Abstract
Allometric growth reflects different allocation patterns and relationships of different components or traits of a plant and is closely related to ecosystem carbon storage. As an introduced species, the growth and carbon storage of Sonneratia apetala are still unclear. To derive allometric relationships of the mangrove S. apetala and to estimate carbon storage in mangrove ecosystems, we harvested 12 individual Sonneratia apetala trees from four different diameter classes in the Futian National Nature Reserve, Guangdong, China. Allometric growth models were fitted. The results showed that diameter at breast height (DBH) and wood density were better variables for predicting plant biomass (including above- and below-ground biomass) than plant height. There were significant power function relationships between biomass and DBH, with a mean allometric exponent of 2.22, and stem biomass accounted for 97% of the variation in S. apetala total biomass. Nearly isometric scaling relationships were developed between stem biomass and other biomass components. To better understand the carbon stocks of the S. apetala ecosystem, we categorized all trees into five age classes and quantified vegetation carbon storage. The S. apetala vegetation carbon storage ranged from 96.48 to 215.35 Mg C ha−1, and the carbon storage significantly increased with stand age. The allometric equations developed in this study are useful to estimate biomass and carbon storage of S. apetala ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Allometry is the study of the relative growth relationships of biological organs in terms of size and functional traits (Huxley 1932; Damuth 2001). Allometric growth is widely used to study the relationship of the biomass and growth rate of two organs or traits and to investigate the effect of individual size on biological processes (Enquist and Niklas 2002; Niklas 2004; Enquist et al. 2007). An allometric model describes the proportionate relationship between the mass of two organs or components (Huxley 1932) and is often described as Y = bXa, where Y and X are the sizes of the two organs or components, parameter a represents the allometric exponent, and parameter b reflects the allometric constant. Generally, when a = 1, there is an isometric relationship between traits in various parts of an organism, but when a ≠ 1, there is an allometric relationship between them (Huxley 1932; Enquist and Niklas 2002). The allometric relationships between different components of plant biomass have been extensively investigated (e.g., Niklas 2004; Cheng and Niklas 2007). For example, Hui et al. (2014) found that below-ground biomass scales near isometrically with above-ground biomass, and the scaling exponent does not vary with tree age and density but increases with tree size, according to a large forest biomass database. In another biomass allocation study, Enquist and Niklas (2002) proposed that the universal allometric scaling factor of leaf biomass versus stem biomass and leaf biomass versus below-ground biomass for a mature tree is 3/4 and that of stem biomass versus below-ground biomass is 1. However, for non-woody plants and juvenile woody plants, allometric scaling between below- and above-ground biomass is an isometric relationship (Enquist and Niklas 2002).
The relationship between plant diameter at breast height (DBH) and tree height is important to explore plant growth. Three models are proposed to describe the relationship, including (1) the constant stress self-similarity model (Dean and Long 1986), which is considered the 1/2 power (H ∝ DBH1/2) allometric growth relationship between DBH and tree height, (2) the elastic self-similarity model (McMahon 1973; Niklas and Enquist 2002a, b), which states that the power exponent is 2/3 (H ∝ DBH2/3), and (3) the geometric self-similarity model (Norberg 1988), which indicates an isometric relationship (H ∝ DBH). There is no consensus on the value of the allometric exponent, as different studies produced different allometric exponents (West et al. 1999; Komiyama et al. 2008; Paul et al. 2016). Therefore, it is considered that the allometric exponent is not invariable but is influenced by individual size, life-type, environmental factors, and species (Hayward et al. 2010; Hui et al. 2014; Enquist et al. 2017). Nevertheless, it remains unclear whether the allometric exponent of mangrove species is invariable, especially that of the introduced species Sonneratia apetala.
Mangrove forests are distributed mostly in tropical and subtropical areas along coastlines. The total mangrove area is 137,760 km2 and covers 0.1% of Earth’s surface area (Giri et al. 2011; Rivera-Monroy et al. 2017). Mangroves provide important ecological and economic services (Vo et al. 2012). Mangrove ecosystems produce and sequester a considerable amount of carbon compared to terrestrial and other wetland ecosystems and are referred to as an important source of “blue carbon” (Donato et al. 2011; McLeod et al. 2011; Migeot and Imbert 2012; Alongi 2014; Hamilton and Friess 2018). However, the total mangrove area is reduced at a rate between 0.16% and 0.39% per year worldwide due to deforestation, and Southeast Asia has the highest loss rate (Hamilton and Casey 2016). Reducing CO2 emissions from deforestation and forest degradation (REDD +) is proposed as an effective approach for forest conservation through afforestation (UNFCCC 2008). Plantations are a common method for restoring mangrove forests in mainland China (Zan et al. 2003), and S. apetala is the main species for mangrove afforestation.
