Abstract
Hybrid composites based on ferrite nanoparticles have shown their effectiveness in the heterogeneous Fenton process due to the multiple properties such as ferromagnetism and biocompatibility. In this work, Magnetite (Fe3O4) nanoparticles are synthesized and dispersed thanks to the anchoring plasma-created sites onto water hyacinth fibers used as the catalytic support. The obtained composites were characterized by XRD, FTIR, SEM/EDX, and the magnetization properties were assessed at different temperatures (20 K, 150 K, and 300 K). The results reveal uniform dispersed nanorod-shaped particles covering the entire surface of the biomass with the saturation magnetization at 15 emu.g−1. The Fenton degradation process was optimized in terms of pH, mass, contact time and pollutant concentration, thus the catalytic performance achieved on two organometallic model pollutants, namely merbromine (MB) and the green complex of naphthol B (NGB) give degradation efficiencies of 97.01 and 99.70% respectively. The Langmuir–Hinshelwood kinetic model better describes the phenomenon where adsorption is the rate-limiting step given the high reactivity of generated species. The trapping species experiments show that besides hydroxyl radicals that contribute mainly to the degradation, other species such as superoxide and hydroperoxyl radicals are involved in the overall degradation mechanism. The obtained material is effective in the plasma-Fenton coupling process and the leaching test confirms their stability after four reuse cycles with the degradation rate greater than 85.10%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heterogeneous Fenton reactions are increasingly used for the oxidation of persistent organic pollutants (POPs) because they allow the generation of a large number of highly reactive hydroxyl radicals (HO•) by the decomposition of hydrogen peroxide (H2O2) in the presence of Iron (II) (Oturan et al., 2001; Yanping et al., 2019). However, the production of ferrous sludge, the concentration of hydrogen peroxide, the acid pH, and the high chemical inputs constitute some drawbacks to their extensive application in real industrial effluent (Mustapha et al., 2019). Therefore, nano-sized ferrites in general and those with magnetic properties in particular have attracted research attention because of their easy recovery and their enhance degradation performances due to the high surface area to volume ratio (Acayanka et al., 2013; Jôse et al., 2020; Xiaoling et al., 2012). Magnetite like all magnetic ferrites has an inverse spinel structure and is widely synthesized in different shapes, sizes, and geometry to ensure various chemical properties (Culita et al., 2015; Lu et al., 2007). However, the chemical synthesis route presents many difficulties related to the toxicity of precursors, the rapid oxidation of Fe2+ into Fe3+, and especially their ability to agglomerate. Therefore the need for alternatives route is growing (Saba, 2015).
The low-temperature plasma obtained at atmospheric pressure, which is based on the in-situ generation of radical species with high oxidation potential (HO•/H2O = 2.85 V/NHE), has been implemented by several authors for the synthesis of oxides or hybrid composites (Dionmbete et al., 2023; Mbouopda et al., 2018). Tiya-Djowe et al. (Tiya-Djowe et al., 2016) developed a way to synthesize the goethite (FeOOH) by treatment of ammonium Iron(II)sulfate hexahydrate (32.5 mM) in humid air plasma. However, according to Ponomar (2018), the goethite is rapidly transformed into hematite in the presence of oxidant species. The plasma usually qualified as the fourth state of matter is generally a strongly oxidizing or reducing medium depending on the nature of the plasma gas used.
The particularity of this work is to carry out the synthesis not in an in-situ mode because of its very oxidizing nature (reactive oxygen and nitrogen species), but rather in spatiotemporal post-discharge mode. Brisset and Pawlat (2016) define this mode as all the reactions occurring after stopping the discharge on an exposed target. This approach gives the advantage of working in a less aggressive environment, only long-lived species remain in solution, particularly hydrogen peroxide (H2O2). This latter, because of its amphoteric character (Eqs, 1 and 2) will partially oxidize part of the Fe2+ to Fe3+ before their precipitation into double oxides. These are the favorable moderate preconditions for obtaining the magnetite (FeO.Fe2O3) sought. Much more interesting, the oxidizing power increases when the pH decreases, which gives the possibility to optimize the process by avoiding at the same the employment of antioxidants such as ascorbic or citric acid usually used to inhibit the oxidation by scavenging the reactive oxygen species (ROS).
The heterogeneous plasma/Fenton coupling here would offer the possibility to optimize the degradation of two model pollutants, and the ability to carry out the Fenton reaction in a wide range of pH, bypassing the difficulty linked to the precipitation of iron hydroxides (He et al., 2020; Marijana et al., 2015).
The two model pollutants are organometallic compounds, and their use in various fields of science has a considerable and non-negligible impact on the environment (Jessica et al., 2020). Organometallics are generally stable in the environment and can remain there for several years. Even in low concentrations, bioaccumulation can quickly occur through their ingestion by microorganisms as has been seen in the past with the Minamata disaster in Japan in the 1950s (Noriyuki, 2006). Organometallic iron complexes such as naphthol green B are used in the industry for stainless wool, paper, etc. (Kunkely and Vogler, 2003), while organomercury is commonly used in hospital centers and the mining industry (Mary et al., 2014; Pfeiffer et al., 1993). Most of these industries or centers do not always have a treatment unit for their effluents. Even if they exist, the methods used (calcination, adsorption, coagulation, etc.) are inadequate and do not eliminate the pollutants; the residues are always discharged into the environment (Kumari et al., 2023). Hence, the need to develop pollution control systems that are reliable and easy to implement using local materials is urgently required.
