Abstract
This is the first study to examine the presence and physicochemical properties of microplastics in mullets (Mugil cephalus) from four coastal areas in East Java, Indonesia. Three locations on the north coast are affected by two largest rivers, Bengawan Solo River and Brantas River, whereas one location in the south coast is slightly impacted by river flow. The abundance, color, shape, and size were examined in the gills, stomach, and intestines of fish samples. The average abundance of microplastics was 10.87 particles/individual in gills, 7.43 particles/individual in stomach, and 4.35 particles/individual in intestines. Black (72.4%) and pellets (62.7%) were the most abundant microplastics found. Most of them are in size of less than 100 μm (71.0%). Microplastics type of polymer was identified with Fourier transform infrared spectroscopy, while the chemical compounds including additives such as plasticizers contained in MPs samples were analyzed using gas chromatography-mass spectrometry. Seven different types of polymers were identified, including polyethylene, polyurethane, polyethylene terephthalate, polypropylene, polyvinyl chloride, polystyrene, and polycarbonate. Cyclohexadiene, one of the plasticizers detected, has the highest concentration of 81.82%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microplastics, tiny pieces of plastic less than 5 mm in size (Thompson, 2008), have accumulated in the ocean on a global scale (Thompson et al., 2004) and are regarded as major dangerous hazards due to their widespread dispersion in different aquatic ecosystems (Nithin et al., 2022), from the sea surface water to the sediment floor (Gomiero et al., 2018). Due to poor waste management, most plastic wastes in the marine environment come from terrestrial sources as a result of anthropogenic activities (Jambeck et al., 2015; Squillante et al., 2023). There are currently 4066 species known to be impacted by marine debris (Zhang et al., 2023), including fish. Comprehensive information on the presence of microplastics in fish all over the world has been provided by some research (Carbery et al., 2018; Jacob et al., 2020). Ingested MPs may physically perforate fish’s guts, obstructing their digestive systems, as well as lowering their feeding activity and nutritional intake while providing them a false sense of satiety (Walkinshaw et al., 2020). The intestine and other tissues can absorb micro-sized particles smaller than 25 µm or 10 µm (Abbasi et al., 2018), which can accumulate in various organs (Ivleva et al., 2017). Moreover, heavy metals (Brennecke et al., 2016; Li et al., 2022), persistent organic contaminants (Bhagat et al., 2022; Verdú et al., 2023), and plastic additives such as plasticizer (Corami et al., 2022) may also be transported by accumulated microplastics. These additives are intentionally added into the plastics to modify its physical and chemical properties and improve its performance, as well as cutting its production costs (United Nations Environment Programme, 2021). Their numbers are numerous in chemistry and exert a variety of functions such as light stabilizer, heat stabilizer, pigments agents, flame retardants, and antistatic (Costa et al., 2023). However, the polymers composed in microplastics, and the presence of unreacted monomers, contaminants, additives, or other compounds within the polymer matrix such as plasticizer, can provide ecotoxicological risks (ECHA, 2019). Additionally, the additives themselves may degrade and produce other harmful compounds while still attached to the plastic products or after leaching, which could stay in the environment and accumulate in biota (Costa et al., 2023), and ultimately can increase the harmful effects on many organisms (Beiras et al., 2021). For instance, di(2-ethylhexyl) phthalate (DEHP) and its byproducts, being the most widely used plasticizer, have been related to endocrine disruption in both human and animal models, as well as having detrimental effects on male reproductive development (Jamarani et al., 2018). Due to this phenomenon, the negative impacts of microplastics on aquatic life and possible risks to the health of humans are receiving more and more attention (Fu et al., 2020; Zhou et al., 2020).
The small particle size of microplastics and their attractive color and buoyancy allow pelagic fish to consume them easily. Fish can deliberately consume microplastics by mistaking them for their natural prey, for example, plankton, or can accidentally ingest microplastics if they are already on or attached to prey (Jovanović, 2017). Fish is a biota that is commonly used as a biomonitor and bioindicator agent for residue or debris in waters because of its wide distribution, ecologically important value, and entrance into the food chain for humans (Su et al., 2019b). For this reason, mullet (Mugil cephalus) was used as a bioindicator agent in several studies (Güven et al., 2017; Zhang et al., 2020a). M. cephalus as a benthopelagic omnivorous fish that eats plankton with a low level of mobility (Ouali et al., 2018; Stancheva & Makedonski, 2013) has potential as a biomonitoring agent for microplastics scattered in water bodies (Zhang et al., 2020a).
One of the factors for plastic contamination in the eastern Java Sea is the majority caused by anthropogenic activities (Utami et al., 2021), especially from organic waste (Yona et al., 2019) or industrial waste (Hanif et al., 2021). Other studies have shown that microplastics found on the sea surface in areas close to intense anthropogenic activity have higher concentrations than areas far from anthropogenic activity (Frère et al., 2017). The North and South coasts of Java have different geographical characteristics that affect the anthropogenic activities of the local community, such as industrial activities and sea transportation. The north coast of Java has semi-enclosed territorial waters which are directly related to Borneo Island and are still influenced by the currents’ movement from Madura Island, while the south coast of Java is exposed to the open sea, the Indian Ocean. In addition, the two longest rivers on the island of Java, namely, the Bengawan Solo River and the Brantas River, flow into Gresik and Sidoarjo Regencies, which are included in the North Coast region of East Java. Meanwhile, Probolinggo Regency is one of the areas that has experienced an increase in mining activities for minerals that are prone to pollution. Previous research has shown that rivers are contaminated with microplastics under various conditions, which will affect the concentration of microplastics that accumulate in seawater (Horton et al., 2017; Mani et al., 2015; Vermaire et al., 2017). Unlike the North Coast, the South Coast of Java, especially the Lumajang area, is not dominated by large rivers. Anthropogenic activity in the southern region of East Java is not as dense as in the northern region. In addition, infrastructure development in the southern region is not as adequate as in the northern region (Hamid, 2014). This indicates that there will likely be differences in the characteristics of microplastic pollution on the North and South Coasts of East Java so that it will affect the conditions of microplastic pollution in the bodies of the mullets that live around them.
Research related to the identification of microplastic abundance in the Indonesian aquatic environment has begun, although it is still partially done. Complete information has not been found regarding the abundance of microplastics in the aquatic biota in East Java (Sari et al., 2021), which means that the characteristics of their pollution in various regions and biota cannot be properly identified and compared. Comprehensive information is needed to map the condition of the marine environment and biota in Indonesia and increase the general public’s knowledge to plan and develop efforts to mitigate microplastic pollution. Therefore, comprehensive information on microplastic pollution including the abundance, color, shape, size, and type of polymer, as well as the chemical compounds in the Indonesian biota, especially mullets (M. cephalus) in East Java, is urgently needed.
2 Materials and Methods
2.1 Study Area
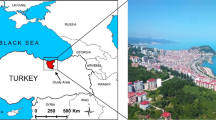
Sampling of mullet fish was conducted on the north coast of East Java, namely, Tambakcemandi, Sidoarjo, Pangkah Wetan Beach, Gresik, Randuputih Beach, and Probolinggo; and also on the south coast of East Java, namely, Wotgalih Beach and Lumajang, as depicted in Fig. 1 with coordinates listed in Table 1. The sample was collected from July to September 2022.
