Abstract
The global significance of carbon storage in Indonesia’s coastal wetlands was assessed based on published and unpublished measurements of the organic carbon content of living seagrass and mangrove biomass and soil pools. For seagrasses, median above- and below-ground biomass was 0.29 and 1.13 Mg C ha−1 respectively; the median soil pool was 118.1 Mg C ha−1. Combining plant biomass and soil, median carbon storage in an Indonesian seagrass meadow is 119.5 Mg C ha−1. Extrapolated to the estimated total seagrass area of 30,000 km2, the national storage value is 368.5 Tg C. For mangroves, median above- and below-ground biomass was 159.1 and 16.7 Mg C ha−1, respectively; the median soil pool was 774.7 Mg C ha−1. The median carbon storage in an Indonesian mangrove forest is 950.5 Mg C ha−1. Extrapolated to the total estimated mangrove area of 31,894 km2, the national storage value is 3.0 Pg C, a likely underestimate if these habitats sequester carbon at soil depths >1 m and/or sequester inorganic carbon. Together, Indonesia’s seagrasses and mangroves conservatively account for 3.4 Pg C, roughly 17 % of the world’s blue carbon reservoir. Continued degradation and destruction of these wetlands has important consequences for CO2 emissions and dissolved carbon exchange with adjacent coastal waters. We estimate that roughly 29,040 Gg CO2 (eq.) is returned annually to the atmosphere–ocean pool. This amount is equivalent to about 3.2 % of Indonesia’s annual emissions associated with forest and peat land conversion. These results highlight the urgent need for blue carbon and REDD+ projects as a means to stem the decline in wetland area and to mitigate the release of a significant fraction of the world’s coastal carbon stores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indonesia, one of the world’s largest countries in area and population, straddles nearly one-tenth (5120 km) of the equator, encompassing 13,466 islandsFootnote 1 of which only about 4000 are inhabited. The coast of Indonesia stretches for more than 95,180 km in length, giving it one of the longest coastlines in the world, and houses some of the world’s richest tropical marine ecosystems, including coral reefs, mangroves and seagrass meadows, within 3.6 million km2 of territorial seas. These ecosystems form a large part of the Coral Triangle of the Indo-Pacific, termed ‘the center of origin’ for many of the world’s tropical marine flora and fauna (Veron et al. 2011).
Indonesia’s coral reefs and coastal wetlands are, however, in trouble. Most of the nation’s coral reefs are destroyed or badly degraded (Tun et al. 2008), and nearly half of the archipelago’s mangroves have been lost mostly to aquaculture and coastal development during the past 50 years (Kusmana 2014). Of the 31,894 km2 of existing mangrove wetland (Spalding et al. 2010), 31 % are in good condition, 27 % are moderately degraded and the remaining 42 % of mangrove forests are heavily degraded (ASCNM 2009). The status of Indonesia’s seagrasses is poorly known; some local seagrass beds are well described as are the distribution of the major seagrass species, but there is no national or regional inventory (Nadiarti et al. 2012). At least one source (UNEP 2008) quotes a decline in Indonesian seagrass cover of 30–40 % since the 1960s and Green and Short (2003) estimate that seagrasses cover 30,000 km2 of Indonesian seas, but there is little quantitative data. What can be said for certain is that the trend is for a decline in seagrass area (Ooi et al. 2011; Short et al. 2014).
Considering the large losses of mangrove and seagrass habitats and the continuing trends throughout Indonesia, it is important to know and conserve the size of their below- and above-ground carbon pools because the carbon from these destroyed and degraded ecosystems is eventually lost to the atmosphere and coastal ocean. Recent calculations (Donato et al. 2011; Pendelton et al. 2012; Alongi and Mukhopadhyay 2014) indicate that the global destruction of mangrove carbon stocks at the current deforestation rate of 1 % results in an annual release of 90–970 Tg C years−1 to the atmosphere/ocean pools, and add an additional 10 % to global CO2 release from deforestation of tropical terrestrial forests. This estimate reflects the fact that mangroves store more carbon (956 t C ha−1; Alongi 2014) than other ecosystems, such as rainforests (481 t C ha−1) and salt marshes (593 t C ha−1); mangrove carbon is stored mostly (75 %) below-ground as it is in seagrass meadows (>90 %). Recent assessments of the carbon sequestration capacity of seagrasses (Fourqurean et al. 2012; Duarte et al. 2013a, b; Lavery et al. 2013; Siikamäki et al. 2013; Macreadie et al. 2014) indicate that, like mangroves, they are among the most effective ecosystems for storing carbon, and that losses of seagrass meadows could contribute an additional 11–90 Tg C years−1 to the atmosphere (Fourqurean et al. 2012; Pendleton et al. 2012).
