Abstract
This review of literature analyses the effectiveness of three commercially available filters, namely the Pall Envirochek filters, IDEXX FiltaMax, and Whatman flatbed membrane, in the process of concentrating Cryptosporidium oocysts and Giardia cysts from water samples. Moreover, several research studies investigated the possibility of using ultrafiltration filters as an affordable substitute for pathogen concentration, and their efficacy for protozoa is reviewed here and compared against the existing commercial filter options. Because of the differences in sampling procedures, no one method for concentrating these protozoa consistently outperforms others. This comparative article summarises our current understanding of the recent water sampling techniques to concentrate waterborne protozoans and highlights the remaining knowledge gaps. While ultrafiltration is a financially practical alternative for the concentration of pathogens, this paper highlights that further investigation is required to optimise their efficacy, particularly for low numbers of oocysts and cysts. These insights play a substantial role in providing information for advancing enhanced water sampling and treatment systems, reducing the dangers connected with waterborne protozoans, and ensuring the protection of public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cryptosporidium and Giardia, and they are abbreviated in this review by oo(cysts), are major protozoan pathogens contributors to waterborne illness, present enormous health hazards on a global scale and resulting in significant morbidity and mortality (Abeywardena et al., 2015; Ahuja, 2021; Luka et al., 2022). These pathogens demonstrate extended viability in aquatic environments (Fayer & Xiao, 2008; Jain et al., 2019; Prasad & Grobelak, 2020) and resistance to traditional disinfection techniques (Abeledo-Lameiro et al., 2018; Dixon, 2014; Xiao et al., 2006), emphasising the necessity for improved approaches to detect their low concentrations in sampled water. The current procedures employed for controlling waterborne protozoans mostly focus on water filtration techniques and the early identification of pathogens. However, an optimal method for concentrating water samples, as a part of sampling process to detect these pathogens has not yet been determined.
The most common method for water sampling and detection is the US Environment Protection Agency US EPA 1623.1 in Fig. 1 (Efstratiou et al., 2017; EPA, 2001; Feng et al., 2003; Omarova et al., 2018; Prystajecky et al., 2014). The UK is following the UK Drinking Water Inspectorate (UKDWI) while Japan, Australia, and New Zealand, in addition to the US EPA method, have their own standards for water sampling and detection. Envirochek® HV Sampling Capsule and Filta-Max® Foam filter are approved by both US EPA and UK DWI (EPA, 2012b; Rhodes et al., 2012) with the UK DWI also endorsing the flat-bed membrane (SCA, 2010b).
These testing methods involve multiple process steps from filtration of a large sample to isolation and identification of oocysts (EPA, 2012b; Luka et al., 2022). Water sampling is an essential process toward further analysis; the small volume of sample that transported to laboratory and subjected for monitoring procedure, should represent the entire part of sampled part (Bridle et al., 2021b; Madrid & Zayas, 2007). Concentration of the water sample is a critical step and aids in detecting low concentrations of Cryptosporidium and Giardia.
In the UK, the average recovery of oocysts during the sampling and analysis stages would be approximately 36% at a concentrate of one oocyst per 10 L (Department of Environment, 2000; Drury & Lloyd, 2003). The UK treatment standard for Cryptosporidium in finished drinking water was not derived from known public health standards because of the inherent difficulty in directly linking specific Cryptosporidium concentrations in water to outbreaks; in addition, published reports on outbreaks often lack precise measurements of oocyst levels at the time of contamination (Howe et al., 2002). Consequently, a treatment standard was established to ensure an optimal margin of safety against outbreaks; it concluded a concentration of less than one oocyst per 10 L would represent a level that was easily achievable using well-operated conventional treatment methods; thus, with this regulatory threshold of more than 1000 L of treated water must be constantly filtered over 24 h (Drury & Lloyd, 2003).
Although there isn't a set legal limit for Cryptosporidium in treated drinking water in the United States, the Environmental Protection Agency (EPA) established treatment technique requirements for unfiltered systems that guarantee at least 99.9% of Cryptosporidium oocysts are inactivated when the mean concentrate level is 0.01 oocysts.L−1 and 99.99% are activated when the mean level is greater than 0.01 oocysts.L−1 (CFR, 2024). Regarding Giardia lamblia cysts, it is required to remove or inactivate 99.9% of cysts (Askenaizer, 2003).
Existing sampling methods are labour-intensive, expensive, and inefficient (Vesey et al., 1993). Scientists and researchers, for the last four decades, have been attempting to develop an ideal method for sampling and detecting the low number of infectious waterborne parasites in selected sample; however, there is no ideal and efficient procedure for these processes (Carraro & Palumbo, 2002). Choosing an appropriate filter is crucial to retain targeted pathogen from the filter (Clancy, 2000); both filter choice and operating procedures, during both sampling and subsequent recovery steps, are critical to meet with accepted recovery efficiency (RE) (Vesey et al., 1993). Many investigations have been done to improve the RE of filters; nevertheless, the difficulties still exist because the complication of water matrix which is unpredictable and can vary from sample to another (Efstratiou et al., 2017; Hassan et al., 2021; Ongerth, 2013; Rhodes et al., 2012).
Waterborne pathogen concentration methods were last studied by (Helmi et al., 2010), for standard filters for both drinking and surface water, for comparison HFUF to USEPA 1623 method by (Hill et al., 2009), for the evaluation of five UF filters with drinking water by (Holowecky et al., 2009), and by (Ferguson et al., 2004)for the comparison of UF and standard filters with raw water in 2004.
More recently, reviews of ultrafiltration have been undertaken and an overview of approaches for all pathogens was given in this book (Bridle et al., 2021b). However, to our knowledge there has not been previous work comparing the traditional techniques with recent ultrafiltration systems for Cryptosporidium and Giardia specifically.
This work gathers the latest data through a semi-systematic literature review, employing keywords like "Cryptosporidium," "Giardia," "protozoan pathogens," "filtration," "sampling," and "sample processing" in databases such as Scopus and Science Direct. Articles were selected based on their focus on the filtration of Cryptosporidium and/or Giardia for water quality monitoring, including both review articles and original studies. Additional sources were identified from the initial articles and manufacturer data sheets. The objective is to contribute to the existing knowledge regarding the current understanding of optimal procedures and methodologies for the concentration of oo(cysts) from water samples, tracking concentration methods and addressing gaps that require additional research. The article concentrates on different methods that rely on filters, considering factors such as the volume of the sample, the filter selection, the initial concentration of oo(cysts), the choice of chemical for filter blocking or elution, and the flow rate. The article aims to investigate how these factors impact recovery rates and, whenever feasible, determine the most optimal choices.
2 Existing Methods
2.1 Detection
The ultimate goal of a sampling process is to enable the detection of Cryptosporidium and Giardia, monitoring the safety of water samples. The standard approach is to use microscopy based on immunofluorescences. This method is widely used in water laboratories, and the number of intact oocysts or cysts can be counted. However, the drawbacks are that viability cannot be determined without using additional dyes, and alternative methods, such as nucleic amplification approaches, are required to obtain further information on species, which is of interest in determining the risk to human health. There are 44 different species and over 120 different genotypes of which 19 species and four genotypes have been identified in humans; the most commonly found species in humans are C. hominis, C. parvum, C. meleagridis, C. canis, and C. felis (Gopfert et al., 2022; Ryan et al., 2021). Additionally, viability and infectivity are not the same, and infectivity measures include excystation assays, tissue culture approaches or animal challenges (Rousseau et al., 2018). Detection methods have been reviewed elsewhere such as in (Cazeaux et al., 2022; Luka et al., 2022), and as such this article focuses on the sampling methods.
2.2 Water Sampling
Water sampling is selecting water specimens for further analysis, following standardised procedures, to ultimately determine their characteristics and ensure they are safe (Gaskin, 2005; Huang et al., 2022; Matamoros, 2012); therefore, it is crucial that the small amount of water, that is subjected to the laboratory for analysis, accurately represents the entire sampled area (Bridle et al., 2021b; Madrid & Zayas, 2007). The detection of low level of waterborne pathogens, particularly those such as Cryptosporidium and Giardia for which amplification by culturing is difficult, demands a sample of 10–50 L of surface (raw) water (Burnet et al., 2021; Robertson & Gjerde, 2000), 10–100 L of surface water of low turbidity (SCA, 2010b), or 1000 L of finished water (Bridle et al., 2015; Bridle et al., 2021a; Gavriilidou & Bridle, 2012; Horton et al., 2018). These samples undergo further filtration and concentration before proceeding to detection procedures (EPA, 2011). Thus, water sampling transcends merely grabbing a portion of water for subsequence analysis; it encompasses a series of processes, including transport, storage, filtration, or concentration, and depending on the purpose of the sampling, a variety of characteristics, including chemical, physical, or biological data, might be measured from the water sample (Barroso et al., 2024; Bridle et al., 2021b; DWQR, 2022; EPA, 2011; Gaskin, 2005; Gule et al., 2023; Madrid & Zayas, 2007; SCA, 2010a; Shepard et al., 2006).
