Abstract
The study was carried out to evaluate hydro geochemistry and the risk of groundwater and surface water pollution in the Kombolcha area. To achieve this, hydrogeochemical analysis, water heavy metal, geospatial data analysis, correlation matrix, principal component analysis, Heavy metal Pollution Index (HPI), and Water Quality Index (WQI) methodologies were employed. A total of 36 samples (both water and effluent samples) had been collected and assessed for major physicochemical variables and heavy metals. Hydrogeochemical methods showed groundwater mineralization due to (1) silicate weathering, (2) cation exchange processes, and (3) anthropogenic sources (i.e., contaminated discharge of sulphate, carbonate, and trace metal effluents). The study result revealed that major ions dominating the area are Ca2+ > Na+ > Mg2+ > K+, HCO3− > SO 2− > Cl− > NO3−, and Fe > Mn > Pb > Cr > Cd for cations, anions and trace metals respectively with all heavy netals had mean concentrations above the WHO recommended limits. Calculated Pollution indices revealed 50.7% of the sample belongs to a low level of pollution, while 35% and 14.3% belong to a medium and high level of pollution respectively which consequently translating the area into high groundwater pollution zones. The correlation matrix revealed that no significant correlation exists between the water quality variables (Cl and NO3− with Fe, Pb, Cr, Mn, and Cd). PCA was applied on the data set to identify the spatial sources of pollution in groundwater and in the first principal component analysis, Mn, Fe, Cr, Pb, and Cd (in descending order) were found in amounts greater than 0.5, confirming that these metals were from anthropogenic sources. The combined assessments based on WQI and HPI, the study showed that water samples in the proximity of industrial sites are polluted by factories effluent and uncontrolled waste disposal due to urbanization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Natural resources are continuously degraded due to populace blast, global climate change, poor management, and misconception of the nature of assets which is resulted from lack of coordination and coordinated approaches (Ahmad & Sankar, 2011; Al-ahmadi, 2013). As of late, due to impromptu development of industrialization and quick urbanization, groundwater quality has become a major concern in the world (Chegbeleh et al., 2020; Zinabu et al., 2019). Urban zones produce both nonpoint and point sources of contaminants, and alongside these industrial effluents, poisonous metals are discharged, as a result the health of rivers worldwide has been impacted by the nation’s economy’s and population’s rapid industrialization. Most rivers that flow through cities are polluted by municipal waste and industrial effluents (Balwant et al., 2018; Tharmar et al., 2022). The contamination of water due to heavy metals (HMs) is a big concern for humankind; however, global studies related to this topic are rare (El-Kholy et al., 2022). Under certainenvironmental conditions, defilement of water by heavy metals (HMs) could accumulate to a toxic concentration level and cause significant ecological impacts and serious health issue (Kumar et al., 2019; Sharp, 2016). Many hydrogeochemical studies have shown that heavy metal toxicity leads to cardiovascular, neurological, and renal problems (Brindha et al., 2020). The main health hazards caused by chemical pollution of water are due to the presence of nitrates, chromium, cadmium, manganese, zinc, lead, and other toxic substances (Sharp, 2016; Yusuf & Sonibare, 2004). Physicochemical water quality checking is basic to characterize the quality of surface water, and groundwater with time and space. It too makes a difference to recognize the source of contamination (Al-sudani, 2019; Kalaivanan et al., 2018). Evaluation of groundwater quality is exceptionally complex that undertaking various parameters capable of causing stresses on groundwater quality (Bawoke, & Anteneh, 2020; Berhe, 2020; Sharma et al., 2016). However, characterization of groundwater quality in Kombolcha by using integrated appropriate methodologies is not carried out yet. Therefore, this study has been aimed to justify an integrated approach that includes heavy metal pollution indices and multivariate statistics to characterize the groundwater quality in the Kombolcha district of north eastern Ethiopia. Heavy metal pollution index (HPI), is used to evaluate the hazardous metal pollution in drinking water purposes (Islam et al., 2016). A few measurable strategies (Several statistical methods) have been utilized for the evaluation of groundwater quality within the world. For example, multivariate statistical methods offer to identify conceivable factors/sources that impact water frameworks and provides as a tool for reliable water resources management as well as quick solution to pollution problems in many part of the world including Kombolcha.
2 Geology and Hydrogeology
Ethiopia is covered by Basement complex, Paleozoic, and Mesozoic sedimentary rock, and Cenozoic volcanism and sediments Geological Survey of Ethiopia (Mengesha et al., 1996). The Kombolcha area occupies the southwestern block of the Afar Depression and the southwestern Afar Depression merges southward with the northeast striking of Main Ethiopian Rift (MER), and eastward with the east striking of Gulf of Aden (Wolfenden et al., 2004). The geological history of the studied area is related to the evolution and development of the Ethiopian Plateau and the Rift system and it is dominated by volcanic rocks of the Eocene to Oligocene to recent or Quaternary sediments (Ebinger et al., 2005). The major lithologic units found in the study area include Basalt, Rhyolitic Ignimbrite, and Alluvial deposits and, the lithologic units are classified into three hydrogeologic groups as very low, Low, and moderately productive group based on transmissivity which ranges from 1.72-59.8 (m2/d).
3 Materials and Methods
3.1 Study Area
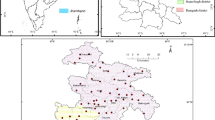
It is industrial district (Fig. 1) and found within the western edge of the Main Ethiopian Rift valley (MER) and center of Borkena River catchment, within projected coordinate system UTM, 37p, 1218460 to 1233940mN, and 571354 to 582900mE, and it covers an area of 136.98 km2. Groundwater is the main source of drinking water in Kombolcha. Subsequently, neighborhood individuals in the study area are fully depended on this groundwater for their daily life.
