Abstract
Leaf litter constitutes a major fraction in total litter production in mangrove forests. Its contribution to NPP of mangrove forests and carbon sequestration is less studied. These aspects were investigated for Kunhimangalam mangrove forest in Kerala, India. We quantified the leaf litter production and estimated the decomposition rates of leaf litter from Aegiceras corniculatum, Avicennia officinalis, Excoecaria agallocha and Rhizophora mucronata. These four species together constituted 92.49% of abundance in Kunhimangalam. The average annual leaf litter production was 8.83 ± 0.95 t ha−1yr−1, 78% of the total litter produced. Leaf litter production was negatively correlated with soil pH (R2 = 0.531) and rainfall (R2 = 0.561). Temperature, salinity and humidity did not show any remarkable influence. The rate of decomposition varied significantly among these species (F = 2497.79, p < 0.01) but as for the mixed leaf litter category (a mixture of leaves from the above four species in equal weight), the rate of decomposition was the highest. The pattern of leaf litter decomposition observed in mixed, R. mucronata and E. agallocha categories best fitted to the exponential decay model indicating an initial phase of faster decomposition followed by a terminal slow phase. The leaf litter of A. corniculatum and A. officinalis categories fitted best to the linear regression model showing a steady pace throughout the period of decomposition. Leaf litter of these four species together contributed 3.56 ± 0.01 t C ha−1 y−1 to NPP. Higher production of leaf litter in Kunhimangalam showed higher potential for carbon sequestration. However, only less than 1% (0.62%) of the leaf litter was decomposed when macrobenthos were excluded from the system. The destiny of the rest 99% appeared critical as this determined the capacity of Kunhimangalam mangrove forest to act as a source or sink for carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high rate of litter production and decomposition combined with the remarkable efficiency in nutrient recycling make mangrove forests one of the most productive ecosystems on the earth (Qasim and Wafar 1990; Kalio 1992; Harrison et al. 1994; Day et al. 1996; Duarte and Cebrian 1996; Kathiresan and Bingham 2001; Bosire et al. 2005; Morrisey et al. 2007; Alongi 2009). Litter constitutes up to one third of the primary production in mangrove forests (Alogi et al. 1992). Nutrients released from litter through decomposition are either retained in the ecosystem to support secondary production or exported to nearby coastal waters by the tides (Alongi et al. 1998). This process is, therefore, the most important source of carbon and nutrients in biogeochemical cycles (Odum and Heald 1975; Alongi et al. 1992; Robertson et al. 1992; Wafar et al. 1997) and thus, vital to both mangrove and adjacent coastal ecosystems. For these reasons, litter production is considered as an indicator of net primary production (NPP) and biogeochemical cycles in mangrove ecosystems.
Litter produced in mangrove forests normally contains leaves, flowers, fruits, bark, needles and twigs (Odum and Heald 1975; Slim et al. 1996; Betoulle et al. 2001). Of them, leaves constitute the major fraction accounting to more than 50% of the total litter production (Wafar et al. 1997). The high productivity of mangrove ecosystem is, thus, attributed to higher rate of leaf production and their rapid decomposition (Park and Kang-Hyun 2003; Mahmood et al. 2011). Leaf litter also plays a major role in enhancement of soil fertility and supply of nutrient materials to animal life in mangrove ecosystems (Srivastava 1980). Hence, leaf litter production as well as decomposition is the key to the nutrient cycling in mangrove sediments (Sherman 2002; Ngoran et al. 2006; Mahmood and Hoque 2008; Mahmood et al. 2009; Triadiati et al. 2011). An assessment of leaf litter production and decomposition indicates the productivity of a mangrove ecosystem (Pool et al. 1975; Snedaker and Snedaker 1984). It also signals the nutrient status of the surrounding coastal ecosystems (Pool et al. 1986). Comparatively, a higher rate of litter production has been observed in tropical mangroves (Mahmood and Hoque 2008).
Production of leaf litter exhibits seasonal variation (Duke 1999; May 1999; Mfilinge et al. 2005a) and is influenced by several factors, viz.: geographical location (Saenger and Snedaker 1993), nutrient concentrations, topography (Cox and Allen 1999), rainfall (Wangondu et al. 2014), temperature (Mchenga and Ali 2017), salinity (Day et al. 1996), solar radiation (Saenger and Snedaker 1993), winds (Ghosh and Banerjee 2013), forest types (Pool et al. 1975), stand structure (Saberi 1989), freshwater drainage (Feller et al. 1999), species composition (Coupland et al. 2005) and anthropogenic influence (Silva et al. 1998). More frequent tides and river inundations induce riverine mangroves to produce higher amount of leaf litter (Pool et al. 1975; Twilley 1995). Decomposition rate of mangrove leaves is influenced by the abundance and diversity of macro benthic fauna and microorganisms (Meentemeyer 1978; Chapin et al. 2002), quality of decomposing substrate (Bosire et al. 2005), physico-chemical nature of the site (Wafar et al. 1997), climatic conditions, particularly the temperature and salinity (Mackey and Smail 1996; Lorıa-Naranjo et al. 2019), tides (Middleton and McKee 2001; Bosire et al. 2005; Imgraben and Dittmann 2008) and hydrocarbon pollution (Frick et al. 1999).
No works exist on litter production and decomposition on Indian mangroves except that of Wafar et al. (1997) on litter decomposition in the Madovi–Zuari Estuaries on the Central West Coast of India, Ghosh and Banerjee (2013) on inter relationship between physico-chemical variables and litter production on mangroves in Sundarbans, Rani et al. (2016) on assessment of NPP of Cochin mangroves and Suresh et al. (2017) on estimation of total carbon stock in Indian mangroves. They correlated litter production positively with temperature, wind velocity, salinity and species diversity but negatively with rainfall. However, the significance of leaf litter decomposition in carbon sequestration and thereby its contribution to NPP was not investigated in Indian context despite the fact that the country harbors 4,952 km2 of mangrove forests (India State of Forest Report 2021) with 57% of true mangrove species in the world (Ragavan et al. 2016).
