Abstract
Wetland communities are shaped by high levels of stress, disturbance and competition. Using South African palmiet wetlands as a case study (Prionium serratum dominated valley-bottom wetlands), we explore whether autogenic or allogenic succession is the dominant process driving community dynamics in valley-bottom wetlands in drylands. Several wetland rehabilitation programmes in South Africa use the dominant wetland species palmiet (P. serratum) as a pioneer to facilitate recolonization. However research is needed on palmiet wetland dynamics and formation to guide these restoration efforts. We explore vegetation patterns by analyzing which environmental parameters drive dominance of palmiet, resulting in the characteristic patchiness of palmiet wetlands, and which plant functional traits account for this. In 20 plots from three palmiet wetlands distributed across the Cape Floristic Region of South Africa, key soil, groundwater and vegetation parameters, as well as community composition were measured. Twenty-two dominant species were selected and 13 functional traits measured. Soil pH and relative groundwater depth were the main environmental parameters driving community assembly in palmiet wetlands. Palmiet-dominated communities were characterized by greater stem diameter, leaf length–width ratio, leaf area and cellulose and lignin concentration compared to fynbos communities. These traits suggest adaptations to disturbances such as fires (thicker stems) and floods (long, thin leaves, flexible shoots and thicker stems). We propose three hypotheses of palmiet wetland development which shed light on palmiet wetland restoration and highlight gaps for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are dynamic ecosystems subjected to high levels of stress (e.g. water inundation), disturbance (e.g. floods or fires) as well as competition (Clement and Proctor 2009; Mitsch and Gosselink 2015; Moor et al. 2017). Wetland landscape patterns are thought to be the combination of varying interactions between abiotic (climate, topography, hydrology, geomorphology) and biotic (productivity, competition, herbivory, peat accumulation) processes (Tooth and McCarthy 2007; Mitsch and Gosselink 2015). However, water level has been proposed to be the single most important determinant for wetland vegetation community dynamics (Clement and Proctor 2009). Extremes are also thought to have more impact on vegetation development in wetlands than average conditions (Clement and Proctor 2009). Indeed, vegetation has been shown to be most sensitive to the mean highest groundwater level, especially during the growing season (Wierda et al. 1997). Others propose that biotic processes can be of great importance, such as in the case of ecosystem engineers: species that significantly modify their environment in their favour, inhibiting return to a previous state (Clement and Proctor 2009).
Due to their transitional nature in both space and time, wetlands are at the center of a debate about the relative roles of autogenic versus allogenic succession (Mitsch and Gosselink 2015). Autogenic succession is the classical ecological theory which suggests that species are organised in recognisable communities, that the community changes through time due to mainly biotic effects, and that these changes are linear and directed towards a stable, mature, climax ecosystem (Odum 1969; Mitsch and Gosselink 2015). Allogenic succession is an individualistic hypothesis (continuum concept) which proposes that the distribution of each species is governed by its response to the environment (abiotic factors), that each species responds differently, no two species occupy the exact same niche and that the observed replacement or invasion sequence is influenced to a large degree by chance (Gleason 1917).
For wetlands that are subjected to severe disturbance that exceeds the capacity of an ecosystem for resilience, the concept of alternative stable states applies. Beyond a certain threshold, typical succession processes will not restore the original state (Suding et al. 2004). It is argued that traditional successional concepts are of limited use in wetlands since community dynamics may not be directional or orderly, or predictable in the long-term (Niering 1989). The alternative concept of progression towards maturity may rather apply (Mitsch and Gosselink 2015). In structuring these communities, the role of chance (competitive lottery; Sale 1977), coincidence and abiotic (allogenic) factors and as well as catastrophic events (floods and droughts) have been suggested to be of more importance (Mitsch and Gosselink 2015).
The exposure of wetland ecosystems to water stress, both in terms of periodic drying and flooding, has led to the evolution of characteristic plant functional traits in wetland species (Mitsch and Gosselink 2015; Moor et al. 2017; Sieben et al. 2017a). A trade-off between competitive ability and stress tolerance limits has been proposed (Wisheu and Keddy 1992). Two trait categories in wetlands are proposed: regenerative or juvenile traits (seed characteristics, dispersal, seedling establishment) and morphological, physiological and phenological adult traits (Clement and Proctor 2009). The juvenile traits are related to success at colonising gaps, whereas adult traits are related to ability to survive stress or compete. There is no evidence that these trait subsets are coupled (Shipley et al. 1989), implying good colonizers may not necessarily be strong competitors.
Soil saturation, resulting in temporary or permanent anoxia in the root zone, and water-table fluctuations, resulting in submergence as well as mechanical disturbance are key challenges to wetland species (Moor et al. 2017). Traits associated with soil saturation include anaerobic respiration in the root zone, development of aerenchyma, higher leaf dry matter content, and lower specific leaf area and leaf nitrogen content (Mitsch and Gosselink 2015; Moor et al. 2017). Traits associated with adaptations to water-table fluctuations and flooding include: high root biomass allocation, extensive rhizomes, high stem flexibility, narrow leaves, and sclerophylly (Colmer and Voesenek 2009; Catford and Jansson 2014; Moor et al. 2017). Leaf mass area was found to be correlated with three components of leaf mechanical resistance, which could have implications for flood resistance: work-to-shear, force-to-punch and force-to-tear (Onoda et al. 2011).