Sampling locations (red circle) of mullets (Mugil cephalus) along the East Java coast, Indonesia (source: https://id.wikipedia.org/wiki/Sungai_Lusi)
2.2 Fish Sampling
Fish were captured by fishermen at chosen sampling sites using gillnets or fishing nets. The captured fish were then placed in glass jars and subsequently transferred to cool boxes, which were labelled based on the location of the fishing activity. The fish samples were subjected to a temperature of − 20 °C prior to conducting subsequent microplastic extraction procedures. A total of 120 fish specimens with an average body length of 17.20 ± 0.32 cm and body weight of 77.57 ± 3.92 g were collected from four distinct locations, with each location obtaining ten specimens that were replicated thrice over a period of three months.
2.3 Microplastics Extraction
The process of identifying microplastics in fish samples involved placing mullet samples on a surgical board and dissecting them from the anus towards the dorsal and anterior direction until the fish organs were visible. The extracted samples were then taken using tweezers and placed in a glass container, where they were subsequently weighed. The extracted organs comprised of the gills, stomach, and intestines. Subsequently, the fish organs underwent desiccation in an oven set at 50 °C for a duration of 24 h. Next, a quantity of 20 mL of Fe solution and 30% H2O2 was incorporated for each gram of the sample, and a period of 48 h was allowed for the decomposition of organic matter (Renzi et al., 2019). Following that, the liquid was subjected to filtration via a vacuum pump utilizing a 1.2 µm Whatman GF/C filter paper.
2.4 Contamination Prevention
During the experiment, the samples were handled using medical gloves, glassware, and metal instruments. Prior to usage, the dissecting tools were consistently washed with distilled water. Throughout the course of the study, the researchers wore laboratory coats made of cotton. The microscopic examination of blank filters was conducted to assess the presence of microplastics (Kılıç and Yücel, 2022). The blank filters exhibited an absence of microplastic particles.
2.5 Microscopic Observation
The examination of filters was conducted using an Olympus CX22 microscope equipped with an attached eyepiece micrometer. The color, type/morphology, and size of MPs were observed. MPs were photographed and subsequently placed onto new filter paper, which was then reserved for Fourier transform infrared (FTIR) analysis in the event that a microplastic particle was detected. The quantification of microplastic abundance was determined by dividing the number of particles detected by the quantity of fish specimens obtained.
The color of microplastics in this study was based on the ISCC-NBS basic color designation system recommended by GESAMP (2019). Meanwhile, the type/morphology of microplastic was identified based on Tanaka and Takada (2016) with several modifications to the following characteristics: (1) Pellets are microplastic granules that are round or cylindrical in shape; (2) fragments are particles produced by the fragmentation of larger materials, irregularly shaped, thick, and have crooked or sharp edges; and (3) fiber is a thread-like particle, both originating from fishing lines and originating from textiles. The category of particle size range in this study is based on the generality of the categories used in many other studies (Rocha-Santos & Duarte, 2015), namely, < 100 μm (20–100 μm), 100–500 μm, 500–1000 μm, dan > 1000 μm (1000–5000 μm).
2.6 Fourier Transform Infrared (FTIR) Analysis
FTIR Bruker Alpha II was used to determine the source of the extracted microplastics. Microplastic samples filtered on Whatman paper were placed on a diamond crystal plate. The spectrum range used is 600 to 4000 cm−1 at a resolution of 4 cm−1. To determine the type of polymer that forms microplastics, the obtained spectrum was checked against to a database of references from the scientific literature.
2.7 Gas Chromatography-Mass Spectrometry (GCMS) Analysis
For the identification of chemical compounds, including plastic additives, contained in the MP particles, GCMS analysis was performed. Compounds identified are characterized by identical mass spectra between the unknown compound and the reference compound (Liu et al., 2021). The list was then cohered with the plastic additives inventory compiled by Costa et al. (2023). For two days at room temperature, one gram of the dried composite sample from every location was ground and pulverized in 20–30 ml of N-hexane (Buwono et al., 2022). A GCMS vial was filled with the filtrate for analysis. Performance, reliability, and productivity of the extract samples were assessed utilizing an Agilent 5977B GC/MSD with a 7890B GC (Agilent Technologies, Inc., USA) using the following settings: the input temperature equal to 200 °C, the column temperature range from 50 °C to 300 °C at 2 min, along with a rise in temperature of 3 °C per minute. The evaluation column is an HP-5 measuring 30 m by 0.32 mm by 0.25 m (Liu et al., 2021).
2.8 Data Analysis
Statistical analysis was conducted by SPSS 25. With the use of the Shapiro–Wilk test, the data’s normality was confirmed. Kruskal–Wallis was employed to investigate the variations in MP abundance between organs and sampling locations with a significance level of p < 0.05. The information on microplastics type of polymer and plastic additives contained in the MP particles presented descriptively.
3 Results
The results of a study of 120 mullet fish caught from 4 different locations in East Java showed that all of them are contaminated by microplastics with a range of 1 to 72 particles per organ. The MPs particles trapped in the gills had an average abundance of 10.87 ± 1.09 particles/individual, while the average abundance of microplastics left in the stomach and intestines is 7.43 ± 0.52 particles/individual and 4.35 ± 0.31 particles/individual respectively. In general, the abundance of microplastics that has accumulated in the gills is greater than the abundance of microplastics that has accumulated in the digestive organs. Upon conducting the Kruskal–Wallis test and its post hoc analysis, the results indicated that there existed statistically significant differences in the abundance of microplastics across the gills and intestines, as well as the stomach and intestines (Fig. 2).
Microplastics found in gills, stomach, and intestines consist of various colors and shapes (Fig. 3). The black color dominated the microplastics found in the fish samples, which was 72.4%, followed by the blue color, which was 13.3%. As many as 62.7% of the microplastics found were in the form of pellets and 29.3% in the form of fibers. Microplastics with a size of < 100 µm and 100–500 µm were the most dominant microplastics found in the organs of mullets in East Java, with a proportion of 71.0% and 21.5% respectively (Fig. 4).
The microplastic constituent polymers found in the composite sample of mullet obtained from the coastal area of East Java were polyethylene (PE), polyurethane (PU), polyethylene terephthalate (PET), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polycarbonate (PC), as can be seen in Table 2, while the GCMS analysis on microplastics composite samples from the gills, stomach, and intestines of mullets (M. cephalus) revealed five different type of additives, namely, plasticizer, flame retardants, antistatic, heat stabilizer, and another stabilizer (Table 3).
4 Discussion
The study reveals a significant prevalence of microplastics in the gills of fish, suggesting that the respiratory pathway serves as a crucial mode of microplastic intake in these organisms (Su et al., 2019a). The findings also underscore the pivotal role of gills in the accumulation of microplastics (Lin et al., 2020). As reported by Abbasi et al. (2018), the abundance of microplastics in the gills is also known to be significantly more than the abundance in the digestive organs. The gills comprise of gill slits, gill arches, and gill filaments which play a role in exchanging respiratory gases and filtering particles for food. The oxygen-rich water that flows through the mouth will then be pumped over their gills. The gill filaments are responsible for filtering sizable particles and plankton from aquatic environments, subsequently redirecting them towards the esophagus then further digested in the stomach. This filtration process also enables the entrapment of microplastics when the gills force the oxygen-poor water out through apertures in the sides of the pharynx. The significant surface area of filtration exhibited by the mullet (M. cephalus) as reported by (Lin et al., 2020) at 862.20 mm2, combined with the narrow inter-filament spaces of 0.048 mm (Eggold & Motta, 1992), can be regarded as the primary factors responsible for the entrapment of plastic particles and other solid materials within the gill structure (Collard, et al., 2017a, 2017b). Nevertheless, it should be noted that the structural features of the gill filaments do not exclusively determine the accumulation of microplastics within the gills (Lin et al., 2020). Entry of microplastics into the gills of fish through water filtration is a non-selective process, potentially accounting for the comparatively high prevalence of microplastics in the gills. At the laboratory level, gills have been identified as the primary organs that are subjected to microplastics (Lin et al., 2020). Elevated concentrations of microplastics have been found to induce harmful effects and pathological alterations in the gill tissue (Ding et al., 2018). The entrapment of microplastics within the gill filaments can lead to a range of negative consequences, including physical damage to the filaments themselves, heightened susceptibility to microplastic infiltration and subsequent infection (Jabeen et al., 2018), diminished respiratory efficacy resulting in hypoxia, and potentially fatal outcomes (Barboza et al., 2020). Various laboratory investigations have exhibited that microplastics can accumulate and disseminate chemical contaminants by ventilation in a way similar to eaten microplastics (Barboza et al., 2018; Zhu et al., 2020). A translocation of microplastics via gills is feasible due to their absorption by the surface of the filament, followed by their entry into cells and the circulatory system via the process of endocytosis (Zhu et al., 2020).
