Abstract
Efficiencies in metal removal (Cd(II), Cr(VI), Ni(II),and Pb(II)) using as sorbent an unmodified sludge generated in the wastewater treatment pond from the wool industry in the city of Trelew (Patagonia, Argentina) were evaluated through batch sorption experiments. Optimal sorption conditions regarding mass of sorbent, contact time, pH, and metal initial concentrations were assessed. Sludge was characterized by a low porosity and scarce organic matter content. In addition, the sorbent presented an important variety of functional groups, necessary for the metal sorption. Kinetic studies revealed that the metal retention onto woolen sludge had better fit of experimental data to the pseudo-second-order model (R2 between 0.956 and 1.000), indicating that the process was jointly controlled by multiple mechanisms dominated by chemisorption. Sorption equilibrium data for Pb and Cd fitted to both Langmuir and Freundlich models, while Ni adjusted better to Langmuir model and Cr to Freundlich model. The maximum sorption capacities of the woolen sludge were 14.16 mg Pb g−1, 29.33 mg Cd g−1, 15.77 mg Ni−1, and 1.10 mg Cr g−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The negative impact on the environment by an inadequate wastewater management containing heavy metals continues to be a topic of study in force around the world (Ahmed et al., 2019; Boateng et al., 2019; Marinho et al., 2017; Zhou et al., 2020). Although there is a natural basal concentration in the environment of certain metals (generally in low concentrations), uncontrolled release of wastewater from activities, such as nuclear energy, chemical manufacturing, mining, and metallurgical industries, negatively affects the quality of water bodies, organisms, and farmlands and, ultimately, through the food chain and human health. In Patagonian coast of Argentina, the environmental presence of heavy metals with different levels of concern has been attributed to natural sources as well as urban, mining, oil, industrial, and port activities (Gil et al., 2019). In Chubut province, a recent study pointed out an anthropic enrichment of Cd, Pb, Cr, and Ni in saltmarsh soils (Idaszkin et al., 2020).
Wastewater treatment technologies have been developed to bring urban and industrial discharges to recommended metal level concentrations, in order to minimize negative consequences. As in many other environmental problems, the technologies that initially emerged such as coagulation/flocculation, ion exchange, flotation, membrane filtration, chemical precipitation, and electrochemical treatment are associated with high economic costs (Carolin et al., 2017). The economic requirement implies that these technologies are beyond the reach of many countries and private companies. In the past few decades, technologies that more environmentally friendly, simpler in operation, and with lower cost began to emerge (Chai et al., 2021; Huang et al., 2020). Among them biosorption stands out, a process is characterized by metal attraction to certain present components onto the cell wall of biological substrates (Beni & Esmaeili, 2020).
Different substrates such as vegetable/agriculture/forestry matrices (Garg et al., 2007; Gupta et al., 2018; Khan et al., 2016; Kiruba et al., 2014), algae (Cui et al., 2019; Demey et al., 2018; Pradhan et al., 2019; Rathinam et al., 2010), yeasts, fungi, and peat (Bartczak et al., 2018; Das et al., 2012; Noormohamadi et al., 2019) have been used as potential sorbents. Semi-solid wastes generated as sub-product in wastewater treatment processes such as activated sludge (Ferro Orozco et al., 2008; Gulnaz et al., 2005; Pagnanelli et al., 2009; Remenárová et al., 2012), municipal pond sludge (Giarratano et al., 2019), and diary industrial sludge (Sassi et al., 2010) have also been evaluated.
Among the main features affecting the potential of biomass in sorption process are pH, temperature, sorbent dosage, metal concentration, and contact time (Huang et al., 2020). The efficiency of a particular sorbent will also depend on the presence and abundance of functional groups on its surface and its affinity for the metal under treatment. In particular, the heavy metal sorption onto sewage sludge surface is usually attributed to the formation of complexes between metals and the carboxyl, hydroxyl, and phenolic surface functional groups of the extracellular polymeric substances (AjayKumar et al., 2009).