South African valley-bottom palmiet wetlands are small, narrow peatlands which are subject to extreme water stress: soil saturation, water table fluctuations, floods as well as droughts and fires (Rebelo 2012; Job 2014). Palmiet wetland vegetation is composed of patches dominated by a single species, Prionium serratum (L.f.) Drège, or palmiet (hereafter palmiet communities), and patches of other plant communities (hereafter fynbos communities). Fynbos is a fire-adapted shrubland that characterizes the Cape Floristic Region, South Africa. Palmiet is a unique competitor and has even been suggested to be an ecosystem engineer (Sieben 2012; Sieben et al. 2017a). Palmiet wetland development is not well understood, though it is suggested that these wetlands have formed in valley-bottoms characterized by repeated cut-and-fill cycles over geological timescales resulting in valley floor-planing (Pulley et al. 2018; Grenfell et al. 2020). It is important to understand palmiet wetland formation and community dynamics, as this could inform restoration and rehabilitation practice (Grenfell et al. 2020). Currently several restoration and rehabilitation programmes are using palmiet as a pioneer species to facilitate recolonization of degraded river and wetland ecosystems. However there is little to no research supporting this work, justifying greater research efforts into understanding palmiet wetland dynamics and formation, with the goal of guiding rehabilitation practices. We aim to compare homogeneous, species-poor palmiet communities with more functionally diverse fynbos communities in palmiet wetlands. We explore vegetation patterns by analysing which environmental parameters drive dominance of palmiet (allogenic vs autogenic succession), resulting in the characteristic patchiness of palmiet wetlands, and which plant functional traits account for its super-dominance. Using these data as a departure point, we hypothesize on the development of palmiet wetlands, and whether palmiet-dominated communities represent climax ecosystems (succession) or whether both communities are ‘mature’ end-stage communities.
Methods
Study region & wetlands
The Cape Floristic Region has a Mediterranean-type climate characterised by summer drought and winter rainfall resulting from the passage of cold fronts (Midgley et al. 2003). The soils of the Cape Floristic Region are mainly nutrient poor, highly leached dystrophic lithosols associated with the sandstone mountains of the Cape Supergroup (Midgley et al. 2003). Vegetation of this region is predominantly fynbos, which is a biodiverse, fire-adapted shrubland composed of three elements: restioids, ericoids and proteoids (Low and Rebelo 1996). Dominant growth forms are shrubs and graminoids with few true trees. For a floristic description and classification of different wetland vegetation communities for the fynbos and other nutrient-poor sandstone and quartzite substrates across South Africa, see Sieben et al. (2017c).
Three palmiet wetlands were selected as study sites within the Cape Floristic Region: the Theewaterskloof and Goukou wetlands (Western Cape) and the Kromme wetland (Eastern Cape) (Table 1). Despite being situated as much as 470 km apart, these wetlands are remarkably similar in vegetation composition. They tend to occur at altitudes of around 400 m; mean annual precipitation is highly variable, highest in the Theewaterskloof catchment and lowest in the Goukou catchment and the global aridity index follows the same trend. Mean annual runoff is also highest in the Theewaterskloof catchment but lowest in the Kromme catchment. In the case of the Kromme and Goukou, most of this runoff occurs over short periods, during flood events (Job 2014; Rebelo et al. 2015). All three wetlands have accumulated peat layers that are between 0.5 and 10 m deep (Table 1).
Study design
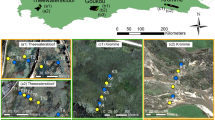
To capture seasonal variation in wetland properties and processes each wetland was sampled twice: once in September 2014, just after winter, and once in March/April 2015, just after summer. Rainfall for the six months preceding the first fieldtrip was average for all sites (approximating 50% of the MAP); however, rainfall for the six months preceding the second fieldtrip was far lower for Theewaterskloof and the Kromme (Table 1). Therefore, for Theewaterskloof and the Kromme, the second campaign represents a drier season, whereas there is little difference for the Goukou wetland. At each of the three wetland sites, least disturbed stretches of wetland were sampled. It should be noted that all wetlands are transformed to some degree, with channelization occurring upstream or downstream of selected fragments. At each site, cross-sectional transects (100–200 m) were made across the wetland, with six plots (3 × 3 m) placed between 20 and 50 m apart, yielding 18 plots (Fig. 1). Transects and plots were chosen in the field to ensure adequate representation of the two plant communities, which we term: palmiet and fynbos, yielding a final sum of 20 plots. Plots were defined as palmiet communities when the percentage cover of palmiet was over 60% (Table 6), and as fynbos communities when the presence of palmiet was less than 60%, yielding sample sizes of 10 and 10 for palmiet and fynbos communities respectively. Piezometers (3 m, PVC) were placed adjacent to every second plot, yielding a total of 12 piezometers (Fig. 1).
The location of the 20 study plots (yellow and blue circles) and three study wetlands within the Cape Floristic Region (green) of South Africa. Blue circles indicate the location of piezometers, and the black shapes indicate the quaternary catchments the wetland are located within. The global aridity index is plotted, with most of the wetlands located in areas classified as semi-arid (Trabucco and Zomer 2018)
Sampling
Plant community composition and vegetation analysis
In each plot, all plants were identified to species level where possible and percentage cover was estimated for each species using the Braun-Blanquet Scale (Mueller-Dombois and Ellenberg 1974). Vegetation (above-ground biomass) was sampled from three randomly selected subplots of 0.28 × 0.28 m within each plot. This above-ground biomass was dried for 48 h at 70 °C, weighed and then ground and homogenised using a mill. Plant total nitrogen and total phosphorus were determined using acid digestion and were measured with a continuous -flow analyzer (CFA) (SKALAR: SAN + +) (Walinga et al. 1989). Potassium, calcium, and magnesium were analyzed by Inductively Coupled Plasma-emission spectrometry (ICP-OES) (Walinga et al. 1989) after acid digestion of approximately 0.3 g of dried and finely ground vegetation with H2SO4–Se-salicylic acid. Thirteen plant functional traits were collected for 22 dominant wetland species. All methods were based on the standardised protocol of Pérez-Harguindeguy et al. (2013), see Table 7 for details.
Soil sampling and chemical analyses
One composite soil sample was taken from 10 points throughout each plot at a depth of 1–10 cm using a hand-held auger of 1 cm in diameter. Soil pH-H2O was measured after adding distilled water to a 10 g soil sample and shaking it for an hour. In each plot one additional undisturbed soil sample was taken of the topsoil using a 100 cm3 metal Kopecky ring to measure bulk density. Samples were weighed after oven drying for 48 h at 70 °C and values expressed as g/cm3. Soil water content was calculated gravimetrically by weighing approximately 20 g of fresh soil before and after drying for 24 h at 105 °C. Soil organic matter content was determined by loss on ignition (4 h at 550 °C). Total phosphorus and nitrogen were analyzed on a CFA. Soft extractions were done on fresh soil to determine NO3−, NH4+ and PO43−. Samples were extracted and preserved for later analysis on a CFA using AA-EDTA (ammonium acetate—ethylenediaminetetraacetic acid) for PO43− and AA–KCl (ammonium acetate—potassium chloride) for NO3− and NH4+ respectively (Houba et al. 1989). Nutrient pools were calculated by multiplying nutrient concentrations by bulk density measurements.
