Abstract
Although the importance of regulating and provisioning services provided by mangroves is widely recognised, our understanding of their role in the maintenance of terrestrial biodiversity is patchy globally and largely lacking for many regions, including conservation priorities such as Madagascar. We carried out the first multi-site bird inventory of mangroves in Madagascar and complemented our data with assessments of local knowledge, in order to broaden our knowledge of which species use this habitat. We directly observed 73 species across three sites in Ambanja and Ambaro Bays, while local respondents indicated the presence of 18 additional species: four observed species are globally threatened, while 37 are endemic to Madagascar or the Malagasy region. Over half the species observed are typically terrestrial, of which 22 have not previously been recorded in mangrove habitats in Madagascar. Local knowledge provided a useful complement to our observed data but we are likely to have underestimated total richness; nevertheless, our findings greatly increased our knowledge of mangrove use by Madagascar’s birds. However, further research is required to investigate the functional role of mangroves in the ecology of the observed species and provide insights into the factors influencing mangrove use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mangroves are vegetated ecosystems growing in intertidal areas of sheltered tropical and subtropical coastlines worldwide. They are amongst the most threatened of all tropical ecosystems (Duke et al. 2007; Valiela et al. 2001) having lost approximately 20–35 % of their global extent since 1980 (FAO 2007; Polidoro et al. 2010; Valiela et al. 2001) as a result of natural and anthropogenic processes including conversion to agriculture and aquaculture, overharvesting, and altered hydrological dynamics arising from upstream land use change (Gilman et al. 2008; Gopal and Chauhan 2006; Primavera 2000, 2006; Walters et al. 2008).
Mangroves have attracted increasing attention from conservation and climate change mitigation programmes in recent years due to the valuable ecosystem services they provide, in particular carbon sequestration and storage (Lafolley and Grimsditch 2009; Nellemann et al. 2009; Ullman et al. 2012): indeed the combined above- and below-ground carbon storage of mangroves greatly exceeds that of many terrestrial tropical forest systems (Donato et al. 2011; Kauffman et al. 2011, 2014; Pendleton et al. 2012; Wang et al. 2013). In addition, mangroves play an important role in coastal protection and erosion prevention (Alongi 2008; Dahdouh-Guebas et al. 2005), and provide breeding and feeding grounds for a range of marine species (Kathiresan and Bingham 2001; Nagelkerken et al. 2008) including commercially important fish and crustaceans (Manson et al. 2005; Naylor et al. 2000). Around the world many human populations in coastal areas depend heavily on mangroves for their subsistence and household income (Glaser 2003; Rasolofo 1997; van Bochove et al. 2014).
Although the socio-economic and ecosystem regulating contributions of mangrove systems are now widely recognised, our understanding of their importance for terrestrial biodiversity remains patchy at the global scale, and even basic knowledge of the species occurring in mangroves is largely lacking for many areas (Nagelkerken et al. 2008). This knowledge gap is important because information on the distribution of biodiversity is fundamental to conservation planning (Ferrier 2002; Pressey et al. 2007). Madagascar is a global conservation priority harbouring an unparalleled combination of diversity and endemism among its terrestrial fauna and flora, particularly at higher taxonomic levels (Brooks et al. 2006; Holt et al. 2013), but is amongst the countries where mangrove use by terrestrial biodiversity remains little researched. With around 213,000 ha of mangroves in 2010, Madagascar possesses approximately 2 % of their global area and is amongst the top 15 most mangrove rich countries in the world (FAO 2007; Giri and Mulhausen 2008; Giri et al. 2011), but despite this we know little about the extent to which these ecosystems are used by the island’s (largely endemic) terrestrial fauna. Knowledge of bird occurrence in Madagascar’s mangroves is limited to two single site inventories (Gardner et al. 2012; Razafindrajao et al. 2002), a small number of single species studies (e.g. Andrianarimisa and Razafimanjato 2012; Razafimanjato et al. 2014) and miscellaneous short reports (e.g. Appert 1970; Woolaver et al. 2004). Since the first step in understanding the use of mangroves by birds is to know which species occur in them, we seek to broaden our knowledge base with a rapid ornithological assessment of three sites in the Ambanja and Ambaro Bays mangrove in north-west Madagascar, which constitutes the largest continuous mangrove system in Madagascar (Jones et al. 2016a). Since rapid inventories may fail to detect rare or seasonal events or species (Anderson et al. 2007; van der Hoeven et al. 2004), we complement our data with an evaluation of the local ecological knowledge (LEK) of fishers and mangrove users in order to provide a more complete picture of the avian diversity of our study system.