Materials and methods
Study area
Kunhimangalam mangrove forest, located between 12° 03′−12° 06′ N and 75° 12′ − 75° 14′ E, was our study area (Fig. 1). It extends to 5 km2 along the banks of Perumba and Pullankodu Rivers. It is the largest and least disturbed mangrove forest in Kerala. It occurs in narrow strips with a width of 50–100 m along the water line and is micro-tidal with a tidal amplitude of one meter. The forest has 10 true mangrove species and 11 mangrove associates. Acanthus ilicifolius L., Aegiceras corniculatum (L.) Blanco., Avicennia officinalis L., Excoecaria agallocha L. and Rhizophora mucronata Lam. are dominant with representations of Avicennia marina (Forssk.) Vierh., Bruguiera cylindrica (L.) Blume, Kandelia candel (L.) Druce, Rhizophora apiculata Blume and Sonneratia caseolaris (L.) Engl. There are three types of plant associations, viz: (i) dominated by A. officinalis, A. marina, E. agallocha and A. corniculatum in the upper intertidal area, and R. mucronata, R. apiculata and K. candel in the lower intertidal area, (ii) dominated by A. officinalis and A. corniculatum in the upper intertidal area, and R. mucronata in the lower intertidal area, and (iii) dominated by A. officinalis and E. agallocha in the upper intertidal area, and A. ilicifolius in the lower intertidal area. A well defined mid-intertidal area cannot be discerned in Kunhimangalam mangrove forest. It harbors 60 species of birds, 42 species of fishes, 14 species of mollusks and 15 species of crabs (Praveen et al. 2015). Kunhimangalam experiences tropical warm and humid climate with pronounced monsoon (June to October), winter (November to February) and summer (March to May).
We focused our study to (i) quantify the leaf litter production in Kunhimangalam mangrove forest and analyze the environmental factors influencing litter production, (ii) measure the rate of leaf litter decomposition, (iii) calculate the carbon (C) content in leaf litter and (iv) assess the relative share of carbon content in leaf litter to NPP.
Study design
We conducted this study in three stages: (i) assessment of the rate of leaf litter production for a period of two years by litter trap method and the influence of environmental factors on leaf litter production, (ii) estimation of organic carbon in leaf litter to understand its share in NPP of the forest and (iii) assessment of the rate of microbial decomposition on the leaf litter of Aegiceras corniculatum, Avicennia officinalis, Excoecaria agallocha and Rhizophora mucronata, the four major mangrove species in Kunhimangalam, by litter bag method. These four species together constituted 92.49% of abundance in the forest (Praveen 2014). The decomposition rate is expressed as percentage loss of initial dry weight per day. The dry weight corresponding to the fresh weight of the litter weighed was estimated using a predetermined fresh weight–dry weight ratio to express all measurements in dry weight.
Quantification of leaf litter production
Quantification of litter production was done using litter trap method (Brown 1984). Twenty litter traps, each made of nylon net (mesh size 1 mm2) and spread over 1 m2 PVC frames (Fig. 2a), were randomly placed in the forest. They were fixed on poles at one meter height above the high tide mark. Hanging traps were also used (Fig. 2b). Trap surface was made in conical shape to facilitate accumulation of litter between collection intervals of one week. Collected litter was sorted as buds, flowers, fruits, twigs and leaves. It was quantified in dry weight. Leaves of each species were segregated and weighed separately. This quantification provided total leaf litter production against total litter production in Kunhimangalam forest day−1 m−2 in dry weight. Litter was collected for a period of two years from August 2008 to July 2010 ( Shanij et al. 2016).
Environmental parameters
Water salinity, water pH, water temperature, soil temperature, soil pH and air temperature were recorded monthly using Erma refractometer Dual scale 0–100 ppt of salinity, Safeseed PHMTR001, mercury thermometer (for water, soil and air), Takemura soil pH meter and moisture tester DM-13 respectively. Data on humidity and rain fall were obtained from Krishi Vigyan Kendra of Kerala Agricultural University at Thaliparamba, about 15 km from Kunhimangalam.
Estimation of organic carbon
We collected freshly fallen senescent leaves from the mangrove forest floor and used for organic carbon estimation. Three samples of 100 g senescent leaves from each of the four species were collected in each season. They were washed with distilled water, oven dried at 60 °C to constant weight, grounded in a mill to pass a 1 mm sieve and analyzed for organic carbon using Walkley and Black method (Jackson 1973). The average value of carbon content in three samples of each species in each season was calculated. The average carbon content of three seasons with respect to the leaf litter of these four species was considered the mean carbon content of leaf litter in the mangrove forest. These data were converted to NPP following Twilley et al. (1992).
Measurement of leaf litter decomposition
We assessed the in situ decomposition rate of the leaf litter employing litter bag method (Harmon et al. 1999). Nylon net of mesh size 1 mm2 was used. The decomposition rate of leaf litter in four mangrove species separately and that in four species mixed were assessed. Thus, altogether five sets of litter bags, each set with 10 replicates were used. Each litter bag carried 50 g freshly fallen senescent leaves. Litter bags were placed randomly in the intertidal zone tied to nearby mangrove trees (Fig. 2c) and allowed to decompose without the influence of any macrobenthos (Fig. 2d, e and f). We inspected the litter bags and weighed the contents weekly, after gently washing off the sediments deposited on them in tidal water. The dry weight corresponding to the wet weight of leaf litter was estimated through predetermined fresh weight - dry weight ratio. This process was continued until the litter was fully decomposed. The loss of weight was plotted against time and decomposition rate of leaf litter was expressed as weight loss in grams day−1.
Arrangements made for litter collection and leaf litter decomposition experiments in Kunhimangalam A A litter trap established on the mangrove forest floor B A hanging litter trap, C A litter bag deployed in the intertidal region, exposed during low tide D The condition of leaf litter at the beginning of decomposition experiment E The condition of leaf litter after 70-80% decomposition during the experiment F Leaf litter at the end of the decomposition experiment
Calculation of fresh weight-dry weight ratio
Freshly fallen leaf litter weighing 5 g, 10 g, 15 g, 20 g, 25 and 30 g was taken from the forest floor and oven dried to constant weight at 70 °C. Their dry weight was plotted on a graph against corresponding fresh weight. The graph provided the dry weight corresponding to the fresh weight of leaf litter.