Cation exchange capacity was determined using the method of Brown (1943) by weighing approximately 8 g of soil before and after air drying in an incubator at 40 °C for 48 h. Samples were then sieved through a 2 mm sieve, 25 ml ammonium acetate solution (1 M) was added to 2.5 g of soil and samples were shaken for one hour. Soil pH was measured and samples were filtered through a 0.45 µm filter before being analyzed for H, Ca, K, Mg, Na, Al, Fe, Mn ions on an ICP-OES. Potassium, calcium, and magnesium were analyzed on the ICP-OES after acid digestion of approximately 0.3 g of dried and finely ground soil with H2SO4–Se-salicylic acid (Walinga et al. 1989). Soil microbial biomass carbon was measured as a proxy for microbial activity in the soil at each site. We used the chloroform fumigation direct extraction protocol for microbial biomass carbon (Martens 1995; Beck et al. 1997).
Groundwater sampling and chemical analyses
Depth to the water table was measured in each piezometer using a sounding device, and standing water emptied using a bailer. Once fresh water had refilled the piezometer, a sample was taken for a pH, and conductivity reading. Six water samples were taken and filtered (0.45 µm) to test for water quality parameters. The concentration of phosphate (PO43−–P), ammonium (NH4+–N), total phosphorus (P-tot), and total Kjeldahl nitrogen were measured on a CFA. Concentrations of sodium, magnesium and calcium were measured on the ICP-OES.
Data analysis
We performed an analysis of similarity (ANOSIM) to determine whether palmiet and fynbos vegetation were in fact distinct, using the Bray–Curtis dissimilarity index. Next, we performed a similarity percentage analysis (SIMPER) to determine which species characterised palmiet and fynbos communities. We used the ‘Vegan’ package for community ecology in R for these two analyses (Warton et al. 2012) as well as to calculate functional diversity indices & community weighted means.
To test the relationship between plant community, wetland (Theewaterskloof, Goukou, Kromme) and soil, groundwater, vegetation tissue chemistry and functional diversity parameters, we fitted linear mixed models taking season into account (winter 2014, summer 2015). Plots were entered as a random effect to account for the dependence between observations from within the same plot. Wetland, plant community, season and the interaction between wetland and plant community were entered as fixed effects. First, the significance of the interaction was tested by comparing the fit of this model to a reduced model with only the three main effects. Where the interaction term was significant, we split the dataset by wetland and tested for the effect of plant community in all three wetlands separately. Where the interaction term was not significant, we excluded it from the model and tested the significance of the main effect: plant community. Significance was tested using an F-test with Kenward-Roger correction for degrees of freedom, as implemented in the “pbKRtest” package of R. All variables, besides pH and ratios, were log(x + 1) transformed prior to analysis to satisfy the assumptions of normality and homoscedasticity of the linear mixed models.
Lastly a detrended correspondence analysis (DCA) was performed on species abundance data, using the “vegan” package in R. Each season was analyzed separately (2014, 2015). Soil and vegetation parameters that differed significantly between palmiet and fynbos communities were correlated to the first and second axes, and overlain on the plot.
Results
Abiotic parameters driving palmiet and fynbos communities
The only measured soil property that differed between fynbos and palmiet communities was pH, where it was marginally higher in fynbos communities (Table 2). Nutrients, nutrient pools and soil buffering capacities showed no significant differences or interesting trends. Relative groundwater depth was significantly different between the two communities, tending to be closer to the surface but more variable for fynbos communities, and deeper below the ground for palmiet communities (Table 3). There was significantly higher Kjeldahl nitrogen in the groundwater of palmiet compared to fynbos communities in the Goukou wetland, however these trends were not observed for other wetlands.
Biotic factors driving palmiet and fynbos communities
Although there was no significant difference in soil or groundwater K and Mg, there was a significantly higher concentration of these cations in palmiet vegetation compared to that of the fynbos vegetation (Table 4). Nutrient concentrations in plant tissues did not differ significantly between communities. There was no significant difference in the number of functional types for the two communities (Table 5). Diversity indices did not differ significantly between communities, however various community weighted means did vary. Stem diameter, leaf length–width ratio, leaf area as well as cellulose and lignin concentration in the leaves were significantly higher in palmiet communities relative to fynbos communities. Conversely, the community weighted mean for percentage of plant silicon concentration was higher in fynbos than palmiet communities.
A comparison of palmiet and fynbos communities
The ANOSIM revealed 62–72% dissimilarity between fynbos and palmiet plant communities. Palmiet communities tended to be characterized by P. serratum (Palmiet, 87–94%) some Cliffortia odorata, and ferns: Todea barbara and Pteridium aquilinum (Table 9). Fynbos communities were distinguished by Restio paniculatus (43–44%), Cliffortia strobilifera (17–23%), and to a lesser extent two graminoids Schoenus gracilis and Isolepis prolifera (Table 9). Based on plant community structure from September 2014 the DCA confirmed that fynbos communities tend to be more diverse than palmiet ones (Fig. 2). Fynbos and palmiet communities cluster separately except for two fynbos plots which clustered closer to the palmiet communities. These two plots were both situated within palmiet communities, though were classified as non-palmiet communities as the cover of palmiet was less than 50%. The results from 2015 showed similar trends and are displayed in Fig. 8.
