Abstract
This study investigated the potential for increased aqueous metals loading due to induced flushing events following removal of beaver dams created on a net alkaline mine drainage (MD) impacted stream. The study reach of an unnamed tributary (UT) to Tar Creek (Ottawa County, Oklahoma, USA) is approximately 1.6 km and has been negatively impacted by two continuous sources of MD containing elevated concentrations of cadmium (Cd), iron (Fe), lead (Pb), and zinc (Zn). The most upstream source is the start of the study reach and the second source is located midway, approximately 0.6 km downstream. The anthropogenic removal of beaver dams resulted in increased aqueous concentrations of Fe and Cd downstream, attributed to the mobilization of metal precipitates stored in the beaver-created wetlands, and loss of the wetland’s capability to retain metals due to deceased storage volumes and retention times. The majority of mobilized Fe (55 kg) occurred at the most upstream wetland. This study showed that the removal of beaver dams on a MD-impacted stream may mobilize metals, but that the mobilized mass load of each metal of interest represented a small proportion of the masses of metals retained in wetlands created by beaver. In abandoned mining areas where beaver-created wetlands represent natural infrastructure addressing water quality degradation, human-induced or storm-event driven destruction would likely have little long-term influence if the beaver dams are rebuilt and can quickly re-establish the wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Castor canadensis, the North American Beaver, are known as ecosystem engineers due to the wetlands created from beaver activity in surface waters (Naiman et al. 1986; Snodgrass and Meffe 1998; Butler and Malanson 2005; Andersen and Shafroth 2010; Hardisky 2011; Law et al. 2016; Puttock et al. 2017). Beaver dams and the resulting wetlands created from the construction of these dams can (1) alter stream channel geomorphology, (2) establish and maintain wetland environments, (3) increase retention of sediments, and (4) influence downstream transportation of water and other materials (Hillman 1998). The physical and chemical processes that occur in these beaver created wetlands also promote metal removal when beaver colonize mine drainage (MD) impacted streams (Dittrich et al. 2020; Shepherd and Nairn 2020).
Mine drainage occurs when mining operations expose the surfaces of geological materials where chemical processes such as dissolution, oxidation, hydrolysis, and precipitation occur (Nordstrom and Alpers 1999). The contaminated water from these mining operations often contain elevated concentrations of metals (e.g., cadmium [Cd], iron [Fe], lead [Pb], and zinc [Zn]), sulfate, and acidity (Younger et al. 2002; Nairn et al. 2009). In underground mining scenarios, MD may surface as artesian flowing discharges negatively impacting receiving water bodies and the ecosystems that rely on them (Taylor et al. 2005; Zalack et al. 2010; Hogsden and Harding 2012; Williams and Turner 2015). One of the most common impacts from MD is lotic habitat loss due to accumulation of metal hydroxide precipitates on the substrate of the water bodies (Letterman and Mitsch 1978; Scullion and Edwards 1980; Zalack et al. 2010; Hogsden and Harding 2012).
Shepherd and Nairn (2020) showed that beaver may be assisting in the treatment of MD through the creation of wetlands behind the constructed dams. One of the beaver-created wetlands in that study (which was conducted on the same stream described herein) with the greatest inflow concentrations of Cd, Fe, Pb, and Zn, showed significant decreases in total metals concentrations in the outflow for net alkaline MD (Table 1). Net alkaline MD occurs when adequate total alkalinity is present in the water due to dissolution of carbonate host rock which exceeds total acidity represented by proton acidity (pH), carbonic acid acidity, and mineral acidity manifested through the oxidation and hydrolysis of dissolved metals (Hedin et al. 1994; Watzlaf et al. 2004; Skousen et al. 2017). In addition, another recent study found similar results in water bodies impacted by net acidic MD (Dittrich et al. 2020). The study analyzed 64 beaver ponds separated into eight separate strands and found a mean decrease in Fe concentrations of 22.5%, while pH increased by an average of 23.5% (Dittrich et al. 2020).