In order to eliminate the chance of error because this phase is so important and delicate, it is imperative to address key questions prior to, during, and after the sampling step to prevent any possibility of errors (Madrid & Zayas, 2007). These considerations are outlined as follows.
-
Is the location of the sample grabbing point identified?
-
Is the equipment for sampling appropriate? Was it stored in an approved condition?
-
Is the delivery of the sample to the laboratory scheduled to prevent exceeding the examination plan?
-
Was the sample kept under standard conditions after it was delivered?
-
Does the chosen analysis method fulfil the analysis’s objectives?
-
Have all procedures, materials utilised, and results been recorded?
Therefore, maintaining a line of communication is important between all involved parties to assure that no mistakes were occurred at any point of the sampling process.
Specifically, the requirements for the sample to detect Giardia and Cryptosporidium are to record the sample location, time and date of collection and processing, the sample volume, the name of the sampler, and the sampling flow rate; samples of up to 50 L of surface water, groundwater, or post-filter water may be filtered on-site by an appropriate method or transported to the laboratory in clean polyethene jerry cans; these reusable containers and any other material that used for sampling shall be disinfected before reuse to prevent any cross contamination (SCA, 2010b).
2.3 Sampling and Detection Methods
The US EPA 1623.1 method, Fig. 1, was developed for Cryptosporidium and Giardia detection (EPA, 2012b) as an extension to the US EPA 1622 method to monitor and detect Cryptosporidium in 1999 (EPA, 2001; Feng et al., 2003). The method requires filtrate 10–50 L of raw water (Burnet et al., 2021; Robertson & Gjerde, 2000) or 1000 L of treated water (Bridle et al., 2015; Bridle et al., 2021a; Gavriilidou & Bridle, 2012; Horton et al., 2018). The primary steps of this method are:
-
I.
Filtrate the sample through an approved filter, Envirochek® HV Sampling Capsule and Filta-Max® Foam Filter; the Continuous Flow Centrifuge (PCFC) is also accepted (EPA, 2012b; Rhodes et al., 2012). In this step, oo(cysts) are captured in the filter's membrane to be recovered by washing the filter and concentrated by centrifugation (Bridle et al., 2021a; Feng et al., 2003).
-
II.
Purify and concentrate the oo(cysts) that have been captured using an approved method, such as immunomagnetic separation (IMS), which uses beads coated with Giardia and Cryptosporidium antibodies to separate the oo(cysts) from other microorganisms or (Bridle et al., 2021a; EPA, 2012b; Feng et al., 2003). The oo(cysts) are then separated from the beads for the next step.
-
III.
Use a fluorescent antibody to stain the oo(cysts) on a microscope slide or membrane, then count them at a magnification of 200–400x (Bridle et al., 2021a; EPA, 2012b; Feng et al., 2003; Jellison et al., 2020).
A quality control (QC) procedure shall be followed to validate the sampling procedure; this includes spiking the filter with a known number of Cryptosporidium oocysts and Giardia cysts as a positive control, which shall be distinguishable from environmental oo(cysts), while counting under the microscope. The US EPA 1623 recommend spiking sampling filter with ~ 100–500 oo(cysts) (EPA, 2012b), and 100 oo(cysts) as per UK regulations (SCA, 2010b). The quantity of oo(cysts) utilised in quality control (QC) can be commercially manufactured or sorted employing in-house flow cytometry. The most widely used commercial oo(cysts) for spiking such as ColorSeed™, supplied by Biopoint Pty Ltd, Australia, which are inactivated and labelled with red fluorescent (Texas-red), and became recognised as red under the microscope with a red filter(Al-Sabi et al., 2015; Jain et al., 2019) and green when they were stained with green fluorescein isothiocyanate (FITC) (Arona et al., 2023; Fradette et al., 2022). The recovery efficiency of sampling can be determined as following:
where:
- R:
-
Recovery efficiency.
- Nred:
-
Number of red (control) oo(cysts) counted.
- T:
-
Number of QC oo(cysts) added. Usually, 100.
As the stained environmental oo(cysts) by FITC would be green to the observer, as well as ColorSeed oo(cysts), the number of environmental oo(cysts) can be determined by subtracting the total number of red oo(cysts) from green ones.
There are many limitations of this method such as:
-
I.
Varying recovery ranging between 21 to 100% and 17 to 100% for Cryptosporidium and Giardia respectively, since not all oo(cysts) in a sample would always be recovered by the technique (Hu et al., 2004; Mccuin & Clancy, 2003). According to the EPA 1623 technique, the accepted recovery rates should not be lower than 8% for Giardia spp and 32% for Cryptosporidium spp (EPA, 2012b; Sammarro Silva & Sabogal-Paz, 2021).
-
II.
The method is not suitable for on-site detection since it is labour- and time-intensive, requires specialised staff, and involves complex operations that call for highly skilled professionals (Luka et al., 2022).
-
III.
Precise equipment and reagents are required, contributing to the procedure's overall expenses and intricacy. The reagents needed for separation and staining can be quite costly, and the process of filtering to detection takes a long time. A single procedure, for example, may cost between $250 and $400 (Efstratiou et al., 2017), and the filter alone may cost as much as $120 which restricts how many sample locations and how frequently sampling can be done with the limited budget available to utilities (Jellison et al., 2020). The equipment that is required to complete this procedure, including maintenance, can cost up to $75 k (Efstratiou et al., 2017).
The filtration process retains unwanted particles which cause difficulties in the detection of the oo(cysts) and increases the false positive likelihood. Uncertain recovery rate, contingent upon factors such as the limited sample amount, turbidity, and concentrate levels of the parasites as the low recovery of oo(cysts), particularly in inferior-quality water, is a significant challenge and several studies have indicated that the recovery rate in severely turbid water was either close to or fell below the minimum acceptable thresholds (Efstratiou et al., 2017; Sammarro Silva & Sabogal-Paz, 2021). The US EPA method lacks the ability to distinguish among the oocyst species without utilising polymerase chain reaction (PCR) based analyses; species level detection is useful since not all Cryptosporidium oocysts or Giardia cysts cause a risk to human health (Allen et al., 2000; Jingyi et al., 2023; Xiao et al., 2006).
3 Comparison of Filters
3.1 Capsule Filters
The main capsule filter type utilised is the Envirochek capsule, Fig. 2-a, which is a polycarbonate capsule enclosing a Supor polyethersulfone membrane witha1 µm porosity with an effective filtration area of 0.13 m2 (Bridle et al., 2021b; Clancy, 2000; Ferguson et al., 2004). Since the capsule is approved by the US EPA method 1623.1 for Giardia and Cryptosporidium detection (EPA, 2012b, 2014), UK DWI (SCA, 2010b), and the ISO 15553 standard (BSI, 2006), it is frequently used to sample as much as 50 L of raw water and 1000 L of finished water.
The operational pressure of Envirochek (EC) is 2.1 bar, and 4.2 bar for the Envirochek HV (EC_HV), the Envirochek HV type of this capsule filter can sample raw and finished water including swimming pool water, and wastewater at flowrate up to 3.4 L.min−1; therefore, the inclusion of an inline regulator is necessary to effectively monitor the pressure during the filtering process (Bridle et al., 2021b; EPA, 2014; SCA, 2010b). The next step is utilisation of a non-ionic detergent elution solution and agitation using a wrist shaker, giving an eluate volume > 200 mL, which then requires concentrating parasite through centrifugation (Bridle et al., 2021b; Palumbo et al., 2002; Pires & Dong, 2014). The main advantage of using this capsule filter is, it is applicable for sampling in both on-field and water treatment plant settings. Since the capsule encloses the filter membrane and does not necessitate placement within a specialised filter housing, unlike the FiltaMax filter, it presents the benefits of eliminating the pre-assembly requirements, facilitating ease and timesaving of collection and elution. For instance, direct inline connection with the sampling pipeline is achievable through its inlet and outlet port. However, it is important to note that the filtering efficiency of this method may be affected by water with a high turbidity of > 20 Nephelometric Turbidity Units (NTU) (Palumbo et al., 2002). Additionally, it is worth considering that the cost of the capsule exceeds £200.00 as offered in 2023 by the Fisher Scientific, UK Lab supplies for catalogue’s items 17,194,221 and 1,717,422. The estimated cost of sampling 10 L of ultrapure water according to the US EPA 1623.1 method, using the EC_HV, was £800 and required six hours to accomplish the test (Fradette & Charette, 2022).