Methods
Study site
The Ambanja and Ambaro Bays in northwest Madagascar are lined with mangroves totalling 45,680 ha, of which 14,015 ha in closed-canopy and 31,665 in open-canopy ecosystems (Jones et al. 2014). The climate is sub-humid tropical with a warm rainy season and frequent cyclones from November–April, and a cooler dry season in May–October (Rasolofo and Ramilijaona 2009). The underlying geology is composed primarily of alluvial and lake deposits, and the relative abundance of rainfall and freshwater contributes to a high stature of mangrove trees compared to equivalent systems in western Madagascar (Giri and Mulhausen 2008; Jones et al. 2014). As with all of Madagascar’s mangroves, the ecosystem is relatively species-poor and is composed of eight true mangrove species: Avicennia marina (white mangrove), Bruguiera gymnorrhiza (orange mangrove), Ceriops tagal (Indian mangrove), Rhizophora mucronata (red mangrove), Sonneratia alba (mangrove apple), Xylocarpus granatum (cannonball mangrove), Lumnitzera racemosa (black mangrove) and Heritiera littoralis (looking-glass mangrove). Mangroves throughout the area are the focus of extensive artisanal fishing and resource extraction activities (Rasolofo 1997) and are threatened by deforestation, having lost 20 % of their area in the period 1990–2010 as a result of timber exploitation and charcoal production (Jones et al. 2014, 2016b).
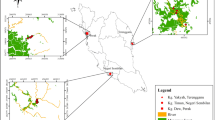
We surveyed three sites (Antsahampano, Ankazomborona and Ankatafa) currently the subject of community-based mangrove management initiatives within conservation programs led by the international non-governmental organisations WWF, l’Homme et l’Environnement and Blue Ventures (Fig. 1). All sites are governed under a GELOSE management transfer contract (see Pollini et al. 2014), and managed by an association of local resource users called a Communauté Locale de Base (CLB).
Map of study sites in north-west Madagascar showing vegetation cover and transect routes followed during rapid bird inventories. Mangrove vegetation cover is derived from Jones et al. (2014), and other vegetation classes from Harper et al. (2007). The background uses a true colour Landsat eight image from 2014, at low tide
Bird surveys
We carried out ornithological surveys at each site towards the end of the rainy season in 2015 (Antsahampano, 11–12th March; Ankazomborona, 18–21st April; Ankatafa, 22–24th April). At each site we attempted to sample different parts of the mangrove (seaward side, small and large channels, back mangrove) approximately equally, but were constrained by tides which restricted accessibility. In order to maximise the diversity of species recorded at each site we also visited areas said by local respondents (mangrove users and CLB members) to be rich in birds or frequented by particular species of interest (e.g. IUCN Red List species). Transects were primarily water based, using a motor boat at Antsahampano and traditional dugout pirogues (lakana) at the other two sites, and were largely carried out during high tides to permit entry into shallow channels. Where possible we also surveyed transects on foot along the terrestrial edge of the mangrove (back mangrove), but we did not penetrate dense mangrove stands on foot (Fig. 1; Table 1). During transects we noted all visual and auditory contacts with birds from within or above mangroves, in mangrove channels or immediately adjacent to mangroves on the seaward side (including on exposed mudflats dotted with mangrove trees, at low tide), but did not record species observed only in terrestrial habitats immediately adjacent to mangroves on the landward side (e.g. dead zones, secondary scrub, grasslands, freshwater wetlands, agriculture and native forests). We scored the relative abundance of each species using an index based on the percentage of transects in which the species was recorded (rare = recorded in <25 % of transects; uncommon = recorded in 25–50 % of transects; frequent = recorded in 50–75 % of transects; common = recorded in >75 % of transects).
Assessment of local knowledge
The expert knowledge of local resource users who spend significant periods of time within a study system can be a reliable and cost effective complement or alternative to directly observed data (Anderson et al. 2007; Danielsen et al. 2014; Turvey et al. 2014; van der Hoeven et al. 2004), particularly given the high costs of, and rapidly diminishing returns from, increased inventorying (Gardner et al. 2008; Grantham et al. 2008). As such, the integration of traditional and scientific knowledge systems to inform environmental management has been widely promoted (Raymond et al. 2010; Sutherland et al. 2014; Tengö et al. 2014; Thaman et al. 2013). In order to provide a fuller picture of bird occurrence in mangroves than can be provided by rapid inventories alone, we ascertained local knowledge using two methods, ‘walking interviews’ (also known as ‘walk-in-the-woods interviews’) (Thomas et al. 2007), and structured focus group interviews (Diamond 1991; Bernard 2006).
