarum GR53 determined their biocontrol effect. In contrast, when compared to healthy controls, plants infected by R. solani had higher DPPH scavenging activity, while plants treated with B. amyloliquefaciens subsp. plantarum GR53 alone did not exhibit any changes. However, infected plants co-treated with B. amyloliquefaciens subsp. plantarum GR53 had lower levels of DPPH scavenging activity than those untreated with the bacteria.
Discussion
The biological control of plant diseases is a promising alternative approach to maintaining plant health and promoting crop yield. In the current study, we showed that GR53, a bacterial isolate from soil, was effective against the fungal pathogen R. solani. GR53 was identified as B. amyloliquefaciens through phylogenetic analysis. Previous studies have also documented the ability of B. amyloliquefaciens to inhibit R. solani fungal growth (Chowdhury et al. 2013; Huang et al. 2012; Ji et al. 2013; Yu et al. 2002) via the production of antifungal substance iturin. Overall, B. amyloliquefaciens has been studied extensively as a producer of α-amylase, subtilisin, barnase, and iturins, all of which can control pathogen growth (Yu et al. 2002). Although seed and root colonization of Bacillus species has been reported in the literature, less consideration has been given to the colonization of diverse plant cultivars. Correa et al. (2009) suggested that B. amyloliquefaciens BNM122 is a probable microbial biocontrol mediator able to restrict the damping-off disease caused by R. solani when inoculated in soybean seeds. In the present study, R. solani significantly reduced the leaf length, leaf width, fresh weight, dry weight, and chlorophyll content in Chinese cabbage plants, but B. amyloliquefaciens subsp. plantarum GR53 effectively mitigated all of these effects. Similar to our results, Solanki et al. (2012) found that damping off disease caused by R. solani inhibited the growth and yield of tomato plants, while B. amyloliquefaciens MB101 application on infected plants caused a severe drop in disease index and significant improvement of plant growth and yield. Additionally, co-treatment of B. amyloliquefaciens 7079 not only significantly suppressed Fusarium-wilt disease in tomatoes and Phytophthora-blight disease in peppers, but the bacteria’s biocontrol effect was actually higher than chemical fungicides (Chung and Kim 2005). The greater efficacy of biological control agents compared to traditional pest control is encouraging, because application of fungicides thiram and carbendazim negatively disturbs both phytopathogenic and non-pathogenic fungi and bacteria (Johnsen et al. 2001; Niewiadomska 2004). Biocontrol approaches thus appear to be extremely favorable alternatives to reduce crop yield loss as limitations on the usage of chemical pesticides increase (Naegely 1997). With biocontrol methods, long-term solutions for pest control potentially exist; Chowdhury et al. (2013) suggested that the inoculating plants with B. amyloliquefaciens FZB42 could allow the bacteria to establish itself effectively in lettuce rhizospheres, reducing the effects of bottom rot without displaying any strong effect on the rhizosphere bacterial community itself.
Stress responsive hormones and antioxidants in plants are major defense factors against diseases. It can accrue through the regulation of plant hormones such as salicylic acid (SA), jasmonic acid (JA), and abscisic acid (ABA), as well as antioxidants. SA is a phenolic compound that mediates the phenylpropanoid pathway in the cytoplasm initiates from phenylalanine, and the isochorismate pathway takes place in the chloroplast (Mercado-Blanco et al. 2001; Vicente and Plasencia 2011), and it has been identified as a key signal for the expression of pathogen resistance proteins during resistance (Loake and Grant 2007). In the current study, SA levels increased in plants co-treated with B. amyloliquefaciens subsp. plantarum GR53 and R. solani. The combined effect of both bacterial and fungal application caused a significant increase of SA levels in plants when compared to their controls. Our results are in agreement with a previous report, which documented that pathogen treatment of Arabidopsis elevated SA accumulation and increased the expression of pathogenesis-related genes, thereby enhancing plant disease resistance (Lee et al. 2006). At the time of infection, resistant plants increase the production of reactive oxygen species (ROS), which may play a role in killing the entering pathogens. Particularly, enhancement of hydrogen peroxide (H2O2) accumulation induces an increase of the peroxidase-catalyzed synthesis of lignin, thus generating a physical barrier against pathogens (Dixon and Harrison 1992). SA attaches to catalase and prevents breakdown of H2O2, promoting an increase in H2O2 cellular concentration, which in turn, might serve as a second messenger for the stimulation of a defense reaction (Chen et al. 1993). Thus, the overproduction of SA stimulated by B. amyloliquefaciens subsp. plantarum GR53 in diseased plants may be one of the important factors to prevent plant damage.