3.2 Sample Collection and Analytical Procedure
To evaluate the quality and pollution status, representative water samples were collected systematically. Samples were taken where industrial effluents were directly mixed with streams/rivers, from nearby wells, and where more municipal wastes were discharged. The sampling device (plastic bottles) were cleaned very well before use for sampling to prevent mixing up of water and contamination if something remains in the bottle before sampling. A total number of 36 samples were collected in February, 2021 from groundwater and surface water sources (hand-dug wells, deep wells, springs, and rivers) across the industrial area in preselected sampling points. The geographical location of each sampling point was determined with a handheld global positioning system (GPS). For further chemical analysis, the samples were sealed tightly and were taken to the laboratory.
During the fieldwork, different water quality data were collected from different water resources such as boreholes, hand-dug wells, springs, and rivers. The data include pH, temperature, EC, and TDS using HANNA HI9813-6 portable multiparametric probe, which was calibrated appropriately before being used each day using calibration solution. The probe was first calibrated with pH 7.0 and then by pH 10 calibration solution. After representative water samples collected from boreholes, springs, hand-dug wells, and rivers in the area, it were sealed tightly and taken to the laboratory for the analysis of major ions like Ca2+ >Na+ > Mg2+ >K+, HCO3- >SO42- > Cl- > NO3-, and heavy metals such as chromium (Cr), cadmium (Cd), iron (Fe), manganese (Mn), and lead (Pb). Calcium (Ca2+), magnesium (Mg2+) Sodium (Na+) and Potassium (K+) were analyzed using a flame photometer. Trace metal analyses were done at Addis Ababa University using ZEEnit 700 P (Analytikjena) flame atomic absorption spectrophotometry (FAAS). Also, Sulfate (SO4-) and bicarbonate (HCO3-) analyzed by spectrophotometer. The FAAS detection limit varies for different trace metals. For heavy metal concentration analysis, 50ml samples of water were digested with 1ml (60-70%) of concentrated nitric acid (HNO3) and 0.5ml (36-40%) concentrated hydrochloric acid (HCl).
The anions of Nitrate (NO3 -) and chloride (Cl-) the samples were identified using ELE international paqualab photometer in Amhara Design and Supervision Enterprise Work (ADSWE) after Nitriphot No1 and Nitriphot No 2 tablets were added with10 ml of water samples. Moreover, pH, EC, TDS, and temperature were measured during the sampling. The water chemistry data were used to determine the water quality/pollution in the study area, and to correlate the water chemistry variation with geology, agricultural inputs, industrial, or other anthropogenic sources. In this case, the most important thing was to achieve the quality control /quality assurance of the laboratory analysis technique, which was by checking the sample container and regents, using blank samples, and run samples in triplicate and the mean values have been taken.
In the use of FAAS for heavy metal analysis optimization of the operating conditions of the instrument is very crucial. Therefore, the analysis of Cd, Cr, Fe, Mn, and Pb began with selection and adjustments of various units of the FAAS (i.e., lamp selection, wavelength selection, slit adjustment, energy, flame adjustment, and detection limit) as shown in Table 1.
3.3 Water Quality Index (WQI)
There are several water quality assessment methods available, and these methods are dependent on the physicochemical parameters which are used in various ways to assess the quality of groundwater for drinking purposes (Chegbeleh et al., 2020). Many studies have adopted the Water Quality Index (WQI) method, and successfully assessed the suitability of water for drinking purposes (Oboh & Egun, 2018). The study adopted the weighted arithmetic index approach (Brown et al., 1972), in conjunction with Piper diagrams in characterizing the chemistry of groundwater in the study area. The World Health Organization (2017) guidelines for drinking water were used for the computation of WQI. According to Ramakrishnaiah et al. (2009), computation of the WQI to assess the suitability of groundwater for drinking purposes is a four-step approach:
Step 1: each of the 15 parameters has been assigned a weight (wi) according to its relative importance in the overall quality of water for drinking purposes and pollution (see Table 6.3). The maximum weight of 5 has been assigned to parameters like total dissolved solids (TDS) and nitrate (NO3), chromium (Cr), cadmium (Cd), lead (Pb), iron (Fe), and manganese (Mn) due to their major importance in water quality assessment in the study area due to industrialization and urbanization. The remaining other parameters were assigned a weight between 1 and 5 depending on their importance in the overall quality of water for drinking purposes (Zakhem & Hafez, 2018; Bawoke & Anteneh, 2020; Chegbeleh et al., 2020).
Step 2: involves the computation of the relative weight (Wi) of each parameter using:
where, Wi is the relative weight, wi is the weight of each parameter and n is the number of parameters
Step 3: computation of the quality rating scale (qi) for each parameter
where, qi, is the quality rating, Ci is the monitored concentration of each parameter in mg/l, and S is the (World Health Organization, 2017) standard for each parameter in mg/l.
Step 4: determination of the sub-index (SI) for each parameter is used to calculate the WQI
Then, the overall WQI was computed by summing up all the sub-index values for each sample as follow
Using the above procedure, the WQI of the sampled water was made (Brown et al., 1972) and it was compared with standards (Table 2).