Microplastics that are quite large in size enter the stomach and intestines of fish through the process of ingesting food, either intentionally because their characteristics are similar to prey or accidentally because their size is too small. The relatively small abundance of microplastics and the significant differences between the stomach and intestines in this study indicate the effectiveness of microplastic elimination from the fish body, as stated by Jovanović et al. (2018) and Grigorakis et al. (2016). The study found that the time required to evacuate of microplastic from the intestinal tract of goldfish was 10 h and 33.4 h for 50% and 90% of the particles, respectively. Furthermore, approximately 90% of Sparus auratus were able to eliminate microplastics from their gastrointestinal tract within a period of 24 h. The elimination of microbeads from the intestinal tract of European seabass larvae was observed to be accomplished within 48 h subsequent to their exposure to microplastics (Mazurais et al., 2015), while microplastic particles exhibited rapid clearance and achieved a state of equilibrium within the intestinal tract of zebrafish within 48 h of initial exposure (Lu et al., 2016). Previous research has indicated that the temporary existence of microplastics within the gastrointestinal tract of fish is evident, as their prevalence does not exhibit a proportional increase in larger and more mature fish (Güven et al., 2017). The mean concentration of microplastics in the digestive tract of mullet (M. cephalus) in an investigation performed by Zhang et al. (2020b) and Guilhermino et al. (2021) proved an amount that was not much different from this study, which is 5.2 particles/individual and 6 particles/individual respectively. The findings suggest that the dietary and environmental characteristics of fish species play a significant role in the accumulation of microplastics within their digestive tracts (Zhang et al., 2020a). Omnivorous fish species that live at the bottom of the water have a greater possibility of ingesting microplastic particles (Zheng et al., 2019). In this case, mullets (M. cephalus) are omnivorous and classified as benthopelagic fish. Adult mullets’ primary sources of food include benthic and epiphytic microalgae living on the bottom, plant debris, inorganic sediment particles, and to a lesser extent, benthic animals (Odum, 1970; Thomson, 1966). They reject the coarser material through a pharyngobranchial organ, which serves as a mechanical and gustatory filter, and prefer fine particles with a diameter between 10 and 200 µm (Brusle, 1981). Food material can be retained for a period of time between 2 and 6 h (Odum, 1970). The careful examination of microplastic particles in the intestine is imperative due to their potential translocation into the liver via direct infiltration into the endothelial cell lining or intestinal lymphatics (Jovanović et al., 2018). Microplastics present in the intestinal tract have the potential to induce detrimental effects such as damage to the intestinal mucosa, heightened intestinal permeability and inflammation, disrupted intestinal metabolism, and dysbiosis of the intestinal microbiota (Qiao et al., 2019). In addition, both microplastics and oxytetracycline exposure in zebrafish (Danio rerio) may interfere with the gut-liver axis and be linked to the development of nonalcoholic fatty liver disease (Zhou et al., 2023).
The findings of this study pertaining to the color preferences of microplastics found in the digestive tract of mullet are consistent with those of previous research reports. Numerous investigations have been conducted thus far regarding the presence of microplastics within the gastrointestinal system of fish. Microplastics, specifically blue and black filaments, as well as blue, green, and black fragments, have been frequently detected in Atlantic horse mackerel (Trachurus trachurus) collected from the central Mediterranean Sea (Chenet et al., 2021). Recent research reveals that the little spotted tabby shark (Scyliorhinus canicula) inhabiting the southwest coast of the UK predominantly ingested gray/transparent microplastics, while green microplastics were comparatively less frequently found in their digestive tract (Morgan et al., 2021). Black and blue microplastic fragments have been frequently detected in the stomach contents of Sardina pilchardus and Engraulis encrasicolus inhabiting the Adriatic Sea in Italy (Renzi et al., 2019). The experimental research carried out by Okamoto et al. (2022) revealed that zebrafish Danio rerio, Indian medaka Oryzias melastigma, and sea anemonefish Amphiprion ocellaris most often exhibited the presence of red, yellow, and green microplastic particles in their gastrointestinal tracts. However, the occurrence of blue and gray particles was comparatively less frequent. This observation suggests that variations in color preferences among organisms may be attributed to differences in environmental and laboratory conditions. However, comparatively in many other studies, as well as in this study, blue and black are the predominated microplastics color found (Zazouli et al., 2022). Blue nylon scraps can be obtained from materials like fishing rope or netting (Abayomi et al., 2017). Fish have been observed to consume microplastics either deliberately or inadvertently, as they may perceive them as natural food sources like plankton. Additionally, the transmission of microplastics can transpire through the ingestion of fish prey that contains microplastics (Jovanović, 2017). Mullets (M. cephalus) are known to have food preferences in the form of Bacillariophyceae and Myxophyceae (Mondal et al., 2015) which have characteristics of golden brown and blue. The color of microplastics is one of the main factors contributing to the consumption and trophic transfer of microplastics by organisms (Au et al., 2017; Avio et al., 2015). The most microplastics color found in the digestive tract of mullet (M. cephalus) in this study was black, blue, and red with a percentage of 72.4%, 13.3%, and 10.7% respectively (Fig. 4A). The color of microplastics in fish shows a pattern similar to the color of microplastics around water bodies, as stated by Morgana et al. (2018) and Wieczorek et al. (2018).
Pellets, fiber, fragments, and films are forms of microplastic found in samples from the digestive tract of mullet (M. cephalus) in the coastal area of East Java. Pellets are the most dominant form of microplastic followed by fiber (Fig. 4B). The presence of these forms of microplastics in mullet’s organs indicates their presence in surrounding environment. Pellets are considered as primary microplastics and serve as precursors for many plastic products (Hidalgo-Ruz et al., 2012). In addition, daily personal care products such as facials and shower gels also contribute to the abundance of pellets or microbeads in aquatic environments (Eerkes-Medrano et al., 2015). In general, fiber is the most common form of microplastic found in global waters (Dai et al., 2018; Zhang et al., 2018). The existence of microplastics in aquatic environments has diverse origins, encompassing landfills, incineration of waste, atmospheric deposition, synthetic textiles such as fishing gear, clothing and household refuse, and environmental abrasion of microplastics (Wu et al., 2018). The prevalence of microplastics that come in the form of fibers (per unit volume) in marine sediments has been observed to be as much as fourfold greater than the prevalence detected on the surface of seawater (Avio et al., 2017; Pham et al., 2014; Tubau et al., 2015). There is a higher probability of accumulation of microplastics in the gastrointestinal tract when they are in the form of fibers (Rist & Hartmann, 2018; Wright et al., 2013). According to a research on domestic washing machine effluent, it has been discovered that each laundry cycle can generate over 1900 fibers from any given clothing item (W. Luo et al., 2019). Furthermore, empirical investigations conducted on sewage derived from a wastewater treatment facility situated in Germany have demonstrated that synthetic fibers were detected in over 80% of the specimens (Mintenig et al., 2017). The utilization of rivers for transport may potentially have an impact on the occurrence and quantity of fiber within urban water systems (Zazouli et al., 2022). The coastline serves as a disposal point for waste, where micro-sized fibers are found in greater abundance. Comparable spatial patterns have also been evidenced in investigations of seawater (Sherman & Van Sebille, 2016; Yonkos et al., 2014).