Sonneratia apetala is a fast-growing, light-demanding, and highly adaptable species that originated in Bangladesh, India, and Sri Lanka (Jayatissa et al. 2002; Peng et al. 2016b). S. apetala was introduced to increase the mangrove area in China. It was first introduced to Dongzhai Port in China from Bangladesh in 1985 and from Dongzhai Port to the Futian National Nature Reserve, Shenzhen, in 1993 (Zan et al. 2003). In order to alleviate the degradation and destruction of mangroves, Kandelia obovata, Sonneratia caseolaris, and S. apetala are widely planted, and the afforestation area of S. apetala exceeds 50% of the total mangrove plantation area (Ren et al. 2009). The growth of S. apetala has attracted wide attention due to its shade intolerance, higher relative growth rate, and lower soil carbon sequestration capability than the same-age native species (Mitra et al. 2012; Peng et al. 2016b; He et al. 2018; Wu et al. 2020). Previous studies focused on the allometric growth of young S. apetala plantations, mostly those aged 10 years or younger (Ren et al. 2010; Lunstrum and Chen 2014), but the growth mechanisms of S. apetala remain unclear. Accordingly, allometric growth equations and allometric exponents of S. apetala from different diameter classes need to be developed.
In this study, we developed allometric growth equations for S. apetala and quantified the carbon storage of S. apetala vegetation in different age classes. Based on different age classes of S. apetala, we hypothesized that its carbon storage increases with age. In this paper, we aimed to (1) develop allometric relationships between shoot biomass and other components of biomass, (2) construct an appropriate allometric equation of biomass with the DBH and wood density of S. apetala, (3) compare the difference in biomass allocation between S. apetala and other mangrove forests, and (4) assess the dynamic changes of carbon storage of S. apetala in different age classes.
Materials and methods
Study sites
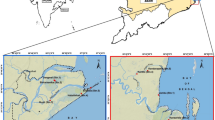
The Futian National Nature Reserve (latitude 22°32′N and longitude 113°45′E) is located in the north of Shenzhen Bay, Shenzhen, Guangdong, China. The reserve is adjacent to the Mai Po Reserve in Hong Kong, which is the smallest mangrove reserve (368 ha) in China. The reserve has a typical subtropical monsoon climate with a mean annual temperature of 22.6 °C and a mean annual precipitation of 1794 mm (Lunstrum and Chen 2014; Yang et al. 2014). The tides in Shenzhen Bay are characterized as irregular semidiurnal tides and are often affected by typhoons from May to October. Species richness of the mangrove community is relatively low in Shenzhen Bay (Ren et al. 2011). The dominant species are K. obovata, Avicennia marina, and Aegiceras corniculatum. Exotic species mainly include S. caseolaris and S. apetala.
Experimental design
In China, S. apetala has been planted for decades; the growth dynamics remain unclear, and the change in its carbon storage in different age classes is also not well understood. In order to better evaluate the growth of S. apetala, we set up the following two experiments in the Futian Natural Reserve.
Experiment 1: Construction of allometric equations to determine the biomass of S. apetala
Tree harvesting was carried out from December 2017 to January 2018. Based on the field investigation, we selected 12 S. apetala individuals from four different diameter classes with similar diameter (mean diameter of 2.97 cm, 11.47 cm, 21.70 cm, and 37.39 cm) and height distribution in the Futian National Nature Reserve, that is, three trees per diameter class (Table S1). Based on the diameter class, trees of similar size were selected randomly. The age of the oldest tree exceeded 20 years. Measurements of DBH, tree height (H), and wood density (p) were undertaken for each selected tree. The average wood density of 12 S. apetala in the Reserve is 0.478 g/cm3. The selected trees were harvested and divided into four components (stem, twigs, leaves, and roots). We excavated the sample trees, with a 1 m radius and up to 2 m in depth, and removed all roots for weighing. The roots included the coarse and fine roots, and their fresh weights were measured, respectively. The total fresh weight of the stems, twigs, leaves, and roots were also measured. A subsample from each component per harvested tree was transported to the laboratory for dry weight measurements following drying in an oven at 80 °C for 72 h until a constant weight was reached. We calculated the dry weight of each sample using the moisture content of the subsample.