3.4 Heavy Metal Pollution Index (HPI)
Heavy metal pollution index (HPI) is a rating method and an effective tool to assess the water quality for heavy metals (Zakhem & Hafez, 2018). HPI is calculated from the point of view of the suitability of ground water for human consumption with respect to metals contamination (Moldovan et al., 2022) using the standards (Table 3) and classified based on (Table 2) and it is important for assigning a weightage (Wi) for each selected parameter, and it is computed using the following equation (Islam et al., 2016).
where, Wi is the relative weight or weighting factor for each chosen parameter defined as equation (6) below; Qi is the sub-index /individual quality rating /for the ith heavy metal ion calculated for each parameter using Equation (7) and n is the number of parameters.
The sub-index, Qi, is computed by:
where, wi means the unit weight factor for the ith heavy metal, which is inversely proportional to the standard value S of the corresponding parameter as defined in Equation (8).
Mi: is the measured concentration value of each parameter in the groundwater and surface water samples; Ii and Si indicate the highest desirable that was taken in according to World Health Organization (World Health Organization, 2017) and maximum permissible value (taken as 1, 0.3, 0.05, 0.01, and 0.05mg/l for Fe, Mn, Pb, Cd, and Cr from Bureau of Indian Standards (Bureau of Indian Standards, 2003) of the ith parameters respectively for this study.
where, S is the standard value, and the proportionality factor k is taken equal to one for all metals in the study (Eldaw et al., 2020) (Tables 4 and 5).
3.5 Principal Component Analysis
In this study, the IBM SPSS v.26 software was used to analyze the dimensional groundwater quality data of the samples including Cl-, NO3-, Fe, Mn, Cr, Pb, and Cd. PCA is a quantification of the significance of variables that explain the observed grouping and patterns of the inherit properties of the individual (Nosrati and Eeckhaut, 2012; Dwivedi & Vankar, 2014). According to Dwivedi & Vankar, (2014), strong, moderate, and weak factor loadings range from >0.75, 0.75 to 0.5, and 0.5 to 0.3, respectively. PCA allow for explaining of related parameters by only one factor (Usman et al., 2014; Egbueri & Onitsha, 2020). It is an important factor responsible for variation in river and groundwater quality and eventually leads to sources identification of water pollution. The general procedure for groundwater quality characterization used in the study is shown in the flow chart below (Fig. 2).
4 Results and Discussion
4.1 Physicochemical Analysis
The result of the physicochemical analysis is presented in (Table 6) and the descriptive statistics in (Table 7).
Cadmium (Cd) variations are explained as the minimum, maximum, and mean values of 0.002, 0.36, and 0.045mg/l, respectively. The mean value of Cd is above the permissible limit of the (World Health Organization, 2017) standard which is 0.003 mg/l.
The variation of manganese (Mn) and iron (Fe) is explained with the mean value of 0.259, and 0.422 mg/l respectively. The mean value of both Fe and Mn variables are above (World Health Organization, 2017) standard limits which are 0.1 and 0.3 mg/l respectively. The groundwater with a higher value of Mn and Fe in spatial variation is closely related to the Steel and Huaxu textile factories the location of industries in (Fig. 1).
The minimum, maximum, and mean values of lead (Pb) and chromium (Cr), are 0.06, 1.071, and 0.146 for lead and 0.01, 1.032 and 0. 099mg/l for chromium. The mean value of both Pb and Cr are above (World Health Organization, 2017) standard limits which are 0.01 and 0.005 mg/l respectively.
Even though, trace metals are found in the earth’s crust, contamination in groundwater could be an outcome of natural and/or anthropogenic sources (Brindha et al., 2020; Rani & Babu, 2008). The aquifer type, the intensity of weathering of minerals from the aquifers, precipitation frequency, quality of the infiltrating water and residence time are the natural factors that control the presence of trace metals in groundwater (Aremu et al., 2010; Brindha et al., 2020). Anthropogenic sources are due to wastes from various industrial activities (e.g., tanning, electroplating, chemicals, and textile manufacturing) (Reyes-toscano et al., 2020). Commonly, trace amounts of metals usually found in freshwater are from the weathering of rocks and soils, and this may not exceed the international standard limit, and also may not deviate from the general trend in the area. However, significant concentration in water can usually be traced to mining, industrial, urban or agricultural sources (Rahman et al., 2020).
From (Table 8), the correlation matrix revealed that no significant correlation exists between the water quality variables (Cl- and NO3- with Fe, Pb, Cr, Mn, and Cd). This indicates variation in the possible sources of the elements in the groundwater. Based on the geology of the study area, the results indicate that the presence of these parameters could not be majorly linked to geogenic origins, but more of anthropogenic origins as there are no geologic or ore deposits, that are rich in these heavy metals. pH has a strong correlation with Cd, Fe, and Pb. A high percentage of TDS and EC are supplied by Cd and Fe with R =0.939, and 0.835 respectively.
4.1.1 Major Cations
Major cations spatial distribution maps were presented in Fig. 3(a–d). Calcium variations is explained as the minimum, maximum, and mean values of 26, 155, and 74.11 mg/l, respectively (Table 7).
The elevated values of Ca2+ are found in the central parts of the area near industrial sites (Fig. 3a) and this deviates from the general trend. According to Piper (1944), if Ca2+/(Ca2++SO42-)>0.5 and TDS>500mg/l the source of Ca is carbonate dissolution/from silicate minerals. In the study area Ca2+/(Ca2++SO42-)> is> 0.5 and TDS is > 500 in ELORA and Tannery well near the Sac and Plastic industry (Table 6 and Appendix 1). Therefore, in the study area in addition to hydrolysis of silicate minerals, the other reason for its elevated value might be from anthropogenic source (i.e., improper use and release of CaCO3 tablet regents) /contaminated discharge of carbonates from Textile, Amir Sac and Plastic factory. The mean value of Ca2+ is within the permissible limit of (World Health Organization, 2017) standards which is 200 mg/l.