Detrended correspondence analysis (DCA) of the plant communities in fynbos and palmiet patches in three South African palmiet wetlands sampled in September 2014. Fynbos sites are in orange, palmiet in green. Symbols: ▲ Theewaterskloof, ■ Goukou, ● Kromme. Species names are given in black, and + indicates species with a lower abundance that are masked by other labels. Parameters that were interesting or significantly different (bold) between palmiet and fynbos wetland patches were overlain and are indicated by the arrows. Soil parameters are in brown, vegetation composition in green, functional diversity indices in blue. CWM community weighted mean, LLWR leaf length–width ratio, Lig lignin, Cel cellulose, SD stem diameter, LA leaf area, Si silicon, No_sp number of species, Veg.K K in vegetation, Veg.Mg Mg in vegetation, MicrobialC microbial carbon, CEC cation exchange capacity, SWC soil water content. For full species names see Table 10. Stippled circles encompass sites from fynbos and palmiet communities
Discussion
Which environmental parameters explain patchiness in palmiet wetlands?
Only two abiotic variables differed significantly between fynbos and palmiet patches in palmiet wetlands. These were soil pH, which was slightly higher in fynbos communities, and relative groundwater depth, which tended to be closer to the surface in one wetland (Goukou) but more variable for another (Theewaterskloof) for fynbos communities. In a study on the Kromme palmiet wetland, Nsor (2007) also found soil pH to be a key environmental variable influencing community assembly. Additionally a study on riparian zones in South Africa confirmed that flow regimes were the key variable determining four different zones of differing plant communities (Reinecke 2013). There is no doubt that hydrological regime and geomorphological processes will play an important role in shaping wetland plant communities (Tooth and McCarthy 2007), however in the case of palmiet wetlands, longer term monitoring of water table depth is needed to yield more insight. In this case it is not clear whether water table depth is driving vegetation dynamics, or whether the vegetation is shaping local water table depth and pH through processes such as transpiration, photosynthesis and decomposition.
Can plant functional traits shed light on the super-dominance of palmiet?
Within palmiet wetlands there are at least two distinct plant communities: palmiet-dominated communities, and somewhat more diverse fynbos communities. The diversity within fynbos communities likely points to more than one community type (e.g. Calopsis paniculata vegetation -Community 1.1, Cliffortia strobilifera vegetation -Community 1.2; see Sieben et al. 2017b). Plants may be grouped into dominant matrix species, interstitial species, and ruderals (Boutin and Keddy 1993), or using a functional approach into dominants, subordinates and transients (Grime 1998). This applies to palmiet communities; P. serratum acting as the dominant matrix species, Cliffortia odorata, ferns Todea barbara and Pteridium aquilinum as well as various Psoralea sp. (depending on the region) acting as the sparse interstitial species. Cliffortia odorata has a creeping form and Psoralea sp. are spindly trees. Vegetation potassium (K) and magnesium (Mg) concentration was significantly higher in the palmiet communities relative to the fynbos; however, these differences are not reflected in the soil or groundwater, therefore it is not clear why this difference should occur. This greater accumulation of K in palmiet could be the result of higher transpiration rates relative to fynbos communities (Brag 1972; Rebelo et al. 2020). Another possibility is that it is indicative of palmiet communities being older than the fynbos ones, and these tissue concentrations reflecting longer term uptake from soil and groundwater (e.g. from high cation flood pulses). Additionally, at low pH, Mg becomes more soluble and therefore in palmiet communities may be more available for plant uptake (Jackman and Black 1951; Lucas and Davis 1961).
While fynbos communities had more plant species than palmiet communities there was no difference in the number of functional types. This suggests that although palmiet communities are more species poor (and less even, with palmiet dominating), the suite of species is optimal for exploiting the niche space provided by the wetland (i.e. there is convergence on the same number of functional types). Community weighted means for stem diameter, leaf length–width ratio, leaf area as well as cellulose and lignin concentration in the leaves were significantly higher in palmiet communities relative to fynbos communities. These mean values in palmiet communities were highly influenced by the dominant species: palmiet. The palmiet community’s overall larger stem diameter than that of the fynbos may be confirmation of the community being fire retardants as opposed to promoters (Rebelo 2001), allowing fires to pass over the crown of the plants, leaving the stems intact. The larger stem diameter may equally confer benefits for withstanding the mechanical disturbance of floods. There is evidence of the co-evolution of rivers and vegetation on geological time-scales that give rise to traits that allow the vegetation to tolerate disturbances (Gibling et al. 2014). The significantly higher leaf length–width ratio (long strap-like leaves) may be an adaptation to the mechanical disturbance of floods (Colmer and Voesenek 2009; Catford and Jansson 2014). Additionally, the higher cellulose and lignin concentration of the leaves, but lower biogenic silica concentration may indicate high stem flexibility (Schoelynck et al. 2010), representing another adaptation to flood events (Colmer and Voesenek 2009; Catford and Jansson 2014). We did not measure below ground traits in this study, however another study on palmiet wetland communities has shown that rhizome internode length (a measure for clonality) was important in explaining vegetation spatial patterns (Sieben 2012).
Ecological hypotheses of palmiet wetland development
Since there is no clear evidence of abiotic conditions explaining patchiness in palmiet wetlands from these results, other explanatory factors for these patterns are possible. Disturbance and competition, or a combination of these, may also shape vegetation patterns. Little is known about how palmiet wetlands have formed over time, but it is acknowledged that pristine, unchanneled valley-bottom palmiet wetlands are typically located on slopes that are steeper than would be expected to be suitable for wetland formation (Pulley et al. 2018; Grenfell et al. 2020). A geomorphological theory for the development of palmiet wetlands suggests that gully cut-and-fill cycles laterally plane valley floors and contribute to gradually widening the valley-bottom and reducing longitudinal slope. Proposed geomorphological drivers for this erosion are impingement of the valley-bottom by alluvial fans, or more commonly the exceedance of slope through aggradation (Grenfell et al. 2010, 2020; Pulley et al. 2018).
Other drivers may potentially include extreme (1:100 year) fires and floods which could cause localized erosion, initiating gully formation in some cases. Whilst Palmiet is fire-adapted (Boucher and Withers 2004), severe fire, especially during drought, could locally damage stem and root stocks. Once these gullies have leveled the longitudinal profile, the shallower slope would encourage deposition of organic matter and clastic sediment (Pulley et al. 2018). This would create an opportunity for competition for establishment (juvenile trait subset) within the potential species pool. Those species with superior colonization ability would have a competitive advantage initially (Shipley et al. 1989).