The present study deals with the development of a cost-effective magnetic-supported catalyst for enhanced Fenton reaction. The singularity of our approach lies in the fact that the plasma plays a triple role here: (i) the pre-functionalization of the biomass to create catalyst attachment sites by photonic bombardment of the biomass surface and by grafting radical species on unsaturated bonds to form new hydrophilic functional groups. These anchoring sites would serve to disperse the Magnetite nanoparticles thereby increasing the surface/volume ratio and avoiding aggregation; (ii) the inhibition of oxidation of Fe2+ during the synthesis of magnetic nanoparticles of magnetite thanks to plasma-activated water (PAW) properties that slow down the oxidation without using additional chemical scavenger; (iii) The plasma-production of Fenton reagent H2O2 by dimerization of the radical HO• and the emission of UV light to enhance the Fenton-like reactions.
Moreover, the kinetic study using pseudo-first, and pseudo-second orders as well as the Langmuir–Hinshelwood model were attempted to better describe the mechanism. Seen from another angle, this study offers the possibility of combining both the basic concerns of a developing country (valorization of local natural resources, development of simple and inexpensive analytical systems, consideration of the protection of the environment) with scientific research issues (preparation, characterization, and application of hybrid materials, new depollution processes). Water Hyacinth used here is well known in tropical regions as an invasive plant that has negative impacts on aquatic ecosystems because of uncontrollable rapid growth and seemingly impossible eradication challenge. Several attempts for their elimination have been made and their presence appears as environmental issues in several countries.
2 Experimental Part
2.1 Pretreatment of Biomass and Preparation of hybrid Material
The experimental Glidarc plasma (GAP) device used for biomass surface modification and the activation of water (PAW) is given in Fig. 1. This device was proposed by Leusueur et al. (Leusueur et al., 1988), for the degradation of volatile compounds and adapted by Brisset et al. (Brisset et al., 1989), for the treatment of industrial wastewater. It consists of producing non-thermal plasma in a cylindrical glass reactor containing two electrodes with diverging profiles brought to a high voltage (10 kV) operating in a direct or alternating mode. At the minimum distance between these electrodes, the electrons are ejected from the cathode towards the anode with high kinetic energy. In contact with a judiciously chosen plasma gas (moist air in our case), part of this energy will be transmitted to the parent molecules of this gas (O2, N2, H2O). It follows a plume of ionized gas containing in addition to electrons and photons, a multitude of reactive nitrosed species (RON) and oxygenated species (ROS). This plume under the effect of the gas pressure will slide and lick the surface of the target exposed (biomass; distilled water and pollutants).
The water hyacinth biomass used as support was sampled in the Wouri River region on the coast of Cameroon. The biomass was cut into slices, cleaned, washed, and then dried at room temperature until constant mass, then crushed and sieved. Aliquots of the fibers were subjected to plasma activation for 1 h (Prola et al., 2013; Takam et al., 2017). The hybrid composite was prepared by co-precipitation in a 2/1 ratio of ferric (FeCl3‚6H2O at 1 mol.L−1, Aldrich 97%) and ferrous ions (FeCl2‚4H2O at 0.5 mol.L−1, Aldrich 99%) mixed with 2.5 g of biomass dispersed in PAW using 50 mL of a NaOH solution (1 mol.L−1, Merck). For comparison, the unsupported catalyst was also prepared following the same route without the biomass. PAW is obtained by exposing 430 ml of distilled water to plasma for 1 h. The latter, thanks to plasma-liquid interactions, will see its physico-chemical properties significantly modified, in particular electrical conductivity, and pH, but also the concentration of long-lived dissolved molecules such as H2O2, HNO2, HNO3 and ONOOH (Brisset and Pawlat, 2016). The resulting hybrid composite (CP) was washed with distilled water and acetone and dried at 70 °C temperature for 6 h.
2.2 Characterization of hybrid Composites
The FTIR, SEM/EDX, DRX, pHpzc, and magnetization measurements were performed. The FTIR spectra were recorded using a Bruker brand AlphaP IR spectrophotometer equipped with a DTGS detector between 4000 and 400 cm−1. The morphology was visualized with a Philips XL 30 brand field emission scanning electron microscope with an electron acceleration voltage of 20 kV and an objective WD: 13 mm, NA: 0.55, using the images produced from the secondary electrons were used. The crystallinity was evaluated using a Siemens D5000 diffractometer equipped with copper Kα radiation (40 kV; 30 mA, k = 1.33 Ǻ) with a scanning speed of 3 deg/min between 10 and 80 (2Ɵ). Measurements of the magnetization at different temperatures were carried out using a vibrating sample magnetometer (VSM) by application of variable magnetic fields. The pH at the point of zero charge (pHpzc) of the composites was determined according to the protocol described by Takam et al. (Takam et al., 2020).
2.3 Experimental Conditions, Chemical Reagents, and Mechanism of Degradation
The organometallic (naphthol green B and merbromin) were obtained from Sigma-Aldrich firm and dissolved at a desired concentration. Distilled water was used throughout the experiments and the reagents were used as received without any further purification. Plasma treatment alone, without a catalyst, was carried out for comparison. For Fenton experiments, the pH of the solutions was adjusted between 3 and 11, the composite dose was varied from 0.1 to 1 g, and the pollutant concentration was from 100 to 500 mg.L−1 for NGB and from 10 to 100 mg.L−1 for MB depending on their solubility. Once the best operating conditions were set, the plasma/Fenton test was carried out for 60 min. The coupling test here could improve the degradation efficiency of organic contaminants thanks to the production of additional radicals HO• through Magnetite-catalysed Fenton and Fenton-like reactions (Eqs. 3, 4).