Statistical analysis
One-way ANOVA was done to find out the variation in monthly leaf litter production, rates of leaf litter production and leaf litter decomposition. Results were considered significant at p ≤ 0.05. We fitted both linear and exponential decay models to the species wise decomposition data to assess the trend of leaf litter decomposition. Model parameters were estimated using least square method and presented in a table format. Monthly average values recorded for rates in leaf litter production, and environmental parameters were analyzed separately using simple linear regression models to identify the dependence behavior between the variables. The fitted model performances were analyzed using R2 values.
Results
Leaf litter production
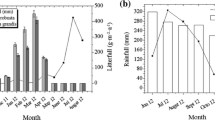
Of the total 3.12 ± 0.18 g m−2 day−1 (11.39 ± 0.66 t ha−1 yr−1) of litter produced (Table 1), leaf litter contributed to 78% (8.83 ± 0.95t ha−1 yr−1). Twigs, fruits, flowers and buds contributed the remaining 22% (Fig. 3). The mean daily production of leaf litter ranged from 1.36 ± 0.02 to 3.93 ± 0.65 g m−2 day−1 (Table 1), the highest was in November followed by April and December. August recorded the lowest followed by July. This variation was significant in ANOVA (F = 7.641, p < 0.01). The pattern of leaf litter production was the same in both the years, 2008–2009 and 2009–2010 (Fig. 4). Season wise, the highest leaf litter production was recorded in winter and the lowest in monsoon (Fig. 5). Major contributions came from Aegiceras corniculatum (17%), Avicennia officinalis (27%), Excoecaria agallocha (18%) and Rhizophora mucronata (22%) (Fig. 6). The rest 16% was contributed by Avicennia marina (4%), Bruguiera cylindrica (3%), Rhizophora apiculata (3%), Kandelia candel (3%) and others (3%).

Source: Shanij et al. 2016
Seasonal variation in the leaf litter production rates in Kunhimangalam mangrove forest from 2008 to 2010.

Source: Shanij et al. 2016
Percentage composition of different mangrove species towards total leaf litter production in Kunhimangalam mangrove forest.
The salinity ranged from 0 ppt (monsoon) to 28 ppt (summer), soil pH from 5.17 (summer) to 5.69 (monsoon), water pH from 5.85 (monsoon) to 7.21 (summer), soil temperature from 23.7 °C (winter) to 27.7 °C (monsoon), water temperature from 25 °C (monsoon) to 28.2 in (summer), air temperature from 25 °C (winter) to 30.4 °C (summer), rainfall from 0 (winter) to 27 cm (monsoon) and Relative Humidity from 84% (summer) to 91% (monsoon).
Organic carbon content in leaf litter
We estimated the average carbon content in leaf litter at 40.14 ± 1.38%. There were no significant seasonal differences in carbon content in leaf litters collected from the four species, though they exhibited significant variation in C content. Aegiceras corniculatum possessed the highest C content (44.62 ± 1.23%) followed by Avicennia officinalis (43.52 ± 0.59%) and Rhizophora mucronata (40.79 ± 1.64%). The least C content was in Excoecaria agallocha (31.63 ± 3.08%). The relative share of leaf litter from these four species to the NPP of Kunhimangalam mangrove forest was calculated at 3.56 ± 0.01 t C ha−1 y−1.
Leaf litter decomposition
Average decomposition rates of leaf litter expressed as percentage of initial dry weight remaining are presented in Figs. 7, 8, 9, 10 and 11. The average rate of decomposition was 0.07 ± 0.01 g d−1 for Aegiceras corniculatum, 0.10 ± 0.01 g d−1 for Avicennia officinalis, 0.11 ± 0.02 g d−1 for Excoecaria agallocha and 0.13 ± 0.02 g d−1 for Rhizophora mucronata. For mixed leaf litter category, it was 0.14 ± 0.04 g d −1 which was 0.62% of 22.5 g dry weight of leaf litter (50 g in fresh weight) deployed in the litterbag (Table 2). Decomposition rates varied significantly (F = 2.536, p < 0.05) among the five categories. The leaf litter of A. corniculatum took 46 weeks, A. officinalis 31 weeks, E. agallocha 26 weeks, R. mucronata 31 weeks and the mixed one 25 weeks to attain 100% decomposition.
Decomposition data of leaf litter from mixed category and Rhizophora mucronata fitted best to the exponential decay model (Figs. 7 and 8; Table 3). This showed that the rate of decomposition was faster for the mixed category (0.27 ± 0.07 g d−1) and R. mucronata (0.26 ± 0.04 g d−1) at the early phase. Thereafter, it took a slower pace, 0.04 ± 0.01 g d−1 and 0.05 ± 0.01 g d−1 respectively. The pattern of decomposition in Aegiceras corniculatum and Avicennia officinalis best fitted to the linear model (Figs. 9 and 10; Table 3) as leaf litter from these two species lost their weight at a steady pace, 0.07 ± 0.01 g d−1and 0.10 ± 0.01 g d−1, respectively during the process of decomposition. Interestingly, leaf litter from Excoecaria agallocha was fitted almost equal to the linear (R2 = 0.923) as well as the exponential (R2 = 0.926) models. However, in all the five experimental treatments, the ‘y intercept’ of the exponential decay model was significantly higher (Table 3) than that of the linear model. This indicated a faster rate in the initial phase of decomposition.
Discussion
Leaf litter production
The annual global litter production in different mangrove ecosystems ranged between 7 and 15 t ha−1 yr−1 (Hossain and Hoque 2008), though an exceptionally higher litter production was recorded in mangrove forests in Australia (34.4 t ha−1 yr−1) (Alongi et al. 2005). The average annual litter production in Kunhimangalam mangrove forest was 11.39 ± 0.66 t ha−1 (Table 1). Rani et al. (2016) reported a mean annual litter production rate in Cochin mangrove forest as 16.57 ± 6.58 t ha−1, about 300 km south of Kunhimangalam.