The Mg2+ distribution map is also shown in Fig. 3b with a mean value of 6.82 mg/l which is within WHO standards (150 mg/l). A higher value of Mg2+ was observed in northern and southwestern parts of the study area in which the area is dominated by basaltic rocks. According to Piper (1944), if Mg2+/ (Ca2++ Mg2+) is <0.5 (i.e., in the study area it is ≤0.29), Mg is supplied from the dissolution of altered ferromagnesian minerals (Appendix 1).
The Na+ and K+ concentrations are also shown in Fig. 3(c and d) respectively. In the area 87.5% of water samples have TDS<500, and 93.8% have Na+/(Na++Cl-)>0.5.
Based on Piper (1944), if Na+/(Na++Cl-) >0.5, the sources of Na are silicate weathering/sodium sources other than halite like albite and ion exchange conditions in thick clay soil. In some wells like ELFORA, the value of Na+ is highly increased. This is related to the infiltration of anthropogenic effluents like NaCl, Na2SO4 regents that used in ELFORA, and Na2S regents in Tannery and Textile factory processing after they dissociate in to Na+, Cl-, S2- when it comes in contacts with the soil moisture. However, 11.76% of water samples have <0.5 in Na+/(Na++Cl-) ratio, and TDS<500mg/l and the sources of these water samples are precipitation (Appendix-1, and Table 6). The value of K+ is higher in wells in the eastern parts of the study area which is covered by Rhyolitic Ignimbrite. This indicates that, the origin of K+ in water is often a result of chemical weathering and the subsequent dissolution of minerals from nearby silicate rocks and clay minerals.
4.1.2 Major Anions
Bicarbonate (HCO3-) which is the major anion is spatially distributed from lower values in the northern and southeastern direction to higher concentrations in the central, southwestern, and to some extent eastern parts (Fig. 4a). The minimum, maximum, and mean values of HCO3- are 1.2, 414, and 189.4 mg/l, respectively (Table 7). Most of the samples (68.8%) are out of the range of (World Health Organization, 2017) standard desirable limits which is 150 mg/l. If HCO3-/sum of anion>0.8, the source of HCO3- is silicate weathering, and if <0.8 and sulphate is high, the source is gypsum/ carbonate dissolution (Piper, 1944). In the study area, 23.5% of water samples have>0.8 and 76.5% of the water samples have values <0.8 HCO3/sum of anion ratio, and higher sulphate content. This indicates that both silicate weathering and contaminated carbonate dissolution from factories played a role in the increment of HCO3- in the northern part of the study area (i.e., non-polluted area) and central parts of the town (polluted zone) respectively (Appendix-1). Due to the use of CaCO3 tablets as a reagent in the processing of the installed factories (i.e., Textile, and Sac and Plastic factories), there might be a contaminated discharge of carbonates from these factories, and this might have a significant effect on HCO3- increment in some wells.
Sulfate (SO42-) is the other anion that is important to be considered in the drinking water suitability analysis where more than 250 mg/l concentrations are not recommended. The minimum, maximum, and mean values that existed in the area are 1.8, 171.9, and 68.6 mg/l, respectively (Table 7).
The most significant source of sulphate is pollution (Mohamed & Zineb, 2015). Dissolved sulphate has its origin from dissolution of sulphate contaminant from industrial discharges and intensive agriculture. Sulphate is also a major constituent in the aerosols which is from smoke coming from industries and automobiles (Dhakate & Lagudu, 2012; Mohamed & Zineb, 2015; Reyes-toscano et al., 2020). In the study area, higher sulfate might have also been derived from the leaching of sodium sulfate.
The maximum value is seen in shallow hand-dug wells like SW-1 and SW-2 where there are extensive agricultural practices, as well as nearby ELFORA and Tannery wells (Table 4) and (Fig. 4b), because these wells are where Na2S and Na2SO4 dissociate into Na+ and S2- and Na+ and SO42- when they come into contact with soil or water, respectively.
The minimum, maximum and mean documented values of chloride (Cl-) are 6.6, 38.7, and 21.7mg/l, respectively (Table 7). From the spatial Cl- map indicated (Fig. 4c), all its values are found below (World Health Organization, 2017) standard. Even if it is below the standard, relatively higher values of Cl- are highly related to industrial sites, urban centers, and irrigation/agricultural areas. If Cl-/Sum anions<0.8, the source is rock weathering or another factor (Piper, 1944). In the study area, the value is<0.8. This indicates rock weathering is the possible source (Appendix-1). Another anthropogenic factor, like domestic effluents, industrial effluents and immense agricultural practice may contribute to its increment in the study area due to an increase in value from the north towards the center of the study area.
Spatial concentration map of nitrate (NO3-) is shown in Fig. 4d, and the minimum, maximum and mean value is found to be 3.5, 34.3 and 13.7 mg/l respectively. As indicated in the spatial concentration NO3- map, all the value is within the recommended WHO limit. The concentration value increases in the urban centers. In Kombolcha town, the community used pit latrine and buried latrine pipes which are directly linked to surface water bodies. This and some other related domestic effluents cause the increment of NO3-.