Given these equations, the limiting reagent H2O2 is directly brought by the plasma without external addition. Also, the plasma discharges produced acidifying species (HNO2 and HNO3) that decreased rapidly the pH around 3 and emits UV radiation (λ > 185 nm) due to the decay of the excited nitrogen molecules (Tarkwa et al., 2021) favourable for such Fenton-like reactions.
After the treatment, the solutions were centrifuged and the supernatant was analyzed using a JENWAY brand spectrophotometer at 715 nm for naphthol green B, and 511 nm for merbromine. The rate of degradation was calculated by applying Eq. 5. Three kinetic models: pseudo-first-order; pseudo-second-order and the Langmuir–Hinshelwood model were introduced to better explain the degradation given by the Eqs. 6-11 (Vasanth et al., 2008). For the degradation mechanism, the trapping of predominant species involved in the degradation was carried out with tert-Butanol (5 mM) for HO• radicals and disodium ethylenediaminetetraacetic acid (5 µM) for h+ holes (Dionmbete et al., 2023).
OM: Organometallic.
3 Results and Discussion
3.1 Characterization of hybrid Composite
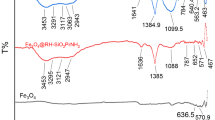
FTIR technique was used to identify the main chemical groups at the surface of obtained composite; the results are depicted in Fig. 2 and the mains bands are gathered in Table 1. Previous work has shown that the treatment of biomass fibers with moist air plasma leads to the grafting of the hydroxyl group (-OH) on the unsaturated bonds (Prola et al., 2013; Takam et al., 2017; Takam et al., 2020). This was observed here by the increase in the absorption band at 3318 cm−1, assigned to the stretching vibration of the hydroxyl group (-OH) of carboxylic acids, phenols, cellulose, hemicellulose, and adsorbed water (Mohanty et al., 2006). The vibrations observed around 2923 cm−1 correspond to the symmetrical and asymmetrical stretching of the C-H of the aliphatic –CH2 group and to the vibration of the C-H group of alkyls in the aliphatic chains of cellulose, lignins, and hemicellulose (Flores et al., 2015). The vibration of the -C = C- group of the aromatic ring was observed at 1560 cm−1 (Mohanty et al., 2006). The absorption band at 1035 cm−1 corresponds to the bond deformation of the C-O group of hemicellulose and lignin in the fibers (Sameer et al., 2019). Finally, the vibration band at 572 cm−1 is assigned to the Fe–O bond in the magnetite Fe3O4 corroborating their incorporation in the organic matrix (Chen et al., 2023; Musa et al., 2010). All these results highlight the fact that even if the magnetite nanoparticles are indeed incorporated into the organic matrix, they do not cover the entire surface like a core–shell. This periodic arrangement means that active sites created by plasma serve as an anchor point for these particles, suggesting a possibility of controlling the distribution by modifying the intrinsic parameters of the plasma.
Given that the shape, size, crystallinity, and dispersion constitute the key parameters that determine their physical and chemical properties, the nanoparticles growth step is, therefore, crucial to avoid uncontrolled aggregation of the particles known as “Ostwald ripening” and causing an undesirable effect of inhomogeneous magnification of the nanoparticles. To obtain uniform sizes, nucleation must be brief and growth must be simultaneous. In this work, we have opted for the use of biomass to confine the particles during their growth. Recent studies have shown that the use of polymers bearing specific functional groups such as polystyrene, biphenol polycarbonate, and polysulfone are likely to interact with nanoparticles and depending on the strength of the interactions would have a direct impact on the size of the nanoparticles obtained (Ergun, 2023; Lee and Hyeon, 2012; Li and Zhu, 2020). These behaviors result from the interaction at the adsorbed polymer–nanoparticle interface and depend on the free energy of the adsorbed layer.
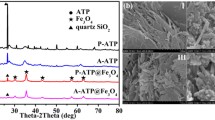
Figure 3 and 4 present the SEM images and the chemical composition respectively. The raw biomass micrograph shows smoother surfaces with irregular and non-compacted sheets, with tunnels and protuberances throughout its length (Fig. 3-a, b). Meanwhile, magnetite unsupported displays compact and highly agglomerated rods (Fig. 3-c, d). SEM images of composite (Fig. 3-e, f) show anisotropic nanorod-size particles randomly dispersed on biomass surface. The aggregates observed have a more rugged structure with repeating motifs offering the possibility of having distinct sites useful for adsorption, a crucial step before oxidation. The main elements from EDX analysis (Table 2) are as expected, iron, carbon, oxygen, chlorine, and sodium. Iron is present in different oxidation states corroborating the formation of magnetite and supporting the inhibition role of plasma-activated water (PAW). The trace presence of chlorine come from the precursor salts and could be eliminated after several washes.