3.2 FiltaMax Compressed Foam Filter
The compressed foam filter was invented by Adrian Parton and developed by IDEXX, Fig. 2-b; It utilises 60 expansible foam rings, as a filter medium, which are compressed between retaining plates; making a nominal equivalent pore size of 1 µm to trap Cryptosporidium and Giardia (BSI, 2006; Helmi et al., 2010; IDEXX, 2023). The filter was assessed against 10–20 L of river water and 100 to 2000 L of tab water at flowrate of 1 to 2 L.min−1 (Sartory et al., 1998). According to the US EPA 1623.1 method, to establish flow through the filter, a head pressure of 0.5 bar is needed; a recommended pressure of 5 bar should result in a flow rate of 3 to 4 L.min−1. It is not advised to operate above the 8.0 bar maximum operating pressure (EPA, 2012b).The captured organisms are subsequently released during the decompression of the medium before the eluting process. This decompression allows for the expansion of pores within the filter (Parton, 1997). Studies investigated the efficiency of the filter by evaluating the recovery rate of oo(cysts) in different level of seeding, water type, and sample volumes (Helmi et al., 2010; Horton et al., 2018; Wohlsen et al., 2004).
One of the considerable drawbacks of the FiltaMax filter is that the passage of oo(cysts) through filters can occur; that could be attributed to either the uneven pore size of the filter medium, which allows a few oo(cysts)to pass through the it, or that the oo(cysts) compressed and reform by the impact of high pressure, or both (Peng et al., 2010; SCA, 2010b). One study installed a 142 mm diameter acrylic copolymer membrane filter in series with the outflow of the filters to count the number of Cryptosporidium oocysts that passed or break through the filter; the maximum breakthrough that were found to be outside the filter was 2.0% of the originally seeded oocysts, and up to 5.1% from the prototype of the original filter (Sartory et al., 1998).
Another constraint linked to the filter is the requirement for specialised IDEXX equipment for elution. IDEXX offers two elution systems: the Filta-Max System and the Filta-Max Xpress System. The primary distinction between Filta-Max and Filta-Max Xpress is in the latter’s utilisation of an automated elution system. Once the desired volume of water samples has been obtained, the filter will be retrieved and extracted from the housing cup to proceed with subsequent elution procedures. The filter would undergo decompression to increase its porosity and facilitate the release of caught parasites, and the final volume of the elute is 600–1200 mL (Bridle et al., 2021b; Sartory et al., 1998).
The cost of the equipment is approximately £26,000 which makes this method expensive for routine samples although it is frequently used for water sampling, waterborne monitoring, and detection (Efstratiou et al., 2017; Jellison et al., 2020).
3.3 Flat-bed Membrane Filtration
In the late 1980s and early 1990s, a new method for water sampling using flat-bed membrane filtration was established because of the high cost and unpredictability of filter cartridge filtrations.
142 mm or larger of the flat-bed membrane with stainless steel support house is approved by UKDWI, Fig. 2-c, (SCA, 2010b), and the US EPA 1623.1 method accepted the use of the Whatman Nuclepore CrypTest™ filter till 2003 when the filter was excluded from the list of permitted filters as a consequence of its discontinuation by the manufacturer (EPA, 2012b). Because of the tendency of high turbid water to block the membrane filter during the filtration process, this filter is appropriate for analysing various sorts of treatment and distribution waters and source waters with relatively low turgidities (SCA, 2010b). When the sample filtration process is accomplished, the filter is backwashed twice by 300 mL of elution buffer, utilising a peristaltic pump and incubated in an ultrasonic bath for two minutes, then the backwash buffer is subjected to further concentration and analyses as per EPA 1623.1 method (Helmi et al., 2010). The design effectively filtered 1–100 L of water at a rate of 2 L.min−1 using a 2 µm porosity filter (Palumbo et al., 2002). The mean recovery rate can be varied depends on the water type and the concentrate of oo(cysts) in the sample (Falk et al., 1998; Wohlsen et al., 2004).
Membrane filtration has two critical advantages over cartridge filtration: it is less expensive and requires less time in the laboratory for processing. On the other hand, this procedure's drawback is the sample volume limitation; turbidity in the water can cause the filter to clog (Palumbo et al., 2002; Sánchez et al., 2018). The standard membrane filter types can filter 10–40 L of low-turbidity water, while they can only filter up to 2 L of highly turbid water (Palumbo et al., 2002; Zarlenga & Trout, 2004). This filter is competitively inexpensive and costs only £8.64 by Avantor Sciences supplier, catalogue number WHAT10418731. In addition, organism can be obtained without backwashing or eluting since the filter's surface is scraped off and then cleaned with phosphate buffer solution (PBS) (Palumbo et al., 2002).
3.4 Hollow Fibre Ultrafiltrate Filter (HFUF)
Ultrafiltration (UF) is a technique of membrane filtration (Efstratiou et al., 2017) that has a surface area of several square metres and a porosity of 1–100 nm (Rhodes et al., 2011). The technology was initially designed for therapeutic purposes like dialysis; however, it has since become utilised in water sample processing research to concentrate several microorganisms simultaneously (Rhodes et al., 2011); even viruses can be retained as well (Bridle et al., 2021b). Hollow fibre ultrafiltration (HFUF) was initially employed in 2001 by a Japanese research group to enhance the concentration of oo(cysts) (Hashimoto et al., 2001). In addition to capturing several microorganisms simultaneously with the HFUF, it has a shorter processing time and lower cost (£10-£25) compared to cartridge filtering, and the recovery is also more efficient than capsule filtration (Efstratiou et al., 2017; Hashimoto et al., 2001, 2002; Rhodes et al., 2011).
Table 1 demonstrates an overall comparison among reviewed filters including costs and mechanical properties.
3.4.1 Filtration Configurations
There are two primary setups for HFUF to concentrate waterborne pathogens from filtered samples: Tangential Flow Filtration (TFF) and Dead-End Ultrafiltration (DEUF), by which they are commonly employed for separating particles that are larger than the pores in a membrane. The TFF, also known as crossflow filtration, continuously circulates the sample through the filter until the desired concentration volume is achieved, often a few hundred millilitres. This filtered sample can then be utilised for further analysis, as shown in Fig. 3-a. The primary benefits of this configuration are that the sample consistently flows alongside the filter surface, resulting in the surface being cleaned and a decreased risk of clogging; hence, the concentrated sample can be obtained without the need for backwashing (Bridle et al., 2021b). Monitoring the pressure during the processing phase is important for the filtration process (Rhodes et al., 2012). This disadvantage can be addressed by installing an in-line pressure regulator and sensor to prevent high-pressure water from harming the filter's membrane. Prior to beginning the sample operation, it is essential to conform to the manufacturing pressure setpoints. The filter must be maintained intact during the high-pressure operation until the sampling is completed. A further limitation of this approach is that concentrating a large volume of samples takes a significant amount of time. For example, concentrating 1000 L of water with this setup is inefficient.
The second configuration is Dead-End Ultrafiltration (DEUF), where the retentate port is sealed while sampling, and the permeate port remains open, as shown in Fig. 3-b. In this setup, the sample is passed through the filter without circulation. Particles, including organisms, will be retained within the filter, and can be retrieved later through backwashing. This configuration is widely used to concentrate substantial quantities of water samples, and it has been demonstrated to be suitable for retrieving many species, including parasites, bacterial spores, vegetative bacteria, and viruses (Smith & Hill, 2009). Therefore, the DEUF is considered as a promising concentration technique (Sučik & Valenčáková, 2023).
3.5 Comparison of EPA 1623.1 and UKDWI Filters, and the HFUF
Figure 4 illustrates a result of an investigation by Helmi et al. in 2010 (Helmi et al., 2010) to compare three filtration systems: Pall (Envirochek HV) and IDEXX (FiltaMax) cartridge filters, as well as Whatman (Cryptest) membrane filter. Cryptosporidium parvum oocysts and Giardia duodenalis cysts were concentrated from a triplicate of two different water sources: 20 L of surface water, turbidity of 7 NTU, and 100 L of drinking water, turbidity of < 2 NTU; the samples were artificially contaminated with 103 oo(cysts). Statistically, FiltaMax had superior performance in the recovery of the Cryptosporidium and Giardia except for Cryptosporidium in surface water. In contrast, Envirochek exhibited recovery rates that were either lower or equivalent in comparison. The Cryptest filter demonstrated recovery rates that lie between those of Envirochek HV and FiltaMax. The study proved the efficacy of using Sodium hexametaphosphate (HMP) in combination with Envirochek HV to enhance the retrieval efficiency of Cryptosporidium from drinking water samples. Moreover, the CrypTest filter was submerged in an ultrasonic bath for two minutes (Helmi et al., 2010).