Despite the provision by beaver created wetlands of many ecosystem services, including water quality improvement, beaver dams are not permanent structures. Beaver dam failure can be caused by a variety of factors such as abandoned and weakened dams, storm events, and active dam removal by humans (Stock and Schlosser 1991; Butler and Malanson 2005; Rosell et al. 2005; Andersen and Shafroth 2010). Beaver dams may be abandoned due to a reduction in food supply, or if the beaver have been removed by humans (Rosell et al. 2005). When dams are abandoned, the slow erosion caused by streamflow will begin to dislodge the building materials, making the dams more likely to fail. A study conducted by Andersen and Shafroth (2010) investigated the impacts of pulse floods on beaver dam structural integrity. The study was conducted at a location where beaver had constructed dams immediately downstream of a man-made dam on a perennial stream with a base flow of 2.6 m3/sec. The man-made dam allowed the research team to release known pulsed volumes of water towards the beaver dams to test their resilience based on dam activity, construction material, and dam height. It was found that herbaceous dams, consisting primarily of Typha spp. (cattails), failed even under the smallest releases of water with a peak discharge of 37 m3/sec. Conversely, the dams constructed with larger woody debris showed minimal damage during the largest pulses of 65 m3/sec peak discharge. Due to the minimal amount of damage, active dams constructed of large woody debris are usually repaired by beaver following any damage (Andersen and Shafroth 2010).
Regardless of the reasons that dams may fail, the rapid release of the stored water may have a drastic impact on both the aquatic community and humans. Stock and Schlosser (1991) found that beaver dam failure caused a 90% decrease in benthic macroinvertebrate density immediately after failure. 60 days later, the benthic macroinvertebrate population experienced a 62% re-establishment. With respect to humans, Butler and Malanson (2005) cited seven articles from 1984 to 1999 stating that beaver dam failures resulted in thirteen deaths and numerous injuries. The most notable of the article is from 1984 where failed dams produced a pulse of water damaging a railroad embankment, causing an Amtrak passenger car to derail, resulting in five deaths (Butler and Malanson 2005). Hillman (1998) reported that a beaver dam failure on a second-order stream resulted in peak flows 3.5 times the maximum discharge recorded on that stream over the previous 23 years.
Knowledge and subsequent literature gaps exist in the investigation of water quality impacts due to the failure of beaver dams, specifically with respect to the potential for metals remobilization. The capacity of beaver-created wetlands to retain metal contaminants under extreme hydrologic conditions, simulated by manual destruction of dams, was unknown prior to this study. The objective of this study was to investigate the potential for remobilization of the captured metals in the sediments behind beaver dams located on a net alkaline MD impacted stream in the event the dams are destroyed. It was hypothesized that total metals concentrations in the stream waters would initially increase with the increase in stream velocity after dam removal but would return to original concentrations as velocities decreased. Two related flushing event studies were conducted: (1) a sequential dam removal experiment where all beaver dams on a stream were destroyed sequentially from downstream to upstream and (2) a single dam removal with extended water quality and quantity monitoring.
Methods
Site description
The unnamed tributary (UT) to Tar Creek is located near Commerce, OK, USA and has been impacted by net alkaline MD from abandoned Pb and Zn mining operations in the Tri-State Mining District. The studied stream reach was approximately 1.6 stream km, and according to the National Wetlands Inventory (NWI), the dominant wetland type, comprising 83% of the area, is classified as a seasonally flooded palustrine system with persistent emergent vegetation. Other wetland types include palustrine forested systems with broad-leaved deciduous trees (16%), and riverine streambed systems (1%), likely from the presence of incised channels along the study reach (Walker 2007; USFWS 2020). However, the NWI classification at the study site was last updated in 2001, while beaver recolonization occurred in late 2013 and into 2014. Since beaver recolonization, an updated NWI classification would likely include the palustrine modifier for beaver activity, with some areas considered continuously saturated.”