The height and wood density of 12 harvested S. apetala trees were correlated with DBH (Fig. S1). The tree height was barely visual in the field survey, and it was difficult to accurately measure the height of the mangrove community; thus, DBH was used to construct allometric equations. Since wood density may be an important determinant of biomass (Chave et al. 2005; Komiyama et al. 2005), we chose DBH and wood density as independent variables for constructing allometric equations for tree biomass in this study. We compared several different DBH and wood density models and selected the best equation for the biomass estimation of S. apetala.
In previous studies, there were more allometric equations for above-ground biomass and fewer equations for below-ground biomass because of the difficulty associated with below-ground sample collection and measurements. In our study, we constructed above- and below-ground biomass allometric equations and compared our results with the allometric equations reported in previous studies.
The below- to above-ground biomass ratios for each S. apetala was calculated and the confidence interval of mean was examined. Then we compared this ratio with the below- to above-ground biomass ratios of other five mangrove species (A. corniculatum, A. marina, Bruguiera gymnorrhiza, K. obovata, and S. caseolaris) published in the literature.
Experiment 2: Assessing the carbon storage in different age classes of S. apetala
According to planting record of the year for S. apetala in the Futian National Nature Reserve, plots of five age classes (13 year, 14 year ,19 year, 21 year, and 26 year) were set up in July 2017 (Fig. S2). Each plot was 10 m × 10 m. There were six plots for the 13-year age class, five plots for the 14-year age class, four plots for the 19-year age class, six plots for the 21-year age class, and four plots for the 26-year age class, that is, a total of 25 plots. At plantation establishment, the stand density of S. apetala was 2000 plants/hm2 in all of the plots. Considering the variation in stand density as plants grow, we first measured the DBH of all mangrove plants in each plot in 2017. In July 2018 and 2019, the DBH of all mangrove plants were re-measured. In order to analyze the growth situation of different age classes of S. apetala, the relative growth rate (RGR) was calculated using the DBH data (Lambers and Poorter 1992). Allometric equations developed above were used to estimate the mangrove biomass. Carbon stocks of the mangrove vegetation were quantified as the vegetation biomass multiplied by the average carbon concentration of all species, that is, 40.5% (Peng et al. 2016a), in 2017, 2018 and 2019.
Data analysis
All of the data were log-transformed to meet the normal distribution requirement before analysis. We calculated the average DBH, average height, and standard deviation (SD) of the 12 harvested trees and tested the significant differences among the four diameter classes using one-way ANOVA followed by least significant difference (LSD) multiple comparisons. Standard major axis (SMA) regressions were used to estimate the allometric constant (a) and exponent (b) using the package “smatr” in R 3.5.0 (Warton et al. 2012).
For the 12 harvested trees of S. apetala in the Futian National Nature Reserve, we constructed allometric equations for the above- and below-ground biomass using DBH and wood density. Before fitting the allometric equations, we examined the relationships between DBH and three biomass components and wood density. Because the data showed heteroscedasticity, natural logarithmic transformations of the data were applied to construct equations. We built additive allometric equations for S. apetala biomass. The allometric equations were developed as follows:
where M represents the biomass of each component, and p is the wood density of each harvested tree. The allometric equation was assessed using the adjusted coefficient of determination (R2), Akaike information criterion (AIC) (Akaike 1974), bias, and root mean square error (RMSE). Adjusted R2 and AIC were used to evaluate model fitness using independent variables. The bias reflected a relative error in the regression function, % error = 100 × (Wpredicted − Wmeasured) /Wmeasured (Chave et al. 2005). ΔW (ΔW = Wpredicted − Wmeasured) was calculated for assessing difference between values of predicted and measured biomass. In view of R2 and model bias, we selected the best model for estimation of biomass of S. apetala.
The ratios of the below- to above-ground biomass of six mangrove plants were analyzed using one-way ANOVA. As the RGR data of different age classes of S. apetala followed a non-normal distribution, the Kruskal–Wallis test was used for comparative analysis. Linear regression analysis was applied to develop relationships between the carbon stocks and five age classes of S. apetala.
Statistical analysis of all data was performed using R 3.5.0 (The R Project for Statistical Computing, www.r-project.org).
Results
Allometric relationships of S. apetala biomass
In S. apetala plantations, the DBH of S. apetala had a strong allometric relationship with its biomass (Fig. 1). The allometric exponent was 2.10 between the DBH and leaf biomass (ML), and the maximum allometric exponent was 2.26 between the DBH and below-ground biomass (MB). The allometric exponent was 2.25 among the DBH and stem biomass (MS), MB, and total biomass (MT). Although the scaling exponents were different, the average was about 2.22 (R2 > 0.88). The allometric exponent was about 2.25 between the DBH and above-ground biomass (MA), MB, and MT, and the coefficient of determination of the equation was greater than 0.97.