Walking interviews were carried out during all survey transects, which were accompanied by 1–4 members of the local CLB management committee, by systematically asking our respondents for the local names of all birds encountered either visually or aurally. We also used these interviews to ascertain the knowledge of respondents and thus their suitability as expert respondents for further enquiries. Subsequently, we carried out focus group interviews with participants selected on the basis of their knowledge of birds and their familiarity with mangrove environments; respondents (n = 3 at Antsahampano, n = 7 at Ankazomborona and n = 4 at Ankatafa) thus largely comprised CLB members and mud crab (Scylla serrata) fishers, who spend more time in the mangroves than fishers targeting other resources. Focus group interviews were facilitated by the use of an illustrated field guide (Sinclair and Langrand 1998) and MP3 recordings of bird calls and song (Huguet and Chappuis 2003). For each species thought to occur in the region and potentially occurring within mangroves, we showed respondents an image of the species and simultaneously played its call/song on a small loudspeaker. If respondents recognised the bird, we asked them to describe aspects of its appearance, behaviour, habitat use or life history in order to corroborate their identification. If the bird was not initially recognised, we prompted respondents by describing aspects of its appearance, size, behaviour or other identifying characteristics (Diamond 1991), or by offering local names already ascertained from walking interviews: if respondents recognised the description, we again sought to corroborate their identification by asking them to describe additional characteristics of the species in question. For all species known to respondents, we asked for its name (specifying that we were interested in the local name rather than that from other villages or regions), and whether they had ever seen it in mangroves; when affirmative responses were provided, we further enquired about its regularity and behaviour within this environment.
Results
We recorded 73 species by direct observation across the three sites, either within or above mangroves or immediately adjacent to them on the seaward side (Table 2). An additional 18 species were not observed but were reported to occur within mangroves by respondents. Four observed species are globally Endangered (EN) or Critically Endangered (CR) (Madagascar fish-eagle Haliaeetus vociferoides, CR; Madagascar heron Ardea humbloti, EN; Madagascar pond-heron Ardeola idae, EN and Madagascar teal Anas bernieri, EN), while two additional EN species were reported by respondents (Madagascar sacred ibis Threskiornis bernieri and Van Dam’s vanga Xenopirostris damii) (IUCN 2015).
In terms of principal habitats utilised, over half of observed species (54.8 %) are terrestrial, i.e. inhabitants of forests, scrublands or open areas rather than seabirds, shorebirds and wetland specialists. Eighteen observed species (24.7 %) are endemic to Madagascar, including four species belonging to endemic genera (Common jery Neomixis tenella, stripe-throated jery N. striatigula, Madagascar starling Hartlaubius auratus and Madagascar mannikin Lepidopygia nana), one belonging to an endemic subfamily (crested coua Coua cristata) and five belonging to the endemic family Vangidae (common newtonia Newtonia brunneicauda, chabert vanga Leptopterus chabert, hook-billed vanga Vanga curvirostris, white-headed vanga Artamella viridis and sickle-billed vanga Falculea palliata). Two further Vangidae and cuckoo roller Leptosomus discolor of the monospecific endemic family Leptosomidae were also reported by informants, as well as two additional endemic species. Nineteen observed species are endemic to the islands of the western Indian Ocean (Madagascar and the Comoros, Seychelles and Mascarene archipelagos) and two are endemic breeders to the region; when added to the strict endemics, 53.4 % of observed species are endemic to some degree.
Discussion
Our data have revealed that a higher diversity of bird species than was previously recognised utilise the mangroves of north-west Madagascar, including a large proportion of terrestrial species that were not known to occur in this habitat. In addition to the 73 species we observed, 14 further species have been recorded in mangrove inventories elsewhere in Madagascar by Razafindrajao et al. (2002) and Gardner et al. (2012) and 12 more were reported by respondents in this study, indicating that at least 99 species (38.7 % of all species regularly occurring in Madagascar, Safford and Hawkins 2014) utilise this habitat. This figure places Madagascar in the lower ranks of global mangrove range states in which bird occurrence has been researched, with a greater richness than Trinidad (84 species, Ffrench 1966) and Surinam (94 species, Haverschmidt 1965), but lower than Guinea-Bissau (125 species, Altenberg and van Spanje 1989), and Peninsular Malaysia (135 species, Nisbet 1968). Australia has the highest diversity of mangrove birds including 186 species in Queensland and 104 species in north-western Australia (Saenger et al. 1977). Species richness at individual sites in Australia has been recorded at 54 and 70 at Darwin Harbour (Noske 1996; Mohd-Azlan et al. 2012) and 47 in Cairns (Kutt 2007); however groups such as migratory shorebirds, herons and aerial insectivores were not included in these studies.