While leaves contributed 78% (8.83 ± 0.95 t ha−1 yr−1) of the average annual litter production (11.39 ± 0.66 t ha−1 yr−1) in Kunhimangalam mangrove forest, their share in Cochin mangrove forest was only 53.9% (8.93 ± 3.49 t ha−1 yr−1) (Rani et al. 2016). Both forests are riverine. Studies of Cintro´n and Schaeffer-Novelli (1983), Lacerda et al. (2001) and Zaldivar Jiménezet al. (2004) have shown that leaves contribute a higher fraction to total litter production than twigs, buds and fruits in young mangrove forests. Wind speed enhances the percentage fraction of twigs, buds and fruits in total litter production. There is a significant positive correlation between litter production and wind speed (Mfilinge et al. 2005a; Mchenga and Ali 2017). Cochin mangrove forest experiences a higher wind speed (11–27 km h−1) than Kunhimangalam mangrove forest (3–7 km h−1) as the former occurs very close to the coast, while the latter is an upstream mangrove area about 15 km off the coast.
The quantity of leaf litter production at global level is estimated between 7 and 15 t ha−1 yr−1. The range increases from higher to lower latitudes (Saenger and Snedaker 1993; Komiyama et al. 2008; Bernini and Rezende 2010). Latitude influences leaf litter production in mangrove forests. But it has not influenced leaf litter production in Kunhimangalam (8.88 ± 0.51 t ha−1 yr−1), though the area is at lower latitude. Soil analysis shows the forest is nutrient poor (Shanij 2017).
Production of leaf litter showed a unimodal pattern (Fig. 4). It was the highest in winter (November to February) and the lowest in monsoon (June to October). Aegiceras corniculatum, Avicennia officinalis and Rhizophora mucronata produced maximum leaf litter in winter. They are evergreen species. Evergreen trees normally shed more leaves before they enter into the reproductive phase. Cumulative effect of stress due to high water salinity, low precipitation, high evapo-transpiration, low contents of nutrients because of reduced fresh water runoff and emergence of new leaves also tend to produce more leaf litter in winter (Ochieng and Erftemeijer 2002; Kamruzzaman et al. 2019). Excoecaria agallocha, a semi-deciduous species, contributed maximum leaf litter in summer (March to May).
Leaf litter production in Kunhimangalam is negatively correlated with soil pH and rainfall in linear regression analysis (Figs. 12 and 13). Temperature, salinity and humidity did not show any remarkable influence on leaf litter production. Rani et al. (2016) reported negative correlation between rainfall and leaf litter production. Clough (1992), Twilley et al. (1997), Wafar et al. (1997), Sherman et al. (2003), Arreola-Lizarraga et al. (2004) and Aké-Castilho et al. (2006) have reported salinity, temperature and rainfall showing positive correlation with leaf litter production in mangrove forests. However, Lopez-Portilho and Ezcurra (1985) and Silva et al. (2006) did not find any such correlation. The above studies indicate that geomorphic and physico-chemical characteristics do not show a uniform pattern of influence on total leaf litter production in mangrove forests.
Leaf litter decomposition
The activity of decomposers, physico-chemical traits of litter and environmental factors influence leaf litter decomposition (Anderson and Swift 1983; Heal et al. 1997; Kavvadias et al. 2001), but the nature of species has more influence on the decomposition rate (Salinas et al. 2011). The leaf litter of Sonneratia alba decomposed faster than that of Rhizophora apiculata, R. mucronata and Bruguiera parviflora in a Malasian mangrove forest (Ashton et al.1999). In mangrove forests of Maputo Bay, Mozambique, the leaf litter of Avicennia marina decomposed faster than that of R. mucronata. The rates of leaf litter decomposition varied from species to species in our experiments. Variations in the rate of decomposition are ascribed to differences in morphology, texture and chemical composition of mangrove leaves (Ashton et al. 1999; Fernando and Bandeira 2009).
Leaf litter decomposition in mangrove forests normally involves two phases: (i) a rapid weight loss phase through leaching and (ii) a breakdown phase by the activities of decomposers (Polunin 1982). This pattern of decomposition occurred in treatments of mixed, Rhizophora mucronata and Excoecaria agallocha categories in our experiments where the decomposition data fitted best to the exponential decay model (Figs. 7, 8 and 9). The initial rapid weight loss is attributed to the high quantity of water soluble phenolic compounds, flavonoids and non-lignified carbohydrates present in the leaves. These compounds leach faster (Mason 1977; Cundell et al. 1979; Mahmood et al. 2007; Ibrahima et al. 2008, 2010; Simali and Roy 2012). The low level of non-leachable structural compounds like lignin, tannin, cellulose etc. in E. agallocha and R. mucronata might have equally contributed to the weight loss. (see Van der Valk and Attiwill 1984; Mfilinge et al. 2005b). The high quantity of non-leachable structural compounds like lignin, tannin, cellulose etc. present in the leaf litter of Avicennia officinalis and Aegiceras corniculatum made the rate of decomposition slower (see Ardon and Pringle 2008; Alvim et al. 2015) and so, their decomposition rate fitted best to the linear model. The decomposition in litter bags occurs in a micro-climatic condition different from that in a natural environment (Boulton and Boon 1991).
Low C/N ratio favored decomposition of mangrove leaf litter (Twilley et al. 1997; Edu et al. 2014) during the break down phase. Low C/N ratio in the leaf litter of Excoecaria agallocha marked high nitrogen concentration and resultant high nutritional value. Both of them promoted faster microbial colonization and decomposition. However, presence of tannin slowed down the decomposition rate, although the C/N ratio in this species is lower than that of Avicennia officinalis and Rhizophora mucronata (Rani et al. 2023). Leaf blades of Aegiceras corniculatum are thinner than those of A. officinalis and R. mucronata but presence of tannin in this species inhibited faster microbial activity and delayed the decomposition process (Cundell et al. 1979; Coen 1988; Steinke et al. 1993; Edu et al. 2014).
Assorted leaf litters accumulated from different species in mangrove forests decomposed faster, when there was remarkable leaching towards the first phase or leaf litters contained leaves of species with low C/N ratio (Mall et al. 1991; Fyles and Fyles 1993; McArthur et al. 1994; Briones and Ineson 1996; McTiernan et al. 1997; Wardle et al. 2003). In our experiments, the rate of leaf litter decomposition was the highest for mixed category. Leaf litters of Excoecaria agallocha and Rhizophora mucronata leached fast during their first phase of decomposition. E. agallocha possessed low C/N ratio. These combinations created a conducive chemical atmosphere for triggering early microbial activities in mixed leaf litter category and enhanced the rate of decomposition. The high nitrogen content in E. agallocha promoted the rate of decomposition. Nutrients released from the fast decomposing E. agallocha, Avicennia officinalis and R. mucronata accelerated action of microbes on the leaves of Aegiceras corniculatum, subduing the effect of high tannin content. The litter of E. agallocha, the fastest decomposing material among the four, quickened microbial actions in the mixed leaf litter category much faster and earlier than the other categories constituted separately by A. officinalis, R. mucronata and A. corniculatum.