4.1.3 Heavy Metals
Heavy metal spatial distribution maps for groundwater samples are presented in Fig. 4(a–e). Cadmium (Cd) variations are explained as the minimum, maximum, and mean values of 0.002, 0.36, and 0.045mg/l, respectively. The elevated values Cd is found in the central parts of the area near Steel and Huaxu textile industrial sites (Fig. 5a). The mean value of Cd is above the permissible limit of the (World Health Organization, 2017) standard which is 0.003 mg/l.
The spatial variation of manganese (Mn), and iron (Fe) is shown in Fig. 5(b, c) with the mean value of 0.259, and 0.422 mg/l respectively. The mean value of both Fe and Mn variables are above (World Health Organization, 2017) standard limits which are 0.1 and 0.3 mg/l respectively. The groundwater with a higher value of Mn and Fe in spatial variation is closely related to the Steel and Huaxu textile factories (see the location of industries in Fig. 1).
In the spatial map of lead (Pb), and chromium (Cr), the elevated values of the variables are closely related to hand-dug wells near Tannery and ELFORA (Fig. 5d & e). The minimum, maximum, and mean values of the variables are 0.06, 1.071, and 0.146 for lead and 0.01, 1.032 and 0. 099mg/l for chromium (Table 7). The mean value of both Pb and Cr are above (World Health Organization, 2017) standard limits which are 0.01 and 0.005 mg/l respectively.
4.2 Pollution Source Identification and Physicochemical Variables of Effluents
4.2.1 Principal Component Analysis (PCA)
Before PCA analysis, the Kaiser Meyer Olkin test (KMO) and Bartlett’s test were performed on the data set. The KMO result was 0.651 and the Bartlett sphericity test was significant (.000, p<0.01), showing that PCA could be considered appropriate and useful to provide significant reduction in data dimensionality (Dwivedi & Vankar, 2014; Mustapha et al., 2011). PCA was applied on the data set to identify the spatial sources of pollution in in groundwater and Borkena River. According to Eigen value criterion, only PC’s with Eigen value greater than one are considered. According to the Kaiser criterion, only the first two factor groups could be used, because subsequent Eigen values are less than 1, the Scree plots is presented in (Fig. 6). Two PC’s were obtained with Eigen value greater than one with total variance of 84.3%, these are considered responsible for the variation in the groundwater, Borkena River and its tributaries system.
In the first principal component analysis, Mn, Fe, Cr, Pb, and Cd (in descending order)were found in amounts greater than 0.5 (Table 9), confirming that these metals were from anthropogenic sources. Since, manganese, iron, chromium, lead and cadmium are not found naturally in this region, enhanced concentration points forward towards industrial activities. After a survey of the industries found in Kombolcha, several iron, chromium, cadmium, carbonate, and lead- processing units were found to have been in operation for over 15 years, and aging machinery with inadequate controls could be sources of hazardous concentrations of heavy metals.
PC2 explains about 30.4% of a variance, has strong positive factor loading on Cl- and NO3. This component group is typical of a combination of both anthropogenic and geogenic origins. The Cl-and NO3- is attributed to organic waste (domestic sewage) origin, and natural processes such as clay deposition and anoxic conditions of the aquifer, nitrification, and denitrification processes (Egbueri & Onitsha, 2020) Different amounts of heavy metals like Fe, Cd, Cr, Cu, Zn,Pb, and physical parameters (measured in-situ) are released along with the industrial effluents (see Table 7). A huge amount of Fe and Zn are discharged from steel factories. The greatest amount of chromium is discharged in Tannery industrial effluents and the concentration of lead is higher in Huaxu industrial effluents. A high percentage of the TDS is contributed by the steel industry, and the Huaxu textile industry. Also, the EC values are influenced by Steel factories, Tannery, and Huaxu textile industry influents. The extremely high value of pH is caused by Steel factory and Tannery factory effluents (Table 10). By relating physicochemical variables of groundwater to the effluents, the pollution source was inferred. The all-over physicochemical variables of factories effluent samples in Kombolcha area are presented in Table 10. The pH of all effluent samples ranged from 8.2 to 12.8 with an average of 8.63, indicating that the effluent in this area was mainly alkaline. In the study area, TDS of industrial effluent varied with a wide range from 1331 to 3517 mg/l.
Therefore, for ground waters polluted by these trace metals, the causes are from a point source (i.e., effluents that contains Cd, Fe, Zn,Pb, and Cu). The main sources of pollution in the area are Steel, Textile, and Tannery factories. BGI brewery and ELFORA discharge heavy metal pollutants in small amounts relative to others (Table 10).
4.2.2 Water Quality Index (WQI)
The computed WQI values and water quality classifications based on WQI ranges of the study area were presented in Table 11. From the table, the five WQI classes were obtained: Excellent water, good water, poor water, very poor water, and unsuitable water for drinking purposes. From the total of 31 water samples, the majority of the samples (N = 19) comprised excellent to good water classes, which covers about 67.9% of the total samples. The next WQI water quality classes fall under poor to very poor water quality covering about 21.4% of the samples (N = 6). The remaining 10.7% of the samples (N=5) covers unsuitable/unfit water type for drinking. The spatial distribution map of groundwater using WQI value for the area is displayed in Fig. 7. The majority of the area is mapped as excellent to good water type. Poor, very poor, and unfit water types are spatially distributed in the central parts of the area close to the factories. Of these samples (N=19), 46.4% (N=13) are below the (World Health Organization, 2017) standard for each water variable and it is suitable for domestic consumption. 21.4% of samples (N=6), including KCTW-2R, BBH-s, KMIW, BH, and UBBH are above the standard limit with HCO3- and HD1 is above the limit with TDS, Fe, and Cd parameters. Even if it falls in excellent to good water types, these water samples are not suitable for domestic consumption.