However, both our observations and our assessment of local knowledge are likely to have underestimated diversity for a number of reasons. First, we carried out our surveys at the end of the breeding season for most species, reducing the detectability of terrestrial birds that were not singing, while many migratory species, particularly shorebirds (Scolopacidae and Charadriidae), would be expected to be absent when surveying was carried out (or present in highly reduced numbers). In addition, although respondents were consistently able to differentiate between terrestrial species on the basis of images and calls, they tended not to differentiate between species in certain species-rich groups of similar looking (and less vocal) species (e.g. shorebirds, terns and other seabirds) and were thus unable to estimate the full richness of these groups that they have observed in mangroves. As a result, our diversity estimates should be considered conservative and further investigations could be expected to reveal additional species.
While the use of mangroves by many coastal and wetland species is well known, our observations of 47 terrestrial species using this habitat is significant because the majority of these species are endemic or regionally endemic, and over half (26 species) have not previously been reported as using mangroves (Safford and Hawkins 2014). Since mangroves are regularly inundated, have low plant species diversity and lower invertebrate diversity and biomass than terrestrial forests (Intachat et al. 2005; Nagelkerken et al. 2008), the use of mangroves by these species is surprising given that Madagascar’s endemic bird species tend to be habitat specialists (Wilmé 1996). However, many of these are relatively common and widespread species that, while forest-dependent, are relatively tolerant of habitat degradation and edge habitats and are therefore not highly threatened (Safford and Hawkins 2014). The most important species for conservation are the six observed or reported birds listed as Endangered or Critically Endangered by the IUCN. Of these none were observed regularly, and all but one (Madagascar heron) were reported as only infrequently seen by respondents; we recorded a pair of Madagascar fish-eagle mating near the village of Andrekareka (Ankatafa), three Madagascar pond-herons roosting among squacco herons (Ardeola ralloides) at Antsahampano, two Madagascar herons feeding in a large channel at Ankatafa, and three Madagascar teal near the village of Ankazomborona. The call of Van Dam’s vanga was recognised by all informants at Ankazomborona and the bird was said to be relatively common in mangroves there, although we cannot rule out possible confusion with white-headed vanga and hook-billed vanga because informants sometimes confused these three species in the field and during interviews. Van Dam’s vanga was also recognised by all informants at Ankatafa and was said to be relatively common in the adjacent terrestrial forests, but was not thought to occur in mangroves at that site (we did not enquire about this species at Antsahampano).
Our data should be interpreted with caution when considering the importance of mangroves for Madagascar’s avifauna because the simple presence of a bird within a mangrove says little about the functional role of this habitat in the ecology of the species. Some largely pelagic species (e.g. terns, frigatebirds) may perch in mangrove trees and/or forage in deeper channels but primarily feed out at sea, while many shorebirds and wetland birds may roost and forage in mangroves but also feed in coastal areas lacking mangrove vegetation. Amongst terrestrial species some may use mangroves for breeding (e.g. grey-headed lovebird Agapornis cana), roosting (e.g. Madagascar mannikin) or perching to sing (e.g. Madagascar hoopoe Upupa marginatus) but are unlikely to feed in this habitat due to their foraging ecology, while others forage over mangroves but are probably unable to roost or breed within them (e.g. swifts and Madagascar nightjar Caprimulgus madagascariensis) (Safford and Hawkins 2014). The persistence of many of species using the mangroves of the region may therefore depend on the maintenance of connectivity between them and adjacent terrestrial habitats (Nagelkerken et al. 2008; Noske 1996; Wells 1999). Overall Madagascar appears to lack any mangrove-dependent species among its terrestrial avifauna, although the Madagascar teal is an obligate mangrove breeder nesting only in holes in Avicennia marina trees (Young 2006; Young et al. 2013), and the habitat provides a stronghold for other threatened endemic species including Madagascar fish-eagle and Madagascar sacred ibis (Andrianarimisa and Razafimanjato 2012; Razafimanjato et al. 2014).