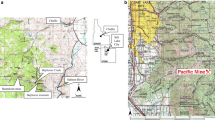
The Center for Restoration of Ecosystems and Watersheds (CREW) at the University of Oklahoma conducts regular water quality sampling events on the UT and the surrounding MD sources in the Tar Creek Superfund Site. All historical water quality data for this research were provided by CREW (Nairn et al. 2012a, b; Nairn 2019). UT receives two continuous MD inputs before it enters Tar Creek, the Southeast Commerce site (SEC) and the Mayer Ranch passive treatment system (MRPTS) (Fig. 1). The most upstream source of MD (SEC) located at the upper end of the study reach, discharged mean total metals concentrations of 133 mg Fe /L, 9.71 mg/L Zn, 0.063 mg/L Pb, 0.031 mg/L Cd, and 0.037 mg/L As, with a flow rate of approximately 375 L/min. The mean total metals values were developed from sixty sampling events conducted over 12 years. The second discharge is located approximately 0.6 stream km downstream, discharging approximately 420 L/min and has been captured and treated using passive treatment technologies since 2008 (Nairn et al. 2009), diluting the untreated MD flowing from the first source to the end of the study reach. Passive treatment is the utilization of ecologically engineered ecosystems to promote physical, biogeochemical, and microbiological processes to remove metals and generate alkalinity (Hedin et al. 1994; Nairn et al. 2009, 2020; Skousen et al. 2017). The mean total metals concentrations for 51 samples of the MRPTS effluent were 0.65 mg Fe /L, 0.46 mg Zn/L, with As, Cd, and Pb below practical quantitation limits of 0.019 mg/L, 0.0007 mg/L, and 0.017 mg/L respectively. In 2013, the presence of beaver on the UT was first noted by the authors, with most of the stream being altered by beaver dams by the end of 2014. Figure 1 shows the locations of the untreated MD discharge from SEC to UT, the MRPTS effluent, and four of eleven beaver dams that were included in this study.
Aerial image of an unnamed tributary to Tar Creek, located in Commerce, OK, USA, showing the sampling locations of destroyed beaver dams and the inflow sources of MD from the untreated SEC discharge and the treated effluent from the MRPTS (Google Earth 2017)
Flushing event sampling and analysis
Manual destruction of beaver dams during base flow conditions led to induced flushing events causing a rapidly flowing pulse of water to move downstream. Four flushing events were conducted in August 2016, and a single event was conducted in January 2017. The manual destruction of dams began at the most downstream dam (BD4) and proceeded to each dam sequentially upstream. Every dam was razed to a base elevation consistent with the upstream bed elevation which was determined to be unaffected by materials accumulated behind the dam. During the destruction events, many additional dams which were originally submerged were discovered, because more recently completed downstream dams had created higher water surface elevations. The newly identified dams were also destroyed, but water samples were not collected due to time constraints; the entire stream needed to be sampled on the same day due to the likelihood of beaver dam reconstruction overnight. In total, eleven dams were destroyed, with water samples collected at four of the eleven sites.
At each water sampling location after dam removal, three time-discrete grab sampling events were conducted. Samples for dissolved metals analyses were filtered on-site immediately following sample collection, using a 0.45-micron filter made of polyethersulfone. Samples for both total (unfiltered) and dissolved metals analyses were then acidified for preservation with trace metal nitric acid. The first metals sample was collected at 5 min post-destruction. Additional grab samples were collected at 35 and 65 min after each dam was destroyed.
The extended monitoring study consisted of a second dam removal event that occurred after beaver rebuilt the dam at the same location as BD1. Extended monitoring included the installation of a flow measuring device (Sontek Argonaut Acoustic Doppler Velocimeter), a recording multiparameter water quality datasonde (YSI 6920 V2), and an autosampler (Sigma 900 Max Portable Sampler). All units were setup at the selected location before the dam was removed. An initial total metals sample was collected prior to dam removal represented at t = 0, while the multiparameter datasonde and flow measuring device did not begin recording values until after dam removal. The datasonde measured pH, dissolved oxygen (DO), specific conductance, temperature, and pH. The flow-measuring device averaged 5 min of continuous flow measurements on 30-min intervals, and the autosampler was programmed to collect grab samples every 30 min for 12 h. The collected water samples were analyzed for total metals and resulting concentrations were plotted to examine relationships with water velocity and remobilization of metals during the destruction event.