4.2.3 Heavy Metal Pollution Index (HPI) of Groundwater
In pollution indexing, metals such as Pb, Cd, Cr, Fe, and Mn were considered. Heavy metal concentrations of groundwater and surface water samples, the guideline values, the maximum permissible (Bureau of Indian Standards, 2003), and desirable limit as defined by (World Health Organization, 2017) were used. Descriptive statistics of the metal concentrations of water samples (in mg/l) were presented in Table 7. The computed HPI values and pollution level classifications based on HPI ranges from (Table 4 and 5) in the study area were presented in Table 8. As shown by the table, three HPI classes are obtained: low (<45), medium (45-90), and highly polluted (>90).
The HPI values for the study area were determined by incorporating the concentration values of recorded heavy metals. 41.7% of the groundwater samples had greater than the standard limit in Fe and Mn parameters. The detection limit of FAAS for Cd, Cr, and Pb are 0.002, 0.01, and 0.06mg/l respectively (Table 1). In the study area, 66.7% of the groundwater samples had >0.002mg/l Cd. The amount of Pb and Cr in 75% of groundwater samples were less than 0.06, and 0.01mg/l, but it could be greater than the standard (World Health Organization, 2017) limit (i.e., 0.01, and 0.005) respectively. The study used the instrument detection limit for HPI calculation and in descriptive summary statics of heavy metals. Based on HPI value 50.7% of the sample belongs to a low level of pollution, while 35% and 14.3% belong to a medium and high level of pollution respectively (Table 12). In the study, it is found that the mean metal concentration in groundwater is in the order of Fe>Mn>Pb> Cr> Cd. The mean concentration values of Fe, Pb, Cd, Cr, and Mn exceeded the drinking water standard (World Health Organization, 2017).
In the present study, the concentration of iron ranges from 0.00754 to 1.586 mg/l with an average value of 0.42223mg/l (Table 6). The highest Fe concentration of 1.586 was found in the center of the studied area near the Steel industrial site. The lowest concentration of Fe was observed in the springs (i.e., UBSP) in the northern part of the studied area. The concentration of cadmium (Cd) for groundwater samples varies between 0.002and 0.36mg/l with an average value of 0.04475 mg/l (Table 6). All groundwater samples in the northern part of the study area had concentrations<0.002mg/l. The low values of Cd indicated that there is no significant source of pollution. The concentration of lead (Pb) in the study area varied between 0.06and 1.071mg/l with an average value of 0.14592mg/l. The concentration of chromium (Cr) ranged from 0.01to 1.032mg/l with an average value of 0.0995mg/l. In groundwater samples, the highest value of chromium with aconcentration of 1.034mg/l was found near ELFORA and Tannery industrial sites. Also, the concentration of manganese (Mn) ranges from 0.00592 to 0.7421mg/l (Fig. 8).
5 Conclusion
The groundwater of Kombolcha city and its environment was analyzed and evaluated for its Heavy metal concentration and pollution status. All of the metals detected and evaluated had mean concentration above the required and permissible limit of WHO standards.
The multivariate statistical analyses (correlation, and principal component analyses) revealed that the release of heavy metals into the groundwater system is much more controlled by human (anthropogenic) activities than on natural (geogenic) processes. The result showed that among the variables studied heavy metals like Cd, Cr, Pb, Mn, and Fe are statistically the most significant parameters that bring variation in water quality in the area. The contribution of anthropogenic activities through discharge of industrial effluent into the groundwater and river is clearly identified by correlation matrix, concentration contour, spatial interpolation, and PCA. Based on the WQI value 67.9% comprised excellent to good water and the remaining 22.6% of the sample covers poor to unfit water types. HPI values revealed that all the groundwater samples in the area grouped under low to medium pollution level. The consumption of water with this elevated metal heavy concentration levels could result in health problems in individual taking such polluted water.
Data Availability
The data used in this study are primary and available after the author’s laboratory analysis.
References
Ahmad, M., & Sankar, D. K. (2011). Major ion chemistry and hydrochemical studies of groundwater of parts of Palar river basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 2011(176), 621–636. https://doi.org/10.1007/s10661-010-1608
Al-ahmadi, M. E. (2013). Hydrochemical characterization of groundwater in wadi Sayyah. Western Saudi Arabia, 3, 721–732. https://doi.org/10.1007/s13201-013-0118
Al-sudani, H. (2019). A Review on Groundwater Pollution. International Journal of Recent Engineering Science, 6(5), 14–22. https://doi.org/10.14445/23497157/IJRES-V6I5P103
Aremu, D. A., Olawuyi, J. F., Sridhar, M. K., & Paul, A. (2010). International Journal of Heavy metal analysis of groundwater from Warri, Nigeria. 37–41. https://doi.org/10.1080/0960312021000001014.
Balwant, H., Baier, K., & Jha, R. (2018). Impact of urbanization on groundwater recharge and urban water balance for the city of Hyderabad, India. International Soil and Water Conservation Research., 6, 51–62.