Filters recovery efficiency of Giardia and Cryptosporidium oo(cysts) from three replicants of drinking and surface water, figure created using data obtained from (Helmi et al., 2010)
In 2004, Wohlsen et al., examined the effectiveness of retrieving oo(cysts) from five different filters: Envirochek (E.C.), Envirochek HV (EC-HV), Filta-Max (F.M.), Millipore flatbed membrane (MFM), and Sartorius flatbed membrane filter (SFM). The study used 10 L of surface water and 10 L of distilled water, both containing 100 oo(cysts). The outcome demonstrated varying levels of pathogens that were successfully restored. As represented in Table 2, none of the results met the EPA's approved recovery rates, except for EC-HV, which only achieved them when using reversed backwash (Wohlsen et al., 2004). Although Wohlsen’s study showed low efficacy in retrieving the target parasites from the selected filters, Helmis's research proved differently, and that may contribute to the use of diverse water matrixes and higher level of parasite seeding. As a result, the sample volume and seeding level affect the filters' efficiency rate.
Regarding the HFUF, multiple works of literature were examined to evaluate the HFUF; like commercial filters authorised by the US EPA and other techniques previously mentioned, the water matrix and sample conditions, including the organism's seeding level, affect the recovery effectiveness of studied filters. However, HFUF has been studied for recovering viruses from 20–100 L of tap water samples since the early 1980s (Hill et al., 2005), and it has proven to be highly effective in eliminating waterborne pathogens for producing uncontaminated water (Herschell Green & Tylla, 1998; Hirata & Hashimoto, 1998). Holowecky and his team conducted a study where they examined five commercially available hollow fibre ultrafiltration cartridges. The purpose was to compare the efficiency of these filters in concentrating drinking water samples; they sampled 100 L of water tangentially filtered through 20–70 kilo Dalton (kDa) cut-off ranges of filters until they obtained a 500 mL concentrate. The study found that out of the 180 combinations of sample conditions tested, no filter consistently performed better or worse than the others for each test microorganism (Holowecky et al., 2009).
Hill and his team employed a Hemoflow F80A UF filter to concentrate a 10-L of tap water sample (Hill et al., 2005), and their study was extended with 100 L of sample utilising a F200NR UF filter by Polaczyk and others; this choice was made because of the filter's cost-effectiveness and larger surface area of 0.2 m2. The study found that there were no significant differences in organism recoveries between the two filters, except for C. parvum and MS2; these two organisms showed considerable differences, with the F80A filter demonstrating the potential for better recovery of protozoa (Polaczyk et al., 2008).
For simultaneous recovery studies, Liu et al., 2012 investigated the RE of three different types of filters: Fresenius F200NR, Minntech HPH, and Baxter Exeltra Plus 210 utilising 10 L of reclaimed water that was seeded by 150 k ± 52 K C. parvum oocysts; filters were pre-treated, or blocked, by filling the filter with 5% calf serum and shaking it for the entire night in a hybridization oven set to 37 °C to improve the UF efficiency. Treating the filter, also known as blocking, gives the filter fibres a negative charge, which increases the repulsion between negatively charged microorganisms and the filter surface, resulting in adhesion reduction with fibres (Polaczyk et al., 2008).
Baxter Exeltra Plus 210 and F200NR showed that under blocking conditions, the recovery efficiency of C. parvum was 10% lower than the result from nonblocking filters, except for the Minntech HPH, the result of blocked filter was only 3% higher in compared to the nonblocked filter. The team repeated their experiment with a non-blocked filter and 500 mL of 0.01% Tween 80, 0.01% sodium polyphosphate (NaPP) elution solution and the RE were increased for both the F200NR and Baxter Exeltra Plus 201 by 8% and 20% respectively(Liu et al., 2012).
3.6 Automated Systems
Kearn, Megna, and Lim introduced the Portable Multiuse Automated Concentration System (PMACS) to concentrate microorganisms in water samples. The PMACS, in Fig. 5 a, employed Hollow Fibre Ultrafiltration (HFUF) and a Dead-End Ultrafiltration (DEUF) configuration to efficiently concentrate microorganisms from 100 L of water. This system was mainly designed for monitoring purposes at water distribution lines. The technology underwent continuous development between 2007 and 2013 and is currently available for commercial use through IntelliSense Design, Inc., located in Tampa, FL, USA (Bridle et al., 2021b; IntelliSense Design, 2021; Leskinen et al., 2012). Although the PMACS system does have automated concentration capabilities for microorganisms, it was not designed initially to concentrate Cryptosporidium and Giardia.
There is a lack of information regarding the system's actuator, sensors, and control scenarios. The available published materials have revealed that the system relies on costly components, such as the Masterflex I/P Precision Brushless peristaltic pump, which is responsible for pumping samples, has an estimated cost of £6000 as priced by the Fisher Scientific supplier (product number 11876883). The PMACS system utilises manual analogue sensors to measure flow rate and pressure. Consequently, there is no continuous recording of hydraulic data throughout the sample procedure. Hence, incidents such as blockages in the filter, excessive pressure, and fluctuations in flow rates cannot be detected without constant supervision and monitoring. Thus, the lack of continuous hydraulic data recording restricts its efficiency in sampling large volumes of water over extended periods, which is essential for effectively monitoring Cryptosporidium and Giardia, as recommended by regulatory agencies.
In 2009, the US EPA implemented an automated water sampling system with a tangential configuration (TFUF) with an HFUF filter. This system concentrated 100 L of water to approximately 500 millilitres, Fig. 5 b. It is noticeable that the materials used for this system are, like PMACS, costly, and the system is operated by computer-based software (EPA, 2012a, 2023).
The US EPA employed 100 Cryptosporidium oocysts and 100 Giardia cysts to evaluate their automated method using the REXEED™ 25-S filter. Nevertheless, this study did not show any statistically significant differences compared to standard EPA methods (EPA, 2011). As stated in the discussion section of this review, a statistical correlation exists between the quantity of oo(cysts) in the sample and the resulting RE, Fig. 7. Thus, further studies are required to prove the efficiency of HFUF at low seeding level at the same circumstances of the results obtained from high seeding degrees. In 2013, Heriot-Watt University developed a semi-automated sampling method using HFUF filters as part of the Aquavalens research project (Norfolk, 2022).The device employs HFUF, demonstrating sensitivity to several factors involved in the sample procedures, such as flow rate and pressure (Bridle et al., 2021b). Therefore, similar to the PMACS, human intervention is necessary to ensure the protection and integrity of filters and systems during filtration. Thus, these systems can’t fill the gap of the need for an autonomous system for the concentration of Cryptosporidium oocysts and Giardia cysts from a large scale of water with complete control and data monitoring facilities.
3.7 Other Approaches
In addition to the standard filters and the HFUFs, other approaches have employed ultrasound waves to improve the oocysts' recovery rate from HFUF or prevent filter clogging. A high ultrasound frequency of 2 MHz (mega sonication) was utilised to enhance current sampling procedures showing that the megasonic technique requires less manual labour to treat filters, avoiding the need for centrifugation and causing less oo(cyst) damage compared to lower frequencies (Al-Sabi et al., 2015; Kerrouche et al., 2015).
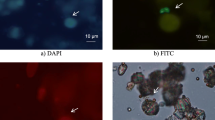
Kerrouche et al., 2015 processed 1000 L of finished water which was artificially contaminated with 100 oocysts of Cryptosporidium and passed through a FiltaMax filter over 24 h.; the standard backwash procedure was replaced with immersing the filter sponges in 600–1200 mL of elution solution in a sealed bag into a 2 MHz sonication bath for 20 min (Kerrouche et al., 2015). By utilising this method, backwashing the filter with the conventional way was no longer necessary. This process lowered the amount of human labour involved in physically extracting the pathogens, even if the RE findings were identical to those of the standard method. It also reduced the final extraction solution from 1200 to 600 mL for filter elution and from 50 to 10 mL for membrane concentration. To enhance the recovery efficiency of Cryptosporidium oocysts from HFUF filters, Kerrouche et al. employed megasonication in conjunction with both TFUF and DEUF configurations. The results of the investigation showed that using megasonic treatment increased the rate of recovery by about 1.5 times. (Bridle et al., 2018). In contrast, a different study conducted in 2018 by Horton et al. (Horton et al., 2018) examined the recovery rate of Giardia cysts when adopting the methodology of Kerrouche et al. Conversely, the outcome showed a decline in the number of cysts that were recovered from the FiltaMax filter with megasonication; as a result, further work needs to be done to establish a filtering method that can reliably work with various species and recover Giardia cysts from samples (Horton et al., 2018). While the studies enhanced several aspects of the sampling processes or the RE, it did not address the impact of directly applying of megasonic waves > 1 MHz on the survivability of oo(cysts). Alternatively, the filter was immersed in a megasonication bath to create waves from the megasonic transducer, which was used to apply the megasonication indirectly to the filter membrane. In this manner, the mechanical waves would confront the sealed bag or the filter's case before reaching the membranes, as the medium in between lessens the influence of the ultrasound's waves on the intact oo (cysts). The ultrasonic frequency and sonication duration are crucial factors in the destruction of oo(cysts). According to Al-Sabi et al., the percentage of nonviable oocysts increased after direct sonication for 20 or 40 s at 40 kHz compared to the average percentage of nonviable when sonicated for 5 or 10 s (Al-Sabi et al., 2015), and 90% of oocysts were inactivated after 90 s at a frequency of 20 kHz (Helmi et al., 2010), where the Cryptosporidium oocysts' shell walls were weakened or damaged because of ultrasonication which were led to release the nuclei from the shell, as they were observed under optical microscope after stained the sample by blue fluorescent DAPI (Ashokkumar et al., 2003).