Figure 5 exhibits the X-ray diffractogram of the hybrids obtained. The most prominent signals observed at 2 \(\theta\) values of 14.76; 21.9 and 30.14 correspond to planes (101), (200) and (004) of cellulose contained in the water hyacinth fibers following JCPDS No 21–1272 (Zhang et al., 2014). The 2 \(\theta\) values at 31.52; 35.34; 45.3; 56.36; 66.04 and 75.14 are assigned to the peaks of magnetite nanoparticles whose respective reflection planes are (220), (311), (400), (422), (511) and (440) according to ICDD card No 19–0629 (Valentina et al., 2015). The shape of these peaks reflects the high crystallinity of the as-prepared hybrid composites. It is observed that the combination of plasma-modified hyacinth fibers with magnetic particles does not alter the crystal structure of magnetite. No extraneous peak was detected, which indicates that the catalyst obtained is pure and the synthesis method used is efficient (Mohamed et al., 2021).
The CP composite was also analyzed using a vibrating sample magnetometer to evaluate the magnetic properties essential for fast and total recovery. Figure 6 (a, b) shows the hysteresis loops at temperatures of 20, 150, and 300 K obtained by applying variable magnetic fields between -6 and 6 T. The saturation magnetization values of CP are 15 emu.g−1, which is less than 92 emu.g−1 for pure magnetite nanoparticles not combined with biomass (Ro et al., 2012). The decrease in the magnetization value could be due to the amorphous nature of biomass that attenuate the magnetization of particles resulting from the co-precipitation. The magnetization curve shows zero coercivity at different temperatures indicating superparamagnetic and temperature-dependent behavior (Shen et al., 2023; Zhan et al., 2018).
3.2 Plasma-Induced Fenton Degradation
3.2.1 Heterogeneous Plasma/Fenton Coupling with CP Composite
The treatment of organometallic solutions in the presence of a catalyst was carried out at pH 3 to avoid the formation of iron hydroxide sludge. From Fig S1 (see supplementary material), a decrease in performance with the increase in pH for the study carried out outside the plasma (adsorption behavior) was observed. This would be due to the electrostatic repulsions given that the point of zero charges of the composite is pHzcn = 7.89 (Fig S2); for pH above pHzcn, both molecules and hybrid composite are negatively charged. This explains the low affinity between the composite and organometallics in a basic medium. The treatment times were varied from 0 to 60 min with a mass optimized at 1 g.L−1 in 430 mL of solution. According to Fig S3, an increase in adsorption efficiency is observed with increasing composite dose given that adsorption is a surface phenomenon, the more the dose of composite increases, the more the sites available for adsorption increase until the saturation inducing an agglomeration of the particles.
Figure 7 shows the UV–Vis spectra of naphthol green B (a) and merbromin (b) during plasma treatment coupled with the heterogeneous Fenton process. An improvement in the efficiency of degradation with the coupling going from 61.27% with the plasma treatment alone to 97.01% for merbromin and from 40.10% to 99.70% for naphthol green B was noticed. This improvement in degradation rate can be explained by the additional action of magnetite through the additional production of HO• and HOO• radicals by direct Fenton reaction and indirect Fenton-like (Wang et al., 2016; Yolanda et al., 2008). For the particular case of the naphthol green B complex, the complete dissociation for 10 min of exposure could not be due to the variations in pH but more probably to the reduction of Iron (III) into Iron (II) according to Laminsi et al.(2012). Once released in solution, the ligand is gradually degraded until reaching a rate of 76% for 60 min. The obtained results were compared with earlier published studies in the literature and Table 3 displays a summary of similar results concerning the elimination of pollutants with different hybrid composites (Clarizia et al., 2017; Mahmoud and Abdelwahab, 2019; Dionmbete et al., 2023; Idrissi et al., 2016; Mahalakshmi et al., 2020; Gomathy and Shanthi, 2020).
3.2.2 Degradation Kinetics Study
The study of the Langmuir–Hinshelwood kinetic model was made by varying the concentrations of organometallics from 100 to 500 mg.L−1 for naphthol green B and from 10 to 100 mg.L−1 for merbromin with a catalyst dose of 1 g.L−1. Figures 8-a and c illustrate the decay kinetics of the initial concentrations of organometallics and Figs. 8-b and d represent the plot of the Langmuir–Hinshelwood kinetics for these two organometallics. Table 4 gives the values of the apparent constants for all the concentrations studied.
It is noted for merbromin, an increase in the apparent constants (0.0287; 0.0311; 0.0356; 0.0456 min−1) with the increase in concentration 10; 20; 50, and 100 mg.L−1, whereas the opposite effect is observed for naphthol green B, the apparent constants decrease (0.0538; 0.0479; 0.0373; 0.028) with the concentrations of 100, 200, 300 and 500 mg.L−1. Globally during the first 15 min, the organometallic concentrations decrease slightly and a pseudo-equilibrium is perceptible, which corresponds to the adsorption–desorption of pollutant molecules on the surface of the hybrid composite (Sun et al., 2018). The Langmuir–Hinshelwood kinetics plot allows us to determine the Kr and Ks for the two organometallics using Eq. 12, where Ks is the equilibrium adsorption constant and Kr is the reaction rate constant. For naphthol green B, the Kr and Ks constants are 0.152 mg.L−1 min−1 and 0.548 L.mg−1 respectively, while are -6.988 mg.L−1 min−1 and -4.04 \(\times\) 10−3 L.mg−1 for merbromin. According to these results, adsorption appears to be the limiting step of the degradation process of both organometallics. Similar results were obtained by Smaali, et al. (2021) for the photocatalytic oxidation of the diclofenac sodium with persulfate in an aqueous medium.