Bawoke, G. T., & Anteneh, Z. L. (2020). Spatial assessment and appraisal of groundwater suitability for drinking consumption in Andasa watershed using water quality index (WQI) and GIS techniques: Blue Nile Basin, Northwestern Ethiopia. Cogent Engineering, 7(1), 1748950. https://doi.org/10.1080/23311916.2020.1748950
Berhe, B. A. (2020). Evaluation of groundwater and surface water quality suitability for drinking and agricultural purposes in Kombolcha town area, eastern Amhara region, Ethiopia. Applied Water Science, 10(6), 1–17. https://doi.org/10.1007/s13201-020-01210-6
Brindha, K., Paul, R., Walter, J., Tan, M. L., & Singh, M. K. (2020). Trace metals contamination in groundwater and implications on human health: Comprehensive assessment using hydrogeochemical and geostatistical methods. Environmental Geochemistry and Health, 42(11), 3819–3839. https://doi.org/10.1007/s10653-020-00637-9
Brown, R. M., Arbor, A., Mcclelland, N. I., Arbor, A., Deininger, R. A., & Connor, M. F. O. (1972). A Water Quality Index-crashing the psychological barrier. Proceedings of the International Conference on Water Pollution Research, 787–797., 173–174.
Bureau of Indian Standards. (2003). Bureau of Indian standard-Indian standard specification for drinking, IS, p 10500.
Chegbeleh, L. P., Aklika, D. K., & Akurugu, B. A. (2020). (2020) Hydrochemical characterization and Suitability Assessment of Groundwater Quality in the Saboba. Hydrology, 7(3), 53. https://doi.org/10.3390/hydrology7030053
Dhakate, R., & Lagudu, S. (2012). Hydrogeochemical parameters for assessment of groundwater quality in a river sub-basin. April. https://doi.org/10.1007/s13762-012-0024
Dwivedi, A. K., & Vankar, P. S. (2014). Source identification study of heavy metal contamination in the industrial hub of Unnao, India. Environmental Monitoring and Assessment, 2014(186), 3531–3539. https://doi.org/10.1007/s10661-014-3636-6
Ebinger, C., Renne, P. R., & Kelley, S. P. (2005). Evolution of a volcanic rifted margin: Southern Red Sea, Ethiopia. Geological Society of America Bulletin, 7, 846–864. https://doi.org/10.1130/B25516.1
Egbueri, J. C., & Onitsha, G. (2020). Heavy Metals Pollution Source Identification and Probabilistic Health Risk Assessment of Shallow Groundwater in Onitsha, Nigeria. Analytical Letters, 1–19. https://doi.org/10.1080/00032719.2020.1712606.
Eldaw, E., Huang, T., Elubid, B., & Mahamed, A. K. (2020). A Novel approach for indexing Heavy Metals Pollution to assess groundwater quality for drinking purposes. International Journal of Environmental Research and Public Health, 17, 1–16. https://doi.org/10.3390/ijerph17041245
El-Kholy, R. A., Zaghlool, E., Isawi, H., Soliman, E. A., Khalil, M. M., El-Aassar, A. H. M., & Said, M. M. (2022). Groundwater quality assessment using water quality index and multivariatestatistical analysis case study: East Matrouh, Northwestern coast, Egypt. Environmental Science and Pollution Research, 29(43), 65699–65722.
Islam, A. R., Ahmed, T., Das, F. S., Saha, S., Rahman, N., & Safiur, M. (2016). Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Science, 30(1), 19–40. https://doi.org/10.1016/j.wsj.2016.05.001
Kalaivanan, K., Gurugnanam, B., Pourghasemi, H. R., Suresh, M., & Kumaravel, S. (2018). Spatial assessment of groundwater quality using water quality index and hydrochemical indices in the Kodavanar sub-basin, Tamil Nadu, India. Sustainable Water Resources Management, 4, 627–641.
Kumar, V., Parihar, R. D., Sharma, A., Bakshi, P., Sidhu, G. P. S., Bali, A. S., ... & Rodrigo-Comino, J. (2019). Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere, 236:124364.
Mengesha, T., Tadiwos, C., & Werkneh, H. (1996). Explanation of the geological map of Ethiopia, Ethiopian institute of geological surveys. Addis Ababa, 3, 79.
Mohamed, C., & Zineb, A. (2015). Geochemistry and hydrogeochemical process of groundwater in the Souf valley of Low Septentrional Sahara, Algeria. 9, 261–273. https://doi.org/10.5897/AJEST2014.1710
Moldovan, A., Török, A. I., Kovacs, E., Cadar, O., Mirea, I. C., & Micle, V. (2022). Metal Contents and Pollution Indices Assessment of Surface Water, Soil, and Sediment from the Aries River Basin Mining Area, Romania. Sustainability, 14(13), 8024.
Mustapha, A., Nabegu, A. B., Putra, U., & State, K. (2011). Surface water pollution source identification using principal component and factor analysis in Getsi River, Kano, Nigeria. Australian Journal of Basic and Applied Sciences, 5(12), 1507–1512.
Nosrati, K., & Van Den Eeckhaut, M. (2012). Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd Plain, Iran. Environmental Earth Sciences, 65, 331–344.
Oboh, I. P., & Egun, N. K. (2018). Utilization of water quality index (WQI) in water quality assessment of groundwater in Agbor metropolis, Delta State Nigeria. International Science and Technology Journal of Namibia, 048–055.
Piper, M. A. (1944). A graphic procedure in the chemical interpretation of water analysis. Transactions American Geophysical Union, 25(6), 914–923. https://doi.org/10.1029/TR025i006p00914
Rahman, M. A. T. M. T., Paul, M., Bhoumik, N., Hassan, M., & Alam, K. (2020). Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices approach. Applied Water Science, 10(8), 1–15. https://doi.org/10.1007/s13201-020-01266-4
Rani, L. A., & Babu, S. (2008). A statistical evaluation of ground water chemistry from the west coast of Tamil Nadu, India. Indian Journal of Geo-Marine Sciences, 37, 186–192.