Consequently, more research is needed to better understand the effects of ultrasonic and megasonic waves on the viability of Giardia cysts and Cryptosporidium oocysts at various frequencies, powers, and times. The results should also be compared with and without the membrane being separated by its enclosure or the washing bags.
Another alternative is the cascade filtration technique, which involves passing the sample through several layers of filters with gradually decreased porosity to separate the particles into different layers. Oda et al. utilised this technique to separate Giardia cysts and Cryptosporidium oocysts from water by filtrate 20 L of finished water through two 47 mm diameter filters, 5.0 to 10.0 and 3.0 um at flowrate of 2 L.min−1. The approach yielded 82.1% and 77.9% recovery rates for Giardia cysts and Cryptosporidium oocysts, respectively (Oda et al., 2000). Nevertheless, a greater sample volume, varied NTU levels, or a more considerable number of oo(cysts) were not used to evaluate the filtration and filters in this procedure.
4 Comparison
The FiltaMax filter (FM), Section 3.1, demonstrated, as in Table 3, a variable recovery rate depending on the seeding and the NTU level, Fig. 6. When 100–150 oocysts were seeded in 10 L of ultrapure water, the optimal recovery rates for Cryptosporidium oocysts were 46% ± 18% and 15% ± 12% for surface water (Simmons Iii et al., 2001). The optimal recovery rates for Giardia cysts were 32.4% ± 23.1% for the EC capsule and 59 ± 11.5% for the Envirochek_HV (EC_HV) capsule when seeded at 100 cysts in 10 L of distilled water (Wohlsen et al., 2004). Notably, the recovery efficiency (RE) was increased to 60% ± 4% and 65% ± 5%, respectively, for Cryptosporidium and Giardia when 1000 oo(cysts) were seeded in 20 L of surface water with a turbidity of 7 NTU, and for 100 L of tap water with a turbidity of < 2 NTU, the RE were 49% ± 12% and 64% ± 22% (Helmi et al., 2010). However, the last results cannot be directly compared to the trials when 100 oocysts were seeded, as evidenced by the correlation between seeding level and recovery efficiency illustrated in Fig. 7.
The relation between the oocysts seeding level and the recovery rate. Data for this Figure has been obtained from the following research articles: (Bridle et al., 2021b; Bridle et al., 2018; Helmi et al., 2010; Hill et al., 2005; Horton et al., 2018; Kuhn & Oshima, 2002; Liu et al., 2012; Oda et al., 2000; Pires & Dong, 2014; Polaczyk et al., 2008; Simmons Iii et al., 2001; Smith & Hill, 2009; Wohlsen et al., 2004)
In contrast, for the FiltaMax filter, Section 3.2, the arrangement of experimental circumstances differed significantly; therefore, it is not easy to deduce the efficiency of the filter accurately unless it is tested under the same parameters in different conditions, for example, if it is seeded with a fixed number of parasites to filter different volumes of samples or vice versa, and also if the turbidity of the water differs between the models under test. Nevertheless, Giardia and Cryptosporidium usually have 40–60% recovery efficiencies and 30–50%, respectively, allowing for detection and quantification at detection levels of 0.2–0.5. L−1 in 10 L (Efstratiou et al., 2017). Despite the foregoing, these investigations indicated that efficiency varies depending on the conditions, and this needs to be considered when sampling as yet there is no clear correlation between any conditions and recovery rates and the influence of seeding level is observed for this data as well.
Like the EC, EC_HV, and FiltaMax filters, the recovery efficiency (RE) of oo(cysts) through membrane filtration can vary depending on the alteration of filtration and detection conditions, such as changes in membrane size or detection technique. For instance, when the filter's surface is scraped off and subsequently cleaned with phosphate buffer solution (PBS), the RE can range from 30 to 90% (Palumbo et al., 2002), whereas the mean RE for detecting Cryptosporidium oocysts using the immunofluorescent antibody-based method was 9%, while for Giardia cysts, it was 49% (Zarlenga & Trout, 2004).
Literature has also demonstrated that ultrafiltration filters, similar to other filters, the variety in the characteristics like the turbidity level, Fig. 6, and filtration conditions, can lead to varying removal efficiencies for the same pathogen. Furthermore, water factors, such as temperature, impact the hydraulic performance of the HFUF. Specifically, lower water temperatures necessitate higher pressure to sustain the desired flow rate (Smith & Hill, 2009). Concerning the flow rate (FR), a lower FR results in a higher RE, as observed in previous studies (Hill et al., 2009; Rhodes et al., 2011). This can be attributed to the increased pressure applied to the filter's medium at higher FR. It is essential to note that increasing pressure to maintain FR can have negative consequences including the destruction of filter fibres, loss of microbes as they pass through the membrane, filter clogging, difficulties in backflushing, or a combination of these issues; furthermore, the larger the filter's surface area, the lower the expected pressure (Smith & Hill, 2009).
It is worth mentioning that there is a lack of sufficient research to establish the RE of HFUF at low levels of oo(cysts). On average, studies utilise 105–106 oo(cysts) for their investigations (Bridle et al., 2021b; Hill et al., 2005; Kuhn & Oshima, 2002; Liu et al., 2012; Polaczyk et al., 2008; Smith & Hill, 2009).
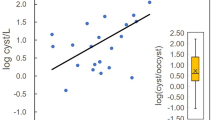
4.1 The Relation Between oo(cysts) Density and the Recovery Rates
The studies reviewed here utilised varying degrees of seeding and filtration, filters, and reagents. The literature reviewed indicates a significant correlation between the recovery rate and the degree of seeding or spiking. It was observed that typically a large number of oo(cysts), 106, were employed as a positive control in studies. Figure 7 demonstrates that the recovery rate dramatically decreased when the filter was spiked or seeded with a low number of oo(cysts), 102–103 compared to 106. Further analysis is necessary to establish the reliability of these results, as they are linked to a drop in pathogens that were not recovered from the filter and system losses, including tubes, valves, pumps, and other components. For instance, the recovery rate would only be 20% if the system was initially seeded with 102 oo(cysts), and 80 of them were lost along the way from the sample tank to the concentrate bottle. By contrast, 90% would be the recovery rate if it started with 106 and lost 105. Therefore, when there is a different concentration of pathogens in the sample, it is challenging to compare different studies, and data obtained with high numbers of (oo)cysts does not necessarily reflect performance at low numbers. In contrast, agents such as the US EPA assessed their procedure with a standard level of oo(cysts). Thus, assessing any new method or enhancement with a low level of parasite concentrate is essential.
5 Conclusion
This comprehensive literature review evaluated four distinct filtration technologies employed for waterborne pathogen detection in water sampling systems: FiltaMax Filter, Envirochek Filter, Flat-bed membrane, and ultrafiltration filters. Several parameters, such as experimental circumstances, seeding levels, water turbidity, filtration rate, and elution chemicals, imparted a significant influence on the effectiveness of filters for detecting waterborne pathogens. Accordingly, the fluctuating recovery rate and filter performance as a response to changes in experimental parameters necessitate careful thoughts before choosing an appropriate sample processing strategy for certain conditions since no single pathogen concentration technique regularly works better than others. The initial results of using ultrafiltration as a cost-effective alternative for pathogen concentration hold potential promises for enhancing automated water sampling systems and reducing the associated public health risks. In addition, the existing automated systems are not yet sufficient to meet the requirement for an independent system specifically intended to concentrate Cryptosporidium oocysts and Giardia cysts from large-scale water sources while providing full control and data monitoring capabilities. It is crucial, when developing systems, to thoroughly examine the data to determine their credibility, especially regarding the loss of pathogens that are not being recovered from the filter and other components of the system, including tubes, valves, pumps, and other parts. Further studies and development are needed to advance waterborne pathogen concentration approaches, to identify filter selection and operational parameters to maximise recovery rates, especially for low oo(cyst) numbers.
Data Availability
This is a review article and all data that visualised in this paper were cited.