Stem biomass was the main biomass component part of S. apetala and accounted for about 97.56% and 81.05% of the variations in the above-ground biomass and total biomass, respectively. Although the allometric exponent of stem biomass and leaf biomass had the smallest value (0.95), the allometric exponent was close to 1 for MS and ML, MA, MB, and MT (Fig. 2). In particular, the allometric exponent was higher than 0.99, which was similar to the isometric growth between ML and MA, MB, and MT.
Bivariate plots between stem biomass and other biomasses of Sonneratia apetala. (a) the allometric relationship between stem biomass (Ms) and leaf biomass (ML), (b) the allometric relationship between stem biomass (Ms) and above-ground biomass (MA), (c) the allometric relationship between stem biomass (Ms) and below-ground biomass (MB), (d) the allometric relationship between stem biomass (Ms) and total biomass (MT). The shaded areas represent the 95% confidence interval
Allometric equations for S. apetala biomass
In this study, we used DBH and wood density to construct allometric equations for the above- and below-ground biomass of S. apetala in the Futian National Nature Reserve (Tables 1, 2 ,3). We found that Eqs. (4) and (5) were better models for above- and below-ground biomass of S. apetala, respectively. Meanwhile, the use of DBH overestimated the total biomass of S. apetala compared to the use of wood density and DBH. Compared to previous allometric equations, we found that DBH and wood density were good predictors for the above- and below-ground biomass of S. apetala in this study (Figs. 3, 4).
The below- to above-ground biomass ratios of S. apetala and other mangrove species
Different mangrove forests had different ratios of below- and above-ground biomass (Fig. 5) (Tam et al. 1995; Khan et al. 2009; Hoque et al. 2011; Pham et al. 2016). The one-way ANOVA results showed that there were significant differences in the ratios between S. apetala and A. marina, K. obovata, and S. caseolaris, but no significant difference between S. apetala and A. corniculatum and B. gymnorrhiza. The average ratio of S. apetala was lower than that of the native species except for B. gymnorrhiza. S. apetala allocated more carbon to above-ground biomass, The ratio of the six mangrove forests ranged from 0.10 to 2.12, with an average of 0.83.
The changes in carbon storage of S. apetala in different age classes
The differences in the RGR of S. apetala between 13 and 14 year, 14 year and 19 year, 19 year and 21 year, and 21 year and 26 year were significant (Fig. 6). S. apetala showed a stable growth overall in the Reserve. We estimated carbon storage using the allometric equations of biomass and RGR. Carbon storage of S. apetala significantly increased with stand age and increased from 2017 to 2019 for each age class (Fig. 7). It is worth mentioning that carbon storage of S. apetala increased rapidly in the 19 year and 21 year age classes from 2017 to 2019. The carbon storage of S. apetala vegetation ranged from 96.48 to 215.35 Mg C ha−1 in the reserve, with an average of 145.59 Mg C ha−1. The stand age effect on the above- and below-ground carbon storage was significant. The mean carbon storage of S. apetala vegetation was 21.61 Mg C ha−1 year−1 across the five age classes.
Discussion
This study systematically analyzed the allometric growth and carbon storage of S. apetala, elucidated the underlying growth mechanism, and provided useful information pertaining to S. apetala plantations.
Allometric growth of S. apetala
The allometric exponent (0.58) between the DBH and height of S. apetala supported the constant stress self-similarity model (Dean and Long 1986) and the elastic self-similarity model (McMahon 1973; Niklas and Enquist 2002a, b), explaining that S. apetala effectively transmitted the wind pressure downward in order to reduce mechanical damage. The taller the S. apetala trees, the larger their diameter, which conformed to the elastic self-similarity model (McMahon 1973). S. apetala can grow up to more than 19 m in the study area, which is similar to the height in the native area. This study showed that the pioneer species S. apetala has a strong adaptability to colonize new environments (Jayatissa et al. 2002; Ren et al. 2009). The average wood density of S. apetala is 0.478 g/cm3 in Shenzhen in China, which is slightly lower than 0.52 g/cm3 in India and 0.537 g/cm3 in South-East Asia (Chave et al. 2009; Zanne et al. 2009). Thus, a change in the wood density of S. apetala may have a limited influence on its biomass.
There were similar allometric exponents between the DBH and the biomass of the different components of S. apetala. The results indicated that the growth of S. apetala conformed to certain models. Stem biomass was the main biomass component of S. apetala, and the proportion of stem to total biomass was higher than 97%. Interestingly, stem biomass was proportional to above-ground biomass, below-ground biomass, and total biomass and showed isometric relationships (Niklas 2004). A 3/4 power function was found between stem biomass and leaf biomass (Enquist and Niklas 2002; Niklas and Enquist 2002a, b), but the relationship between stem biomass and root biomass was isometric, which is consistent with our study.