Net primary productivity
Primary productivity of Avicennia officinalis, Aegiceras corniculatum. Excoecaria agallocha and Rhizophora mucronata by way of leaf litter in Kunhimangalam mangrove forest was estimated at 3.56 ± 0.01 t C ha−1 y−1. In Cochin mangrove forest, Rani et al. (2016) estimated the litter share of NPP at 7.12 ± 2.81 t C ha−1 y−1. This difference, though the two mangrove forests are only about 300 km apart, could be due to the fact that they estimated the C content of the litter which included flowers, propagules and twigs apart from leaves. Carbon contents are more in mangrove propagules than in leaf litter. Alongi (2009), who estimated the mean global aboveground mangrove NPP rate at 11.1 t C ha−1 y−1, noted that there was considerable scatter in data owing to variations in tree age, structure and species composition of mangrove forests, besides variations in climatic and other environmental conditions. Estimates have shown that 32% of the Gross Primary Productivity (GPP) in mangrove plants is shunted to NPP of the canopy (Alongi 2014). In Kunhimangalam almost 30% of the NPP was constituted by leaves. So, it is logical to think that our estimates of NPP from leaf litter have rightly reflected the total NPP of Kunhimangalam mangrove forest. This shows its higher potential for carbon sequestration.
The mangrove ecosystem has high potential for carbon assimilation. It is an established fact. However, it is still not clear whether they function as a significant carbon sink in the coastal ocean (Alongi 2014). Less than 1% (0.62%) of the leaf litter only was decomposed in Kunhimangalam mangrove forest when macrobenthos were excluded from the system. The slow rate of leaf litter decomposition indicates that the release of sequestered carbon is very slow. What did happen to the major portion of leaf litter (99%)? The possibilities are: (i) it might have been washed off to coastal waters supplying organic matter to ‘near shore’ consumers (Teal 1962; Odum and Heald 1975; Odum 1980), (ii) entered into higher consumer trophic levels through the detritus food chain (Lee 1997; Nicholson 2009) or (iii) remained on the forest floor, later to be converted to the extensive peat deposit (Middleton and McKee 2001). Quantification of the amount of leaf litter exported to coastal waters, detritus food chain or peat formation is critical to know the entire pathways of carbon. Precisely, not the decomposed leaf litter constituting an insignificant 0.62% but the balance 99.38% determines the potential of Kunhimangalam mangrove forest to act as a source of or sink for carbon. Thus, without taking these factors into account, equating the litter production directly to the carbon sequestration potential of a mangrove forest may lead to erroneous conclusion.
Conclusions
Leaf litter formed the major fraction of total litter produced in Kunhimangalam mangrove forest. Geomorphological and physico-chemical characters of mangrove forests influence leaf litter production. Higher productions of leaf litter in Kunhimangalam mangrove forest shows its higher potential for carbon sequestration. The results of our decomposition study show that it is the destiny of 99% of the leaf litter that determines the capacity of this forest to act as a source or sink for carbon. The role of detritivores like crabs is highly significant in converting leaf litter directly to particulate organic carbon or soil organic carbon by feeding on leaves or shredding them into small pieces suitable for microorganisms to act upon. The efficiency of a mangrove forest as a principal carbon sequestering ecosystem does not depend merely on its primary productivity, but on the delicate balance existing among different ecosystem functions operating directly or indirectly on the litter produced.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
NA.
References
Ake-Castilho JA, Vazques G, Lopez-Portilho J (2006) Litterfall and decomposition of Rhizophora mangle L. in a coastal lagoon in the southern Gulf of Mexico. Hydrobiologia 559:101–111
Alongi DM (2009) Paradigm shifts in mangrove biology. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier Publishing, Amsterdam, pp 615–640
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6(1):195–219
Alongi DM, Boto KG, Robertson AI (1992) Nitrogen and phosphorus cycles. In: Robertson AI, Alongi DM (eds) Tropical Mangrove ecosystems. American Geophysical Union, Washington DC, pp 251–292
Alongi DM, Ayukai T, Brunskill GJ, Clough BF, Wolanski E (1998) Sources, sinks, and export of organic carbon through a tropical, semi-enclosed delta (Hinchinbrook Channel, Australia. Mangroves and Salt Marshes 2:237–242
Alongi DM, Clough BF, Robertson AI (2005) Nutrient-use efficiency in arid-zone forests of the mangroves Rhozophora stylosa and Avicennia marina. Aquat Bot 82(2):121–131
Alvim EACC, Medeiros AO, Rezende RS, Goncalves JF Jr (2015) Leaf breakdown in a natural open tropical stream. J Limnol 74(2):248–260. https://doi.org/10.4081/jlimnol.2014.982
Anderson JM, Swift MJ (1983) Decomposition in Tropical Forest. In: Sulton SL, Chadwick AC, Whitemore TC (eds) The tropical rain forest. Ecology and Management Blackwell, Oxford, pp 289–309
Ardon NM, Pringle CM (2008) Do secondary compounds inhibit microbial- and insect-mediated leaf breakdown in a tropical rainforest stream. Costa Rica? Oecologia 155:311–323
Arreola-Lizarraga JS, Flores-Verdugo FJ, Ortego-Rubio A (2004) Structure and litterfall of an arid mangrove stand on the Gulf of California, Mexico. Aquat Bo 79:137–143
Ashton EC, Hogarth PJ, Ormond R (1999) Breakdown of mangrove leaf litter in a managed mangrove forest in. Peninsular Malaysia Hydrobiologia 413:77–88