In addition to this Langmuir–Hinshelwood kinetic model, the pseudo-first and pseudo-second-order models are evaluated (Fig S4). The results reveal based on the values of the different correlation coefficients that the pseudo-first-order described better than the pseudo-second-order model. Therefore, the degradation of organometallics is controlled by the diffusion of pollutant molecules (Wang et al., 2016).
3.2.3 Trapping Species for Mechanism Elucidation
The free radical species trapping experiment was carried out to stand out the role of each reactive species in the degradation mechanism. The tests were carried out under optimal experimental conditions with EDTA-2Na (5 µM) to trap h+ holes and with tert-Butanol (5 mM) to trap hydroxyl radicals. The results obtained were compared with those obtained in the absence of scavengers and given in Fig. 9, the corresponding UV–Vis spectra are provided in Fig S5 (supplementary material).
It is observed that tert-BuOH inhibits significantly the degradation of naphthol green B as shown in Fig. 9-a, whereas EDTA-2Na has no significant effect, which means that hydroxyl radicals are the species that contribute mainly to the degradation of naphthol green B. For merbromin, the addition of t-BuoH and EDTA-2Na only halve the degradation rate for the first 15 min, meaning that there could be other species in the reaction medium that are involved. It is known that plasma is a mixture of several species such as photons, electrons, charged particles, free radicals (HO•, NO•, O2•−, HOO•), and long-life molecules (H2O2, ONOOH). Superoxide radicals can be produced according to Eq. 13 and in an acid medium can be protonated to give hydroperoxyl radicals according to Eq. 14, the latter can then be responsible for the degradation of merbromin when the hydroxyl radicals are trapped by t-BuOH. Moreover, other compounds such as ONOOH can be photolyzed in a plasma medium to produce the radicals NO2• (Eq. 15) and contribute to the degradation reaction (Voufouo et al., 2022).
3.2.4 Leaching Behavior and Reusability Test
The Leaching tests consisted in removing the catalyst from the solution previously stirred in the dark for 60 min to monitor any degradation in a plasma medium. The tests report a curve appearance almost similar to that obtained by plasma alone (Fig. 10-a). This result would mean that there is no longer an additional supply of reactive species and that only the species generated by the plasma are responsible for the observed degradation. This result attests that the particles are firmly attached to the support. The integrity of the composite is preserved after processing (see supplementary Fig S6). The ability of the particles dispersed on the support to detach in the solution was also evaluated by following the concentration of Fe ions (total iron) dissolved in the solution. The results are shown in Fig. 10-a. It emerges about the concentration of iron an increase in the first 15 min followed by a gradual decrease. The resulting interpretation would come from the dissociation of the complex preceding the effective degradation of the organic ligand, thus releasing the Fe ions in solution. The observed decrease after 15 min could imply that once released these ions are complexed or precipitated on the catalyst.
The study of the reuse cycles of CP composites was carried out under optimal conditions. After each test, the composite is filtered, washed several times in ethanol and water, and afterward dried at room temperature for further reuse test. Figure 10-b illustrates the band diagram showing the degradation rate as a function of each reuse cycle. Four reuse cycles were carried out.
The results obtained during the test show that the catalyst remains stable and active. Indeed, the degradation efficiency decreases slightly as shown with the degradation rate moving to 85.10% for the fourth cycle compared to 97.01% for the first cycle. This decrease may be due to: (a) the loss of composite mass during filtration; (b) the clogging due to the precipitation of metal release after organometallic degradation, and (c) the difficulty to regenerate the catalyst due to the multiple chemical interactions. It is therefore useful for subsequent use to find adequate eluent to desorb the spent composite.
4 Conclusion
This work reports the development of a cost-effective magnetic-supported catalyst for enhanced removal of two organometallics from wastewater through Fenton reactions in low-temperature plasma conditions. The composite obtained (CP) was characterized to assess its size, morphology, crystallinity, and magnetization behavior. Herein we have taken advantage of the extraordinary properties of the non-thermal plasma of the glidarc type obtained at atmospheric pressure. This plasma is a plume of radical and ionized species containing electrons and molecular species in excited states. This is a favorable environment for undertaking several chemical reactions. Firstly, it consisted in functionalizing natural biomass by grafting hydroxyl radicals on the \(\pi -\pi\) bonds. These functional groups served as binding or anchoring sites for the magnetite nanoparticles’ dispersion and thereby controlling their growth. The twin aim was the production by plasma of Fenton’s’ reagents (H2O2) without external input obtained by dimerization of radial HO•. Moreover, since the plasma also emits electromagnetic waves in the UV, these photons will boost thanks to the Fenton-like reaction the degradation. The results obtained report a hybrid material containing magnetite nanorods periodically dispersed on the surface of the biomass. No leaching behavior was observed during the treatment. Catalytic tests report a significant improvement in degradation rates from 61% with the plasma treatment alone to 97% for merbromin and from 40 to 99% for naphthol green B. Langmuir–Hinshelwood kinetics revealed that the diffusion and adsorption of pollutants on the catalyst surface was the rate-determining step. This is an interesting result that attests to the positive synergetic effect of combining the heterogeneous Fenton process with plasma even after 4 reuse cycles ensured by the superparamagnetic properties of the composite that allow easy removal.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Acayanka, E., Tiya-Djowe, A., Laminsi, S., Tchoumkwe, C. C., Nzali, S., Mbouopda, P.A., Ndifon, P. T., Gaigneaux, E. M. (2013) Plasma-Assisted Synthesis of TiO2 Nanorods by Gliding Arc Discharge Processing at Atmospheric Pressure for Photocatalytic Applications. Plasma Chemistry and Plasma Processing, 33. https://doi.org/10.1007/s11090-013-9455-7.