We constructed allometric equations for S. apetala biomass using DBH and wood density. Wood density is an important trait indicative of mechanical and physiological properties (Swenson and Enquist 2007). Previous studies developed allometric equations with DBH and wood density to determine the growth of mangroves (Chave et al. 2005; Komiyama et al. 2005). In this study, we found that Eq. (4) for the total biomass and above-ground biomass with DBH and wood density had a higher R2 and lower bias compared to other functions, indicating that Eq. (4) was the most suitable estimate of the total biomass and above-ground across different sizes of S. apetala. For the below-ground biomass of S. apetala, Eq. (5) was a better model for the estimation.
Several equations have been developed to estimate biomass for S. apetala. For example, Komiyama et al. (2005)’s model overestimated above-ground biomass and below-ground biomass, and Lunstrum and Chen (2014) reported an underestimate of above-ground biomass in this study, which was influenced by 6-year-old S. apetala forests. For the diameter class larger than 10 cm, the published equations (Chave et al. 2005, 2014; Ren et al. 2010; Lunstrum and Chen 2014) underestimated the above-ground biomass in this study. Based on a comparison of common equations of below-ground biomass, the estimation of Ren et al. (2010) produced the lowest below-ground biomass estimates, which might have been caused by regional environmental differences. The other equations basically overestimated the below-ground biomass (Komiyama et al. 2005; Lunstrum and Chen 2014). The different allometric equations with different estimates of biomass were affected by region, environmental conditions, and age.
Allocation of S. apetala biomass
The ratio of below-ground to above-ground biomass reflects the plant carbon allocation strategy (Wilson 1988). The ratio varied among different mangrove forests (Fig. 4). The average ratio of the mangrove forests was higher than that of upland forests (Cairns et al. 1997), indicating greater carbon allocation to below-ground biomass in the mangrove forests. The ratio of S. apetala was lower than that of other mangrove forests but still higher than that of the upland forests. The greater allocation to above-ground biomass by S. apetala was related to its the biological characteristics of the greater height and DBH and strong colonization ability (Jayatissa et al. 2002; Ren et al. 2009; Peng et al. 2016b).
Carbon storage of S. apetala
In the Reserve, S. apetala still grow, further showed that it has strong adaptability. Mangrove ecosystems have a high carbon sequestration potential (Alongi 2012, 2014) and can be effective in mitigating climate change (Murdiyarso et al. 2015). In our study, we found that the average carbon storage of S. apetala plantations was 145.59 Mg C ha−1, which is equivalent to the average total carbon storage in a subtropical forest (Liu et al. 2018) and is consistent with a previous study (Li et al. 2019). Based on three years of field measurements, carbon storage of S. apetala vegetation significantly increased with stand age class. The carbon storage of S. apetala vegetation in Futian is comparable to that in other locations (Liu et al. 2014; Schile et al. 2017) but is higher than that of terrestrial forest ecosystems (Pregitzer and Euskirchen 2004; Donato et al. 2011; Alongi 2014) and other wetland ecosystems (McLeod et al. 2011; Migeot and Imbert 2012), and is lower than that reported for Micronesia and the Atlantic coast (Kauffman et al. 2011; Kauffman and Bhomia 2017). Previous studies reported that carbon stocks of live mangrove biomass ranged from 75.8 to 150.3 Mg ha−1, which was lower than our estimate of the S. apetala (Simard et al. 2019; Ouyang and Lee 2020). Mangrove ecosystem carbon storage had large variations. The reasons for the difference in carbon storage of mangrove forests include stand age, tree species, geographical environment, tree richness, and species diversity (Pregitzer and Euskirchen 2004; Kauffman et al. 2011; Kauffman and Bhomia 2017; Schile et al. 2017; Liu et al. 2018). Meanwhile, some studies found that external environment factors (e.g., upstream rivers and adjacent waters) contributed more to mangrove carbon storage than the production of the mangrove trees (Li et al. 2018). Carbon sink of S. apetala may be affected by the external environment. Overall, S. apetala is an ideal species that can improve mangrove restoration and carbon storage. For carbon sinks study of S. apetala, we also need to consider sediment organic carbon and dead biomass component due to their potential carbon storages (Ouyang and Lee 2020).