Bernini E, Rezende CE (2010) Estrutura Da vegetação em florestas de mangue do estuário do rio Paraíba do sul, Estado do Rio De Janeiro, Brasil. Acta Bot Bras 18:491–502
Betoulle JL, Fromard F, Fabre A, Puig H (2001) Characterisation of litter and its contributions to soil nutriment in a mangrove of French Guiana. Can J Bot 79:238–249
Bosire JO, Dahdouh-Guebas F, Kairo JG, Kazungu J, Dehairs F, Koedam N (2005) Litter degradation and CN dynamics in reforested mangrove plantations at Gazi Bay, Kenya. Biol Conserv 126:287–295
Boulton AJ, Boon PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments: time to turnover a new leaf? Aust J Mar Freshwater Res 42:1–43
Briones MJI, Ineson P (1996) Decomposition of eucalyptus leaves in litter mixtures. Soil Biol Biochem 28(10–11):1381–1388
Brown SM (1984) Mangrove Litter Production and Dynamics in Snedaker, C.S and Snedaker, G.J. 1984. The Mangrove Ecosystem: Research Methods. On Behalf of The Unesco/SCOR, Working Group 60 on Mangrove Ecology. Page 200–208
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Cintron G, Schaeffer-Novelli Y (1983) Introduccio´n a la ecologı´a del manglar. Oficina Regional de Cienca y Tecnologı´a de la UNESCO para Ame´rica Latina y el Caribe ROSTLAC, Montevideo, Uruguay
Clough BF (1992) Primary productivity and growth of mangrove forests. In: Robertson AI, Alongi DM (eds) Tropical Mangrove ecosystems-Coastal and Estuarine Series 41. American Geophysical Union, Washington, pp 225–249
Coen LD (1988) Herbivory by crabs and the control of algal epibionts on Caribbean host corals. Oecologia 75(2):198–203
Coupland GT, Paling EI, McGuinness KA (2005) Vegetative and reproductive phenologies of four mangrove species from northern Australia. Aust J Bot 53:109–117
Cox EF, Allen JA (1999) Stand structure and productivity of the introduced Rhizophora mangle in Hawaii. Estuaries 22:276–284
Cundell AM, Brown MS, Stanford R, Mitchell R (1979) Microbial degradation of Rhizophora mangle leaves immersed in the sea. Estuar Coast Marine Sci. https://doi.org/10.1016/0302-3524(79)90041-0
Day JW Jr, Coronado-Molina C, Vera-Herrera FR, Twilley R, Rivera-Monroy VH, Alvarez-Guillen H, Day R, Conner W (1996) A seven year record of above-ground net primary production in a southeastern Mexican mangrove forest. Aquat Bot 55:39–60
Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Duke NC (1999) Phenological trends with latitude in the mangrove tree Avicennia marina. J Ecol 78:113–133
Edu EAB, Nsirim LEW, Martins OO (2014) Monitoring and assessment of leaf litter dynamics in a mixed mangal forest of the Cross River Estuary, Nigeria. Int J Environ Monit Anal 2(3):163–174
Feller IC, Whigham DF, O’Neill JP, McKee KM (1999) Effects of nutrient enrichment on within-stand nutrient cycling in mangrove ecosystems in Belize. Ecology 80:2193–2205
Fernando SMC, Bandeira SO (2009) Litterfall and decomposition of mangrove species Avicennia marina and Rhizophora mucronata in Maputo Bay, Mozambique, Western Indian Ocean. J Mar Sci 8(2):173–182
Frick CM, Farrell RE, Germida JJ (1999) Assessment of phytoremediation as an in situ technique for cleaning oil-contaminated sites. PTAC Petroleum Technology Alliance, Canada, Calgary
Fyles JW, Fyles IH (1993) Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J for Res 23:358–361
Ghosh R, Banerjee K (2013) Inter-relationship between physico-chemical variables and litter production in mangroves of Indian Sundarbans. J Mar Sci Res Dev S 11:001. https://doi.org/10.4172/2155-9910.S11-001
Harmon ME, Nadelhoffer KJ, Blair JM (1999) Measuring decomposition, nutrient turnover, and stores in plant litter. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long term ecological research. Oxford University Press, New York, pp 202–240
Harrison PJ, Snedaker SC, Ahmed SI, Azam F (1994) Primary producers of the arid climate mangrove ecosystem of the Indus River Delta, Pakistan: an overview. Trop Ecol 35(2):155–184
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: an historical overview. CAB International, Oxon
Hossain M, Hoque AKF (2008) Litter production and decomposition in mangroves - a review. Indian J for 31(2):227–238
Ibrahima A, Biyanzi P, Halima M (2008) Changes in organic compounds during leaf litter leaching: laboratory experiment on eight plant species of the Sudano-guinea of Ngaoundere. Cameroon for 1:27–33
Ibrahima A, Gillon D, Joffre R (2010) Leaf litter decomposition of Mediterranean tree species in relation to temperature and initial water imbibitions under microcosm experiment. Res J Agric Biol Sci 6:32–39
Imgraben S, Dittmann S (2008) Leaf litter dynamics and litter consumption in two temperate south Australian mangrove forest. J Sea Res 59:83–93
India State of Forest Report (2021) Forest Survey of India, Ministry of Environment Forest and Climate Change, Government of India, New Delhi, India
Jackson ML (1973) Soil Chemical Analysis. Constable and Company Ltd. Prentice Hall of India Pvt. Ltd., New Delhi, pp 10–114
Kalio ANJ (1992) A pilot study of mangrove litter production in the Bonny Estuary of Southern Nigeria. Discov Innov 4(3):71–78
Kamruzzaman Md, Basak K, Paul SK, Ahmed S, Osawa A (2019) Litterfall production, decomposition and nutrient accumulation in Sundarbans mangrove forests, Bangladesh. For Sci Technol 15(1):24–32. https://doi.org/10.1080/21580103.2018.1557566
Kathiresan K, Bingham BL (2001) Biology of mangrove and mangrove ecosystems. Adv Mar Biol 40:81–251
Kavvadias VA, Alifragis DA, Tsiontsis A, Brofas G, Stamatelos G (2001) Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For Ecol Manage 144:113–127
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Lacerda LD, Conde JE, Kjerfve B, Alvarez-Leon R, Polanı´a J (2001) American mangroves. In: En Lacerda LD (ed) Mangrove ecosystems: function and management. Springer, Berlin, pp 1–62