References
Abeledo-Lameiro, M. J., Ares-Mazás, E., & Goméz-Couso, H. (2018). Use of ultrasound irradiation to inactivate Cryptosporidium parvum oocysts in effluents from municipal wastewater treatment plants. Ultrasonics Sonochemistry, 48, 118–126. https://doi.org/10.1016/j.ultsonch.2018.05.013
Abeywardena, H., Jex, A. R., & Gasser, R. B. (2015). Chapter six - A perspective on Cryptosporidium and Giardia, with an emphasis on bovines and recent epidemiological findings. In D. Rollinson & J. R. Stothard (Eds.), Advances in parasitology (Vol. 88, pp. 243–301). Academic Press. https://doi.org/10.1016/bs.apar.2015.02.001
Ahuja, S. (2021). Overview: modern water purity and quality. In Handbook of water purity and quality (pp. 1–18). Elsevier. https://doi.org/10.1016/B978-0-12-821057-4.00014-8
Allen, M. J., Clancy, J. L., & Rice, E. W. (2000). The plain, hard truth about pathogen monitoring. Journal AWWA, 92(9), 64–76. https://doi.org/10.1002/j.1551-8833.2000.tb09005.x
Al-Sabi, M. N. S., Gad, J. A., Riber, U., Kurtzhals, J. A. L., & Enemark, H. L. (2015). New filtration system for efficient recovery of waterborne Cryptosporidium oocysts and Giardia cysts. Journal of Applied Microbiology, 119(3), 894–903. https://doi.org/10.1111/jam.12898
Arona, J. C., Hall, T. J., Mckinnirey, F., & Deng, F. (2023). Comparison of four commercial immunomagnetic separation kits for the detection of Cryptosporidium. Journal of Water and Health, 21(10), 1580–1590. https://doi.org/10.2166/wh.2023.217
Ashokkumar, M., Vu, T., Grieser, F., Weerawardena, A., Anderson, N., Pilkington, N., & Dixon, D. R. (2003). Ultrasonic treatment of Cryptosporidium oocysts. Water Science and Technology, 47(3), 173–177. https://doi.org/10.2166/wst.2003.0191
Askenaizer, D. (2003). Drinking water quality and treatment. In R. A. Meyers (Ed.), Encyclopedia of physical science and technology (third edition) (pp. 651–671). Academic Press. https://doi.org/10.1016/B0-12-227410-5/00186-1
Barroso, G. R., Pinto, C. C., Gomes, L. N. L., & Oliveira, S. C. (2024). Assessment of water quality based on statistical analysis of physical-chemical, biomonitoring and land use data: Manso River supply reservoir. Science of the Total Environment, 912, 169554. https://doi.org/10.1016/j.scitotenv.2023.169554
Bridle, H., Kerrouche, A., & Desmulliez, M. (2018). Megasonic elution of waterborne protozoa enhances recovery rates. Matters. https://doi.org/10.19185/matters.201712000007
Bridle, H., Gilbride, K., Green, J., & Lee, S. (2021a). Chapter 3 - Existing methods of detection. In H. Bridle (Ed.), Waterborne pathogens (2nd ed., pp. 41–60). Academic Press. https://doi.org/10.1016/B978-0-444-64319-3.00003-4
Bridle, H., Jacobsson, K., & Schultz, A. C. (2021b). Chapter 4 - Sample processing. In H. Bridle (Ed.), Waterborne Pathogens (2nd ed., pp. 63–109). Academic Press. https://doi.org/10.1016/B978-0-444-64319-3.00004-6
Bridle, H., Balharry, D., Gaiser, B., & Johnston, H. (2015). Exploitation of nanotechnology for the monitoring of waterborne pathogens: State-of-the-art and future research priorities. Environmental Science & Technology, 49(18), 10762–10777. https://doi.org/10.1021/acs.est.5b01673
BSI. (2006). BS ISO 15553:2006: Water quality. Isolation and identification of Cryptosporidium oocysts and Giardia cysts from water. In: British Standards Institute.
Burnet, J.-B., Habash, M., Hachad, M., Khanafer, Z., Prévost, M., Servais, P., Sylvestre, E., & Dorner, S. (2021). Automated targeted sampling of waterborne pathogens and microbial source tracking markers using near-real time monitoring of microbiological water quality. Water (Basel), 13(15), 2069. https://doi.org/10.3390/w13152069
Carraro, E., & Palumbo, F. (2002). An overview of identification methods in use for Giardia and Cryptosporidium. In F. Palumbo, G. Ziglio, & A. Van der Beken (Eds.), Detection methods for algae, protozoa and helminths in fresh and drinking water. Wiley. https://books.google.co.uk/books?id=2voo3NPGi9gC. Accessed 15 Apr 2024.
Cazeaux, C., Lalle, M., Durand, L., Aubert, D., Favennec, L., Dubey, J. P., Geffard, A., Villena, I., & La Carbona, S. (2022). Evaluation of real-time qPCR-based methods to detect the DNA of the three protozoan parasites Cryptosporidium parvum, Giardia duodenalis and Toxoplasma gondii in the tissue and hemolymph of blue mussels (M. edulis). Food Microbiology, 102, 103870–103870. https://doi.org/10.1016/j.fm.2021.103870
CFR. (2024). Title 40—Protection of Environment, Part 141 Subpart W. Code of Federal Regulation. https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-141/subpart-W. Accessed 15 Apr 2024.
Clancy, J. L. (2000). New approaches for isolation of Cryptosporidium and Giardia. AWWA Research Foundation and American Water Works Association. https://books.google.co.uk/books?id=8U3scoKxcaQC. Accessed 15 Apr 2024.
EPA. (2011). Comparison of ultrafiltration techniques for recovering biothreat agents in water (Vol. EPA/600/R-11/103).
Department of Environment. (2000). The water supply (water quality) regulations 2000. The official home of UK legislation. https://www.legislation.gov.uk/uksi/2000/3184/regulation/29/made. Accessed 15 Apr 2024.
Dixon, B. R. (2014). Protozoa: Cryptosporidium spp. In Y. Motarjemi (Ed.), Encyclopedia of food safety (pp. 18–22). Academic Press. https://doi.org/10.1016/B978-0-12-378612-8.00132-3
Drury, D., & Lloyd, A. (2003). Chapter 46 - Drinking water regulations for Cryptosporidium in England and Wales. In R. C. A. Thompson, A. Armson, & U. M. Ryan (Eds.), Cryptosporidium (pp. 349–351). Elsevier. https://doi.org/10.1016/B978-044451351-9/50049-5
DWQR. (2022). Public water supplies annual report.
Efstratiou, A., Ongerth, J., & Karanis, P. (2017). Evolution of monitoring for Giardia and Cryptosporidium in water. Water Research, 123, 96–112. https://doi.org/10.1016/j.watres.2017.06.042
EPA. (2001). Method 1622: Cryptosporidium in water by Filtration/IMS/FA [Method]. U.S. Environmental Protection Agency.
EPA. (2012b). Method 1623.1: Cryptosporidium and Giardia in water by Filtration/IMS/FA [method]. U.S. Environmental Protection Agency. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100J7G4.PDF?Dockey=P100J7G4.PDF. Accessed 15 Apr 2024.
EPA. (2012a). Automated ultrafiltration device (Full length video). https://www.youtube.com/watch?v=kKg_gbh3DFI. Accessed 15 Apr 2024.
EPA. (2014). Method 1693: Cryptosporidium and Giardia in disinfected wastewater by Concentration/IMS/IFA. In: U.S. Environmental Protection Agency.
EPA. (2023). Portable device to concentrate water samples for microorganism analysis. Retrieved 12 June from https://www.epa.gov/ftta/portable-device-concentrate-water-samples-microorganism-analysis
Falk, C. C., Karanis, P., Schoenen, D., & Seitz, H. M. (1998). Bench scale experiments for the evaluation of a membrane filtration method for the recovery efficiency of Giardia and Cryptosporidium from water. Water Research, 32(3), 565–568. https://doi.org/10.1016/S0043-1354(97)00313-8
Fayer, R., & Xiao, L. (2008). Cryptosporidium and cryptosporidiosis (2nd ed.). CRC.