Qualitatively, there were 8 types of microplastic constituent polymers that could be identified from the gill and digestive tract composite samples of mullet in this study (Table 1), namely, polyethylene (PE), polyurethane (PU), polyethylene terephthalate (PET), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polycarbonate (PC). In general, in many other studies, PE is the most dominant microplastic polymer found in the digestive tract of fish (Zazouli et al., 2022). A meta-analysis shows that PE is the most dominant type of plastic found in marine waters (Erni-Cassola et al., 2019). PE and PP are widely utilized plastic polymers in daily human activities, which results in their significant prevalence in natural habitats owing to their robustness and widespread application (Erni-Cassola et al., 2019; Tang et al., 2018). The prevalence of these two polymers in marine samples is attributed to their comparatively lower densities. However, their occurrence diminishes with increasing depth of the seawater (Zazouli et al., 2022). Furthermore, it is noteworthy that a significant proportion, up to 62%, of the worldwide demand for plastic is attributed to PP. It is also worth mentioning that these two polymer types constitute the primary polymers present in the Mediterranean waters (Andrady, 2015). Comparable findings were also observed in piscine specimens located along the shoreline of Kochi, India (James et al., 2020). Polyester is a frequently employed substance in the production of commercial marine supplies and devices, such as fishing gear (Steer et al., 2017). PET is a commonly utilized material that takes the form of fibers for clothing and storage containers for solutions and a food item (Naji et al., 2017).
The majority of microplastic sizes found in mullets in this study (Fig. 4C) were in the range of < 100 µm (71.0%) and 100–500 µm (21.5%). The impact of microplastics on organisms is significantly influenced by their size characteristics. Therefore, it is crucial to assess the capacity of microplastics of different sizes of interacting with organisms (Lee et al., 2013; Rodríguez-Seijo & Pereira, 2017). Entry of microplastics with larger sizes into organisms may lead to harm to their gastrointestinal tract or gill filaments as they traverse these internal organs (Ryan, 2019; Su et al., 2019a; Von Moos et al., 2012). Given the propensity of tiny black microplastics from highly industrialized areas to engage with chemical contaminants (Wang et al., 2018) such as plasticizers, polybrominated diphenyl ethers (PBDEs), heavy metals, and polychlorinated biphenyls (PCBs), these microplastics of smaller dimensions exert a more pronounced influence on organisms, particularly at the cellular level (Prinz & Korez, 2020). Microplastics measuring less than 1 mm in diameter have the ability to traverse the cellular barrier and exit through the digestive tract or gills, resulting in detrimental consequences such as oxidative harm, reproductive capacity, and immune reactions in living organisms (Abbasi et al., 2018; Browne et al., 2008; Collard, et al., 2017a, 2017b; Prokić et al., 2019).
Various chromatogram peaks have been detected as additives in microplastics, including plasticizers, flame retardants, antistatic agents, heat stabilizers, and other stabilizers. A database of chemical substances found in microplastics found in mullet fish is shown in Table 2. Mesitylene is one of a benzene derivatives (Sobhani et al., 2021) which functions as a stabilizer and typically has a concentration of 0.5–2.5% in polyefin-I, polyefin-II, or PA (Costa et al., 2023). Phenol and 2,4-cyclohexadien-1-one, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, which act as plasticizers, have the highest concentration in total among other chemical compounds, while benzene,4-ethyl-1,2-dimethyl- and benzene,1-ethyl-3,5-dimethyl- are considered flame retardants. Flame retardants refers to a group of such inorganic and/or organic compounds that specifically make polymers, wood, and wood-based materials, as well as textiles, flame-resistant (Wensing et al., 2005). 3-Quinolinecarboxylic acid, 6,8-difluoro-4-hydroxy-, ethyl ester, and 4-chloro-6-methoxy-2-methylquinolin-8-amine are heat stabilizers which play an important role in improving PVC thermal stability (Gao et al., 2022).
Despite the widely use of plastic additives, many industrial producers do not offer thorough information on chemical additions added to plastic polymers, including their concentrations. Some additives are classified as chemicals of very high concern, since they have been shown to be endocrine disruptors, mutagenic, or carcinogenic to aquatic biota (Groh et al., 2018; Wagner & Schlummer, 2020). For instance, skeletal development, metabolism, and immune response can be interfered by the highly hazardous additive, phthalate esters (Luo et al., 2022). Currently, the European Chemicals Agency (ECHA) is reviewing regulations on additives for use in the European Common Market, while phthalates, type of plastic additives, have undergone an EU risk assessment, and much research has been done on their impact on the environment, human health, and their application in diverse products (Campanale et al., 2020). Results from studies on the ecotoxicity of numerous common plastic chemical additives in microplastics leachate point to the possibility of carcinogenic consequences, neurotoxicity, inflammation, and changes to lipid metabolism (Capolupo et al., 2020; Jeong & Choi, 2020). The identification and analysis of the chemical constituents of microplastics is crucial due to their potential impact on the health of organisms and the environment.
5 Conclusion
The gills of mullets (Mugil cephalus) are more likely to contain microplastics than intestines due to the non-selective filtration through the wide surface area and the narrow inter-filament spaces of the gills that cause the microplastics to become entrapped, while the microplastics presence in the stomach and intestines is impacted by numerous factors including their feeding habit. Black and blue as well as pellet and fiber shaped microplastics are the characteristics of microplastics that are abundantly found in mullets organ possibly because they mimic the fish’s natural prey. Furthermore, the predominant discovery pertains to microplastics measuring less than 100 µm in size. The organ of mullets was found to contain seven distinct plastic polymers through the use of FTIR analysis. Additionally, plastic additives were detected through GCMS analysis. The study’s findings advance our understanding of the degree of microplastic pollution in marine fish, and the presence of plastic additives in fish could be concerning; hence, it is necessary to develop a risk assessment of microplastics in fish in relation to fish consumption.
Data Availability
Anyone who wishes to obtain the data of this research can request it from the corresponding author.
References
Abayomi, O. A., Range, P., Al-Ghouti, M. A., Obbard, J. P., Almeer, S. H., & Ben-Hamadou, R. (2017). Microplastics in coastal environments of the Arabian Gulf. Marine Pollution Bulletin, 124(1), 181–188. https://doi.org/10.1016/j.marpolbul.2017.07.011
Abbasi, S., Soltani, N., Keshavarzi, B., Moore, F., Turner, A., & Hassanaghaei, M. (2018). Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere, 205, 80–87. https://doi.org/10.1016/j.chemosphere.2018.04.076
Andrady, A. L. (2015). Plastics and environmental sustainability. John Wiley & Sons.