Despite the worldwide importance of wool industry, it accounts for less than 3% of total world fiber supply, while synthetic fibers represent more than 50% (Simpson, 2002). In 2018, the global wool production was 1.141 million tons (American Sheep Industry Association, 2018). In the 2018–2019 harvest, Argentina was the third world producer of wool with 40,696 tons. Of that output, 31.77% corresponded to Chubut province (AWF, 2019), being entirely processed in the woolen industrial park of Trelew city. Wool processing involves several chemical and mechanical steps, among which it is possible to mention: scouring, carbonizing, bleaching, dyeing, oiling, fulling, and finishing operations (Starovoytova, 2012). From these processes, an unusual and extremely complex type of wastewater is generated, which includes the natural impurities of the fibers used, and the processing chemicals (Singh & Yadav, 2014). In particular, the liquid presents high concentration of organic material, wool grease, detergent, lanolin, short wool, silt, and dyes. At the physical–chemical level, values of BOD5 15 to 40 g L−1, COD 30 to 150 g L−1, total grease 9 to 50 g L−1, suspended solids 15 to 80 g L−1, and alkaline pH were described (Peláez et al., 2001). Treatment by means of stabilization ponds for both sewage and industrial wastewater is widely used due to its simplicity and low cost (Verbyla et al., 2017). A relevant aspect is that the sludge accumulation can have a negative impact on treatment efficiency, resulting in short-circuiting of flow and reduced mean hydraulic residence time (Coggins et al., 2019). The extraction, adequate treatment of sludge, and its subsequent reuse in various purposes represent a widely beneficial management strategy, mainly for the generation of agronomic fertilizer (Dubis et al., 2020; Nunes et al., 2021; Scaglia et al., 2018) and alternative energies (Kwon et al., 2012; Liu et al., 2021; Rulkens, 2008).
The main goal of this research was to investigate the potential use as sorbent of metals of a sludge generated in a wastewater treatment system from a wool industry. The removal of Cd(II), Ni(II), Pb(II), and Cr(VI) from each monometallic aqueous solution using batch systems was tested. The influence on sorption of pH, sorbent mass, and contact time was evaluated.
2 Materials and Methods
2.1 Study Site–Sorbent Collection
Wastewater from the wool company “La Peinaduría” in Trelew city (latitude: 43° 14′ 57.486″ S; longitude: 65° 18′ 27.486″ W) (Fig. 1) is pretreated to extract yarn waste and lanolin, and then, it is conducted to a system of settling, stabilization, and evaporation ponds, located 4.5 km from the factory. The whole system is comprised by 6 ponds, occupying an area of 16 Ha. Periodically, the bottom sludge is extracted and accumulated in open air-drying piles in the surroundings of the pond. Dry sludge samples were taken from these piles for the metal sorption experiments.
2.2 Sludge Sorbent Characterization
Once in the laboratory, the sorbent under study was analyzed for moisture content, after drying in an oven at 105 °C. In order to determine the content of organic matter, a fraction of dry sludge was calcinated at 450 °C for a period of 4 h (Baird et al., 2017). Enumeration of culturable heterotrophic fungi and bacteria serial dilution was done by triplicate (Wollum II, 1982) using the rose Bengal chloramphenicol agar (Martin, 1950) and the plate count agar (Baird et al., 2017), respectively. The following biomolecules concentrations were measured: proteins (Lowry et al., 1951), carbohydrates (Gerchakov & Hatcher, 1972), and lipids (Bligh & Dyer, 1959). Chlorophyll “a” and phaeophytin were measured according to the fluorometric technique of Lorenzen and Jeffrey (1980).
A granulometry assay was performed using mesh size sieves of 0.063 mm, 0.125 mm, 0.425 mm, and 2 mm. About 100 g of dried sludge was placed on the top sieve, being the individual fractions separated by shaking in a vibratory sieve shaker (Zonytest) for 15 min at 150 rpm. Particles trapped on each sieve were removed and weighed, being the results expressed as the percentage retained in each sieve.
The bulk mineralogy was analyzed by X-ray diffraction (XRD) using a PANalytical X’Pert Powder diffractometer, which is fitted with a copper tube, operating at 45 kV and 40 mA and a post-diffraction graphite monochromator. Semi-quantification of the major mineralogical components was performed after processing the bulk sediment XRD scans in the software package X’Pert High-Score Plus (PANalytical) using the Rietveld full-pattern fitting method.
Surface morphology of sludge was analyzed with a JEOL 6460LV low vacuum scanning electron microscope (SEM) with a back-scattered electrons (BSE), an accelerating voltage of 20 kV, and a spot size of 60 (probe diameter). The instrument is equipped with an energy dispersive X-ray spectrometer (EDS) system to determine the elemental composition during SEM observations. Elements were automatically identified and quantified by the INCA software, and results were normalized to 100%. Copper was used as standard for the quantitative analysis.
In addition, the initial contents of Cd, Cr, Ni, and Pb in sludge were analyzed by optical emission spectrometry (ICP-OES, Agilent-720), according to the EPA Method 3051A (USEPA, 2007). Briefly, about 0.5 g of dried sludge was placed in quartz microwave vessels with nitric and hydrochloric acids. Vessels were sealed and heated in a NovaWave SPC SCIENCE microwave at 180 °C for 10 min. After cooling, the vessel contents were diluted to 50 mL and then analyzed by ICP-OES.
The presence of surface functional groups was analyzed by using a Fourier transform infrared (FTIR) spectrometer (Thermo Scientific NICOLET 6700). Sludge was dried at 60 °C and rubbed in agate mortar. Then, a pellet was made with 1% (w/w) of the sample dispersed in KBr. The FTIR spectra were obtained from 400 to 4000 cm−1 with a resolution of 4 cm−1 and 75 single scans.