Although an autosampler was set to collect samples every 30 min for 12 h, after 6 h of sampling, water levels decreased below the intake line of the autosampler, approximately 8 cm from the stream bottom. The recording multiparameter datasonde began to show signs of the sensors being out of the water column at 2 and 1.5 h. The sensors were determined to be out of the water when the specific conductivity dropped from 2.8 to 0.03 mS/cm.
Samples for aqueous total and dissolved metals determinations were first hot nitric acid microwave digested following USEPA SW-846 Method 3015a (USEPA 2014). These digested samples were analyzed following USEPA SW-846 Method 6010c using a Varian Vista-PRO simultaneous axial inductively coupled plasma-optical emission spectrometer (ICP-OES) (USEPA 2014).
Physical stream measurements
Stream cross-sections were measured before and after removal of beaver dams. The stream cross-sections were measured every 30 m for the entire length of the 1.6-km study reach. Assuming base flow conditions, the cross-section data and the average flow rates of the two MD discharges were used to determine approximate water, surface areas (1), volumes (2), and hydraulic retention times (HRT) (3) of each beaver impoundment before and after dam removal.
The estimated volume of water behind each beaver dam was calculated from the difference in water depth and width from the cross-sections generated before and after beaver dam removal. This estimated volume was then multiplied by the mean total metals concentrations to calculate the mass of metals mobilized.
Results and discussion
Beaver-created wetlands on the UT were dominated by a mix of emergent (Typha latifolia and Juncus effuses) and floating (Potamogeton natans) herbaceous vegetation with stands of small trees (Salix nigra) dominant in riparian edges. Hydric indicators were not found in soils due to the effects of disturbance by mine tailings and precipitation of orange-red iron oxyhydroxides from the mine water. Although the UT likely supported beaver-created wetlands in the past, it had undergone substantial physical, chemical, and biological degradation due to mining impacts for most of the twentieth century.
Measurements of stream physical attributes before and after beaver dam removals documented changes in wetland condition due to beaver activity. Portions of the UT influenced by beaver activity showed wetted surface area increases of nearly 300% (Table 2), converting the flowing stream into a series of natural treatment wetlands with low velocity flow. Data collected from the cross-sections at one of the larger dams, BD1, showed the portion of stream affected by the beaver-created wetland increased the HRT by over 600% and surface area by over 350% (Table 2).
Sequential dam removal
Total and dissolved Fe concentrations increased from 5 to 65 min after dam removal, while total and dissolved Zn and Pb concentrations did not show consistent trends (Table 3). At BD1, total and dissolved Zn concentrations decreased with respect to time after dam removal, while at BD3 and BD4, they remained unchanged over the 1-hour sampling period (Table 3). Pb concentrations showed a slight increase in total and dissolved fractions over time at BD1 but remained unchanged at the three remaining dams during sequential destruction events (Table 3).
During the sequential dam removal process, attempts were made to collect water quality data on as many beaver dam locations as possible in a single day to limit potential alterations due to environmental factors if the sampling was extended over numerous days (e.g., rapid dam rebuilding by beaver). However, collecting only three grab samples per dam over a 1-hour period did not allow for development of meaningful long-term trends. In some instances, the sequential dam removal events showed increasing total and dissolved concentrations of Fe and Cd (Table 3). Fe and Cd were expected to have similar trends since Cd has been shown to sorb to Fe oxides in previous studies (Carroll et al. 1998). However, the remobilization of Pb and Zn was inconclusive due to inconsistent trends among each of the four dam locations (Table 3). The lack of consistent trends for Pb and Zn was attributed to the minimal changes in Pb and Zn aqueous concentrations at the inflow and outflow of the dams when the dams were intact, leading to lesser Pb and Zn accumulation in the stream sediments of the beaver created wetlands (Table 1).