Conclusions
In this study, we developed allometric relationships among DBH and height, wood density, and the biomass of the different components of S. apetala. Our results showed DBH and wood density were better predictors of S. apetala biomass. The average allometric exponent was 2.20 between DBH and biomass. Near-isometric scaling relationships were found among different components of biomass, which is supported by the growth-hydraulic model (Niklas 2004). Stem biomass accounted for more than 97% of the variation in total S. apetala biomass. The ratio of below- to above-ground biomass of S. apetala was lower than that of other mangroves and higher than that of upland forests. Mangrove forest ecosystems appear to store more carbon than terrestrial ecosystems and other wetland ecosystems, and the carbon storage of S. apetala vegetation increased with stand age. In this study, the allometric equations of S. apetala were developed for the Futian National Nature Reserve and applications to other regions need to be verified. The sample capacity of S. apetala was relatively small in this study. But nevertheless, our results showed that the restoration of mangrove forests will alleviate land degradation, mitigate climate change, and increase carbon storage over time.
Data availability
The datasets generated during and/or analysed during the current study is available in the Appendix A.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Alongi DM (2012) Carbon sequestration in mangrove forests. Carbon Manag 3:313–322
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Ann Rev Mar Sci 6:195–219
Basuki TM, van Laake PE, Skidmore AK, Hussin YA (2009) Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. For Ecol Manage 257:1684–1694
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Fölster H, Fromard F, Higuchi N, Kira T, Lescure J-P, Nelson BW, Ogawa H, Puig H, Riéra B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Chave J, Rejou-Mechain M, Burquez A, Chidumayo E, Colgan MS, Delitti WB, Duque A, Eid T, Fearnside PM, Goodman RC, Henry M, Martinez-yearizar A, Mugasha WA, Muller-Landau HC, Mencuccini M, Nelson BW, Ngomanda A, Nogueira EM, Ortiz-Malavassi E, Pelissier R, Ploton P, Ryan CM, Saldarriaga JG, Vieilledent G (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Change Biol 20:3177–3190
Cheng D, Niklas K (2007) Above- and below-ground biomass relationships across 1543 forested communities. Ann Bot 99:95–102
Damuth J (2001) Scaling of growth: plants and animals are not so different. Proc Natl Acad Sci USA 98:2113–2114
Dean TJ, Long JN (1986) Validity of constant-stress and elastic-instability principles of stem formation in Pinus contorta and Trifolium pratense. Ann Bot 58:833–840
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297
Enquist BJ, Bentley LP, Shenkin A, Maitner B, Savage V, Michaletz S, Blonder B, Buzzard V, Espinoza TEB, Farfan-Rios W, Doughty CE, Goldsmith GR, Martin RE, Salinas N, Silman M, Díaz S, Asner GP, Malhi Y (2017) Assessing trait-based scaling theory in tropical forests spanning a broad temperature gradient. Global Ecol Biogeogr 26:1357–1373
Enquist BJ, Brown JH, West GB (1998) Allometric scaling of plant energetics and population density. Nature 395:163–165
Enquist BJ, Kerkhoff AJ, Stark SC, Swenson NG, McCarthy MC, Price CA (2007) A general integrative model for scaling plant growth, carbon flux, and functional trait spectra. Nature 449:218–222
Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295:1517–1520
Franco M, Kelly CK (1998) The interspecific mass–density relationship and plant geometry. Proc Natl Acad Sci USA 95:7830–7835
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, Masek J, Duke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr 20:154–159
Hamilton SE, Casey D (2016) Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob Ecol Biogeogr 25:729–738
Hamilton SE, Friess DA (2018) Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat Clim Change 8:240–244
Hayward A, Kolasa J, Stone JR (2010) The scale-dependence of population density–body mass allometry: Statistical artefact or biological mechanism? Ecol Complex 7:115–124
He Z, Peng Y, Guan D, Hu Z, Chen Y, Lee SY (2018) Appearance can be deceptive: shrubby native mangrove species contributes more to soil carbon sequestration than fast-growing exotic species. Plant Soil 432:425–436