Although our pooled observations indicate that a high diversity of bird species utilise the mangroves of Ambanja and Ambaro Bays, our data cannot be used to infer the relative value of the three sites for bird conservation or prioritise between them because we were unable to ensure comparable research effort between sites. Since our transects were primarily carried out by boat our access into mangroves was limited by tides; we therefore spent variable amounts of time in different parts of the mangrove (e.g. small channels, main channels and the seaward edge) at each site, and this during different parts of the day when birds show variable activity and detectability. As a result, we are unable to produce rarefaction curves to estimate the completeness of sampling at each site. Observed differences in species diversity may be the result of differences in mangrove habitat structure or their proximity to terrestrial forests, but may also have arisen partially as a result of methodological differences: water-based surveying in Antsahampano was carried out in a motor boat rather than a pirogue, which greatly reduced the detectability of terrestrial species (such as parrots, pigeons and passerines) which were often observed by call. However, this site was also surveyed a month earlier than the others, with the result that several migratory wader species were recorded which may already have been absent by the time Ankazomborona and Ankatafa were surveyed.
Our assessments of local knowledge of mangrove utilisation by birds provided a complementary data source to our direct observations and enabled us to generate a more complete picture of local mangrove bird diversity than would otherwise have been possible from a rapid inventory alone. For example, local respondents reported the presence of two Endangered species (Van Dam’s vanga and Madagascar sacred ibis) that we did not observe directly. In addition, data from the bird survey alone may have suggested that Ankatafa was more important than the other sites as both Madagascar fish-eagle (CR) and Madagascar heron (EN) were recorded only there, though these species in fact occur at all three sites, as revealed by LEK. The method was rapid and cheap compared to boat-based field surveys, and we are confident in the reliability of the data collected in this way because we systematically sought corroborating evidence from our informants (Diamond 1991). However, use of this approach is dependent on the use of audio recordings of bird calls as well as visual aids since many species were more readily identified by respondents by their vocalisations than by images. The relative lack of distinctive vocalisations among seabirds and shorebirds compared to terrestrial species may partly explain why the former two groups tended to be lumped and known only by generic names, while the latter tended to be individually distinguished as species; thus the method appears more valid for some species groups than for others. In addition, the method requires an excellent knowledge of local birds on the part of the interviewer, because corroborating enquiries involving species’ behaviour and other identifying characteristics are necessary to ensure correct identification and thus the viability of respondent data (Diamond 1991).
In conclusion, we have carried out the most comprehensive assessment to date of mangrove utilisation by Madagascar’s birds, and revealed that a previously unrecognised diversity of species use this habitat to some extent. Although these data are preliminary and tell us little about the functional importance of mangroves for the maintenance of species populations, the records of 39 species not previously reported from mangroves demonstrates that these ecosystems may support diverse bird communities in Madagascar and provides the first indication of the potential importance of mangroves for the species in question. Further research should build on these findings to better understand the conservation importance of mangroves for the country’s avifauna. This should include (i) further inventories of an expanded range of sites and in different seasons; (ii) ecological research to better understand the functional role of mangroves in the maintenance of species populations (focused particularly on endemics and species of conservation concern); and (iii) habitat selection studies focused on mangroves and adjacent terrestrial habitats, to understand differences in the ecological traits of bird species that do and do not utilise mangrove habitats. Such research would provide valuable insights into the ecological and behavioural factors influencing mangrove use by birds in Madagascar and worldwide.