Destruction of BD1 showed greater changes in metals concentrations than those seen at the remaining dams due to its location upstream of the treated MD effluent from MRPTS. The treated effluent from MRTPS dilutes the untreated MD from SEC at the downstream dams (BD2, BD3, and BD4). At BD1, Fe precipitates were visible on the bottom of the stream. However, particulate Fe accounted for a small proportion of the increase in total Fe concentrations compared to the dissolved fraction (Table 3). The dissolved fraction of Fe at BD1 showed an increasing trend with respect to time, initially accounting for 65% of total Fe and increasing to 85% an hour later. Similarly, 1 h after the removal of BD1, dissolved Cd account for 81% of the total Cd concentration. The increase in dissolved Fe and Cd at BD1 is likely due to the decrease in HRT, resulting in insufficient time to retain Fe and enabling the remobilization of reduced sediments.
The mean total metals concentrations are likely an underestimate because Fe (at BD1, BD2, BD3 and BD4) and Cd (at BD1) concentrations after 1 h showed increasing trends with no indication of reaching steady-state conditions (Table 3 However, the subsequent calculations of the mass of metals mobilized may provide modest overestimations because they did not account for metals flux through each dam at base flow in a 1-hour period. Greater than 98% of the mobilized metal mass occurred upstream of MRPTS effluent, where BD1 contributed 55 kg of the 56 kg total mobilized Fe mass (Table 4).
A separate study conducted on these dams found that the Fe removal rate behind BD1 was approximately 4.12 g Fe/m2/day (Shepherd and Nairn 2020). Using the surface area of this impoundment (2990 m2), it was calculated that the BD1 impoundment retained approximately 12.3 kg of Fe/day. Therefore, the mobilized mass of Fe at BD1 represents approximately 4.5 days of Fe retention. However, since the dams were destroyed beginning downstream, the study does not address how far downstream the metals remained mobilized. The downstream distance that the metals may have been transported depends on many factors, including if the dams were anthropogenically destroyed, as they were in this study, or if the dams were washed out by a single high flow event, and how quickly dams may have been reconstructed by beaver. Fletcher et al. (2019) found headwater streams with beaver dams would accumulate trace metals in the sediments, but after a record rainy period, the trace metals were remobilized causing increased metals concentrations downstream in the sediment and biota.
Single dam removal event with extended monitoring
After completing the sequential dam removal in August 2016, the dam at BD1 was rebuilt by beaver. Therefore, BD1 was used to further evaluate a single dam removal event in January 2017. BD1 received the greatest aqueous metals concentrations due to the untreated MD from SEC and therefore was most likely to show noticeable trends. By destroying a single dam and collecting data over an extended time period, relationships between changing velocities and metals concentrations were able to be examined.
Similar to the sequential dam removal event, Pb and Zn concentrations did not change over the 6-hour period during which samples were collected (Table 5). Fe and Cd concentrations increased significantly from the 30 min to 120 min time period, then increased at a decreasing rate between the second and fifth hours (Fig. 2). Based on the findings of the sequential dam removal at BD1, the increasing Fe and Cd concentrations over the 6-hour sampling period were expected to be primarily in the dissolved form, largely attributed to HRT decreases and remobilization of reduced sediments. The turbidity data collected during the event provide evidence of sediment mobilization, shown by the high turbidity immediately following dam removal (Table 5).
The effect of decreased HRT and surface area on DO was also seen following the removal of BD1. Before MD discharges to the surface, the water is anoxygenic, and all Fe is in the dissolved Fe(II) form. Once MD reaches the surface, most oxygen diffused into the water is immediately consumed to oxidize, hydrolyze and precipitate Fe(III), maintaining low DO for extended periods of time. Over time, the amount of oxygen diffusing into the water will exceed the amount of oxygen consumed during Fe oxidation and thus the measured net DO will increase. This process has been studied and documented in many other MD settings (e.g., Kirby et al. 1999; Dempsey et al. 2001).
At this study site, the untreated MD containing low DO and nearly 100% dissolved Fe(II), entered UT at the inflow of BD1. It is hypothesized that before the removal of BD1, the HRT (68 h) and surface area (2990 m2) allowed substantial oxygen diffusion into the water to exceed the amount of oxygen consumed during Fe oxidation. However, despite increased stream velocities following the removal of BD1, the decrease in HRT (11 h) and surface area (850 m2) decreased the amount of oxygen transferred into the water column, resulting in insufficient oxygen to oxidize the majority of the dissolved Fe. DO data support this hypothesis, as the initial DO was 85% saturation 30 min after dam removal, and decreased to 33% saturation 2 h later, indicating insufficient DO diffusion due to shorter HRT (Table 5).