Feng, Y. Y., Ong, S. L., Hu, J. Y., Song, L. F., Tan, X. L., & Ng, W. J. (2003). Effect of particles on the recovery of Cryptosporidium oocysts from source water samples of various turbidities. Applied and Environmental Microbiology, 69(4), 1898–1903. https://doi.org/10.1128/AEM.69.4.1898-1903.2003
Ferguson, C., Kaucner, C., Krogh, M., Deere, D., & Warnecke, M. (2004). Comparison of methods for the concentration of Cryptosporidium oocysts and Giardia cysts from raw waters. Canadian Journal of Microbiology, 50(9), 675–682. https://doi.org/10.1139/w04-059
Fradette, M.-S., & Charette, S. J. (2022). Working toward improved monitoring of Cryptosporidium and Giardia (oo)cysts in water samples: Testing alternatives to elution and immunomagnetic separation from USEPA Method 1623.1. BMC Research Notes, 15(1). https://doi.org/10.1186/s13104-022-06118-9
Fradette, M.-S., Culley, A. I., & Charette, S. J. (2022). Detection of Cryptosporidium spp. and Giardia spp. in environmental water samples: A journey into the past and new perspectives. Microorganisms, 10(6), 1175. https://doi.org/10.3390/microorganisms10061175
Gaskin, J. E. (2005). QUALITY ASSURANCE | Water applications. In P. Worsfold, A. Townshend, & C. Poole (Eds.), Encyclopedia of analytical science (second edition) (pp. 511–531). Elsevier. https://doi.org/10.1016/B0-12-369397-7/00515-X
Gavriilidou, D., & Bridle, H. (2012). Comparison of immobilization strategies for Cryptosporidium parvum immunosensors. Biochemical Engineering Journal, 68, 231–235. https://doi.org/10.1016/j.bej.2012.07.024
Gopfert, A., Chalmers, R. M., Whittingham, S., Wilson, L., Van Hove, M., Ferraro, C. F., Robinson, G., Young, N., & Nozad, B. (2022). An outbreak of Cryptosporidium parvum linked to pasteurised milk from a vending machine in England: A descriptive study, March 2021. Epidemiology and Infection, 150, 1–9. https://doi.org/10.1017/s0950268822001613
Gule, T. T., Lemma, B., & Hailu, B. T. (2023). Evaluation of the physical, chemical, and biological characteristics of surface water in urban settings and its applicability to SDG 6: The case of Addis Ababa, Ethiopia. Scientific African, 21, e01744. https://doi.org/10.1016/j.sciaf.2023.e01744
Hashimoto, A., Hirata, T., & Kunikane, S. (2001). Occurrence of Cryptosporidium oocysts and Giardia cysts in a conventional water purification plant. Water Science and Technology, 43(12), 89–92. https://doi.org/10.2166/wst.2001.0717
Hashimoto, A., Kunikane, S., & Hirata, T. (2002). Prevalence of Cryptosporidium oocysts and Giardia cysts in the drinking water supply in Japan. Water Research, 36(3), 519–526. https://doi.org/10.1016/s0043-1354(01)00279-2
Hassan, E. M., Ormeci, B., DeRosa, M. C., Dixon, B. R., Sattar, S. A., & Iqbal, A. (2021). A review of Cryptosporidium spp. and their detection in water. Water Science and Technology, 83(1), 1–25. https://doi.org/10.2166/wst.2020.515
Helmi, K., Skraber, S., Leblanc, L., Hoffmann, L., & Cauchie, H. M. (2010). Comparison of three methods to concentrate Giardia cysts and Cryptosporidium oocysts from surface and drinking waters. Water Science and Technology, 62(1), 196–201. https://doi.org/10.2166/wst.2010.311
Herschell Green, J., & Tylla, M. (1998). A comparison of ultrafiltration on various river waters. Desalination, 119(1), 79–83. https://doi.org/10.1016/S0011-9164(98)00114-3
Hill, V. R., Polaczyk, A. L., Hahn, D., Narayanan, J., Cromeans, T. L., Roberts, J. M., & Amburgey, J. E. (2005). Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants [Article]. Applied and Environmental Microbiology, 71(11), 6878–6884. https://doi.org/10.1128/aem.71.11.6878-6884.2005
Hill, V. R., Polaczyk, A. L., Kahler, A. M., Cromeans, T. L., Hahn, D., & Amburgey, J. E. (2009). Comparison of hollow-fiber ultrafiltration to the USEPA VIRADEL technique and USEPA method 1623. Journal of Environmental Quality, 38(2), 822–825. https://doi.org/10.2134/jeq2008.0152
Hirata, T., & Hashimoto, A. (1998). Experimental assessment of the efficacy of microfiltration and ultrafiltration for Cryptosporidium removal. Water Science and Technology, 38(12), 103–107. https://doi.org/10.1016/S0273-1223(98)00809-9
Holowecky, P. M., James, R. R., Lorch, D. P., Straka, S. E., & Lindquist, H. D. A. (2009). Evaluation of ultrafiltration cartridges for a water sampling apparatus [Article]. Journal of Applied Microbiology, 106(3), 738–747. https://doi.org/10.1111/j.1365-2672.2008.04019.x
Horton, B., Katzer, F., Desmulliez, M. P. Y., & Bridle, H. L. (2018). Towards enhanced automated elution systems for waterborne protozoa using megasonic energy. Journal of Microbiological Methods, 145, 28–36. https://doi.org/10.1016/j.mimet.2017.12.002
Howe, A. D., Forster, S., Morton, S., Marshall, R., Osborn, K. S., Wright, P., & Hunter, P. R. (2002). Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak [Journal Article]. Emerging Infectious Diseases, 8(6), 619–624. https://stacks.cdc.gov/view/cdc/13808. Accessed 15 Apr 2024.
Hu, J., Feng, Y., Ong, S. L., Ng, W. J., Song, L., Tan, X., & Chu, X. (2004). Improvement of recoveries for the determination of protozoa Cryptosporidium and Giardia in water using method 1623. Journal of Microbiological Methods, 58(3), 321–325. https://doi.org/10.1016/j.mimet.2004.04.013
Huang, S., Yoshitake, K., Watabe, S., & Asakawa, S. (2022). Environmental DNA study on aquatic ecosystem monitoring and management: Recent advances and prospects. Journal of Environmental Management, 323, 116310. https://doi.org/10.1016/j.jenvman.2022.116310
IDEXX. (2023). Filta-Max System. Retrieved November from https://www.idexx.co.uk/en-gb/water/water-products-services/filta-max/. Accessed 15 Apr 2024.
IntelliSense Design, I. (2021). IntelliSense design PMACS 1000. IntelliSense Design, Inc. Retrieved December from https://intellisensedesign.com/product-r%26d. Accessed 15 Apr 2024.
Jain, S., Costa Melo, T. G., Dolabella, S. S., & Liu, J. (2019). Current and emerging tools for detecting protozoan cysts and oocysts in water. TrAC Trends in Analytical Chemistry, 121. https://doi.org/10.1016/j.trac.2019.115695
Jellison, K., Cannistraci, D., Fortunato, J., & McLeod, C. (2020). Biofilm sampling for detection of Cryptosporidium oocysts in a Southeastern Pennsylvania Watershed. Applied and Environmental Microbiology, 86(23), 1–12. https://doi.org/10.1128/AEM.01399-20
Jingyi, J., Ping, Y., Jian, X., Jia, C., Xujian, M., Qiong, L., Bowen, T., & Fengming, W. (2023). Efficacy of a membrane concentration method combined with real-time PCR for detection of Giardia and Cryptosporidium in drinking water. Letters in Applied Microbiology, 76(10). https://doi.org/10.1093/lambio/ovad121
Kerrouche, A., Desmulliez, M. P. Y., & Bridle, H. (2015). Megasonic sonication for cost-effective and automatable elution of Cryptosporidium from filters and membranes. Journal of Microbiological Methods, 118, 123–127. https://doi.org/10.1016/j.mimet.2015.09.001
Kuhn, R. C., & Oshima, K. H. (2002). Hollow-fiber ultrafiltration of Cryptosporidium parvum oocysts from a wide variety of 10-L surface water samples. Canadian Journal of Microbiology, 48(6), 542–549. https://doi.org/10.1139/w02-049
Leskinen, S. D., Kearns, E. A., Jones, W. L., Miller, R. S., Bevitas, C. R., Kingsley, M. T., Brigmon, R. L., & Lim, D. V. (2012). Automated dead-end ultrafiltration of large volume water samples to enable detection of low-level targets and reduce sample variability [Article]. Journal of Applied Microbiology, 113(2), 351–360. https://doi.org/10.1111/j.1365-2672.2012.05345.x
Liu, P., Hill, V. R., Hahn, D., Johnson, T. B., Pan, Y., Jothikumar, N., & Moe, C. L. (2012). Hollow-fiber ultrafiltration for simultaneous recovery of viruses, bacteria and parasites from reclaimed water [Article]. Journal of Microbiological Methods, 88(1), 155–161. https://doi.org/10.1016/j.mimet.2011.11.007
Luka, G., Samiei, E., Tasnim, N., Dalili, A., Najjaran, H., & Hoorfar, M. (2022). Comprehensive review of conventional and state-of-the-art detection methods of Cryptosporidium. Journal of Hazardous Materials, 421, 126714. https://doi.org/10.1016/j.jhazmat.2021.126714
Madrid, Y., & Zayas, Z. P. (2007). Water sampling: Traditional methods and new approaches in water sampling strategy. TrAC, Trends in Analytical Chemistry (Regular Ed.), 26(4), 293–299. https://doi.org/10.1016/j.trac.2007.01.002
Matamoros, V. (2012). 1.13 - Equipment for water sampling including sensors. In J. Pawliszyn (Ed.), Comprehensive sampling and sample preparation (pp. 247–263). Academic Press. https://doi.org/10.1016/B978-0-12-381373-2.00013-2
Mccuin, R. M., & Clancy, J. L. (2003). Modifications to United States environmental protection agency methods 1622 and 1623 for detection of Cryptosporidium oocysts and Giardia cysts in water. Applied and Environmental Microbiology, 69(1), 267–274. https://doi.org/10.1128/AEM.69.1.267-274.2003
Norfolk. (2022). Horizon 2020. Retrieved Apr. 12 from https://www.norfolk.gov.uk/business/grants-and-funding/european-funding/eu-programmes/eu-wide-funds/horizon-2020. Accessed 15 Apr 2024.