In cases where this gully erosion exceeds the scale that the system has evolved to cope with (e.g. with the introduction of European-style farming and irrigation, railways, dams and roads), it is possible that the system exceeds a threshold and switches to another stable state (Suding et al. 2004; Brown et al. 2017; Tooth 2018; Grenfell et al. 2020). This is evidenced by cases where the alluvium of the entire valley-floor is washed downstream for hundreds of meters (e.g. parts of the Kromme and Duivenhoks rivers), or the unprecedented synchronous phase of incision across the wetland in Pietersielieskloof palmiet wetland (Grenfell et al. 2020). Recruitment in this harsh environment of floods, droughts, grazing and fire, is challenging and therefore success is limited in the short-to-medium term (Rebelo 2017). Though the erosion process itself is “natural” (Pulley et al. 2018), in many cases this process has accelerated (Brown et al. 2017) and intensified in the Anthropocene to a point that is not beneficial for nature and society. On geological time-scales these wetland ecosystems will probably self-restore, but in the interim it represents a large loss of ecosystem services to society, justifying rehabilitative intervention (Tooth 2018; Grenfell et al. 2020). Little is known about which species are best to use for palmiet wetland rehabilitation, as little is known about the genesis of palmiet wetlands. We present three possible hypotheses from an ecological perspective, which may guide future research and rehabilitation initiatives.
Hypothesis 1
Succession towards palmiet-dominated communities.
One hypothesis to explain patchiness in palmiet wetland vegetation is that fynbos communities (possibly with Palmiet interspersed, but not dominated by it) are an earlier successional stage, and that autogenic succession is at work, propelling the system towards a climax community, dominated by palmiet (Mitsch and Gosselink 2015). This is based on the findings that (1) fynbos communities tend to be more species rich, characteristic of the pioneer stage, and (2) Palmiet-dominated communities had higher tissue K and Mg, possibly due to being older. A conceptual diagram for this process is given in Fig. 3. This hypothesis is underpinned by the following two assumptions: (1) fynbos species are superior juvenile competitors and have higher success establishing from the potential seed pool, and (2) Palmiet is a superior adult competitor, able to take over by predominantly clonal growth (vegetative reproduction) by expanding existing patches. These clonal root systems are likely to be very old and analogous to the ‘underground forests’ formed by species in tropical Africa (Maurin et al. 2014). Palmiet is cited to be an ecosystem engineer (Sieben 2012), due to its ability to modify its environment to its advantage. One way in which Palmiet may modify its environment is through the production of thick above-ground roots which form a convoluted mass sometimes as much 20 cm to 1 m above the ground surface (Plate 1: b, c, e). In fynbos communities within palmiet wetlands there are no such structures, and fynbos grows straight from the peat or soil. It is possible that this root formation is an adaptation for flood events: the root system prevents uprooting during flood events, and the height of the plants from the ground may result in a decrease in anoxia in the root zone.
Photographs of palmiet wetlands showing a one of the preferential flow paths through the wetland (dispersed flow), b an impression of the length/height of the raised stems of palmiet, c drone imagery showing the patchy nature of the palmiet wetlands at Theewaterskloof, d a thick palmiet root (hand indicating scale), e the complex palmiet root system, f the tussock-like structure of palmiet, g drone imagery of the raised/mounded nature of palmiet patches in the Vyeboom wetlands, lower at the edges, raised in the center, h vegetative reproduction of palmiet, i a patch of palmiet growing in a recently formed gully in the Kromme River. Photos are from the Theewaterskloof, Vyeboom, Goukou and Kromme wetland systems
Patches of palmiet (e.g. looking at a cross-section across the valley) also appear to be slightly raised in the center as a result of this root development at scale (Fig. 4). Possibly an adaptation to maximize light exposure, or a result of accumulation of sediment from diffuse flows (e.g. Plate 1: g), this phenomenon is known in other wetlands in drylands, and thought to be critical for chemical sedimentation (Tooth and McCarthy 2007). These raised mounds eventually alter the pathway of water through the valley-bottom, creating preferential flow paths (Pulley et al. 2018). The sediment deposition (aggradation) may also be the phenomenon that accounts for the steeper longitudinal slope of Palmiet wetlands relative to the bedrock (Pulley et al. 2018; Grenfell et al. 2020). Literature suggests that this would be likely to result in localized erosion (Pulley et al. 2018; Grenfell et al. 2020), which would restart the cycle of vegetation colonization and succession in these highly dynamic wetland ecosystems.
Conceptualized cross section through a wetland dominated by palmiet, and that of a more diverse fynbos community in the mid-region of the Goukou catchment. The gray stippled line represents the peat bed of an average transect in the Goukou. In the fynbos community: yellow represents graminoids, green: shrubs, brown: ferns, black: trees with some individual palmiet plants. In the case of the palmiet-dominated wetland, the brown root zone and raised center is apparent. In this case, it is hypothesized that the fynbos community may be the result of disturbance and recent colonization, and there is no presence of peat
Hypothesis 2
Succession towards fynbos communities.
A second hypothesis is that fynbos communities are the climax community, with the palmiet-dominated community as the pioneer, an alternative form of autogenic succession (Mitsch and Gosselink 2015). Following severe gully erosion, it has been noted that palmiet establishes in small patches within these gullies (Plate 1). It has been proposed that palmiet traps sediments and organic material, and that as these patches expand, the wetland would be raised, until the gully was refilled (Barclay 2016, Fig. 5). Palmiet patches have also been observed to close up over water-filled gullies, or water-bodies, by means of their root system (Fig. 6). As sediment accumulates and the habitat becomes more suitable for other species, it is possible that fynbos species could establish and outcompete Palmiet. It is well known that palmiet does not tolerate shade (e.g. following invasion by alien trees), so could be out-competed by other tall, dense shrubs (Boucher and Withers 2004). This hypothesis is underpinned by the following two assumptions: (1) palmiet is a superior juvenile competitor, especially in extreme conditions (e.g. following gully erosion), and (2) fynbos species are able to outcompete palmiet once it has reached a mature state. In this situation, Palmiet transforms a habitat from one that is inhospitable to other species, to one that is ideal, through the trapping of sediment: an altruistic ecosystem engineer.