Initial increased water velocities created by the induced flushing event did not correlate to total metals concentrations. Water velocity decreased from initial data collection until it was below the detection limits of the flow sensor at 2 h when the velocities were reported as negative values (Fig. 2).
Although it was necessary to destroy the beaver dams and drain these natural treatment wetlands in order to complete this study, the beaver ensured many of these wetlands were quickly recreated. Beaver began repairing dams shortly after the study was completed. The rapid reconstruction was noted at one of the dams from data collected with a depth sensor that was deployed on the upstream side of one of the destroyed dams, logging values every 15 mins. Not only had the beaver re-established the original water elevation of the wetland within 48 h of completion of the study, the water elevation continued to increase over the next 4 days, plateauing approximately 15 cm above the water elevation measured before the study was conducted (Fig. 3).
Conclusions
Beaver dam failure is well documented in the literature (Stock and Schlosser 1991; Hillman 1998; Butler and Malanson 2005; Rosell et al. 2005; Andersen and Shafroth 2010). It is not a matter of if a beaver dam will fail, it is a matter of when and how. This study investigated anthropogenic beaver dam failure in a MD impacted stream and the resulting potential for accumulated metal precipitate remobilization. Sequential dam removal experiments supported the hypothesis that selected total metals concentrations would increase over the 65-min duration after dam removal. Fe and Cd concentrations showed increasing trends over time, but Zn and Pb concentrations did not show noticeable changes. The increasing Fe and Cd concentrations were attributed to two factors: remobilization of precipitated metals stored in the beaver created wetlands, and decreased metal removal capabilities due to decreased hydraulic retention times after the dams were removed. The hypothesis, which stated the total metals concentrations in the stream waters would initially increase with the increase in stream velocity after dam removal, was rejected because the metals concentrations did not track trends in velocity. The recorded velocities during the single dam removal experiment continually decreased while Fe and Cd concentrations showed increasing trends over the same time period. Pb and Zn concentrations did not show clear trends with respect to remobilization. The lack of consistent trends was attributed to the minimal retention of Pb and Zn when dams were intact (Table 1). The overall findings of this study demonstrate a potential for metals mobilization due to beaver dam removal, but the mass of mobilized metals will likely be a small fraction of the metals retained in beaver created wetlands. In abandoned mining areas where beaver-created wetlands may represent the only natural infrastructure addressing water quality degradation, human-induced or storm-event driven destruction would likely have minimal long-term influence if the beaver dams are rebuilt and can quickly re-establish the wetlands.
Data availability
Data is available upon request from the first author.
Code availability
Not applicable.
References
Andersen DC, Shafroth PB (2010) Beaver dams, hydrological thresholds, and controlled floods as a management tool in a desert riverine ecosystem, Bill Williams River, Arizona. Ecohydrology 3:325–338. https://doi.org/10.1002/eco.113
Butler DR, Malanson GP (2005) The geomorphic influences of beaver dams and failures of beaver dams. Geomorphology 71:48–60. https://doi.org/10.1016/j.geomorph.2004.08.016
Carroll SA, O’Day PA, Piechowski M (1998) Rock-Water interactions controlling zinc, cadmium, and lead concentrations in surface waters and ediments, US Tri-State Mining District. 2. Geochemical interpretation. Environ Sci Technol 32:956–965. https://doi.org/10.1021/es970452k