Hoque A, Sharma S, Hagihara A (2011) Above and belowground carbon acquisition of mangrove Kandelia obovata trees in Manko Wetland, Okinawa, Japan. Int J Environ 1:7–13
Hui D, Wang J, Shen W, Le X, Ganter P, Ren H (2014) Near isometric biomass partitioning in forest ecosystems of China. PLoS ONE 9:e86550
Huxley JS (1932) Problems of relative growth. Methuen and Co Ltd, London
Jayatissa LP, Dahdouh-Guebas F, Koedam N (2002) A review of the floral composition and distribution of mangroves in Sri Lanka. Bot J Linn Soc 138:29–43
Kauffman JB, Bhomia RK (2017) Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: global and regional comparisons. PLoS ONE 12:e0187749
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC (2011) Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 31:343–352
Khan MNI, Suwa R, Hagihara A (2009) Biomass and aboveground net primary production in a subtropical mangrove stand of Kandelia obovata (S, L) Yong at Manko Wetland, Okinawa, Japan. Wetl Ecol Manag 17:585–599
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21:471–477
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research. Academic Press, New York, pp 187–261
Li S-B, Chen P-H, Huang J-S, Hsueh M-L, Hsieh L-Y, Lee C-L, Lin H-J (2018) Factors regulating carbon sinks in mangrove ecosystems. Glob Change Biol 24:4195–4210
Li Z, Zan Q, Yang Q, Zhu D, Chen Y, Yu S (2019) Remote estimation of mangrove aboveground carbon stock at the species level using a low-cost unmanned aerial vehicle system. Remote Sens 11:1018
Liu H, Ren H, Hui D, Wang W, Liao B, Cao Q (2014) Carbon stocks and potential carbon storage in the mangrove forests of China. J Environ Manag 133:86–93
Liu X, Trogisch S, He J, Niklaus PA, Bruelheide H, Tang Z, Erfmeier A, Scherer-Lorenzen M, Pietsch KA, Yang B, Kühn P, Scholten T, Huang Y, Wang C, Staab M, Leppert KN, Wirth C, Schmid B, Ma K (2018) Tree species richness increases ecosystem carbon storage in subtropical forests. Proc R Soc B 285:20181240
Lunstrum A, Chen L (2014) Soil carbon stocks and accumulation in young mangrove forests. Soil Biol Biochem 75:223–232
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
McMahon T (1973) Size and shape in biology: elastic criteria impose limits on biological proportions, and consequently on metabolic rates. Science 179:1201–1204
Migeot J, Imbert D (2012) Phenology and production of litter in a Pterocarpus officinalis (Jacq) swamp forest of Guadeloupe (Lesser Antilles). Aquat Bot 101:18–27
Mitra A, Sengupta K, Banerjee K (2012) Spatial and temporal trends in biomass and carbon sequestration potential of Sonneratia apetala Buch.-Ham in Indian Sundarbans. Proc Natl Acad Sci India Sect B 82:317–323
Murdiyarso D, Purbopuspito J, Kauffman JB, Warren MW, Sasmito SD, Donato DC, Manuri S, Krisnawati H, Taberima S, Kurnianto S (2015) The potential of Indonesian mangrove forests for global climate change mitigation. Nat Clim Change 5:1089–1092
Niklas KJ (2004) Plant allometry: is there a grand unifying theory? Biol Rev 79:871–889
Niklas KJ, Enquist BJ (2002a) Canonical rules for plant organ biomass partitioning and annual allocation. Am J Bot 89:812–819
Niklas KJ, Enquist BJ (2002b) On the vegetative biomass partitioning of seed plant leaves, stems, and roots. Am Nat 159:482–497
Norberg RA (1988) Theory of growth geometry of plants and self-thinning of plant populations: geometric similarity, elastic similarity, and different growth modes of plant parts. Am Nat 131:220–256
Ouyang X, Lee SY (2020) Improved estimates on global carbon stock and carbon pools in tidal wetlands. Nat Commun 11:317
Paul KI, Roxburgh SH, Chave J, England JR, Zerihun A, Specht A, Lewis T, Bennett LT, Baker TG, Adams MA, Huxtable D, Montagu KD, Falster DS, Feller M, Sochacki S, Ritson P, Bastin G, Bartle J, Wildy D, Hobbs T, Larmour J, Waterworth R, Stewart HT, Jonson J, Forrester DI, Applegate G, Mendham D, Bradford M, O’Grady A, Green D, Sudmeyer R, Rance SJ, Turner J, Barton C, Wenk EH, Grove T, Attiwill PM, Pinkard E, Butler D, Brooksbank K, Spencer B, Snowdon P, O’Brien N, Battaglia M, Cameron DM, Hamilton S, McAuthur G, Sinclair J (2016) Testing the generality of above-ground biomass allometry across plant functional types at the continent scale. Glob Chang Biol 22:2106–2124
Peng C, Qian J, Guo X, Zhao H, Hu N, Yang Q, Chen C, Chen L (2016a) Vegetation carbon stocks and net primary productivity of the mangrove forests in Shenzhen, China. Chin J Appl Ecol 27:2059–2065