Lee SY (1997) Potential trophic importance of the faecal material of the mangrove crab Sesarma messa. Mar Ecol Prog Ser 159:275–284
Lopez-Portilho J, Ezcurra E (1985) Litterfall of Avicennia germinans L. in a one-year cycle in a mudflat at the Laguna De Mecoacan. Tabasco Mexico Biotropica 17:186–190
Lorıa-Naranjo M, Sibaja-Cordero JA, Cortes J (2019) Mangrove leaf litter decomposition in a seasonal tropical environment. J Coast Res 35(1):122–129. https://doi.org/10.2112/JCOASTRES-D-17-00095.1
Mackey AP, Smail G (1996) The decomposition of mangrove litter in a subtropical mangrove forest. Hydrobiologia 332:93–98
Mahmood H, Saberi O, Misri K, Japar Sidik B (2007) Nutrients dynamics associated with leaf litter degradation of Bruguieria parviflora (Whight and Arnold) at Kuala Selangor Mangrove Forest, Malaysia. Indian J for 30:325–330
Mahmood H, Limon SH, Rahman MS, Azad AK, Islam MS, Khairuzzaman M (2009) Nutrients (N, P and K) dynamics associated with the leaf litter of two agro forestry tree species of Bangladesh. I for 2:183–186
Mahmood H, Siddique MRH, Rahman MS, Hossain MZ, Hasan MM (2011) Nutrient dynamics associated with leaf litter decomposition of three agro forestry tree species (Azadirachta indica, Dalbergia sissoo and Melia azadirachta) of Bangladesh. J for Res 22:577–582
Mall LP, Singh VP, Garge A (1991) Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric mangrove and mixed mangrove forests of Andaman Islands. Trop Ecol 32:144–152
Mason FC (1977) Decomposition, the Institute of Biology’s studies in Biology. Edward Arnold Ltd., London, UK
May JD (1999) Spatial variation in litter production by the mangrove Avicennia marina var. Australasica in Rangaunu Harbour, New Zealand. New Zeal J Mar Fresh 33:163–172
McArthur JV, Aho JM, Rader RB, Mills GL (1994) Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. J N Amer Benthol Soc 13:57–67
Mchenga I, Ali AI (2017) Mangrove litter production and seasonality of dominant species in Zanzibar, Tanzania. J East Afr Nat Hist 106(1):5–18
McTiernan KB, Ineson P, Coward PA (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78(3):527–538
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Mfilinge PL, Meziane T, Bachok Z, Tsuchiya M (2005a) Litter dynamic and particulate organic matter out welling from a subtropical mangrove in Okinawa Island, South Japan. Estuar Coast Shelf Sci 63:301–313
Mfilinge PL, Meziane T, Bachok Z, Tsuchiya M (2005b) Total lipid and fatty acid classes in decomposing mangrove leaves of Bruguiera gymnorhiza and Kandelia Candel: significance with respect to lipid input. J Oceanogr 61:613–622
Middleton BA, McKee KL (2001) Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J Ecol 89:818–828
Morrisey D, Beard C, Morrison M, Craggs R, Lowe M (2007) The New Zealand mangrove: review of the current state of knowledge. Auckland Regional Council Technical Publication No. 325. Auckland, New Zealand
Ngoran A, Zakra N, Ballo K, Kouamé C, Zapata F, Hofman G, Van CO (2006) Litter decomposition of Acacia auriculiformis Cunn. Ex Benth. And Acacia mangium Willd. Under coconut trees on quaternary sandy soils in Ivory Coast. Biol Fertil Soils 43:102–106
Nicholson C (2009) Mangroves and crabs as ecosystem engineers in Zanzibar. Independent Study Project (ISP) Collection Paper 760. (http://digitalcollections.sit.edu/isp_collection/760, Accessed on 22/11/2022
Ochieng CA, Erftemeijer PLA (2002) Phenology, litterfall and nutrient resorption in Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya. Trees. https://doi.org/10.1007/s00468-001-0146-2
Odum EP (1980) The status of three ecosystem level hypotheses regarding salt marshes: tidal subsidy, outwelling and the detritus based food chain. In: Kennedy VS (ed) Estuarine perspectives. Academic Press, New York, pp 485–496
Odum WE, Heald EJ (1975) The detritus-based food web of an estuarine mangrove community. In: Cronin LE (ed) Estuarine Research. Academic Press, New York, pp 265–286
Park S, Kang-Hyun C (2003) Nutrient leaching from leaf litter of emergent macrophyte (Zizania latifolia) and the effects of water temperature on the leaching process. Korean J Biol Sci 7:289–294
Polunin NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in fresh water. Arch Hydrobiol 94:182–209
Pool DJ, Lugo AE, Snedaker SC (1975) Litter production in mangrove forests of southern Florida and Puerto Rico. In: Walsh G, Snedaker S, Teas H (eds) Proceedings of the International Symposium on the Biology and Management of Mangroves. University of Florida, Gainesville, Florida, pp 213–237
Pool DJ, Lugo A, Snedaker SC (1986) Litter production in mangrove forests of Southern Florida and Puerto Rico. Reprint 14462 UPR-RUM, 213–237
Praveen VP (2014) The role of brachyuran crabs in structure, composition and recruitment of mangrove forests in Kerala, PhD Thesis, University of Kerala, Thiruvananthapuram, India
Praveen VP, Shanij K, Suresh S, Balakrishnan P (2015) Kunhimangalam, the largest mangrove in Kerala needs immediate conservation attention. SACON ENVIS Newsletter - Sarovar Saurabh 11(2):1–2
Qasim SZ, Waffar MVW (1990) Marine resource in the tropic. Resource Manage Optim 7:141–169
Ragavan P, Saxena A, Jayaraj RSC, Moha PM, Ravichandran K, Saravanan S, Vijayaraghavan A (2016) A review of the mangrove floristics of India. Taiwania 61(3):224–242. https://doi.org/10.6165/tai.2016.61.224
Rani V, Sreelekshmi S, Preethy CM, Bijoy Nandan S (2016) Phenology and litterfall dynamics structuring ecosystem productivity in a tropical mangrove stand on South West coast of India. Reg Stud Mar Sci 8:400–407. https://doi.org/10.1016/j.rsma.2016.02.008