Brisset, J. L., Doubla, A., Amouroux, J., Lelievre, J., & Goldmann, M. (1989). Chemical reactivity of the gaseous species in a plasma discharge in air: An acid-base study. Applied Surface Science, 36(1–4), 530–538. https://doi.org/10.1016/0169-4332(89)90947-1
Brisset, J. L., & Pawlat, J. (2016). Chemical effects of air plasma species on aqueous solutes in direct and delayed exposure modes: Discharge, post-discharge and plasma activated water. Plasma Chemistry and Plasma Processing, 36, 355–381. https://doi.org/10.1007/s11090-015-9653-6
Chen, X., Wang, S., Li, D., et al. (2023). Fabrication of Adsorption-reactive composite PAM-AMPS/Fe3O4 hydrogel based on persulfate advanced oxidation processes for organic pollutants degradation. Water Air Soil Pollution, 234, 533. https://doi.org/10.1007/s11270-023-06561-9
Clarizia, L., Russo, D., DiSomma, I., Marotta, R., & Andreozzi, R. (2017). Homogeneous photo-Fenton processes at near neutral pH: A review. Applied Catalysis b: Environmental, 209, 358–371. https://doi.org/10.1016/j.apcatb.2017.03.011
Culita, D. C., Simonescu, C. M., Dragne, M., Stanica, N., Munteanu, C., Preda, S., & Oprea, O. (2015). Effect of surfactant concentration on textural, morphological and magnetic properties of CoFe2O4 nanoparticles and evaluation of their adsorptive capacity for Pb(II) ions. Ceramics International, 41, 13553–13560. https://doi.org/10.1016/j.ceramint.2015.07.150
Dionmbete, G., Miloh, N., Tarkwa, J. B. E., Acayanka, A. B., Mbouopda, P., Boyom-Tatchemo, F.-W., & Kamgang, G. Y. (2023). Hybrid composite with lignocellulosic biomass supported titania obtained under plasma conditions for photocatalytic application. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-023-04246-1
Ergun, C. (2023). A current review on conducting polymer-based catalysts: Advanced oxidation processes for the removal of aquatic pollutants. Water Air Soil Pollution, 234, 524. https://doi.org/10.1007/s11270-023-06526-y
Flores, R. N., Sanchez, H. Y., & Cruz, J. (2015). Composites from water hyacinth (Eichhornea crassipe) and polyester resin. Fibers and Polymers, 16, 196–200. https://doi.org/10.1007/s12221-015-0196-5
Gomathy, J., & Shanthi, M. (2020). An efficient nanocomposite CdS-ZnWO4 for the degradation of Naphthol Green B dye under UV-A light illumination. Nano-Structures & Nano-Objects, 22, 100452. https://doi.org/10.1016/j.nanoso.2020.100452
He, G., Zhen, L., Ying, Z., Nan, J., Huijuan, W., & Jie, L. (2020). Degradation of chloramphenicol by pulsed discharge plasma with heterogeneous Fenton process using Fe3O4 nanocomposites. Separation and Purification Technology, 253, 117540. https://doi.org/10.1016/j.seppur.2020.117540
Idrissi, M., Miyah, Y., Benjelloun, Y., & Chaouch, M. (2016). Degradation of crystal violet by heterogeneous Fenton-like reaction using Fe/Clay catalyst with H2O2. Journal of Materials and Environmental Science, 7, 50–58.
Jessica, B., Emmanuel, S., & Renald, B. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e04691
Jôse, R. A. L., Douglas, L. F., Thalles, H. S. M., Rhayza, V. M. O., Amanda, A. S., Graziele, C. C., & Luciane, P. C. R. (2020). Potential of a magnetic hybrid material produced using water hyacinth (Eichhornia crassipes) for removal of inorganic and organic pollutants from aqueous media. Journal of Environmental Chemical Engineering, 8, 104100. https://doi.org/10.1016/j.jece.2020.104100
Kumari, H., Sonia, Suman, et al. (2023). A review on photocatalysis used for wastewater treatment: Dye degradation. Water Air Soil Pollution, 234, 349. https://doi.org/10.1007/s11270-023-06359-9
Kunkely, H., & Vogler, A. (2003). Photolysis of Naphthol Green B in aqueous solution. Photoreduction of Fe(III) induced by ligand-to-metal charge transfer excitation. Zeitschrift fur Naturforschung, 58B, 922–924. https://doi.org/10.1515/znb-2003-0914
Laminsi, S., Acayanka, E., Ndifon, P. T., Tiya, A. D., & Brisset, J. L. (2012). plasma-chemical dissociation and degradation of naphthol green B complex. Environmental Engineering and Management Journal, 11, 1461–1466.
Lee, N., & Hyeon, T. (2012). Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chemical Society Reviews, 41, 2575–2589. https://doi.org/10.1039/C1CS15248C
Lesueur, H., Czernichowski, A., Chapelle, J., (1988) Dispositif de génération de plasmas basse température par formation de décharges électriques glissantes. French Patent, 2639172.