Our quantification results of stress hormone JA in Chinese cabbage revealed that R. solani-infected samples had higher levels of JA when compared to controls, but B. amyloliquefaciens subsp. plantarum GR53 treatment effectively reduced JA concentration in diseased plants. JA and SA can act either antagonistically or synergistically in adjusting stress reactions (Rao et al. 2000). Unfortunately, to date, we have only a limited understanding of complex regulatory systems where several hormonal pathways interrelate and affect plant defense responses (Bari and Jones 2009). Elevated levels of JA in plants responding to infection have been demonstrated by several studies (Bari and Jones 2009; Lorenzo and Solano 2005; Wassternack 2007). Thus, since B. amyloliquefaciens subsp. plantarum GR53 modulates the relative abundance of JA, its presence may reprogram the communication of defense-related genes and organize multifaceted interactions between defense signaling pathways to trigger an active defense response against R. solani infection.
ABA is another stress hormone involved in numerous aspects of plant development, including the regulation of stomatal aperture and adaptive responses to environmental conditions, but the role of ABA in plant disease resistance is not well defined (Mauch-Mani and Mauch 2005). In this study, ABA accumulation was significantly higher in R. solani-infected plants than in controls, and co-treatment with B. amyloliquefaciens subsp. plantarum GR53 reduces ABA levels. Previous research studies have shown that high accumulation of ABA in plants under disease conditions correlated with increased susceptibility (Mohr and Cahill 2003; Thaler and Bostock 2004). ABA inhibits the transcription of a basic β-1,3-glucanase that can destroy β-1,3-glucan callose, which regulates plasmodesmata to establish physical blocks against invading pathogens (Rezzonico et al. 1998). ROS production, Ca2+ signaling, and mitogen-activated protein kinase are inter-related with ABA signaling for disease resistance. Therefore, the alteration of these components and a high concentration of ABA all affect the plant’s response to disease causing agents, controlled by the signaling pathway of SA (Mauch-Mani and Mauch 2005). This result suggests that the mechanism behind the ability of B. amyloliquefaciens subsp. plantarum GR53 to heighten plant resistance against R. solani might be a reduction of ABA and an increase of SA content.
Another, non-mutually exclusive possibility is that B. amyloliquefaciens subsp. plantarum GR53 affects DPPH scavenging levels in diseased plants. Plants under stress conditions, such as fungal infection, produce antioxidants to scavenge ROS. In this experiment, we observed that DPPH scavenging activity of diseased plants was higher than healthy controls, but DPPH scavenging capacity was effectively normalized by the activity of B. amyloliquefaciens subsp. plantarum GR53. The alteration in ROS-scavenging enzymes could be a vital process in phytopathogen defense (Gara et al. 2003). Non-pathogenic microbial inoculation can heighten the resistance of host plants against their pathogens by an increase of phenolic compounds (Gasoni and Gurfinkel 2009; Sneh et al. 1989). In support of this, we found that the phenolic content of R. solani-infected plants was less than that of the controls, but again, B. amyloliquefaciens subsp. plantarum GR53 treatment attenuated that consequence. Thimmaiah (1999) and Chatterjee and Ghosh (2008) suggested that the inhibition of phenolic compounds synthesis in plants during pathogen infection indicates the susceptibility of plants. The lower level of phenolic content in R. solani-infected Chinese cabbage plants revealed that this cultivar was susceptible to R. solani induced disease. A different rhizobacteria strain, B. pumilus SE34, had a similar effect in bean plants against F. oxysporum by triggering the release of callose and phenolic substances for inhibiting pathogenic symptoms (Benhamou et al. 1996).