The carbon stored in these vegetated ecosystems (including salt marshes that are mainly temperate) has been termed ‘blue carbon’ (Pendleton et al. 2012). A growth of schemes to conserve and restore wetland blue carbon (but not solely as part of the REDD+Footnote 2 umbrella) has emerged over the past few years. Their objective is to enhance carbon sequestration both as a means to ameliorate the rise in carbon emissions and to protect and conserve these rapidly disappearing habitats—partly to avoid the return of these considerable carbon sinks to the atmosphere and coastal ocean (Mcleod et al. 2011; Pendleton et al. 2012). Naturally, Indonesia stands out as a high-priority nation for such blue carbon projects, owing to the areal extent and size of its mangrove forests and seagrass meadows, but to date only one assessment of mangrove carbon stocks (Murdiyarso et al. 2015) has been made.
This paper addresses this lack of information by using published and unpublished measurements of the organic carbon content (Corg) of living seagrass and mangrove biomass and soil pools to estimate the size and significance of Indonesia’s blue carbon stocks compared to the global wetland C stores. We also estimate the magnitude of the continuing losses and return of Indonesia’s wetland carbon to the atmosphere/ocean pools compared with the current rates of deforestation. We hope that these estimates will highlight the global importance of Indonesia’s coastal wetlands.
Data sources, assumptions and carbon estimates
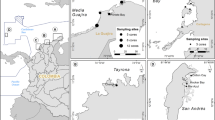
Data on seagrass and mangrove Corg content of living biomass and Corg content and dry bulk density (DBD) of soils were compiled from a number of locations (Fig. 1) to deliver preliminary estimates of carbon stocks of these Indonesian ecosystems. These data are from published sources obtained using literature values and augmented by unpublished data, when available. Different data sources invariably use different methods, but we treated all data equally and standardized all nomenclature across the data. Units are in Mg C ha−1 except where noted.
Seagrasses
An additional criterion for data selection was that each source contains empirical data from individual sites for at least two of the three carbon pools: above-ground biomass, below-ground biomass and the soil pool. The total soil Corg pool was standardized to a soil depth of 1 m. Soil surface values were extrapolated to 1 m using the formulae derived by Fourqurean et al. (2012) from all known seagrass soil Corg data: for Corg, −0.005 ± 0.003log10 (Corg + 1) cm−1; for DBD, 8.6 ± 4.0 (mg (dry weight) ml−1) cm−1, except in one study (Alongi et al. 2008a) where the known maximum depth of core penetration was less (Fig. 2). Seagrass biomass (DW) was converted to Corg using the Corg content measured for the dominant species in each reference listed in Table 1.