Dempsey BA, Roscoe HC, Ames R, Hedin R, Jeon B (2001) Ferrous oxidation chemistry in passive abiotic systems for the treatment of mine drainage. Geochem Explor Environ Analysis 1:81–88. https://doi.org/10.1144/geochem.1.1.81
Dittrich PG, Swab RM, Nolan AK (2020) Acid mine drainage treatment by beaver impoundments in southeast Ohio. J Am Soc Mining Reclam 9(3):1–13. https://doi.org/10.21000/JASMR20030001
Fletcher DE, Lindell BE, Lindell AH, Stankus PT, Fletcher ND, McArthur JV, Seaman JC (2019) Basins, beaver ponds, and the storage and redistribution of trace elements in an industrially impacted coastal plain stream on the Savannah River site, SC, USA. Environ Int 133:105174. https://doi.org/10.1016/j.envint.2019.105174
Google Earth 7.3 (2017) Commerce, Oklahoma, USA 36.920864°N, 94.869388°W, elevation 1600m. Available at http://www.google.com/earth/index.html. Accessed 13 Aug 2017
Hardisky T (2011) Beaver management in Pennsylvania. Pennsylvania game commission
Hedin RS, Nairn RW, Kleinmann RLP (1994) Passive treatment of coal mine drainage. US Department of the Interior. Bureau of Mines Information Circular 9389, pp 37
Hillman GR (1998) Flood wave attenuation by a wetland following a beaver dam failure on a second order boreal stream. Wetlands 18(1):21–34. https://doi.org/10.1007/BF03161439
Hogsden KL, Harding JS (2012) Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshwater Science 31:108–120. https://doi.org/10.1899/11-091.1
Kirby CS, Thomas HM, Southam G, Donald R (1999) Relative contributions of abiotic and biological factors in Fe(II) oxidation in mine drainage. Appl Geochem 14:511–530. https://doi.org/10.1016/S0883-2927(98)00071-7
Law A, McLean F, Willby NJ (2016) Habitat engineering by beaver benefits aquatic biodiversity and ecosystem processes in agricultural streams. Freshw Biol 61:486–499. https://doi.org/10.1111/fwb.12721
Letterman RD, Mitsch WJ (1978) Impact of mine drainage on a mountain stream in Pennsylvania. Environ Pollut 17:53–73. https://doi.org/10.1016/0013-9327(78)90055-1
Naiman RJ, Melillo JM, Hobbie JE (1986) Ecosystem alteration of boreal forest streams by beaver (Castor canadensis). Ecology 67(5):1254–1269. https://doi.org/10.2307/1938681
Nairn RW, Beisel T, Thomas RC, LaBar JA, Strevett KA, Fuller D, Strosnider WH, Andrews WJ, Bays J, Knox RC (2009) Challenges in design and construction of a large multi-cell passive treatment system for ferruginous lead-zinc mine waters. J Am Soc Mining Reclam. https://doi.org/10.21000/JASMR09010871
Nairn RW, Strevett KA, and Basara J (2012a) Remediation and restoration monitoring at the Tar Creek superfund site, Oklahoma: source, transport and fate of mine waste contaminants. Final Deliverable to US Geological Survey, DOI-USG 04HQAG0131, pp 11151
Nairn RW, Strevett KA, Knox RC, and Matthews WJ (2012b) Design, construction and evaluation of a passive treatment system for contaminated mine waters at the Tar Creek superfund site. Final Deliverable to US Environmental Protection Agency, FY04 104(b)(3) Project X7-97682001-0, pp 566
Nairn RW (2019) Passive treatment of contaminated mine waters: design, construction, and initial evaluation of the southeast commerce passive treatment system. Oklahoma Department of Environmental Quality Agreement PO2929019163, pp 98
Nairn RW, LaBar JA, Oxenford LR, Shepherd NL, Holzbauer-Schweitzer BK, Arango JG, Tang Z, Dorman DM, Folz CA, McCann JI, Ingendorf JD, Stanfield HT, Knox RC (2020) Toward sustainability of passive treatment in legacy mining watersheds: operational performance and system maintenance. Conference Proc. International Mine Water Association, Christchurch, New Zealand, p 123–128
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the iron mountain superfund site, California. Proc Natl Acad Sci USA 96(7):3455–3462. https://doi.org/10.1073/pnas.96.7.3455
Puttock A, Graham HA, Cunliffe AM, Elliot M, Brazier RE (2017) Eurasian beaver activity increases water storage, attenuates flow and mitigates diffuse pollution from intensively-managed grasslands. Sci Total Environ 576:430–443. https://doi.org/10.1016/j.scitotenv.2016.10.122