Li, H., & Zhu, Y. J. (2020). Liquid-phase synthesis of iron oxide nanostructured materials and their applications. Chemistry – A European Journal, 26, 9180–9205. https://doi.org/10.1002/chem.202000679
Lu, A. H., Salabas, E. L., & Schüth, F. (2007). Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angewandte Chemie International Edition, 46, 1222–1244. https://doi.org/10.1002/anie.200602866
Mahalakshmi, G., Rajeswari, M., & Ponnarasi, P. (2020). Synthesis of few-layer g-C3N4 nanosheets-coated MoS2/TiO2 heterojunction photocatalysts for photo-degradation of methyl orange (MO) and 4-nitrophenol (4-NP) pollutants. Inorganic Chemistry Communications, 120, 108146. https://doi.org/10.1016/j.inoche.2020.108146
Mahmoud, M. E., & Abdelwahab, M. S. (2019). Fabricated and functionalized magnetite/phenylenediamine for adsorptive removal of methylene blue. International Journal of Biological Macromolecules, 128, 196–203. https://doi.org/10.1016/j.ijbiomac.2019.01.102
Marijana, M., Milica, J., Dalibor, S., Vesna, K., Goran, R., Gordana, G. C., & Dragan, M. (2015). Application of non-thermal plasma reactor and Fenton reaction for degradation of ibuprofen. Science of the Total Environment, 505, 1148–1155. https://doi.org/10.1016/j.scitotenv.2014.11.017
Mary, C., Sheehan, A., Burke, A., Patrick, N., Breysse, J. M., Mary, A., (2014) Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review, Bulletin of the World Health Organization, 92. https://doi.org/10.2471/BLT.12.116152
Mbouopda, P. A., Acayanka, E., Nzali S., Kamgang, Y. G., Njoyim, T. E.B., Laminsi, S., Dominique, R., (2018) Comparative study of plasma-synthesized and commercial-P25 TiO2 for photocatalytic discoloration of Reactive Red 120 dye in aqueous solution. Desalination, and Water Treatment, 1–9. https://doi.org/10.5004/dwt.2018.23118.
Mohamed, E. G., Nader, H., Ahmed, S., Akram, E., & Ashraf, E. A. (2021). Synthesis and Characterization of Porous Magnetite Nanosphere Iron Oxide as a Novel Adsorbent of Anionic Dyes Removal from Aqueous Solution. Biointerface Research in Applied Chemistry, 11, 13377–13401. https://doi.org/10.33263/BRIAC115.1337713401
Mohanty, K., Jha, M., Meikap, B. C., & Biswas, M. N. (2006). Biosorption of Cr (VI) from aqueous solutions by Eichhornia crassipes. Chemical Engineering Journal, 117, 71–77. https://doi.org/10.1016/j.cej.2005.11.018
Musa, M. C., Sadan, O., Abdullah, C., & Tezer, F. (2010). Effect of milling time on the synthesis of magnetite nanoparticles by wet milling. Materials Science and Engineering B, 172, 72–75. https://doi.org/10.1016/j.mseb.2010.04.019
Mustapha, M. B., Abdul, A. A. R., & Anam, A. (2019). A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Safety and Environmental Protection, 126, 119–140. https://doi.org/10.1016/j.psep.2019.03.028
Noriyuki, H., (2006) The history and the present of Minimata disease. Japan Medical Association Journal 49:112–118. https://www1.med.or.jp/english/journal/pdf/jmaj/v49no03.
Oturan, M. A., Oturan, N., Lahitte, C., & Trevin, S. (2001). Production of hydroxyl radicals by electrochemically assisted Fenton’s reagent: Application to the mineralization of an organic micropollutant, pentachlorophenol. Journal of Electroanalytical Chemistry, 507(1–2), 96–102. https://doi.org/10.1016/S0022-0728(01)00369-2
Pfeiffer, W. C., Lacerda, L. D., Salomons, W., & Malm, O. (1993). Environmental fate of mercury from gold mining in the Brazilian Amazon. Environmental Reviews. https://doi.org/10.1139/a93-00
Ponomar, V. P. (2018). Thermomagnetic properties of the goethite transformation during high-temperature treatment. Minerals Engineering, 127, 143–152. https://doi.org/10.1016/j.mineng.2018.08.016
Prola, L. D. T., Acayanka, E., Lima, E. C., Umpierres, C. S., Vaghetti, J. C. P., Wmekson, O. S., Laminsi, S., & NDjifon, P. T. (2013). Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of Reactive Red 120 textile dye from aqueous solution. Industrial Crops and Products, 46, 328–340. https://doi.org/10.1016/j.indcrop.2013.02.018
Ro, U., Mohapatra, S., & Ahmad, S. (2012). Fe3O4 inverse spinal superparamagnetic nanoparticles. Materials Chemistry and Physics, 132, 96–202. https://doi.org/10.1016/j.matchemphys.2011.11.032
Saba, H. (2015). A review on nanoparticles: Their synthesis and types. Research Journal of Recent Sciences, 4, 1–3.