Au, S. Y., Lee, C. M., Weinstein, J. E., van den Hurk, P., & Klaine, S. J. (2017). Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs. Integrated Environmental Assessment and Management, 13(3), 505–509. https://doi.org/10.1002/IEAM.1907
Avio, C. G., Gorbi, S., & Regoli, F. (2015). Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Marine Environmental Research, 111, 18–26. https://doi.org/10.1016/J.MARENVRES.2015.06.014
Avio, C. G., Gorbi, S., & Regoli, F. (2017). Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Marine Environmental Research, 128, 2–11. https://doi.org/10.1016/J.MARENVRES.2016.05.012
Barboza, L.G.A., Vieira, L.R., Branco, V., Carvalho, C., & Guilhermino, L. (2018). Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Scientific Reports, 8, 15655. https://doi.org/10.1038/s41598-018-34125-z
Barboza, L. G. A., Lopes, C., Oliveira, P., Bessa, F., Otero, V., Henriques, B., Raimundo, J., Caetano, M., Vale, C., & Guilhermino, L. (2020). Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Science of The Total Environment, 717, 134625. https://doi.org/10.1016/J.SCITOTENV.2019.134625
Beiras, R., Verdejo, E., Campoy-López, P., & Vidal-Liñán, L. (2021). Aquatic toxicity of chemically defined microplastics can be explained by functional additives. Journal of Hazardous Materials, 406, 124338. https://doi.org/10.1016/J.JHAZMAT.2020.124338
Bhagat, K., Barrios, A. C., Rajwade, K., Kumar, A., Oswald, J., Apul, O., & Perreault, F. (2022). Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere, 298, 134238.
Brennecke, D., Duarte, B., Paiva, F., Caçador, I., & Canning-Clode, J. (2016). Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coastal and Shelf Science, 178, 189–195.
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., & Thompson, R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental Science & Technology, 42(13), 5026–5031. https://doi.org/10.1021/es800249a
Brusle, J. (1981). Food and feeding in grey mullet. In O. H. Oren (Ed.), Aquaculture of grey mullets (pp. 185–217). Cambridge University Press.
Buwono, N. R., Risjani, Y., & Soegianto, A. (2022). Spatio-temporal patterns of occurrence of microplastics in the freshwater fish Gambusia affinis from the Brantas River. Indonesia. Environmental Pollution, 311, 119958. https://doi.org/10.1016/j.envpol.2022.119958
Campanale, C., Dierkes, G., Massarelli, C., Bagnuolo, G., & Uricchio, V. F. (2020). A relevant screening of organic contaminants present on freshwater and pre-production microplastics. Toxics, 8, 100. https://doi.org/10.3390/toxics8040100
Capolupo, M., Sørensen, L., Jayasena, K. D. R., Booth, A. M., & Fabbri, E. (2020). Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Research, 169, 115270. https://doi.org/10.1016/j.watres.2019.115270
Carbery, M., O’Connor, W., & Palanisami, T. (2018). Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environment International, 115, 400–409.
Chenet, T., Mancia, A., Bono, G., Falsone, F., Scannella, D., Vaccaro, C., Baldi, A., Catani, M., Cavazzini, A., & Pasti, L. (2021). Plastic ingestion by Atlantic horse mackerel (Trachurus trachurus) from central Mediterranean Sea: A potential cause for endocrine disruption. Environmental Pollution, 284, 117449. https://doi.org/10.1016/j.envpol.2021.117449
Collard, F., Gilbert, B., Compère, P., Eppe, G., Das, K., Jauniaux, T., & Parmentier, E. (2017a). Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environmental Pollution, 229, 1000–1005. https://doi.org/10.1016/J.ENVPOL.2017.07.089
Collard, F., Gilbert, B., Eppe, G., Roos, L., Compère, P., Das, K., & Parmentier, E. (2017b). Morphology of the filtration apparatus of three planktivorous fishes and relation with ingested anthropogenic particles. Marine Pollution Bulletin, 116(1–2), 182–191. https://doi.org/10.1016/J.MARPOLBUL.2016.12.067
Corami, F., Rosso, B., Sfriso, A. A., Gambaro, A., Mistri, M., Munari, C., & Barbante, C. (2022). Additives, plasticizers, small microplastics (<100 μm), and other microlitter components in the gastrointestinal tract of commercial teleost fish: Method of extraction, purification, quantification, and characterization using Micro-FTIR. Marine Pollution Bulletin., 177, 113477. https://doi.org/10.1016/J.MARPOLBUL.2022.113477
da Costa, J. P., Avellan, A., Mouneyrac, C., Duarte, A., & Rocha-Santos, T. (2023). Plastic additives and microplastics as emerging contaminants: Mechanisms and analytical assessment. TrAC Trends in Analytical Chemistry, 158, 116898. https://doi.org/10.1016/J.TRAC.2022.116898
Dai, Z., Zhang, H., Zhou, Q., Tian, Y., Chen, T., Tu, C., Fu, C., & Luo, Y. (2018). Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environmental Pollution, 242, 1557–1565. https://doi.org/10.1016/J.ENVPOL.2018.07.131
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H., & Zhu, W. (2018). Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environmental Pollution, 238, 1–9. https://doi.org/10.1016/J.ENVPOL.2018.03.001
ECHA. (2019). Annex XV restriction report proposal for a restriction substance: intentionally added microplastics. European Chemicals Agency (ECHA), Helsinki, Finland.
Eerkes-Medrano, D., Thompson, R. C., & Aldridge, D. C. (2015). Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Research, 75, 63–82. https://doi.org/10.1016/J.WATRES.2015.02.012
Eggold, B. T., & Motta, P. J. (1992). Ontogenetic dietary shifts and morphological correlates in striped mullet, Mugil cephalus. Environmental Biology of Fishes, 34, 139–158. https://doi.org/10.1007/BF00002390
Erni-Cassola, G., Zadjelovic, V., Gibson, M. I., & Christie-Oleza, J. A. (2019). Distribution of plastic polymer types in the marine environment; A meta-analysis. Journal of Hazardous Materials, 369, 691–698. https://doi.org/10.1016/J.JHAZMAT.2019.02.067
Frère, L., Paul-Pont, I., Rinnert, E., Petton, S., Jaffré, J., Bihannic, I., Soudant, P., Lambert, C., & Huvet, A. (2017). Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environmental Pollution, 225, 211–222. https://doi.org/10.1016/j.envpol.2017.03.023
Fu, W., Min, J., Jiang, W., Li, Y., & Zhang, W. (2020). Separation, characterization and identification of microplastics and nanoplastics in the environment. Science of the Total Environment, 721, 137561.
Gao, X., Jiang, P., Zhang, Z., Li, Z., Li, Y., Leng, Y., & Zhang, P. (2022). Synthesis of bismaleate pentaerythritol ester and its application as PVC auxiliary heat stabilizer. Journal of Applied Polymer Science, 139(12), 51820.
GESAMP. (2019). Guidelines or the monitoring and assessment of plastic litter and microplastics in the ocean (Kershaw P.J., Turra A. and Galgani F. editors), (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Reports and Studies. GESAMP, 99, 130p. Accessed date: 5-9-2023. http://www.gesamp.org/site/assets/files/2002/rs99e.pdf
Gomiero, A., Strafella, P., & Fabi, G. (2018). From macroplastic to microplastic litter: occurrence, composition, source identification and interaction with aquatic organisms. Experiences from the Adriatic Sea. In A. Gomiero (Ed.), Plastics in the Environment (pp. 1–20). IntechOpen.