Peng Y, Diao J, Zheng M, Guan D, Zhang R, Chen G, Lee SY (2016b) Early growth adaptability of four mangrove species under the canopy of an introduced mangrove plantation: Implications for restoration. For Ecol Manag 73:179–188
Pham TD, Yoshino K, Bui DT (2016) Biomass estimation of Sonneratia caseolaris (l) Engler at a coastal area of Hai Phong city (Vietnam) using ALOS-2 PALSAR imagery and GIS-based multi-layer perceptron neural networks. Gisci Remote Sens 54:329–353
Pregitzer KS, Euskirchen ES (2004) Carbon cycling and storage in world forests: biome patterns related to forest age. Glob Change Biol 10:2052–2077
Ren H, Chen H, Li Z, Han W (2010) Biomass accumulation and carbon storage of four different aged Sonneratia apetala plantations in Southern China. Plant Soil 327:279–291
Ren H, Lu H, Shen W, Huang C, Guo Q, Li Z, Jian S (2009) Sonneratia apetala BuchHam in the mangrove ecosystems of China: an invasive species or restoration species? Ecol Eng 35:1243–1248
Ren H, Wu X, Ning T, Huang G, Wang J, Jian S, Lu H (2011) Wetland changes and mangrove restoration planning in Shenzhen Bay, Southern China. Landsc Ecol Eng 7:241–250
Rivera-Monroy VH, Lee SY, Kristensen E, Twilley RR (2017) Mangrove ecosystems: a global biogeographic perspective: structure, function, and services. Springer, Berlin
Schile LM, Kauffman JB, Crooks S, Fourqurean JW, Glavan J, Megonigal JP (2017) Limits on carbon sequestration in arid blue carbon ecosystems. Ecol Appl 27:859–874
Simard M, Fatoyinbo L, Smetanka C, Rivera-Monroy VH, Castañeda-Moya F, Tomas N, Stocken TV (2019) Mangrove canopy height globally related to precipitation, temperature and cyclone frequency. Nat Geosci 12:40–45
Swenson NJ, Enquist BJ (2007) Ecological and evolutionary determinants of a key plant functional trait: Wood density and its community-wide variation across latitude and elevation. Am J Bot 94:451–459
Tam NFY, Wong YS, Lan CY, Chen GZ (1995) Community structure and standing crop biomass of a mangrove forest in Futian Nature Reserve, Shenzhen, China. Hydrobiologia 295:193–201
UNFCCC (2008) In: Report of the conference of the parties on its thirteenth session. Bali, Indonesia, 3–15 December, 2007 (Decision 1/CP.13)
Vo QT, Kuenzer C, Vo QM, Moder F, Oppelt N (2012) Review of valuation methods for mangrove ecosystem services. Ecol Indic 23:431–446
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smatr 3-an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
West GB, Brown JH, Enquist BJ (1999) A general model for the structureand allometry of plant vascular systems. Nature 400:664–667
Wilson JB (1988) A review of evidence on the control of shoot: root ratio, in relation to models. Ann Bot 61:433–449
Wu M, He Z, Fung S, Cao Y, Guan D, Peng Y, Lee SY (2020) Species choice in mangrove reforestation may influence the quantity and quality of long-term carbon sequestration and storage. Sci Total Environ 714:136742
Yang Q, Lei A, Li F, Liu L, Zan Q, Shin PKS, Cheung SG, Tam NFY (2014) Structure and function of soil microbial community in artificially planted Sonneratia apetala and S. caseolaris forests at different stand ages in Shenzhen Bay. China Mar Pollut Bull 85:754–763
Zan Q, Wang B, Wang Y, Li M (2003) Ecological assessment on the introduced Sonneratia caseolaris and Sapetala at the mangrove forest of Shenzhen Bay, China. Acta Bot Sin 45:544–551
Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J (2009) Data from: towards a worldwide wood economics spectrum. Dryad, Dataset. https://doi.org/10.5061/dryad.234
Acknowledgements
We thank Stuart Hamilton for his constructive revision that greatly improved the paper, and the Shenzhen Mangrove Wetlands Conservation Foundation (MCF) for help in the field works.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant 31770513), Shenzhen Science and Technology Innovation Committee (Project no. JCYJ20180504170040910), Urban Administration and Law Enforcement Bureau of Shenzhen Municipality (201802), and the Zhang-Hongda Science Foundation at Sun Yat-sen University.
Author information
Authors and Affiliations
Contributions
DZ, SY designed the experiment; DZ, MW, QY, ZL, ZH, HY performed the field experiments; DZ analyzed data; DZ, SY, DH wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, D., Hui, D., Wang, M. et al. Allometric growth and carbon storage in the mangrove Sonneratia apetala. Wetlands Ecol Manage 29, 129–141 (2021). https://doi.org/10.1007/s11273-020-09772-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09772-7