References
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76:1–13
Altenberg W, van Spanje T (1989) Utilization of mangroves by birds in Guinea-Bissau. Ardea 77:57–74
Anderson J, Rowcliffe JM, Cowlishaw G (2007) Does the matrix matter? A forest primate in a complex agricultural landscape. Biol Conserv 135:212–222
Andrianarimisa A, Razafimanjato G (2012) Madagascar sacred ibis Threskiornis bernieri: current population status, distribution, and implications for conservation. In: Harebottle DM, Craig AJFK, Anderson MD, Rakotomanana H, Muchai M (eds) Proceedings of the 12th Pan-African Ornithological Congress. Anim Demogr Unit, Cape Town, pp 120–130
Appert O (1970) Zurbiologie der vangawurger (Vangidae) sudwest-Madagaskars. Ornithol Beob 67:101–133
Bernard H (2006) Research methods in anthropology: qualitative and quantitative approaches, 4th edn. Altamira Press, Lanham
Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL (2006) Global biodiversity conservation priorities. Science 313:58–61
Dahdouh-Guebas F, Jayatissa LP, di Nitto D, Bosire JO, Lo Seen D, Koedam N (2005) How effective were mangroves as a defence against the recent tsunami? Curr Biol 15:R443–R447
Danielsen F, Jensen PM, Burgess ND, Coronado I, Holt S, Poulsen MK, Rueda RM, Skielboe T, Enghoff M, Hemmingsen LH, Sørensen M, Pirhofer-Walzl K (2014) Testing focus groups as a tool for connecting indigenous and local knowledge on abundance of natural resources with science-based land management systems. Conserv Lett 7:380–389
Diamond JM (1991) Interview techniques in ethnobiology. In: Pawley A (ed) Man and a half: essays in Pacific anthropology and ethnobiology in honour of Ralph Bulmer. The Polynesian Society, Auckland, pp 83–86
Donato DC, Kauffman JB, Murdiyarso D, Kumianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297
Duke NC, Meynecke JO, Dittmann S, Ellison AM, Anger K, Berger U, Cannicci S, Diele K, Ewel KC, Field CD, Koedam N, Lee SY, Marchand C, Nordhaus I, Dahdouh-Guebas F (2007) A world without mangroves? Science 317:41–42
Food and Agricultural Organization (FAO) (2007) The world’s mangroves 1980–2005. FAO, Rome
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51:331–363
Ffrench RP (1966) The utilization of mangroves by birds in Trinidad. Ibis 108:423–424
Gardner CJ, De Ridder C, De Ridder B, Jasper LD (2012) Birds of Ambondrolava mangrove complex, southwest Madagascar. Check List 8:1–7
Gardner TA, Barlow J, Araujo IS, Ávila-Pires TC, Bonaldo AB, Costa JE, Esposito MC, Ferreira LV, Hawes J, Hernandez MI, Hoogmoed MS, Leite RN, Lo-Man-Hung NF, Malcolm JR, Martins MB, Mestre LA, Miranda-Santos R, Overal WL, Parry L, Peters SL, Ribeiro-Junior MA, da Silva MN, da Silva Motta C, Peres CA (2008) The cost-effectiveness of biodiversity surveys in tropical forests. Ecol Lett 11:139–150
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options: a review. Aquat Bot 89:237–250
Giri C, Muhlhausen J (2008) Mangrove forest distributions and dynamics in Madagascar (1975–2005). Sensors 8:2104–2117
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, Masek J, Duke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr 20:154–159
Glaser M (2003) Interrelations between mangrove ecosystem, local economy and social sustainability in Caeté Estuary, North Brazil. Wetl Ecol Manag 11:265–272
Gopal B, Chauhan M (2006) Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat Sci 68:338–354
Grantham HS, Moilanen A, Wilson KA, Pressey RL, Rebelo TG, Possingham HP (2008) Diminishing return on investment for biodiversity data in conservation planning. Conserv Lett 1:190–198
Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F (2007) Fifty years of deforestation and forest fragmentation in Madagascar. Environ Conserv 34:325–333
Haverschmidt F (1965) The utilization of mangroves by South American birds. Ibis 107:540–542
Holt BG, Lessard JP, Borregaard MK, Fritz SA, Araújo MB, Dimitrov D, Fabre PH, Graham CH, Graves GR, Jønsson KA, Nogués-Bravo D, Wang Z, Whittaker RJ, Fjeldså J, Rahbek C (2013) An update of Wallace’s zoogeographic regions of the world. Science 339:74–78
Huguet P, Chappuis C (2003) Oiseaux de Madagascar, Mayotte, Comoros, Seychelles, Réunion. Maurice. Société d’Etudes Ornithologiques de France, Paris
Intachat J, Holloway JD, Speight MR (2005) A preliminary assessment of the diversity of geometroid moths within different types of forests in Peninsular Malaysia. Malay Nat J 57:1–28
IUCN (International Union for Conservation of Nature and Natural Resources) (2015). The IUCN Red List of Threatened Species 2015.2. http://www.iucnredlist.org/ Accessed 17 Mar 2015