Grigorakis, S., Mason, S. A., & Drouillard, K. G. (2016). Determination of the Gut Retention of Plastic Microbeads and Microfibers in Goldfish (carassius Auratus). https://doi.org/10.1016/j.chemosphere.2016.11.055
Groh, K. J., Backhaus, T., Carney-Almroth, B., Geueke, B., Inostroza, P. A., Lennquist, A., Leslie, H. A., Maffini, M., Slunge, D., Trasande, L., Warhurst, A. M., & Muncke, J. (2018). Overview of Known Plastic Packaging-Associated Chemicals and Their Hazards Editor: Damia Barcelo. https://doi.org/10.1016/j.scitotenv.2018.10.015
Guilhermino, L., Martins, A., Lopes, C., Raimundo, J., Vieira, L. R., Barboza, L. G. A., Costa, J., Antunes, C., Caetano, M., & Vale, C. (2021). Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Marine Pollution Bulletin, 173, 113008. https://doi.org/10.1016/J.MARPOLBUL.2021.113008
Güven, O., Gökdağ, K., Jovanović, B., & Kıdeyş, A. E. (2017). Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environmental Pollution, 223, 286–294. https://doi.org/10.1016/J.ENVPOL.2017.01.025
Hamid, A. (2014). Investment potential in Southern Cross Lane East Java. Jurnal Bina Praja, 6(3), 197–204.
Hanif, K. H., Suprijanto, J., & Pratikto, I. (2021). Identifikasi Mikroplastik di Muara Sungai Kendal, Kabupaten Kendal. Journal of Marine Research, 10(1), 1–6. https://doi.org/10.14710/jmr.v9i2.26832
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., & Thiel, M. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environmental Science and Technology, 46(6), 3060–3075. https://doi.org/10.1021/es2031505
Horton, A. A., Svendsen, C., Williams, R. J., Spurgeon, D. J., & Lahive, E. (2017). Large microplastic particles in sediments of tributaries of the River Thames, UK – Abundance, sources and methods for effective quantification. Marine Pollution Bulletin, 114(1), 218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Ivleva, N. P., Wiesheu, A. C., & Niessner, R. (2017). Microplastic in aquatic ecosystems. Angewandte Chemie International Edition, 56(7), 1720–1739. https://doi.org/10.1002/anie.201606957
Jabeen, K., Li, B., Chen, Q., Su, L., Wu, C., Hollert, H., & Shi, H. (2018). Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere, 213, 323–332. https://doi.org/10.1016/j.chemosphere.2018.09.031
Jacob, H., Besson, M., Swarzenski, P. W., Lecchini, D., & Metian, M. (2020). Effects of virgin micro-and nanoplastics on fish: Trends, meta-analysis, and perspectives. Environmental Science & Technology, 54(8), 4733–4745.
Jamarani, R., Erythropel, H. C., Nicell, J. A., Leask, R. L., & Marić, M. (2018). How green is your plasticizer? Polymers,10, 834. https://doi.org/10.3390/polym10080834
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., Narayan, R., & Law, K. L. (2015). Plastic waste inputs from land into the ocean. Science, 347(6223), 768–771.
James, K., Vasant, K., Padua, S., Gopinath, V., Abilash, K. S., Jeyabaskaran, R., Babu, A., & John, S. (2020). An assessment of microplastics in the ecosystem and selected commercially important fishes off Kochi, south eastern Arabian Sea, India. Marine Pollution Bulletin, 154, 111027. https://doi.org/10.1016/j.marpolbul.2020.111027
Jeong, J., & Choi, J. (2020). Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCastTM and deep learning models combined approach. Environment International, 137, 105557. https://doi.org/10.1016/j.envint.2020.105557
Jovanović, B. (2017). Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integrated Environmental Assessment and Management, 13(3), 510–515. https://doi.org/10.1002/IEAM.1913
Jovanović, B., Gökdağ, K., Güven, O., Emre, Y., Whitley, E. M., & Kideys, A. E. (2018). Virgin microplastics are not causing imminent harm to fish after dietary exposure. Marine Pollution Bulletin, 130, 123–131. https://doi.org/10.1016/J.MARPOLBUL.2018.03.016
Jung, M. R., Horgen, F. D., Orski, S. V., Rodriguez, V. C., Beers, K. L., Balazs, G. H., Jones, T. T., Work, T. M., Brignac, K. C., Royer, S. J., Hyrenbach, K. D., Jensen, B. A., & Lynch, J. M. (2018). Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Marine Pollution Bulletin, 127, 704–716. https://doi.org/10.1016/j.marpolbul.2017.12.061
Lee, K.-W., Shim, W. J., Kwon, O. Y., & Kang, J.-H. (2013). Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environmental Science & Technology, 47(19), 11278–11283. https://doi.org/10.1021/es401932b
Li, W., Zu, B., Yang, Q., Huang, Y., & Li, J. (2022). Adsorption of lead and cadmium by microplastics and their desorption behavior as vectors in the gastrointestinal environment. Journal of Environmental Chemical Engineering, 10(3), 107379.
Lin, L., Ma, L.-S., Li, H.-X., Pan, Y.-F., Liu, S., Zhang, L., Peng, J.-P., Fok, L., Xu, X.-R., & He, W.-H. (2020). Low level of microplastic contamination in wild fish from an urban estuary. Marine Pollution Bulletin, 160, 111650. https://doi.org/10.1016/j.marpolbul.2020.111650
Liu, Y., Li, R., Yu, J., Ni, F., Sheng, Y., Scircle, A., Cizdziel, J. V., & Zhou, Y. (2021). Separation and identification of microplastics in marine organisms by TGA-FTIR-GC/MS: A case study of mussels from coastal China. Environmental Pollution, 272, 115946. https://doi.org/10.1016/j.envpol.2020.115946
Lu, Y., Zhang, Y., Deng, Y., Jiang, W., Zhao, Y., Geng, J., Ding, L., & Ren, H. (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environmental Science and Technology, 50(7), 4054–4060. https://doi.org/10.1021/acs.est.6b00183
Luo, W., Su, L., Craig, N. J., Du, F., Wu, C., & Shi, H. (2019). Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environmental Pollution, 246, 174–182. https://doi.org/10.1016/J.ENVPOL.2018.11.081
Luo, H., Liu, C., He, D., Sun, J., Li, J., & Pan, X. (2022). Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Science of The Total Environment, 849, 157951. https://doi.org/10.1016/j.scitotenv.2022.157951
Mani, T., Hauk, A., Walter, U., & Burkhardt-Holm, P. (2015). Microplastics profile along the Rhine River. Scientific Reports, 5(1), 17988. https://doi.org/10.1038/srep17988
Mazurais, D., Ernande, B., Quazuguel, P., Severe, A., Huelvan, C., Madec, L., Mouchel, O., Soudant, P., Robbens, J., Huvet, A., & Zambonino-Infante, J. (2015). Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Marine Environmental Research, 112, 78–85. https://doi.org/10.1016/j.marenvres.2015.09.009
Mintenig, S. M., Int-Veen, I., Löder, M. G. J., Primpke, S., & Gerdts, G. (2017). Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Research, 108, 365–372. https://doi.org/10.1016/J.WATRES.2016.11.015
Mondal, A., Chakravortty, D., Mandal, S., Bhattacharyya, S. B., & Mitra, A. (2015). Feeding ecology and prey preference of grey mullet, Mugil cephalus (Linnaeus, 1758) in extensive brackish water farming system. J Marine Sci Res Dev, 6, 178. https://doi.org/10.4172/2155-9910.1000178
Morgan, E., Hutchinson, D., & Gaion, A. (2021). Plastic ingestion by the small-spotted catshark (Scyliorhinus canicula) from the South West Coast of the United Kingdom. Bulletin of Environmental Contamination and Toxicology, 106(6), 910–915. https://doi.org/10.1007/S00128-021-03129-3/FIGURES/3
Morgana, S., Ghigliotti, L., Estévez-Calvar, N., Stifanese, R., Wieckzorek, A., Doyle, T., Christiansen, J. S., Faimali, M., & Garaventa, F. (2018). Microplastics in the arctic: A case study with sub-surface water and fish samples off Northeast Greenland. Environmental Pollution, 242, 1078–1086. https://doi.org/10.1016/J.ENVPOL.2018.08.001
Naji, A., Esmaili, Z., Mason, S. A., & Dick Vethaak, A. (2017). The occurrence of microplastic contamination in littoral sediments of the Persian Gulf. Iran. Environmental Science and Pollution Research, 24(25), 20459–20468. https://doi.org/10.1007/S11356-017-9587-Z/FIGURES/5
Nithin, A., Sundaramanickam, A., Iswarya, P., & Babu, O. G. (2022). Hazard index of microplastics contamination in various fishes collected off Parangipettai, Southeast coast of India. Chemosphere, 307, 136037. https://doi.org/10.1016/j.chemosphere.2022.136037
Odum, W. E. (1970). Utilization of the direct grazing and plant detritus food chains by the striped mullet Mugil cephalus. In J. J. Steele (Ed.), Marine food chains (pp. 222–240). Oliver and Boyd Ltd.