Oda, T., Sakagami, M., Ito, H., Yano, H., Rai, S. K., Kawabata, M., & Uga, S. (2000). Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Research, 34(18), 4477–4481. https://doi.org/10.1016/S0043-1354(00)00205-0
Omarova, A., Tussupova, K., Berndtsson, R., Kalishev, M., & Sharapatova, K. (2018). Protozoan parasites in drinking water: A system approach for improved water, sanitation and hygiene in developing countries. International Journal of Environmental Research and Public Health, 15(3), 495. https://doi.org/10.3390/ijerph15030495
Ongerth, J. E. (2013). The concentration of Cryptosporidium and Giardia in water – The role and importance of recovery efficiency. Water Research, 47(7), 2479–2488. https://doi.org/10.1016/j.watres.2013.02.015
Palumbo, F., Ziglio, G., & Van der Beken, A. (2002). Detection methods for algae, protozoa and helminths in fresh and drinking water. Wiley. https://books.google.co.uk/books?id=2voo3NPGi9gC. Accessed 15 Apr 2024.
Parton, A. (1997). Filtration method and apparatus. U. S. Patent.
Peng, X., Murphy, T. M., & Holden, N. M. (2010). Physical straining of Cryptosporidium parvum oocysts through saturated soils.
Pires, N. M. M., & Dong, T. (2014). A cascade-like silicon filter for improved recovery of oocysts from environmental waters [Article]. Environmental Technology (United Kingdom), 35(6), 781–790. https://doi.org/10.1080/09593330.2013.851280
Polaczyk, A. L., Narayanan, J., Cromeans, T. L., Hahn, D., Roberts, J. M., Amburgey, J. E., & Hill, V. R. (2008). Ultrafiltration-based techniques for rapid and simultaneous concentration of multiple microbe classes from 100-L tap water samples [Article]. Journal of Microbiological Methods, 73(2), 92–99. https://doi.org/10.1016/j.mimet.2008.02.014
Prasad, M. N. V., & Grobelak, A. (2020). Waterborne pathogens : Detection and treatment. Elsevier Science & Technology.
Prystajecky, N., Huck, P. M., Schreier, H., & Isaac-Renton, J. L. (2014). Assessment of Giardia and Cryptosporidium spp. as a microbial source tracking tool for surface water: application in a mixed-use watershed. Applied and Environmental Microbiology, 80(8), 2328–2336. https://doi.org/10.1128/aem.02037-13
Rhodes, E. R., Villegas, L. F., Shaw, N. J., Miller, C., & Villegas, E. N. (2012). A modified EPA method 1623 that uses tangential flow hollow-fiber ultrafiltration and heat dissociation steps to detect waterborne Cryptosporidium and Giardia spp. Journal of Visualized Experiments (65). https://doi.org/10.3791/4177
Rhodes, E. R., Hamilton, D. W., See, M. J., & Wymer, L. (2011). Evaluation of hollow-fiber ultrafiltration primary concentration of pathogens and secondary concentration of viruses from water. Journal of Virological Methods, 176(1–2), 38–45. https://doi.org/10.1016/j.jviromet.2011.05.031
Robertson, L. J., & Gjerde, B. (2000). Effect of sample holding time on recovery of Cryptosporidium oocysts and Giardia cysts from water samples. Applied and Environmental Microbiology, 66(4), 1724–1725. https://doi.org/10.1128/aem.66.4.1724-1725.2000
Rousseau, A., La Carbona, S., Dumètre, A., Robertson, L. J., Gargala, G., Escotte-Binet, S., Favennec, L., Villena, I., Gérard, C., & Aubert, D. (2018). Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: A review of methods. Parasite, 25, 14. https://doi.org/10.1051/parasite/2018009
Ryan, U., Zahedi, A., Feng, Y., & Xiao, L. (2021). An update on zoonotic Cryptosporidium species and genotypes in humans. Animals, 11(11), 3307. https://doi.org/10.3390/ani11113307
Sammarro Silva, K. J., & Sabogal-Paz, L. P. (2021). Giardia spp. cysts and Cryptosporidium spp. oocysts in drinking water treatment residues: Comparison of recovery methods for quantity assessment. Environmental Technology, 42(20), 3144–3153. https://doi.org/10.1080/09593330.2020.1723712
Sánchez, C., López, M. C., Galeano, L. A., Qvarnstrom, Y., Houghton, K., & Ramírez, J. D. (2018). Molecular detection and genotyping of pathogenic protozoan parasites in raw and treated water samples from southwest Colombia. Parasites & Vectors, 11(1), 563–563. https://doi.org/10.1186/s13071-018-3147-3
Sartory, D. P., Parton, A., Parton, A. C., Roberts, J., & Bergmann, K. (1998). Recovery of Cryptosporidium oocysts from small and large volume water samples using a compressed foam filter system. Letters in Applied Microbiology, 27(6), 318–322. https://doi.org/10.1046/j.1472-765X.1998.00459.x
SCA. (2010a). The microbiology of drinking water (2010) - Part 2 - Practices and procedures for sampling. In UK: Standing Committe of Analysis.
SCA. (2010b). The microbiology of drinking water (2010) - Part 14-Methods for the isolation, identification and enumeration of Cryptosporidium oocysts and Giardia cysts. In (Vol. Blue Book 234). Standing Committe of Analysis.
Shepard, J. R. E., Cirino, N. M., Egan, C., & Aryel, R. M. (2006). CHAPTER 9 - Water suppliers. In M. M. Wagner, A. W. Moore, & R. M. Aryel (Eds.), Handbook of biosurveillance (pp. 143–159). Academic Press. https://doi.org/10.1016/B978-012369378-5/50011-9
Simmons Iii, O. D., Sobsey, M. D., Heaney, C. D., Schaefer Iii, F. W., & Francy, D. S. (2001). Concentration and detection of Cryptosporidium oocysts in surface water samples by method 1622 using ultrafiltration and capsule filtration [Article]. Applied and Environmental Microbiology, 67(3), 1123–1127. https://doi.org/10.1128/AEM.67.3.1123-1127.2001
Smith, C. M., & Hill, V. R. (2009). Dead-End hollow-fiber ultrafiltration for recovery of diverse microbes from water. Applied and Environmental Microbiology, 75(16), 5284–5289. https://doi.org/10.1128/aem.00456-09
Sučik, M., & Valenčáková, A. (2023). Methods used for concentrating oocysts of Cryptosporidium spp., spores Encephalitozoon spp. and Enterocytozoon spp. and their occurrence in Slovak water samples [Review]. Frontiers in Environmental Science, 11. https://doi.org/10.3389/fenvs.2023.1161465
Vesey, G., Slade, J. S., Byrne, M., Shepherd, K., & Fricker, C. R. (1993). A new method for the concentration of Cryptosporidium oocysts from water. Journal of Applied Bacteriology, 75(1), 82–86. https://doi.org/10.1111/j.1365-2672.1993.tb03412.x
Wohlsen, T., Bates, J., Gray, B., & Katouli, M. (2004). Evaluation of five membrane filtration methods for recovery of Cryptosporidium and Giardia isolates from water samples. Applied and Environmental Microbiology, 70(4), 2318–2322. https://doi.org/10.1128/AEM.70.4.2318-2322.2004
Xiao, L., Alderisio, K. A., & Jiang, J. (2006). Detection of Cryptosporidium oocysts in water: Effect of the number of samples and analytic replicates on test results. Applied and Environmental Microbiology, 72(9), 5942–5947. https://doi.org/10.1128/AEM.00927-06
Zarlenga, D. S., & Trout, J. M. (2004). Concentrating, purifying and detecting waterborne parasites. Veterinary Parasitology, 126(1), 195–217. https://doi.org/10.1016/j.vetpar.2004.09.001
Acknowledgements
With thanks to the Heriot-Watt University James Watt Scholarship and Biopoint advancing Microbiology, Sydney, Australia for providing the PhD funding for the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Mshelesh, H., Bridle, H. & Vesey, G. Comparative Evaluation of Commercial Filters and Ultrafiltration for Concentrating Cryptosporidium Oocysts and Giardia Cysts from Water Samples. Water Air Soil Pollut 235, 696 (2024). https://doi.org/10.1007/s11270-024-07511-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07511-9