Jones TG, Ratsimba HR, Ravaoarinorotsihoarana L, Cripps G, Bey A (2014) Ecological variability and carbon stock estimates of mangrove ecosystems in northwestern Madagascar. Forests 5:177–205
Jones TG, Glass L, Gandhi S, Ravaoarinorotsihoarana L, Carro A, Benson L, Ratsimba HR, Giri C, Randriamanatena D, Cripps G (2016a) Madagascar’s mangroves: quantifying nation-wide and ecosystem specific dynamics, and contemporary mapping of distinct ecosystems. Remote Sens 8:106
Jones TG, Ratsimba HR, Carro A, Ravaoarinorotsihoarana L, Glass L, Teoh M, Benson L., Cripps G, Giri C, Zafindrasilivonona B, Raherindray R, Andriamahenina Z, Andriamahefazafy M (2016b) The mangroves of Ambanja and Ambaro Bays, northwest Madagascar: historical dynamics, current status and deforestation mitigation strategy. In: Diop S et al (eds) Estuaries: a lifeline of ecosystem services in the western Indian Ocean. Springer, Cham. 10.1007/978-3-319-25370-1_5
Kathiresan K, Bingham B (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC (2011) Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 31:343–352
Kauffman JB, Heider C, Norfolk J, Payton F (2014) Carbon stocks of intact mangroves and carbon emissions arising from their conversion in the Dominican Republic. Ecol Appl 24:518–527
Kutt AS (2007) Bird assemblage in a dune-mangrove mosaic, Cairns, Queensland. Aust Zool 34:158–164
Laffoley D, Grimsditch G (2009) The management of natural coastal carbon sinks. IUCN, Gland
Manson FJ, Loneragan NR, Skilleter GA, Phinn SR (2005) An evaluation of the evidence for linkages between mangroves and fisheries: a synthesis of the literature and identification of research directions. Oceanogr Mar Biol 43:483–513
Mohd-Azlan J, Noske RA, Lawes MJ (2012) Avian species assemblage structure and indicator bird species of mangroves in the Australian monsoon tropics. Emu 112:287–297
Nagelkerken I, Blaber SJ, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Moony H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Nellemann C, Corcoran E, Duarte CM, Valdés L, de Young C, Fonseca L, Grimsditch G (2009) Blue carbon: the role of healthy oceans in binding carbon. United Nations Environment Programme and GRID-Arendal, Arendal
Nisbet ICT (1968) The utilization of mangroves by Malayan birds. Ibis 110:348–352
Noske R (1996) Abundance, zonation, and foraging ecology of birds in mangroves of Darwin Harbour, Northern Territory. Wildl Res 23:443–474
Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, Sifleet S, Craft C, Fourqurean JW, Kauffman JB, Marba N, Megonigal P, Pidgeon E, Herr D, Gordon D, Baldera A (2012) Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7:e43542
Polidoro BA, Carpenter KE, Collins L, Duke NC, Ellison AM, Ellison JC, Farnsworth EJ, Fernando ES, Kathiresan K, Koedam NE, Livingstone SR, Miyagi T, Moore GE, Nam VN, Ong JE, Primavera JH, Salmo SG III, Sanciangco JC, Sukardjo S, Wang Y, Yong JWH (2010) The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS One 5:e10095
Pollini J, Hockley N, Muttenzer FD, Ramamonjisoa BS (2014) The transfer of natural resource management rights to local communities. In: Scales IR (ed) Conservation and environmental management in Madagascar. Routledge, London, pp 172–192
Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA (2007) Conservation planning in a changing world. Trends Ecol Evol 22:583–592
Primavera JH (2000) Development and conservation of Philippine mangroves: institutional issues. Ecol Econ 35:91–106
Primavera JH (2006) Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast Management 49:531–545
Rasolofo MV (1997) Use of mangroves by traditional fishermen in Madagascar. Mangroves Salt Marshes 1:243–253
Rasolofo MV, Ramilijaona O (2009) Variability in the abundance and recruitment of Fenneropenaeus indicus and Metapenaeus monoceros postlarvae and juveniles in Ambaro Bay mangroves of Madagascar. Nat Faune 24:103–109
Raymond CM, Fazey I, Reed MS, Stringer LC, Robinson GM, Evely AC (2010) Integrating local and scientific knowledge for environmental management. J Environ Management 91:1766–1777
Razafimanjato G, Seing Sam T, Rakotondratsima M, Réné de Roland LA, Thorstrom R (2014) Population status of the Madagascar fish eagle Haliaeetus vociferoides in 2005–2006. Bird Conserv Int 24:88–99
Razafindrajao F, Lewis R, Nichols R, Woolaver L (2002) Discovery of a new breeding population of Madagascar teal Anas bernieri in north-west Madagascar. Dodo 37:60–69