Vertical profiles of mean (±1 SE) organic carbon concentrations (as % soil dry weight) with increasing soil depth in three seagrass meadows in Sulawesi, Indonesia. Site SB1 was a meadow dominated by Enhalus acoroides and located a few meters from the mouth of a mangrove-lined creek, with very fine sandy soil (40 % CaCO3). Site SB2 was a meadow dominated by E. acoroides, Halodule uninervis, and Cymodocea rotundata and located further seawards, composed of very fine sandy soil (70 % CaCO3). Site SB3 was a meadow located adjacent to a coral reef, composed of fine carbonate (83 % CaCO3) sand, and dominated by the above species as well as C. serrulata and Thalassia hemprichii. Alongi et al. (2008a) provides a full description of these sites and soil C methods. Briefly, triplicate sediment samples were taken using a hand-held stainless steel corer. Dried sub-samples were processes for determination of total C (TC) on a Perkin-Elmer 2400 CHNS/O Series II Analyzer and for total organic carbon (TOC) on a Shimadzu TOC Analyzer with solid sampler. Total inorganic carbon was assumed CaCO3, as determined by difference between the TC and TOC concentrations
All seagrass meadows sampled in Indonesia were composed of sandy soils, or in some cases, were growing on coral rubble (Table 1), and were dominated by Enhalus acoroides, Thalassia hemprichii, Halodule uninervis, Cymodocea rotundata, or Cymodocea serrulata. The data from all sites (Fig. 3) showed asymmetric distribution with very few values greater than 0.6 Mg C ha−1 for above-ground carbon biomass (AGCB) and 3 Mg C ha−1 for below-ground carbon biomass (BGCB). AGCB ranged from 0.01 to 1.15 Mg C ha−1 with a median of 0.29 and a mean (±1 SE) of 0.32 ± 0.03 Mg C ha−1 (Table 1). BGCB was greater than ABCG at all locations and ranged from 0.02 to 4.84 Mg C ha−1 with a median of 1.13 and a mean (±1 SE) of 1.23 ± 0.11 Mg C ha−1 (Table 1). The largest carbon pool was the soil, ranging from 31.3 to 293.3 Mg C ha−1 with a median of 118.1 and a mean (±1 SE) of 129.9 ± 9.6 Mg C ha−1 (Table 1). Seagrass AGCB and BGCB correlated positively (Pearson’s r = 0.501; P < 0.00000167) but biomass did not correlate with soil carbon. The sum of these three pools is the median carbon storage for an Indonesian seagrass meadow, which is 119.5 Mg C ha−1. Extrapolating this value to the total area of seagrasses (30,000 km2) gives a total seagrass carbon storage value in Indonesia of 368.5 ± 19.5 Tg C or 0.3685 ± 0.002 Pg C.
Frequency distribution of reported observations of above-ground carbon biomass (AGCB, top histogram), below-ground carbon-biomass (BGCB, middle histogram) and soil carbon (bottom histogram) from individual sample sites in Indonesian seagrass meadows (mean values for locations are in Table 1)
Mangroves
An additional criterion for the acceptance of mangrove data sources was documentation in appropriate detail of above- and below biomass and of soil C stocks to a soil depth of 1 m or down to bedrock; other biomass data exist for Indonesia, but these sources had inadequate methodological details, lack of replicate sample plots, and/or insufficient soil data, so they were excluded from our analysis. We realize that soil carbon stocks of mangroves are frequently >1 m (see Donato et al. 2011), but in order to make comparisons we limited to this depth. Similarly, references containing only soil C data but no or insufficient above- and below-ground biomass data were also excluded. These criteria resulted in the acceptance of only two sources (Table 2). Nevertheless, both of these published sources used identical methods (Howard et al. 2014) and contain data from 37 different mangrove forests in Sumatra, Sulawesi, Java, West Papua and Kalimantan and encompass all known forest types (estuarine, riverine, fringing, carbonate settings).
The data from all forest plots (Fig. 4) showed asymmetric distribution especially for above-ground carbon biomass (AGCB) with very few values greater than 350 Mg C ha−1. AGCB ranged from 8.2 to 478.4 Mg C ha−1 with a median of 159.1 and a mean (±1 SE) of 191.2 ± 20.2 Mg C ha−1 (Table 2). Below-ground carbon biomass (BGCB) was less than AGCB at all locations, ranging from 2.5 to 58.6 Mg C ha−1 with a median of 16.7 and a mean (±1 SE) of 21.1 ± 2.7 Mg C ha−1(Table 2). The largest reservoir was the soil pool, ranging from 18.8 to 1569.3 Mg C ha−1 with a median of 774.7 and a mean (±1 SE) of 761.3 ± 73.6 Mg C ha−1 (Table 2). In one Sumatran forest growing atop a relict coral reef, the largest C pool was the mangrove forest canopy (Alongi et al. 2008b). Mangrove AGCB and BGCB correlated positively (Pearson’s r = 0.436; P < 0.007) but neither biomass pool correlated with soil carbon.