Rani V, Sreelakshmi C, Bijoy Nandan S, Santu KS, Preethy CM (2023) Feeding ecology of Parasesarma plicatum and its relation to carbon structuring in mangrove ecosystem. Hydrobiologia 850 (4):911–927 Robertson AI, Alongi DM, Boto KG (1992) Food chains and carbon fluxes. In: Robertson AI, Alongi DM (eds) Tropical Mangrove Ecosystems. American Geophysical Union, Washington DC, pp 293–326
Saberi O (1989) The rate of litter production in mangrove forest at Siar beach. Lundu Sarawak Pertanika 12(1):47–51
Saenger P, Snedaker SC (1993) Pantropical trends in mangrove above ground biomass and annual litterfall. Oecologia 96:293–299
Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta R, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M, Farfan F (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Shanij K (2017) Sesarmid crabs and nutrient cycling in a selected mangrove ecosystem of Kerala. Ph. D. Thesis, University of Kerala, Thiruvananthapuram, Kerala, India
Shanij K, Praveen VP, Suresh S, Oommen MM, Nayar TS (2016) Leaf litter translocation and consumption in mangrove ecosystems: the key role played by the sesarmid crab neosarmatium malabaricum. Curr Sci 110(10):1969–1976
Sherman PM (2002) Effects of land crabs on seedling densities and distributions in a mainland neotropical rain forest. J Trop Ecol 18(1):67–89
Sherman RE, Fahey TJ, Martinez P (2003) Spatial patterns of biomass and above- ground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 6:384–398
Silva CAR, Mozeto AA, Ovalle ARC (1998) Distribution and fluxes as macrodetritus of phosphorus in red mangroves, Sepetiba Bay, Brazil. Mangroves and Salt Marshes 2(1):37–42
Silva R, Silva AP, Oliveira SR (2006) Concentration, stock and transport rate of heavy metals in a tropical red mangrove, Natal, Brazil. Mar Chem 99:2–11
Simlai A, Roy A (2012) Analysis of and correlation between phytochemical and antimicrobial constituents of Ceriops Decandra, a medicinal mangrove plant, from Indian Sundarban estuary. J Med Plants Res 6:4755–4765
Slim FJ, Gwada PM, Kodjo M, Hemminga MA (1996) Biomass and litterfall of Ceriops tagal and Rhizophora mucronata in the mangrove forest of Gazi bay, Kenya. Mar Freshw Res 73:25–38
Snedaker SC, Snedaker JG (eds) (1984) The mangrove ecosystem: research methods. Monographs on Oceanographic Methodology, UNESCO, United Kingdom
Srivastava PBL (1980) Research proposals for mangrove vegetation in Malaysia. In: Srivastava PBL, Kadir RA (eds) Proceedings of Workshop on Mangrove and Estuarine Vegetation, Forest Department, Kuala Lumpur, pp 64–75
Steinke TD, Holland AJ, Singh Y (1993) Leaching losses during decomposition of mangrove leaf litter. S Afr J Bot 59:21–25
Suresh HS, Bhat DM, Ravindranath NH, Sukumar R (2017) Carbon stocks and sequestration potential of. Indian Mangroves Trop Ecol 58(3):547–553
Teal JM (1962) Energy-flow in salt-marsh ecosystem of Georgia. Ecology 43:614–624
Triadiati S, Tjitrosemito E, Sundarsono G, Qayim I, Leuschner C (2011) Litterfall production and leaf-litter decomposition at natural forest and Cacao agro forestry in Central Sulawesi, Indonesia. Asian J Biol Sci 4:221–234
Twilley RR (1995) Properties of mangrove ecosystems related to the energy signature of coastal environments. In: Hall C (ed) Maximum Power. University of Colorado Press, Boulder, Colorado, pp 43–62
Twilley RR, Chen RH, Hargis T (1992) Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut 64:265–288
Twilley RR, Pozo M, Garcia VH, Rivera-Monroy VH, Zambrano R, Bodero A (1997) Litter dynamics in riverine mangrove forests in the Guayas River Estuary. Ecuador Oecologia 111:109–122
Van der Valk AG, Attiwill PM (1984) Decomposition of leaf and root litter of Avicennia marina at Westernport bay, Victoria, Australia. Aquat Bot 18:205–221
Wafar S, Untawale AG, Wafar M (1997) Litterfall and energy flux in a mangrove ecosystem. Estuar Coast Shelf Sci 44:111–124
Wangondu VW, Bosire JO, Kairo JG, Koedam N (2014) Litter fall dynamics of restored mangroves (Rhizophora mucronata Lam. And Sonneratia alba sm.) In Kenya. Restor Ecol 22(6):824–831
Wardle DA, Nilsson MC, Zackrisson O, Gallet C (2003) Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35(6):827–835
Zaldivar Jimenez A, Herrera Silveira J, Coronado Molina C, Alonzo Parra D (2004) Estructuray Productividad De Los manglares en la reserva de biosfera Ría Celestún, Yucatán, México. Maderay Bosques Special Issue 10(1):25–35
Acknowledgements
We thank the Ministry of Environment, Forest and Climate Change, Government of India, New Delhi for financial support(D O No. 22/16/2004-CS (M) dated 09/06/2005). Dr. P. P. Moosa, Scientist in charge, Krishi Vigyan Kendra, Kerala Agricultural University, Thaliparamba, Kerala is gratefully acknowledged for providing us with data on humidity and rainfall of the study area.
Funding
The research work was funded by the Ministry of Environment, Forest and Climate Change, Government of India, New Delhi (D O No. 22/16/2004-CS (M) dated 09/06/2005).
Author information
Authors and Affiliations
Contributions
KS, SS and TSN conceptualized and designed the study and collected data in the field. KS and VJ analyzed the data. KS and SS prepared the first draft. TSN revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Permission
The data and samples for the study were collected from an area under the authority of Payyannur Municipality and Kunhimangalam Gramapanchayat and the study was conducted with the knowledge and permission of the stakeholders.
Ethical approval
NA.
Consent to participate
NA.
Consent for publication
NA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shanij, K., Suresh, S., Jilesh, V. et al. Leaf litter production and decomposition in a Riverine Mangrove forest in India. Wetlands Ecol Manage 32, 59–77 (2024). https://doi.org/10.1007/s11273-023-09961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09961-0