Conceptual diagram of palmiet recolonization following disturbance (after Barclay 2016). The gray stippled area indicates peat, blue: water, green: palmiet and interstitial vegetation, brown: sediment and organic build-up from palmiet. a intact palmiet wetland with raised center, stimulating gully formation at the edge of the wetland, b alluvium washed out and gully eroded to bed-rock, c Palmiet starts to colonize the gully from the edges, using its superior root stock, d sediment and organic matter build up, until e the gully is refilled and the wetland is “intact” once more. As a result of sediment deposition, the habitat becomes more suitable for other facultative wetland species, which may start to invade and outcompete palmiet in patches
Aerial photograph time series showing expansion of palmiet patches behind the Jonkershoek dam from 2005 to 2018. The light green region in the map of South Africa represents the Cape Floristic Region. White arrows indicate change in palmiet patches. The values in m2 show the area of the palmiet wetland in each time-step, calculated from Google Earth Imagery©
Hypothesis 3
Non-directional maturity.
An alternative hypothesis is that all communities may be ‘mature’ palmiet wetland communities and that there is no temporal succession towards a ‘climax’ palmiet community (Niering 1989; Mitsch and Gosselink 2015). This hypothesis would rely on the assumption that both Palmiet and other palmiet wetland fynbos species have equal or similar juvenile colonization abilities (Shipley et al. 1989), depending on conditions or challenges to establishment. Evidence to support this hypothesis is provided in Fig. 7 which shows the stability of patches of palmiet-dominated communities over a 60-year period in the Goukou wetland. The Palmiet-dominated patches do not appear to expand nor shrink over this time. Conversely, palmiet patch expansion where there is no competition from other communities seems to be much more rapid (e.g. establishing across a channel) (Fig. 5). From the change in the small patches of Palmiet at Jonkershoek over the past 13 years (Fig. 6), it appears that palmiet patches could potentially expand at a rate of as much as 15 m2 per month. It is, however, possible that clonal expansion of palmiet communities into existing fynbos communities, or vice versa, take place on much greater timescales than can be captured by aerial photographic history. If this were the case, this would have important consequences for restoration using the species Palmiet.
Aerial photograph time series of the Goukou palmiet wetland from 1954–2014. The green region in the map of South Africa represents the Cape Floristic Region. Arrows represent four large palmiet patches which tend to be lighter than the adjacent fynbos communities. The darkest areas on the photographs are invasive alien trees, invading tributaries and the periphery of the wetland
Implications for wetland restoration/rehabilitation
Several restoration programmes seek to use Palmiet as a pioneer species in replanting efforts on the basis that it is an ecosystem engineer, and therefore would be able to colonize a degraded river or wetland system, creating habitat for other species to follow at a later stage. However, only one of our three hypotheses suggest that Palmiet would be appropriate for use as a pioneer (Hypothesis 2), and one hypothesis implies that it may be appropriate in certain conditions (Hypothesis 3). It is also possible that all three hypotheses hold in certain circumstances, and do not in others. For example, fynbos species may be superior juvenile competitors in palmiet wetlands after severe fire, where there is still alluvium to colonize, whereas Palmiet may be a superior competitor where there is an open body of water to colonize, or a water-filled gully. Either way it seems clear that palmiet expanding from existing patches with healthy root beds, or ‘islands’, into open water takes place rapidly, whereas palmiet recruiting from seedlings takes place much more slowly, which is the opposite of some other dominant wetland macrophytes (Terer et al. 2014).
This has critical implications for restoration activities. Firstly, perhaps palmiet may not be the best species to use for initial stages of palmiet wetland restoration compared to other fynbos wetland species. More research is needed on the efficacy of different palmiet wetland species in rehabilitation efforts in cases where rapid growth and ground cover is needed. Secondly, if palmiet is planted, it may experience better establishment and higher survival if planted in patches, rather than individually. Further research is needed into these dynamics as well as the long-term impacts of groundwater level fluctuations and the influence of fire, and their interactions. Furthermore, these three hypotheses remain to be interrogated from a geomorphological and hydrological perspective. An interdisciplinary approach combining ecology, hydrology and geomorphology is recommended.
Conclusion
Soil pH and relative groundwater depth were two key environmental parameters that were correlated with plant community assembly in palmiet wetlands. Long-term monitoring is needed to understand the relationship between relative groundwater depth and plant community assembly in palmiet wetlands. Palmiet communities were characterized by higher community weighted means for stem diameter, leaf length–width ratio, leaf area as well as cellulose and lignin concentration. These suggest adaptations to fire (thicker stems—fire retardants) and floods (long, thin leaves, flexible shoots and thicker stems). We propose three hypotheses to explain the presence of patchiness in palmiet wetland vegetation. The hypotheses shed light on palmiet restoration strategies and highlight gaps for future research.
Data availability
Data will be made available upon acceptance.
Code availability
Code will be made available upon acceptance.