Frequency distribution of reported observations of above-ground carbon biomass (AGCB, top histogram), below-ground carbon-biomass (BGCB, middle histogram) and soil carbon (bottom histogram) from individual sample sites in Indonesian mangrove forests (mean values for locations are in Table 2)
Summing the median AGCB, BGCB and soil pools (limited to 1 m depth), we derive a median carbon storage value for an Indonesian mangrove forest of 950.5 ± 29.9 Mg C ha−1. Extrapolating this value to the total area of mangroves (31,894 km2) results in a total mangrove carbon storage in Indonesia of 3.0 ± 0.1 Pg C.
Discussion
The sum of seagrass and mangrove carbon storage in Indonesia is 3.4 Pg C and can be compared to the global estimate of carbon storage for all coastal wetlands of 20 Pg C (10 PgC estimated by Chmura et al. (2003) for tidal marshes and mangroves, plus an equivalent Corg storage (Fourqurean et al. 2012) for seagrasses). If accurate, we can estimate that Indonesia’s estuarine and marine wetlands store roughly 17 % of the world’s blue carbon. Unfortunately, due to the lack of sufficient data on mass sediment accumulation of mangrove and seagrass soils in Indonesia, it is not possible to derive an annual carbon burial rate. Such data is urgently needed. Sequestration rates can be derived for plant biomass (assuming such stocks are not burned or clear-felled), but these are wholly inadequate because soils account for 82 and 99 % of the total carbon reserves for the archipelago’s mangroves and seagrasses, respectively (Tables 1, 2). One empirical estimate indicates carbon sequestration (estimated from sediment accretion using surface elevation tables) in mangroves in Bali of 2.2 ± 0.6 Mg C ha−1 years−1 (Sidik 2014) which is very close to the global mean burial rate of 1.7 Mg C ha−1 years−1. Using the Bali estimate, this means that approximately 7 Tg of carbon is buried in mangrove soils throughout Indonesia annually.
Refinement of the carbon storage estimates in the future may result in an even higher percentage of the world’s total, especially for Indonesia’s seagrasses, for several reasons. First, the areal coverage of Indonesia’s seagrass meadows is most likely greater than 30,000 km2 because many of the shallow coastal waters of the country’s 13,466 islands remain unsurveyed (Ooi et al. 2011). Green and Short (2003) themselves maintained that their figure was a likely underestimate. Second, only the senior author has made any attempt to sample Indonesian seagrass deposits to maximum depth of penetration; most of the available studies (Table 1) sampled only surface soils. Third, in other areas of Southeast Asia experiencing increased river runoff due to deforestation (Terrados et al. 1998; Gacia et al. 2003), many seagrass meadows in Indonesia likely inhabit finer sediment deposits than those locations cited in Table 1. Thus, the average soil carbon pool in an Indonesian seagrass meadow is probably much greater than estimated here, considering also that recent seagrass carbon studies (Fourqurean et al. 2012) have been able to penetrate seagrass meadows across the globe to a soil depth of 1 meter. The mangrove soil data are also underestimates of the true soil stocks as a number of studies (Donato et al. 2011; Murdiyarso et al. 2015) have found significant carbon stocks deeper than 1 meter. Further, it has been demonstrated that when mangroves are disturbed carbon losses from depth >1 m have been measured (Ong 1993; Kauffman et al. 2014).
The data reported here for Indonesia’s mangroves is likely more spatially representative than that for the seagrasses, but nevertheless, many islands remain unsurveyed (Fig. 1). Alongi et al. (2008b) discovered that many coral reefs throughout the archipelago have been smothered by catchment soils due to enhanced land erosion and subsequent transport, leading to colonization and development of mangroves. Although no quantitative data exist, sedimentation has increased since the 1970s to the extent that fringing reefs are rapidly disappearing and being replaced by mangroves and seagrasses (Budiman et al. 1986; Yulianto et al. 2004; Alongi et al. 2008b; Sekiguchi and Aksornkoae 2008). Therefore, despite ongoing deforestation, there are some areas where mangrove and seagrass ecosystems area are expanding at the expense of the archipelago’s coral reefs, providing that increased sediment loading does not decrease the amount of light reaching the bottom to fuel seagrass photosynthesis. The net result of enhanced land erosion may well be enhanced coastal carbon storage.