Saenger P, Specht MM, Specht RL, Chapman VJ (1977) Mangal and coastal salt-marsh communities in Australasia. In: Chapman VJ (ed) Ecosystems of the World 1. Elsevier, Amsterdam, pp 293–345
Safford R, Hawkins F (2014) Birds of Africa vol VIII: the Malagasy region. Christopher Helm, London
Sinclair I, Langrand O (1998) Birds of the Indian Ocean Islands. Struik, Cape Town
Sutherland WJ, Gardner TA, Haider LJ, Dicks LV (2014) How can local and traditional knowledge be effectively incorporated into international assessments. Oryx 48:1–2
Tengö M, Brondizio E, Elmqvist T, Malmer P, Spierenberg M (2014) Connecting diverse knowledge systems for enhanced ecosystem governance - the multiple evidence base approach. Ambio 43:579–591
Thaman R, Lyver P, Mpande R, Perez E, Cariño J, Takeuchi K (eds) (2013) The contribution of indigenous and local knowledge systems to IPBES: building synergies with science. UNESCO, Paris
Thomas E, Vandebroek I, Van Damme P (2007) What works in the field? A comparison of different interviewing methods in ethnobotany with special reference to the use of photographs. Econ Bot 61:376–384
Turvey ST, Fernández-Secades C, Nuñez-Miño JM, Hart T, Martinez P, Brocca JL, Young RP (2014) Is local knowledge a useful conservation tool for small mammals in a Caribbean multicultural landscape? Biol Conserv 169:189–197
Ullman R, Bilbao-Batista V, Grimsditch G (2012) Including blue carbon in climate market mechanisms. Ocean Coast Manage 83:15–18
Valiela I, Bowen JL, York JK (2001) Mangrove forests: one of the world’s threatened major tropical environments. Bioscience 51:807–815
van Bochove J, Sullivan E, Nakamura T (eds) (2014) The importance of mangroves to people: a call to action. United Nations Environment Programme-World Conservation Monitoring Centre, Cambridge
van der Hoeven CA, de Boer WF, Prins HHT (2004) Pooling local expert opinions for estimating mammal densities in tropical rainforests. J Nat Conserv 12:193–204
Walters BB, Rönbäck P, Kovacs JM, Crona B, Hussain SA, Badola R, Primavera JH, Barbier E, Dahdouh-Guebas F (2008) Ethnobiology, socio-economics and management of mangrove forests: a review. Aquat Bot 89:220–236
Wang G, Dongsheng G, Peart MR, Chen Y, Peng Y (2013) Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of south China. Forest Ecol Manag 310:539–546
Wells (1999) The birds of the Thai-Malay Peninsula, Vol. 1 non-passerines. Academic Press, London
Wilmé L (1996) Composition and characteristics of bird communities in Madagascar. In: Lourenço W (ed) Biogéographie de Madagascar. Editions ORSTOM, Paris, pp 349–362
Woolaver L, Nichols R, Razafindrajao F, Hawkins F (2004) Sighting of Van Dam’s Vanga Xenopirostris damii (Schlegel, 1866) in mangroves of north-west Madagascar. Bull BOC 124:69–71
Young HG (2006) Madagascar teal Anas bernieri: the ecology and conservation of a short distance migrant. In: Boere GC, Galbraith CA, Stroud DA (eds) Waterbirds around the world. The Stationery Office, Edinburgh, pp 252–254
Young HG, Razafindrajao F, Bin Aboudou AI, Woolaver LG, Lewis RE (2013) Madagascar teal Anas bernieri: a mangrove specialist from Madagascar’s west coast. In: Gleason G, Victor TR (eds) Mangrove ecosystems: biogeography, genetic diversity and conservation strategies. Nova, New York, pp 157–166
Acknowledgments
We are grateful to Raymond Raherindray, Christian Randimbiarison, Bienvenue Zafindrasilivonona and Ferdinand Botsy for logistical support and assistance in the field, and to the members of Fizamiti, Ankameva and Tsy Omenkavana CLB and villagers of Andrekareka (Ankatafa), Ankazomborona and Antsahampano for facilitating our research and sharing their knowledge of the mangroves and their birds. We also thank WWF and l’Homme et l’Environnement for logistical support, and the Waterloo Foundation, Darwin Initiative and Global Environment Facility who funded the research. Leah Glass and three anonymous reviewers provided comments which improved an earlier version of the manuscript.
Funding
This research was carried out within the framework of mangrove conservation projects funded by The Waterloo Foundation (Award number 449-1421), The Darwin Initiative (Award number 19-016) and Global Environment Facility (Award number 4452). All awards were made to Blue Ventures Conservation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gardner, C.J., Andriamahenina, Z., Carro, A. et al. Rapid assessments and local knowledge reveal high bird diversity in mangroves of north-west Madagascar. Wetlands Ecol Manage 25, 45–58 (2017). https://doi.org/10.1007/s11273-016-9501-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-016-9501-3