Ramakrishnaiah, C. R., Sadashivaiah, C., & Ranganna, G. (2009). Assessment of Water Quality Index for the Groundwater in Tumkur Taluk , Karnataka State , India. 6(2), 523–530.
Reyes-Toscano, Claudia Alejandra, Alfaro-Cuevas-Villanueva, Ruth, Cortés-Martínez, Raúl., Morton-Bermea, Ofelia, Hernández-Álvarez, Elizabeth, Buenrostro-Delgado, Otoniel, & Ávila-Olivera, Jorge Alejandro. (2020). Hydrogeochemical characteristics and assessment of Drinking Water Quality in the Urban area of Zamora, Mexico. Water, 12, 556. https://doi.org/10.3390/w12020556
Sharma, S., Sharma, G., & Yadav, R. K. (2016). Impact of Industrial Development on Groundwater Quality- A Case Study of Impact of Effluent From Vishwakarma Industrial Area, Jaipur On Ground Water. Bulletin of Environment, Pharmacology and Life Sciences, 5(7), 12–15.
Sharp, J. M. (2016). The impacts of urbanization on groundwater systems and recharge. Journal of African Earth Sciences, January 2010, 1–7. https://doi.org/10.4409/Am-004-10-0008.
Tharmar, E., Abraham, M., Prakash, R., Sundaram, A., Flores, E. S., Canales, C., & Alam, M. A. (2022). Hydrogeochemistry and water quality assessment in the Thamirabarani River stretch by applying GIS and PCA techniques. Sustainability, 14(24), 16368.
Usman, U. N., Toriman, M. E., Juahir, H., Abdullahi, M. G., Rabiu, A. A., & Isiyaka, H. (2014). Assessment of groundwater quality using multivariate statistical techniques in Terengganu. Science and Technology, 4(3), 42–49.
Wolfenden, E., Ebinger, C., Yirgu, G., Deino, A., & Ayalew, D. (2004). Evolution of the northern Main Ethiopian rift: birth of a triple junction. Earth and Planetary Science Letters, 224, 213–228. https://doi.org/10.1016/j.epsl.2004.04.022
World Health Organization. (2017). Guidelines for Drinking‑ water Quality. Incorporating the first addendum (Fourth edition). Switzerland.
Yusuf, R. O., & Sonibare, J. (2004). Characterization of textile industries. Effluents in Kaduna, Nigeria and pollution implications. Global Nest: The International Journal, 6(3), 212–221.
Zakhem, B. A., & Hafez, R. (2018). Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environmental Earth Sciences, 73(10), 6591–6600. https://doi.org/10.1007/s12665-014-3882-5
Zinabu, E., Irvine, P., Kelderman, J., & Van Der Kwast, K. (2019). Impacts and policy implications of metals effluent discharge into Rivers within industrial zones: A Sub-Saharan perspective from Ethiopia. Environmental Management, 61, 700–715. https://doi.org/10.1007/s00267-017-0970-9
Acknowledgements
We thank Wollo University, Kombolcha Institute of Technology (KIoT) for supporting and facilitating in this study and Dr. Sileshi from Department of Geology, School of Earth Sciences, Bahir Dar University for his revising work.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
The study conception, design, material preparation, data collection, laboratory analysis and original draft of the manuscript were performed by A.Y.A. A.B.N and A.E.A involved in writing, reviewing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Informed Consent
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Institutional Review Board Statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Source-rock deduction summary from (Piper, 1944)
Appendix 1: Source-rock deduction summary from (Piper, 1944)
Parameter | Value | Conclusion |
|---|---|---|
HCO3−/SiO2 | > 10 | Carbonate weathering |
< 5 | Silicate weathering | |
SiO2/Na+ + K+-Cl− | < 1 | Cation exchange |
> 1 and < 2 | Albite weathering | |
> 2 | Ferromagnesian minerals | |
Na+/Na+ + Cl− | > 0.5 | Sodium source other than Halite- Albite, Ion Exchange |
< 0.5, TDS > 500 | Sea water | |
< 0.5, TDS < 500 | Rain water | |
= 0.5 | Halite solution | |
Mg2+/Ca2+ + Mg2+ | < 0.5 | Ferromagnesian minerals |
> 0.5 | Granite weathering | |
= 0.5 | Lime stone- dolomite weathering | |
Ca2+/Ca2+ + SO42− | = 0.5 | Gypsum dissolution |
< 0.5 and pH < 5.5 | Pyrite oxidation | |
< 0.5 and pH neutral | Calcium removal-ion exchange or calcite precipitation | |
> 0.5 | Calcium source other than gypsum-carbonate or silicate | |
TDS | > 500 | Carbonate weathering or brine or seawater |
< 500 | Silicate weathering | |
Cl-/Sum Anions | > 0.8 and TDS > 500 | Seawater or brine or evaporites |
> 0.8 and TDS < 100 | Rainwater | |
< 0.8 | Rock weathering or related factors | |
HCO3-/Sum Anions | > 0.8 | Silicate weathering |
< 0.8, sulfate high | Gypsum/ carbonate dissolution | |
< 0.8, sulfate low | Seawater or brine |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adamu, A.Y., Nigussie, A.B. & Amognehegn, A.E. Hydrogeochemical Study and Geospatial Analysis of Water Quality Using GIS based Water Index and Multivariate Statistics in Kombolcha City, Ethiopia. Water Air Soil Pollut 235, 637 (2024). https://doi.org/10.1007/s11270-024-07438-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07438-1