That Indonesia’s coastal wetlands store approximately one-fifth of the world’s blue carbon is not totally unexpected if one considers that the archipelago’s mangroves and seagrasses account for about 21 % of the global total coverage of mangroves and at least 10 % of seagrasses (Green and Short 2003; Spalding et al. 2010). Further, the carbon stocks of mangroves located at lower latitudes are higher than those of higher latitudes (Alongi 2009). Indonesian seagrasses may or may not store more carbon in soils as we only report on the top 50 cm; data currently in hand would suggest that Indonesian seagrasses may store less Corg in above- (mean = 0.32 Mg C ha−1) and below-ground biomass (mean = 1.23 Mg C ha−1) than the global averages of 0.76 and 1.76 Mg ha−1 recorded by Fourqurean et al. (2012). The ‘average’ mangrove forest in Indonesia stores roughly as much Corg (mean = 950.5 Mg C ha−1) as they do globally (mean = 956 Mg C ha−1), but slightly more proportionally below-ground (83 vs 75 %) in soil and roots (Alongi 2014). But these differences with the global averages may be more representative of the small sample size in Indonesia compared with the global dataset rather than of true differences in the distribution of carbon.
While Indonesia likely has at least one-fifth of the world’s blue carbon stores, continuing and alarming destruction and degradation of the nation’s mangrove forests and seagrass meadows does have important consequences. This is not limited only to coastal stability and sustainability, but also in terms of greenhouse gas emissions. CO2 emissions have been both estimated (Alongi et al. 1998; Pendleton et al. 2012; Siikamäki et al. 2012) and observed after degradation of mangrove forests (Lovelock et al. 2011; Sidik and Lovelock 2013; Kauffman et al. 2014). Given the continuing mangrove deforestation rate of 1 % of area annually, and assuming that 88 % of the entire carbon store in above- and below ground biomass as well as in soil to a depth of 1 meter is oxidized to CO2 during the destruction process (Kauffman et al. 2014), the amount of CO2 returned annually to the atmosphere/ocean pools is roughly 26,400 Gg CO2 or 29,040 Gg CO2 if we make the identical assumptions for seagrasses (see Kennedy et al. (2014) for level of uncertainty and validity of these assumptions). This estimate is equivalent to about 3.2 % of the annual CO2 emissions associated with forest and net forest and peatland conversion (906,874 Gg CO2 years−1) in Indonesia (FAO 2012), which is roughly proportional to the total area of coastal wetlands to forest area (World Bank 2012). Our emissions estimate is only a small proportion of the 0.15–1.02 Pg CO2 that may be released by land-use change of all of the world’s coastal wetlands (Pendleton et al. 2012).
Nevertheless, REDD+ and other climate mitigation projects that conserve the present carbon stores as well as those activities that restore mangrove and seagrass habitats are urgently required. This is not simply to maintain and restore Indonesia’s carbon stocks and the other important ecosystem services that arise from these ecosystems, but to prevent a significant fraction of the world’s marine carbon reserves from being lost to the atmosphere and ocean carbon pools.
Notes
GIS surveys beginning in 2011 have reduced the widely-accepted island total from 17,080. This difference is due to the increased accuracy of GIS as well as to not including inlets under the definition of island in the UN Law of the Sea Convention (https://www.unstats.un.org/unsd/geoinfo/UNGEGN/docs/10th-uncsgn-doc/E_CONF.101_134_The%20Naming%20Procedures%20%Of%20/Indonesia%20%20Islands.pdf).
REDD+ stands for efforts to reduce emissions from deforestation and degradation, and the role of conservation, sustainable management of forests, and enhancement of forest carbon stocks in developing countries (+).