Okamoto, K., Nomura, M., Horie, Y., & Okamura, H. (2022). Color preferences and gastrointestinal-tract retention times of microplastics by freshwater and marine fishes. Environmental Pollution, 304, 119253. https://doi.org/10.1016/J.ENVPOL.2022.119253
Ouali, N., Belabed, B.-E., & Chenchouni, H. (2018). Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from north African coasts of the Mediterranean Sea. Science of the Total Environment, 639, 156–174. https://doi.org/10.1016/j.scitotenv.2018.04.377
Pham, C. K., Ramirez-Llodra, E., Alt, C. H. S., Amaro, T., Bergmann, M., Canals, M., Company, J. B., Davies, J., Duineveld, G., Galgani, F., Howell, K. L., Huvenne, V. A. I., Isidro, E., Jones, D. O. B., Lastras, G., Morato, T., Gomes-Pereira, J. N., Purser, A., Stewart, H., Tojeira I., Tubau, X., Rooij, D.V., & Tyler, P. A. (2014). Marine litter distribution and density in European seas, from the shelves to deep basins. PLoS ONE, 9(4), e95839. https://doi.org/10.1371/journal.pone.0095839
Prinz, N., & Korez, Š. (2020). Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: A review. In S. Jungblut, V. Liebich, & M. Bode-Dalby, (Eds.), YOUMARES 9 - The oceans: Our research, our future (pp. 101–120). Cham, Springer. https://doi.org/10.1007/978-3-030-20389-4_6
Prokić, M. D., Radovanović, T. B., Gavrić, J. P., & Faggio, C. (2019). Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC - Trends in Analytical Chemistry, 111, 37–46. https://doi.org/10.1016/J.TRAC.2018.12.001
Qiao, R., Deng, Y., Zhang, S., Wolosker, M. B., Zhu, Q., Ren, H., & Zhang, Y. (2019). Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere, 236, 124334. https://doi.org/10.1016/J.CHEMOSPHERE.2019.07.065
Renzi, M., Specchiulli, A., Blašković, A., Manzo, C., Mancinelli, G., & Cilenti, L. (2019). Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environmental Science and Pollution Research, 26(3), 2771–2781. https://doi.org/10.1007/s11356-018-3762-8
Rist, S., & Hartmann, N. B. (2018). Aquatic ecotoxicity of microplastics and nanoplastics: Lessons learned from engineered nanomaterials. Handbook of Environmental Chemistry, 58, 25–49. https://doi.org/10.1007/978-3-319-61615-5_2/TABLES/1
Rocha-Santos, T., & Duarte, A. C. (2015). A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends in Analytical Chemistry, 65, 47–53. https://doi.org/10.1016/J.TRAC.2014.10.011
Rodríguez-Seijo, A., & Pereira, R. (2017). Morphological and physical characterization of microplastics. Comprehensive Analytical Chemistry, 75, 49–66. https://doi.org/10.1016/BS.COAC.2016.10.007
Ryan, P. G. (2019). Ingestion of plastics by marine organisms. Handbook of Environmental Chemistry, 78, 235–266. https://doi.org/10.1007/698_2016_21/COVER
Sari, G., Kasasiah, A., Rahmawati Utami, M., & Trihadiningrum, Y. (2021). Microplastics contamination in the aquatic environment of Indonesia: A comprehensive review. Journal of Ecological Engineering, 22(10), 127–140. https://doi.org/10.12911/22998993/142118
Sherman, P., & Van Sebille, E. (2016). Modeling marine surface microplastic transport to assess optimal removal locations. Environmental Research Letters, 11(1), 014006. https://doi.org/10.1088/1748-9326/11/1/014006
Sobhani, Z., Panneerselvan, L., Fang, C., Naidu, R., & Megharaj, M. (2021). Chronic and transgenerational effects of polystyrene microplastics at environmentally relevant concentrations in earthworms (Eisenia fetida). Environmental Toxicology and Chemistry, 40(8), 2240–2246. https://doi.org/10.1002/etc.5072
Squillante, J., Scivicco, M., Ariano, A., Nolasco, A., Esposito, F., Cacciola, N. A., Severino, L., & Cirillo, T. (2023). Occurrence of phthalate esters and preliminary data on microplastics in fish from the Tyrrhenian sea (Italy) and impact on human health. Environmental Pollution, 316, 120664. https://doi.org/10.1016/J.ENVPOL.2022.120664
Stancheva, M., & Makedonski, L. (2013). Determination of heavy metals (Pb, Cd, As and Hg) in Black Sea grey mullet (Mugil cephalus). Bulgarian Journal of Agricultural Science, 19 (Supplement 1), 30–34.
Steer, M., Cole, M., Thompson, R. C., & Lindeque, P. K. (2017). Microplastic ingestion in fish larvae in the western English Channel. Environmental Pollution, 226, 250–259. https://doi.org/10.1016/J.ENVPOL.2017.03.062
Su, L., Deng, H., Li, B., Chen, Q., Pettigrove, V., Wu, C., & Shi, H. (2019a). The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. Journal of Hazardous Materials, 365, 716–724. https://doi.org/10.1016/j.jhazmat.2018.11.024
Su, L., Nan, B., Hassell, K. L., Craig, N. J., & Pettigrove, V. (2019b). Microplastics biomonitoring in Australian urban wetlands using a common noxious fish (Gambusia holbrooki). Chemosphere, 228, 65–74. https://doi.org/10.1016/J.CHEMOSPHERE.2019.04.114
Tanaka, K., & Takada, H. (2016). Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Scientific Reports, 6, 34351. https://doi.org/10.1038/srep34351
Tang, G., Liu, M., Zhou, Q., He, H., Chen, K., Zhang, H., Hu, J., Huang, Q., Luo, Y., Ke, H., Chen, B., Xu, X., & Cai, M. (2018). Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Science of the Total Environment, 634, 811–820. https://doi.org/10.1016/J.SCITOTENV.2018.03.336
Thomson, J. M. (1966). The Grey Mullets. Oceanography and Marine Biology: An Annual Review, 4, 301–335.
Thompson, R.C. (2008). How concerned should we be about microplastics? In C. Arthur, J. Baker, & H. Bamford (Eds.), Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris (pp. 22–76). NOAA Technical Memorandum NOS-OR&R-30.
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., McGonigle, D., & Russell, A. E. (2004). Lost at sea: Where is all the plastic? Science, 304(5672), 838.
Tubau, X., Canals, M., Lastras, G., Rayo, X., Rivera, J., & Amblas, D. (2015). Marine litter on the floor of deep submarine canyons of the Northwestern Mediterranean Sea: The role of hydrodynamic processes. Progress in Oceanography, 134, 379–403. https://doi.org/10.1016/J.POCEAN.2015.03.013
United Nations Environment Programme. (2021). Drowning in Plastics – Marine Litter and Plastic Waste Vital Graphics. United Nations Environment Programme (UNEP), Secretariats of the Basel, Rotterdam and Stockholm Conventions (BRS) and GRID-Arendal.