In conclusion, although reports exist on the biocontrol effects of B. amyloliquefaciens against R. solani infection, to our knowledge, this is the first study to examine the mechanism behind protective qualities of B. amyloliquefaciens with plant hormones and antioxidants analysis. The current findings reveal that B. amyloliquefaciens regulates levels of plant stress hormones and antioxidant activity by increasing total phenolic content. In this way, B. amyloliquefaciens mitigated the biotic stress effects in R. solani infected Chinese cabbage. Thus, B. amyloliquefaciens application to agricultural fields can be a sustainable alternative to chemical fungicides, acting as an effective biocontrol agent to prevent diseases caused by R. solani.
References
Adachi M, Sako Y, Ishida Y (1996) Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8 S ribosomal DNA and internal transcribed spacer regions. J Phycol 32:424–432
Amerine MA, Ough CS (1980) Methods for analysis of musts and wine. Wiley, New York, pp 205–206
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Benhamou N, Kloepper JW, Quadt-Hallman A, Tuzon S (1996) Induction of defense-related ultrastructural modification in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112:919–929
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Buonaurio R, Scarponi L, Ferrara L, Sidoti P, Bertona A (2002) Induction of systemic acquired resistance in pepper by acibenzolar-S-methyl against bacterial spot disease. Eur J Plant Pathol 108:41–49
Chatterjee A, Ghosh SK (2008) Alterations in biochemical components in mesta plants infected with yellow vein mosaic disease. Braz J Plant Physiol 20:267–275
Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886
Choudhary DK (2011) Plant growth promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen, Macrophomina phaseolina. Biotechnol Lett 33:2287–2295
Chowdhury SP, Dietel K, Randler M, Schmid M, Junge H, Borriss R, Hartmann A, Grosch R (2013) Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 8:e68818
Chung SH, Kim SD (2005) Biological control of phytopathogenic fungi by Bacillus amyloliquefaciens 7079; suppression rates are better than popular chemical fungicides. J Microbiol Biotechnol 15:1011–1021
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:49–51
Correa OS, Montecchia MS, Berti MF, Ferrari MCF, Pucheu NL, Kerber NL, Garcia AF (2009) Bacillus amyloliquefaciens BNM122, a potential microbial biocontrol agent applied on soybean seeds, causes a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol 41:185–194
Dixon RA, Harrison MG (1992) Activation, structure and organization of genes involved in microbial defense in plants. Adv Genet 28:165–234
Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA 89:2480–2484
Gara LD, de-Pinto MC, Tommasi F (2003) The antioxidant systems vis-a-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol Biochem 41:863–870
Gasoni L, Gurfinkel BSD (2009) Biocontrol of Rhizoctonia solani by the endophytic fungus Cladorrhinum foecundissimum in cotton plants. Australas J Plant Pathol 38:389–391
Gonzalez-Garcia V, Portal-Onco MA, Rubio-Susan V (2006) Review: biology and systematics of the form genus Rhizoctonia. Span J Agric Res 4:55–79
Hadar Y, Chet I, Henis Y (1979) Biological control of Rhizoctonia solani damping-off with wheat bran culture of Trichoderma harzianum. Phytopathology 69:64–68
He H, Silo-Suh LA, Handelsman J, Clardy J (1994) Zwittermicin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett 35:2499–2502
Huang X, Zhang N, Yong X, Yang X, Shen Q (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res 167:135–143
Ji SH, Paul NC, Deng JX, Kim YS, Yun BS, Yu SH (2013) Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 41:234–242
Johnsen K, Jacobsen C, Torsvik V, Sorensen J (2001) Pesticide effects on bacterial diversity in agricultural soils—a review. Biol Fertil Soils 33:443–453
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ (2014) Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol 54:427–433
Lee J, Nam J, Park HC, Na G, Miura K, Jin JB, Yoo CY, Baek D, Kim DH, Jeong JC, Kim D, Lee SY, Salt DE, Mengiste T, Gong Q, Ma S, Bohnert HJ, Kwak SS, Bressan RA, Hasegawa PM, Yun DJ (2006) Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J 49:79–90