References
Barclay A (2016) Ecosystem engineering by the wetland plant palmiet: does it control fluvial form and promote diffuse flow in steep-sided valleys of the Cape Fold Mountains. MSc Dissertation. Rhodes University, Grahamstown
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biogeochem 29:1023–1032
Boucher C, Withers M (2004) Palmiet: Prionium serratum a Cape river plant. Veld & Flora 90:26–28
Boutin C, Keddy PA (1993) A functional classification of wetland plants. J Veg Sci 4:591–600. https://doi.org/10.2307/3236124
Brag H (1972) The influence of potassium on the transpiration rate and stomatal opening in Triticum aestivum and Pisum sativum. Physilogia Plant 26:250–257
Brown IC (1943) A rapid method of determining exchangable hydrogen and total exchangable bases of soils. Soil Sci 56:353–357
Brown AG, Tooth S, Bullard JE, Thomas DSG, Chiverrell RC, Plater AJ, Murton J, Thorndycraft VR, Tarolli P, Rose J, Wainwright J, Downs P, Aalto R (2017) The geomorphology of the Anthropocene: emergence, status and implications. Earth Surf Process Landf 42:71–90. https://doi.org/10.1002/esp.3943
Catford JA, Jansson R (2014) Drowned, buried and carried away: effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytol 204:19–36. https://doi.org/10.1111/nph.12951
Clement B, Proctor MCF (2009) Ecological dynamics I: vegetation as bioindicators and dynamic community. In: Maltby E, Barker T (eds) The wetlands handbook, 1st edn. Wiley, Oxford, pp 282–303
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681. https://doi.org/10.1071/FP09144
Gibling MR, Davies NS, Falcon-Lang HJ, Bashforth AR, DiMichele WA, Rygel MC, Ielpi A (2014) Palaeozoic co-evolution of rivers and vegetation: a synthesis of current knowledge. Proc Geol Assoc 125:524–533. https://doi.org/10.1016/j.pgeola.2013.12.003
Gleason HA (1917) The structure and development of the plant assosciation. Torrey Bot Club Bull 44:463–481
Grenfell SE, Ellery WN, Grenfell MC, Ramsay LF, Fluegel TJ (2010) Sedimentary facies and geomorphic evolution of a blocked-valley lake: lake Futululu, northern Kwazulu-Natal, South Africa. Sedimentology 57:1159–1174
Grenfell SE, Mamphoka MF, Grenfell MC, Job N (2020) Evaluating the potential for natural ecosystem recovery in cut-and-fill wetlands: case study of Pietersielieskloof palmiet wetland, South Africa. Wetl Ecol Manag 28:863–882. https://doi.org/10.1007/s11273-020-09756-7
Grime J (1998) Benefits of plant diversity to ecosystems immediate, filter and founder effects. J Ecol 86:902–910
Houba R, Van der Lee JJ, Novozamsky I, Wallinga I (1989) Soil and plant analysis, a series of syllabi. Part 5. Wageningen Agricultural University, Netherlands
Jackman RH, Black CA (1951) Solubility of iron, aluminium, calcium, and magnesium inositol phosphates at different pH values. Soil Sci 72:179–186
Job N (2014) Geomorphic origin and dynamics of deep, peat-filled, valley bottom wetlands dominated by palmiet (Prionium serratum)—a case study based on the Goukou Wetland, Western Cape. MSc Dissertation, Rhodes University, Eastern Cape
Kotze DC (2015) A survey of organic soils in the upper Riviersonderend catchment. World Wildlife Fund Report, South Africa
Low AB, Rebelo AG (1996) Vegetation of South Africa, Lesotho and Swaziland. National Biodiversity Institute, Pretoria
Lucas RE, Davis JF (1961) Relationships between pH values of organic soils and availabilities of 12 plant nutrients. Soil Sci 92:177–182. https://doi.org/10.1097/00010694-196109000-00005
Martens R (1995) Current methods for measuring microbial biomass C in soil: potentials and limitations. Biol Fertil Soils 19:87–99
Maurin O, Davies TJ, Burrows JE, Daru BH, Yessoufou K, Muasya AM, van der Bank M, Bond WJ (2014) Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytol 204:201–214. https://doi.org/10.1111/nph.12936
Middleton BJ, Bailey AK (2008) Water resources of South Africa 2005 study (WR2005). Water Research Commission, Pretoria, South Africa
Midgley GF, Hannah L, Millar D, Thuiller W, Booth A (2003) Developing regional and species-level assessments of climate change impacts on biodiversity in the Cape Floristic Region. Biol Conserv 112:87–97. https://doi.org/10.1016/S0006-3207(02)00414-7
Mitsch WJ, Gosselink JG (2015) Wetland vegetation and succession. Wetlands. Wiley, New Jersey, pp 215–258
Moor H, Rydin H, Hylander K, Nilsson MB, Lindborg R, Norberg J (2017) Towards a trait-based ecology of wetland vegetation. Int J Lab Hematol. https://doi.org/10.1111/ijlh.12426
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New Jersey, pp 93–135
Niering WA (1989) Wetland vegetation development. In: Majumdar S, Brooks R, Brenner F, Tiner J (eds) Wetlands ecology and conservation: emphasis in Pennsylvania. Pennsylvania Academy of Science, Easton, pp 103–113
Nsor AC (2007) Plant community distribution and diversity, and threats to vegetation of the Kromme River Peat Basins, Eastern Cape Province, South Africa. MSc Dissertation. Rhodes University, South Africa
Odum EP (1969) The strategy of ecosystem development. Science 164:262–270
Onoda Y, Westoby M, Adler PB, Choong AMF, Clissold FJ, Cornelissen JHC, Díaz S, Dominy NJ, Elgart A, Enrico L, Fine PVA, Howard JJ, Jalili A, Kitajima K, Kurokawa H, McArthur C, Lucas PW, Markesteijn L, Pérez-Harguindeguy N, Poorter L, Richards L, Santiago LS, Sosinski EE, Van Bael SA, Warton DI, Wright IJ, Joseph Wright S, Yamashita N (2011) Global patterns of leaf mechanical properties. Ecol Lett 14:301–312. https://doi.org/10.1111/j.1461-0248.2010.01582.x
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, De VAC, Buchmann N, Funes G, Hodgson JG, Thompson K, Morgan HD, Steege H, Van Der HMGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Pulley S, Ellery WN, Lagesse JV, Schlegel PK, McNamara SJ (2018) Gully erosion as a mechanism for wetland formation: an examination of two contrasting landscapes. L Degrad Dev 29:1756–1767
Rebelo AG (2001) SASOL proteas: a field guide to the proteas of South Africa. Fernwood Press (Pty) Ltd, South Africa
Rebelo AJ (2012) An ecological and hydrological evaluation of the effects of restoration on ecosystem services in the Kromme River system, South Africa. MSc Dissertation, Stellenbosch University, Western Cape