References
Alongi DM (2009) The energetics of mangrove forests. Springer, Amsterdam
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6:195–219
Alongi DM, Mukhopadhyay SK (2014) Contribution of mangroves to coastal carbon cycling in low latitude seas. Agric For Meteorol. doi:10.1016/j.agrformet.2014.10.005
Alongi DM, Sasekumar A, Tirendi F, Dixon P (1998) The influence of stand age on benthic decomposition and recycling of organic matter in managed mangrove forests of Malaysia. J Exp Mar Biol Ecol 225:197–218
Alongi DM, Trott LA, Undu MC, Tirendi F (2008a) Benthic microbial metabolism in seagrass meadows along a carbonate gradient in Sulawesi, Indonesia. Aquat Microb Ecol 51:141–152. doi:10.3354/ame01191
Alongi DM, Trott LA, Rachmansyah TF, Mckinnon AD, Undu MC (2008b) Growth and development of mangrove forests overlying smothered coral reefs, Sulawesi and Sumatra, Indonesia. Mar Ecol Prog Ser 370:97–109
ASCNM (2009) Peta mangroves Indonesia. Pusat Survey Sumber Daya Alam Laut Badam Koordinasi Survey dan Pemetaan Nasional, Cibinong
Booij K, Hillebrand TJ, Nolting RF, van Ooijen J (2001) Nutrients, trace metals, and organic contaminants in Banten Bay, Indonesia. Mar Pollut Bull 42:1187–1190
Budiman A, Kartawinata K, Prowiroatmodjo S, Sapulete D (1986) Coral reef-associated mangrove communities in Indonesia. In: Soemodihardjo S (ed) Proceedings of MAB-COMAR regional workshop on coral reef ecosystems: their management practices and research/training needs. UNESCO, Jakarta, pp 12–119
Campbell JE, Lacey EA, Decker RA, Crooks S, Fourqurean JW (2015) Carbon storage in seagrass beds of Abu Dhabi, United Arab Emirates. Estuar Coasts 38:242–251
Chmura GL, Anisfield SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycles 17:1111–1123
Christianen MJA, Govers LL, Bouma TJ, Kiswara W, Roelofs JGM, Lamers LPM, van Katwijk MM (2012) Marine megaherbivore grazing may increase seagrass tolerance to high nutrient loads. J Ecol 100:546–560
Christianen MJA, van Belzen J, Herman PMJ, van Katwijk MM, Lamers LPM, van Leent PJM, Bouma TJ (2013) Low-canopy seagrass beds still provide important coastal protection services. PLoS One 8:e62413
Delongh HH, Wenno BJ, Meelis E (1995) Seagrass distribution and seasonal biomass changes in relation to dugong grazing in the Moluccas, east Indonesia. Aquat Bot 50:1–19
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297
Duarte CM, Kennedy H, Marbà N, Hendriks I (2013a) Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies. Ocean Coast Manag 83:32–38
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013b) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Change 3:961–968
Erftemeijer PLA (1994) Differences in nutrient concentrations and resources between seagrass communities on carbonate and terrigenous sediments in south Sulawesi, Indonesia. Bull Mar Sci 54:403–419
Erftemeijer PLA, Herman PMJ (1994) Seasonal changes in environmental variables, biomass, production, and nutrient contents in two contrasting tropical intertidal seagrass beds in South Sulawesi, Indonesia. Oecologia 99:45–59
Erftemeijer PLA, Middelburg JJ (1993) Sediment-nutrient interactions in tropical seagrass beds: a comparison between a terrigenous and a carbonate sedimentary environment in South Sulawesi (Indonesia). Mar Ecol Prog Ser 102:187–198
FAO (2012) Statistics database. http://www.faostat3.fao.org/home/E
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krausse-Jensen D, McGlathery SO (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci. doi:10.1038/NGEO1477
Gacia E, Duarte CM, Marbá N, Terrados J, Kennedy H, Fortes MD, Tri NH (2003) Sediment deposition and production in SE Asia seagrass meadows. Estuar Coast Shelf Sci 56:909–919