Sameer, F., Nicola, S., Joel, P., & Rodenburg, C. (2019). Exploiting plasma exposed, natural surface nanostructures in ramie fibers for polymer composite applications. Materials, 12, 1631–1646. https://doi.org/10.3390/ma12101631
Shen, J., Wang, B., & Cai, L. (2023). Propriétés magnétiques et stabilité thermique des composites magnétiques doux à base de fer amorphe/carbonyle. Journal of Materials Science: Materials in Electronics, 34, 1169. https://doi.org/10.1007/s10854-023-10512-9
Smaali, A., Berkani, M., Merouane, F., Vasseghian, Y., Rahim, N., & Kouachi, M. (2021). Photocatalytic-persulfate-oxidation for diclofenac removal from aqueous solutions: Modeling, optimization, and biotoxicity test assessment. Chemosphere, 266, 129158. https://doi.org/10.1016/j.chemosphere.2020
Sun, P., Jun, Z., Wenxiu, L., Qi, W., & Wenbin, C. (2018). Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2. Catalysts, 8, 326. https://doi.org/10.3390/catal8080326
Takam, B., Acayanka, E., Kamgang, Y. G., Pedekwang, M., Laminsi, S., (2017) Enhancement of sorption capacity of cocoa shell biomass modified with non-thermal plasma for removal of both cationic and anionic dyes from aqueous solution. Environmental Sciences and Pollution Research. https://doi.org/10.1007/s11356-017-9328-3.
Takam, B., Tarkwa, J. B., Acayanka, E., Nzali, S., Chesseu, D. M., Kamgang, G. Y., & Laminsi, S. (2020). Insight into the removal process mechanism of pharmaceutical compounds and dyes on plasma-modified biomass: The key role of adsorbate specificity. Environmental Science and Pollution Research, 27, 20500–20515. https://doi.org/10.1007/s11356-020-08536-3
Tarkwa, J. B., Acayanka, E., Sop, B. T., et al. (2021). Effect of Gliding Arc Plasma-Induced UV Light During the Photo-Fenton Oxidation of 4-Chlorophenol in Aqueous Solution. Plasma Chemistry and Plasma Processing. https://doi.org/10.1007/s11090-021-10171-w
Tiya-Djowe, A., Nzali, S., Njoyim, T. E., Laminsi, S., & Gaigneaux, M. E. (2016). Thermal treatment of plasma-synthesized goethite improves Fenton-like degradation of orange II dye. Environmental Chemistry Letters, 14, 515–519. https://doi.org/10.1007/s10311-016-0578-y
Valentina, G., Ecaterina, A., Alina, M. H., Laurenţiu, M., George, M., Alexandru, M., Grumezescu, A., Gabriel, S., Florin, I., Horia, M., & Mariana, C. (2015). MAPLE fabrication of thin films based on kanamycin functionalized magnetite nanoparticles with anti-pathogenic properties. Applied Surface Science, 336, 188–195. https://doi.org/10.1016/j.apsusc.2014.10.177
Vasanth, K., Porkodi, K., & Rocha, F. (2008). Langmuir-Hinshelwood kinetics – A theoretical study. Catalysis Communications, 9, 82–84. https://doi.org/10.1016/j.catcom.2007.05.019
Voufouo, A. S., Tarkwa, J. B., Acayanka, E., Momeni, N., Nzali, S., Kamgang, Y. G., & Laminsi, S. (2022). Photocatalytic performance of N-TiO2@SiO2 composite obtained under gliding arc plasma processing at atmospheric pressure. Results in Engineering, 15, 100516. https://doi.org/10.1016/j.rineng.2022.100516
Wang, J., Sun, Y., Jiang, H., & Feng, J. (2016). Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. Journal of Saudi Chemical Society. https://doi.org/10.1016/j.jscs.2016.08.002
Xiaoling, L., Yuanhong, Z., Sanyuan, Z., Li, G. M., Peg, Y., Jianxi, Z., Hongping, H., & Zheng, J. (2012). The contribution of vanadium and titanium on improving methylene blue decolorization through heterogeneous Uv-Fenton reaction catalyzed by their co-doped magnetite. Journal of Hazardous Materials, 200, 247–254. https://doi.org/10.1016/j.jhazmat.2011.11.007
Yanping, Z., Runliang, Z., Yunfei, X., Jianxi, Z., Gangqiang, Z., & Hongping, H. (2019). Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Applied Catalysis b: Environmental, 255, 117739. https://doi.org/10.1016/j.apcatb.2019.05.041
Yolanda, F., Roberto, F., & Alberto, A. G. (2008). Heterogeneous catalysis in the Fenton-type system reactive black 5/H2O2. Journal of Molecular Catalysis a: Chemical, 281, 184–191. https://doi.org/10.1016/j.molcata.2007.10.019
Zhan, H., Bian, Y., Yuan, Q., Ren, B., Hursthouse, A., & Zhu, G. (2018). Preparation and potential applications of superparamagnetic Nano-Fe3O4. Processes, 6, 33. https://doi.org/10.3390/pr6040033
Zhang, J., Wang, Y., Zhang, L., Zhang, R., Liu, G., & Cheng, G. (2014). Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresource Technology, 151, 402–405. https://doi.org/10.1016/j.biortech.2013.10.009
Acknowledgements
The authors are grateful to International Foundation for Science (IFS) for equipment support (Grant no: W/4219-1).
Author information
Authors and Affiliations
Contributions
Nehemie Miloh: Investigation, Original draft preparation; Verdiane K. Kengne: Investigation; Acayanka Elie: Conceptualization, Writing- Reviewing and Editing; Patrick M. Kouotou: Writing, data Curation; Kamgang Youbi Georges: Formal analysis, Writing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miloh, N., Kengne, V.K., Acayanka, E. et al. Plasma-assisted Synthesis of Supported Superparamagnetic Oxides for Enhanced Fenton Reactions. Water Air Soil Pollut 235, 631 (2024). https://doi.org/10.1007/s11270-024-07446-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07446-1