Rosell F, Bozser O, Collen P, Parker H (2005) Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mamm Soc 35:248–276. https://doi.org/10.1111/j.1365-2907.2005.00067.x
Scullion J, Edwards RW (1980) The effects of coal industry pollutants on the macroinvertebrate fauna of a small river in the South Wales coalfield. Freshw Biol 10:141–162. https://doi.org/10.1111/j.1365-2427.1980.tb01189.x
Shepherd NL, Nairn RW (2020) Metals retention in a net alkaline mine drainage impacted stream due to the colonization of the North American Beaver (Castor canadensis). Sci Total Environ 731:139203. https://doi.org/10.1016/j.scitotenv.2020.139203
Skousen J, Zipper CE, Rose A, Ziemkiewicz PZ, Nairn RW, McDonald LM, Kleinmann RL (2017) Review of passive treatment systems for acid mine drainage treatment. Mine Water Environ 36:133–215. https://doi.org/10.1007/s10230-016-0417-1
Snodgrass JW, Meffe GK (1998) Influence of beavers on stream fish assemblages: effects of pond age and watershed position. Ecol Soc Am 79:928–942
Stock JD, Schlosser IJ (1991) Short-term effects of a catastrophic beaver dam collapse on a stream fish community. Environ Biol Fishes 31:123–129. https://doi.org/10.1007/BF00001012
Taylor J, Pape S, Murphy N (2005) A summary of passive and active treatment technologies for acid and metalliferous drainage (AMD). Proceedings of Fifth Australian Workshop on Acid Drainage
United States Environmental Protection Agency (2014) Test methods for evaluating solid waste (SW-846), Update version, Revision 8, USEPA
US Fish and Wildlife Service (2020) National Wetlands Inventory website. US Department of the Interior, Fish and Wildlife Service, Washington. http://www.fws.gov/wetlands/. Accessed 28 Dec 2020
Walker K (2007) Stream restoration design of a tributary in the Tar Creek watershed, Ottawa county, Oklahoma. University of Oklahoma Master’s thesis. pp 290
Watzlaf GR, Schroeder KT, Kleinmann RLP, Kairies CL, Nairn RW (2004) The passive treatment of coal mine drainage. Unites States department of energy, national energy technology laboratory. Report DOE/NETL-2004/1202
Williams KM, Turner AM (2015) Acid mine drainage and stream recovery: effects of restoration on water quality, macroinvertebrates, and fish. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2015014
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, remediation. Kluwer Academic Publishers, Dordrecht
Zalack JT, Smucker NJ, Vis ML (2010) Development of a diatom index of biotic integrity for acid mine drainage impacted streams. Ecol Ind 10:287–295. https://doi.org/10.1016/j.ecolind.2009.06.003
Acknowledgements
Field sample collection and beaver dam removal were completed with the assistance of Brandon Holzbauer-Schweitzer (University of Oklahoma Center for Restoration of Ecosystems and Watersheds). The laboratory analyses for this research were conducted at the University of Oklahoma Center for Restoration of Ecosystems and Watersheds, in the School of Civil Engineering and Environmental Science. This research project was supported by the Grand River Dam Authority Ecosystems and Watershed Management Projects 100052 and A15-0240 and Oklahoma Department of Environmental Quality Land Protection Division Agreement PO2929019163.
Funding
Funding for this research was provided by the Oklahoma Department of Environmental Quality (Agreement PO 2929019163 SRA FY15-ORA4-14) and the Grand River Dam Authority (Agreements GRDA 100052 and GRDA A15-0240).
Author information
Authors and Affiliations
Contributions
NLS: Conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, Visualization. RWN: Conceptualization, methodology, validation, resources, data curation, writing—review & editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shepherd, N.L., Nairn, R.W. Induced mobilization of stored metal precipitates from beaver (Castor canadensis) created wetlands on a mine drainage impacted stream. Wetlands Ecol Manage 30, 127–137 (2022). https://doi.org/10.1007/s11273-021-09839-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-021-09839-z