Green EP, Short FT (2003) World atlas of seagrasses. University of California Press, Berkeley
Howard J, Hoyt S, Isensee K, Telszewski M, Pidgeon E, eds (2014) Coastal blue carbon: methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrasses. Conservational International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature, Arlington. http://www.thebluecarboninitiative.org/manual/
Kauffman JB, Heider C, Norfolk J, Payton F (2014) Carbon stocks of intact mangroves and carbon emissions arising from their conservation in the Dominican Republic. Ecol Appl 24(3):518–527
Kennedy H, Alongi DM, Karim A, Chen G, Chmura GL, Crooks S, Kairo JG, Liao B, Lin G (2014) Coastal Wetlands, Chapter 4. In: Hiraishi T, Krug T, Tanabe T, Srivastava N, Baasansuren J, Fukuda M, Troxler TG (eds) (2013) Supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: wetlands. IPCC, Switzerland, pp 4.1–4.55. http://www.ipcc-nggip.iges.or.jp/public/wetlands/index.html
Kiswara W (1992) Community structure and biomass distribution of seagrasses at Banten Bay, West Java, Indonesia. In: Chou LM, Wilkinson CR (eds) Third ASEAN science and Technology work conference proceedings, Marine science: living coastal resources, vol 6. Department of Zoology, National University of Singapore and National Science and Technology Board, Singapore, pp 241–250
Kusmana C (2014) Distribution and current status of mangrove forests in Indonesia. In: Faridah-Hanum I, Latiff A, Hakeem KR, Ozturk M (eds) Mangrove ecosystems of Asia. Springer, New York, pp 37–60
Lavery PS, Mateo M-Á, Serrano O, Rozalmi M (2013) Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service. PLoS One 8(9):e73748. doi:10.1371/journal.pone.0073748
Lovelock CE, Feller IC, Ruess RW (2011) CO2 efflux from cleared mangrove peat. PLoS One 6:e21279. doi:10.1371/journal.pone.0021279
Macreadie PI, Baird ME, Trevathan-Tackett SM, Larkum AWD, Ralph PJ (2014) Quantifying and modelling the carbon sequestration capacity of seagrass meadows—a critical assessment. Mar Pollut Bull 83:430–439
Mcleod E, Chmura GL, Bouillon S, Salm R, Björk DC, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Murdiyarso D, Purbopusito J, Kauffman JB, Warren MW, Sasmito SD, Donato DC, Manuri S, Krisnawati H, Taberima S, Kurnianto S (2015) The potentials of Indonesian mangrove forests for global change mitigation. Nat Clim Change. doi:10.1038/nclimate2734
Nadiarti RE, Djuwita B, Purbayanto A, Asmus H (2012) Challenging for seagrass management in Indonesia. J Coast Dev 15:234–242
Nienhuis PH, Coosen J, Kiswara W (1989) Community structure and biomass distribution of seagrasses and macrofauna in the Flores Sea, Indonesia. Neth J Sea Res 23:197–214
Ong JE (1993) Mangroves—a carbon source and sink. Chemosphere 27:1097–1107
Ooi JLS, Kendrick GA, Van Niel KP, Affendi YA (2011) Knowledge gaps in tropical Southeast Asian seagrass systems. Estuar Coast Shelf Sci 92:118–131
Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, Sifleet S, Craft C, Fourqurean JW, Kauffman JB, Marba N, Megonigal P, Pidgeon E, Herr D, Gordon D, Baldera A (2012) Estimating global “blue carbon’ emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7:e43542. doi:10.1371/journal.pone.0043542
Pratono T, Razak H, Gunawan I (2009) Organochlorine pesticides in the coastal sediment of Citarum estuary, Jakarta Bay: the important role of fine fraction sediment as their agent in transport and processes of their early diagenesis. J I Teknologi Kelautan Tropis 1:23–26
Priosambodo D (2006) Growth rate and production of tropical seagrass Enhalus acoroides (L.) f. Royle in Awerange and Labuange Bays, Barru Regency, south Sulawesi. Torani Bull Mar Sci 16:334–345