Lee MK, Chun JH, Byeon DH, Chung SO, Park SU, Park S, Arasu MV, Al-Dhabif NA, Limg YP, Kim SJ (2014) Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT Food Sci Technol 58:93–101
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol 10:466–472
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signaling network. Curr Opin Plant Biol 8:532–540
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant–pathogen interactions. Curr Opin Plant Biol 8:409–414
McCloud ES, Baldwin IT (1997) Herbivory and caterpillar regurgitants amplify the wound induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 203:430–435
Mercado-Blanco J, van-der-Drift KM, Olsson PE, Thomas-Oates JE, van-Loon LC, Bakker PA (2001) Analysis of the pmsCEAB gene cluster involved in biosynthesis of salicylic acid and the siderophore pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J Bacteriol 183:1909–1920
Michael RY, Nannette YY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30:461–469
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naegely SK (1997) Greenhouse vegetables: business is booming. Greenh Grow 15:14–18
Nagarajkumar M, Bhaskaran R, Velazhahan R (2004) Involvement of secondary metabolites and extracellular lytic enzymes produced by Pseudomonas fluorescens in inhibition of Rhizoctonia solani, the rice sheath blight pathogen. Microbiol Res 159:73–81
Niewiadomska A (2004) Effect of carbendazim, imazetapir and thiram on nitrogenase activity, the number of microorganisms in soil and yield of red clover (Trifolium pratense L.). Pol J Environ Stud 13:403–410
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Osman MS, Sivakumar D, Korsten L (2011) Effect of biocontrol agent Bacillus amyloliquefaciens and 1-methylcyclopropene on the control of postharvest diseases and maintenance of fruit quality. Crop Prot 30:173–178
Pavlo A, Leonid O, Iryna Z, Natalia K, Maria PA (2011) Endophytic bacteria enhancing growth and disease resistance of potato (Solanum tuberosum L.). Biol Control 56:43–49
Qi QG, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very long chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol 117:979–987
Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY (2013) IAA-producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol 23:856–863
Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12:1633–1646
Reithner B, Schuhmacher R, Stoppacher N, Pucher M, Brunner K, Zeilinger S (2007) Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet Biol 44:1123–1133
Rezzonico E, Flury N, Meins FJ, Beffa R (1998) Transcriptional downregulation by abscisic acid of pathogenesis-related beta-1,3-glucanase genes in tobacco cell cultures. Plant Physiol 117:585–592
Seskar M, Shulaev V, Raskin I (1998) Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol 116:387–392
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Shiau FL, Chung WC, Huang JW, Huang HC (1999) Organic amendment of commercial culture media for improving control of Rhizoctonia damping-off of cabbage. Can J Plant Pathol 21:368–374
Sneh B, Ichielevich-Auster M, Plaut Z (1989) Mechanisms of seedling protection by a hypovirulent isolate of Rhizoctonia solani. Can J Bot 67:2135–2141
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocont Sci Technol 22:203–217
Stabb EV, Jacobson LM, Handelsman J (1994) Zwittermicin A producing strains of Bacillus cereus from diverse soils. Appl Environ Microbiol 60:4404–4412
Thaler SJ, Bostock RM (2004) Interactions between abscisic-acid mediated responses and plant resistance to pathogens and insects. Ecology 85:48–58
Thimmaiah SR (1999) Standard method of biochemical analysis. Kalyani Publishers, New Delhi, pp 230–231
Vicente MR, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Wassternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Acknowledgments
This work was financially supported by National Research Foundation of Korea (NRF), Ministry of Science, ICT and Future Planning through Basic Science Research Program (2014R1A1A1004918).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, SM., Radhakrishnan, R. & Lee, IJ. Bacillus amyloliquefaciens subsp. plantarum GR53, a potent biocontrol agent resists Rhizoctonia disease on Chinese cabbage through hormonal and antioxidants regulation. World J Microbiol Biotechnol 31, 1517–1527 (2015). https://doi.org/10.1007/s11274-015-1896-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1896-0