Rebelo AJ (2017) Ecosystem services of palmiet wetlands: the role of ecosystem composition & function. Doctoral Thesis, University of Antwerp
Rebelo AJ, Le Maitre DC, Esler KJ, Cowling RM (2015) Hydrological responses of a valley-bottom wetland to land-use/land-cover change in a South African catchment: making a case for wetland restoration. Restor Ecol 23:829–841. https://doi.org/10.1111/rec.12251
Rebelo AJ, Morris C, Meire P, Esler KJ (2019) Ecosystem services provided by South African palmiet wetlands: a case for investment in strategic water source areas. Ecol Indic 101:71–80. https://doi.org/10.1016/j.ecolind.2018.12.043
Rebelo AJ, Jarmain C, Esler KJ, Cowling RM, Le Maitre DC (2020) Water-use characteristics of palmiet (Prionium serratum), an endemic south African wetland plant. WSA 46:558–572. https://doi.org/10.17159/wsa/2020.v46.i4.9069
Reinecke MK (2013) Links between riparian vegetation and flow. PhD Dissertation. Stellenbosch University, South Africa
Sale PF (1977) Maintenence of high diversity in coral reef fish communities. Am Nat 111:337–359
Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E (2010) Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytol 186:385–391. https://doi.org/10.1111/j.1469-8137.2009.03176.x
Shipley B, Keddy PA, Moore DR, Lemky K (1989) Regeneration and establishment strategies of emergent macrophtyes. J Ecol 77:1093–1110
Sieben EJJ (2012) Plant functional composition and ecosystem properties: the case of peatlands in South Africa. Plant Ecol 213:809–820. https://doi.org/10.1007/s11258-012-0043-3
Sieben EJJ, Glen RP, Muasya AM (2017a) The wetland flora of South Africa: occurrence patterns, frequency and dominance across a diverse landscape. Aquat Bot. https://doi.org/10.1016/j.aquabot.2017.03.003
Sieben EJJ, Khubeka SP, Sithole S, Job NM, Kotze DC (2017b) The classification of wetlands: integration of top-down and bottom-up approaches and their significance for ecosystem service determination. Wetl Ecol Manag. https://doi.org/10.1007/s11273-017-9585-4
Sieben EJJ, Kotze DC, Job NM, Muasya AM (2017c) The sclerophyllous wetlands on quartzite substrates in South Africa: floristic description, classification and explanatory environmental factors. South African J Bot 113:54–61. https://doi.org/10.1016/j.sajb.2017.07.008
Suding KN, Gross KL, Houseman GR (2004) Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol 19:46–53
Terer T, Muasya AM, Higgins S, Gaudet JJ, Triest L (2014) Importance of seedling recruitment for regeneration and maintaining genetic diversity of Cyperus papyrus during drawdown in Lake Naivasha, Kenya. Aquat Bot 116:93–102. https://doi.org/10.1016/j.aquabot.2014.02.008
Tooth S (2018) The geomorphology of wetlands in drylands: resilience, nonresilience, or …? Geomorphology 305:33–48. https://doi.org/10.1016/j.geomorph.2017.10.017
Tooth S, McCarthy TS (2007) Wetlands in drylands: geomorphological and sedimentological characteristics, with emphasis on examples from southern Africa. Prog Phys Geogr 31:3–41. https://doi.org/10.1177/0309133307073879
Trabucco A, Zomer R (2018) Global aridity index and potential evapotranspiration (ET0) climate database v2.CGIAR consortium for spatial information (CGIAR-CSI). https://cgiarcsi.community. Accessed 10 Nov 2021
Walinga I, Van Vark W, Houba VJG, Van Der Lee JJ (1989) Plant analysis procedures. Soil and plant analysis. Part 7. Wageningen Agricultural University, NL
Warton DI, Wright TW, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101
Wierda A, Fresco L, Grootjans A, Van Diggelen R (1997) Numerical assessment of plant species as indicators of the groundwater regime. J Veg Sci 8:707–716
Wisheu IC, Keddy PA (1992) Competitive and centrifugal organization of plant communities: theory & tests. J Veg Sci 3:147–156
Acknowledgements
A.J.R. gratefully acknowledges the following organizations for funding: The Erasmus Mundus Programme (European Commission), Research Fund of the University of Antwerp (Belgium) and the National Research Foundation (South Africa). KJE acknowledges support by the National Research Foundation of South Africa (Grant Number 103841). Thank you to Byron-Mahieu van der Linde for assistance in the field. Research was conducted under permit number: 0052-AAA008-00019 (CapeNature).
Funding
Partial financial support was received from the Erasmus Mundus Programme (European Commission), the PhD Research Fund of the University of Antwerp (Belgium), an international PhD Scholarship through the National Research Foundation (South Africa) and the National Research Foundation of South Africa (Grant Number 103841).
Author information
Authors and Affiliations
Contributions
AJR, KJE, PM conceived the study, AJR performed data analysis, AJR wrote the manuscript and KJE, PM, ES, contributed and edited it.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table
6,
7,
8,
9,
10.
See Figure
Detrended correspondence analysis (DCA) of the plant communities in fynbos and palmiet patches in three South African palmiet wetlands sampled in March 2015. Fynbos sites are in orange, palmiet in green. Symbols: ▲ Theewaterskloof, ■ Goukou, ● Kromme. Species names are given in black, and + indicates species with a lower abundance that are masked by other labels. Parameters that were interesting or significantly different (bold) between palmiet and fynbos wetland patches were overlain and are indicated by the arrows. CWM community weighted mean, LLWR leaf length–width ratio, Lig lignin, Cel cellulose, SD stem diameter, LA leaf area, Si silicon, No_sp number of species, Veg.K K in vegetation, Veg.Mg Mg in vegetation, MicrobialC microbial carbon, CEC cation exchange capacity, SWC soil water content. Stippled circles encompass sites from fynbos and palmiet communities. Soil parameters are in brown, vegetation composition in green, functional diversity indices in blue. For full species names see Table 9
8.
Rights and permissions
About this article
Cite this article
Rebelo, A.J., Sieben, E., Meire, P. et al. What drives patchiness in palmiet wetlands?. Wetlands Ecol Manage 30, 785–811 (2022). https://doi.org/10.1007/s11273-021-09853-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-021-09853-1