Sekiguchi H, Aksornkoae S (2008) Environmental problems in the coastal zone. In: Mimura N (ed) Asia-Pacific coasts and their management: state of the environment. Springer, Dordrecht, pp 65–171
Short FT, Coles R, Fortes MD, Victor S, Salik M, Isnain I, Andrew J, Seno A (2014) Monitoring in the Western Pacific region shows evidence of seagrass decline in line with global trends. Mar Pollut Bull 83:408–416
Sidik F (2014) Mangrove forest responses to environmental change in Indonesia. PhD dissertation, University of Queensland
Sidik F, Lovelock CE (2013) CO2 efflux from shrimp ponds in Indonesia. PLoS One 8:e66329. doi:10.1371/journal.pone.0066329
Siikamäki J, Sanchirico JN, Jardine SL (2012) Global economic potential for reducing carbon dioxide emissions from mangrove loss. Proc Natl Acad Sci USA 109:14369–14374. doi:10.1073/pnas.1200519109
Siikamäki J, Sanchirico JN, Jardine SL, McLaughlin D, Morris D (2013) Blue carbon: coastal ecosystems, their carbon storage, and potential for reducing emissions. Environment 55:6. doi:10.1080/00139157.2013.843981
Spalding M, Kainuma M, Collins L (2010) World atlas of mangroves. Earthscan, London
Stapel J, Hemminga MA, Bogert CG, Maas YEM (2001) Nitrogen (15N) retention in small Thalassia hemprichii seagrass plots in an offshore meadow in South Sulawesi, Indonesia. Limnol Oceanogr 46:24–37
Supriadi KR, Bengen DG, Hutomo M (2014) Carbon stock of seagrass community in Barranglompo Island, Makassar. ILMU Kelautan 19:1–10
Terrados J, Duarte CM, Fortes MD, Borum J, Agawin NSR, Bach S, Thampanya U, Kamo-Nielsen KW, Geertz-Hanse O, Vermaat J (1998) Changes in community structure and biomass of seagrass communities along gradients of siltation in SE Asia. Estuar Coast Shelf Sci 46:757–768
Tun K, Ming CJ, Yeemin T, Phongsuwan N, Amri AY, Ho N, Sour K, Long NV, Nanola C, Lane D, Tuti Y (2008) Status of coral reefs in Southeast Asia. In: Wilkinson CR (ed) Status of coral reefs of the world: 2008. Global coral reef monitoring network and reef and rainforest research centre, Townsville, pp 131–144
UNEP (2008) National reports on seagrass in the South China Sea. UNEP/GEF/SCS Technical Publication No. 12
Van Katwijk MM, van der Welle MEW, Lucassen E, Vonk JA, Christianen MJA, Kiswara W, al Hakim I II, Arifin A, Bouma TJ, Roelofs JGM, Lamers LPM (2011) Early warning indicators for river nutrient and sediment loads in tropical seagrass beds: a benchmark from a near-pristine archipelago in Indonesia. Mar Pollut Bull 62:1512–1520
Veron JEN, Devantier LM, Turak E, Green AL, Kininmonth S, Stafford-Smith M, Peterson N (2011) The Coral Triangle. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 47–55
Vonk JA, Christianen MJA, Stapel J (2010) Abundance, edge effect, and seasonality of fauna in mixed-species seagrass meadows in southwest Sulawesi, Indonesia. Mar Biol Res 6:282–291
World Bank (2012) National statistics. http://www.data.worldbank.org/indicator/AG.LND.FRST.ZS
Yulianto E, Supkapti WS, Rahardjo AT, Noeradi D, Siregar DA, Suparan P, Hirakawa K (2004) Mangrove shoreline responses to Holocene environmental change, Makassar Strait, Indonesia. Rev Paleobot Palynol 131:251–268
Acknowledgments
This is contribution no. 736 from the Southeast Environmental Research Center at Florida International University and is a publication of the Blue Carbon Initiative, Washington DC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alongi, D.M., Murdiyarso, D., Fourqurean, J.W. et al. Indonesia’s blue carbon: a globally significant and vulnerable sink for seagrass and mangrove carbon. Wetlands Ecol Manage 24, 3–13 (2016). https://doi.org/10.1007/s11273-015-9446-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-015-9446-y