Utami, D. A., Reuning, L., Konechnaya, O., & Schwarzbauer, J. (2021). Microplastics as a sedimentary component in reef systems: A case study from the Java Sea. Sedimentology, 68(6), 2270–2292. https://doi.org/10.1111/sed.12879
Veerasingam, S., Ranjani, M., Venkatachalapathy, R., Bagaev, A., Mukhanov, V., Litvinyuk, D., Mugilarasan, M., Gurumoorthi, K., Guganathan, L., Aboobacker, V. M., & Vethamony, P. (2021). Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Critical Reviews in Environmental Science and Technology, 51(22), 2681–2743. https://doi.org/10.1080/10643389.2020.1807450
Verdú, I., Amariei, G., Rueda-Varela, C., González-Pleiter, M., Leganés, F., Rosal, R., & Fernández-Piñas, F. (2023). Biofilm formation strongly influences the vector transport of triclosan-loaded polyethylene microplastics. Science of the Total Environment, 859, 160231.
Verleye, G. A. L., Roeges, N. P. G., & De Moor, M. O. (2001). Easy identification of plastics and rubbers. Rapra Technology, Shrewsbury, UK.
Vermaire, J. C., Pomeroy, C., Herczegh, S. M., Haggart, O., & Murphy, M. (2017). Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. FACETS, 2, 301–314. https://doi.org/10.1139/facets-2016-0070
Von Moos, N., Burkhardt-Holm, P., & Köhler, A. (2012). Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environmental Science and Technology., 46(20), 11327–11335. https://doi.org/10.1021/ES302332W/SUPPL_FILE/ES302332W_SI_001.PDF
Wagner, S., & Schlummer, M. (2020). Legacy additives in a circular economy of plastics: Current dilemma, policy analysis, and emerging countermeasures. Resources, Conservation & Recycling, 158, 104800. https://doi.org/10.1016/j.resconrec.2020.104800
Walkinshaw, C., Lindeque, P. K., Thompson, R., Tolhurst, T., & Cole, M. (2020). Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicology and Environmental Safety, 190, 110066.
Wang, F., Wong, C. S., Chen, D., Lu, X., Wang, F., & Zeng, E. Y. (2018). Interaction of toxic chemicals with microplastics: A critical review. Water Research, 139, 208–219. https://doi.org/10.1016/j.watres.2018.04.003
Wensing, M., Uhde, E., & Salthammer, T. (2005). Plastics additives in the indoor environment - Flame retardants and plasticizers. Science of the Total Environment, 339(1–3), 19–40. https://doi.org/10.1016/j.scitotenv.2004.10.028
Wieczorek, A. M., Morrison, L., Croot, P. L., Allcock, A. L., Macloughlin, E., Savard, O., Brownlow, H., & Doyle, T. K. (2018). Frequency of microplastics in mesopelagic fishes from the Northwest Atlantic. Frontiers in Marine Science, 5, 39. https://doi.org/10.3389/fmars.2018.00039
Wright, S. L., Thompson, R. C., & Galloway, T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environmental Pollution, 178, 483–492. https://doi.org/10.1016/J.ENVPOL.2013.02.031
Wu, C., Zhang, K., & Xiong, X. (2018). Microplastic pollution in inland waters focusing on Asia. Handbook of Environmental Chemistry, 58, 85–99. https://doi.org/10.1007/978-3-319-61615-5_5/TABLES/2
Yona, D., Sari, S. H. J., Iranawati, F., Bachri, S., & Ayuningtyas, W. C. (2019). Microplastics in the surface sediments from the eastern waters of Java Sea, Indonesia. F1000Research, 8, 98. https://doi.org/10.12688/f1000research.17103.1
Yonkos, L. T., Friedel, E. A., Perez-Reyes, A. C., Ghosal, S., & Arthur, C. D. (2014). Microplastics in four estuarine rivers in the chesapeake bay, U.S.A. Environmental Science and Technology, 48(24), 14195–14202. https://doi.org/10.1021/ES5036317/SUPPL_FILE/ES5036317_SI_001.PDF
Zazouli, M., Nejati, H., Hashempour, Y., Dehbandi, R., Nam, V. T., & Fakhri, Y. (2022). Occurrence of microplastics (MPs) in the gastrointestinal tract of fishes: A global systematic review and meta-analysis and meta-regression. Science of The Total Environment, 815, 152743. https://doi.org/10.1016/J.SCITOTENV.2021.152743
Zhang, K., Shi, H., Peng, J., Wang, Y., Xiong, X., Wu, C., & Lam, P. K. S. (2018). Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Science of the Total Environment, 630, 1641–1653. https://doi.org/10.1016/J.SCITOTENV.2018.02.300
Zhang, C., Wang, S., Pan, Z., Sun, D., Xie, S., Zhou, A., Wang, J., & Zou, J. (2020). Occurrence and distribution of microplastics in commercial fishes from estuarine areas of Guangdong. South China. Chemosphere, 260, 127656. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127656
Zhang, C., Wang, S., Sun, D., Pan, Z., Zhou, A., Xie, S., Wang, J., & Zou, J. (2020). Microplastic pollution in surface water from east coastal areas of Guangdong, South China and preliminary study on microplastics biomonitoring using two marine fish. Chemosphere, 256, 127202. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127202
Zhang, C., Wang, S., Sun, D., Pan, Z., & Zou, J. (2023). Investigation of microplastics in surface water and estuarine mullet Mugil cephalus from 23 Estuary Areas. South China. Sustainability, 15(5), 4193. https://doi.org/10.3390/su15054193
Zheng, K., Fan, Y., Zhu, Z., Chen, G., Tang, C., & Peng, X. (2019). Occurrence and species-specific distribution of plastic debris in wild freshwater fish from the pearl river catchment. China. Environmental Toxicology and Chemistry, 38(7), 1504–1513. https://doi.org/10.1002/ETC.4437
Zhou, W., Han, Y., Tang, Y., Shi, W., Du, X., Sun, S., & Liu, G. (2020). Microplastics aggravate the bioaccumulation of two waterborne veterinary antibiotics in an edible bivalve species: Potential mechanisms and implications for human health. Environmental Science & Technology, 54(13), 8115–8122.
Zhou, W., Shi, W., Du, X., Han, Y., Tang, Y., Ri, S., Ju, K., Kim, T., Huang, L., Zhang, W., Yu, Y., Tian, D., Yu, Y., Chen, L., Wu, Z., & Liu, G. (2023). Assessment of nonalcoholic fatty liver disease symptoms and gut–liver axis status in zebrafish after exposure to polystyrene microplastics and oxytetracycline, alone and in combination. Environmental Health Perspectives, 131(4), 47006. https://doi.org/10.1289/EHP11600
Zhu, X., Qiang, L., Shi, H., & Cheng, J. (2020). Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Marine Pollution Bulletin, 154, 111079. https://doi.org/10.1016/j.marpolbul.2020.111079
Acknowledgements
The first author is appreciative to the Indonesian Ministry of Education, Culture, Research, and Technology for the scholarship. This study was funded by Ministry of Education and Culture of Indonesia under contract number 916/UN3.15/PT/2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Indriyasari, K.N., Soegianto, A., Irawan, B. et al. The Presence of Microplastics and Plasticizers in Different Tissues of Mullet (Mugil cephalus) Along the East Java Coast in Indonesia. Water Air Soil Pollut 234, 600 (2023). https://doi.org/10.1007/s11270-023-06623-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06623-y