2.3 Batch Sorption Experiments
2.3.1 Synthetic Solution Preparation
Stock solutions of Ni, Cd, Pb, and Cr were prepared from Ni(NO3)2.6H2O, CdCl2.H2O, Pb(NO3)2, and K2Cr2O7, respectively, and dissolved in deionized water. Reagents used to prepare the stock solutions were of analytical grade.
2.3.2 Study of Optimal Sorption Conditions
The optimal sorption conditions were examined, and the removal efficiencies were measured under: (1) different pH conditions, (2) different contact times, and (3) various sorbent masses together with different initial concentrations of metal.
-
1)
To evaluate the influence of pH, 0.10 g of sorbent were stirred in 100 mL of solution containing metal concentration of 50 mg L−1 for a period of 180 min, varying the pH in the following values: 2, 3, 4, 5, and 6.
-
2)
For testing the effect of the sorbent mass, experiments were carried out by stirring a metal solution of 50 mg L−1 with 0.10, 0.25, 0.50, and 1.00 g of sludge at pH 4 and a contact time of 180 min.
-
3)
Finally, the contact time was tested with the following intervals: 15, 30, 45, 60, 90, 120, 180, and 240 min; for each of the following metal concentrations: 10, 25, 50, and 100 mg L−1. Regarding the fixed variables, pH was of 6 for Cd, Ni, and Pb, and 2 for Cr, and sorbent mass was 1.00 g.
In all cases, sorption tests were conducted in 250-mL Erlenmeyer glass flasks, mixing a known weight of sludge in 100 mL of a monometallic solution in duplicates, keeping the temperature fixed at 20 °C, and shaking speed of 200 rpm. After completing each experiment, 10 mL of the mixture was extracted from the glass flasks and centrifuged at 3000 rpm for 5 min, and then, the measurements of final metal concentrations were made on the supernatant liquid using an ICP-OES Agilent-720.
Sorption efficiency of sludge was calculated in function of the sorption capacity at equilibrium state (qe), according to Eqs. 1 and 2.
where qe is the solute mass adsorbed per unit adsorbent mass at equilibrium (mg g−1), C0 is the initial concentration of solute in the solution (mg L−1), Ce is the solute concentration at equilibrium (mg L−1), V is the solution volume (L), and m is the adsorbent mass (g).
2.3.3 Sorption Isotherm and Kinetic Modeling
The sorption isotherms, through the calculation of their constants, allow knowing surface and affinity properties of sorbents. At the same time, they are a useful tool to compare the sorption efficiency among different sorbents.
In order to assess the equilibrium and kinetic conditions, experiences were carried out with 100 mL of synthetic solution of each metal in contact with 1.00 g of sludge and stirring rate of 200 rpm for 240 min. Samples of 10 mL were centrifuged at 3000 rpm for 5 min. The pH was fixed at 6 for Cd, Ni, and Pb, and at 2 for Cr, the temperature was maintained at 20 °C. Initial metal concentrations of 10, 25, 50, and 100 mg L−1 were experimented.
The obtained experimental data were fitted with the Langmuir and Freundlich models according to Eqs. 3 and 4, respectively. The Langmuir model proposes a single layer, superficial sorption with an infinite and equal number of sites for the sorption, while Freundlich model is based on the fact that sorption is multilayer and on a heterogeneous surface (Sari & Tuzen, 2008).
where qmax relates to the maximum adsorption capacity (mg g−1), b is the constant of the Langmuir isotherm (L mg−1), KF is the Freundlich constant (mg g−1), and 1/n is the degree of heterogeneity (mg−1).
Several models can be used to express the mechanism of solute sorption onto a sorbent. The sorption kinetics of the metals onto the industrial sludge were tested using pseudo-first-order, pseudo-second-order, and intra-particle models according to Eqs. 5, 6, and 7, respectively.
where qt is amount of solute sorbed on the surface of the sorbent at time t (mg g−1); k1 is the Lagergren first-order constant (min−1); k2 is the Lagergren second-order constant (g mg−1 min−1), and Kid is the intra-particle constant (mg g−1 min−0.5).
2.3.4 Experiences of Exchangeable Cations
In order to investigate the ion exchange contribution in metal desorption, batch experiments were performed with 1 g of sludge in 100 mL of solution with a concentration of 50 mg L−1 of Cd, Cr, Ni, and Pb. The fixed parameters were pH 6 for Cd, Ni, and Pb and pH 2 for Cr; temperature of 20° C; stirring speed of 200 rpm; and contact time of 15, 30, 45, 60, 90, 120, 180, and 240 min. For each time interval, concentrations of Ca, Na, K, and Mg were also analyzed by ICP-OES Agilent-720.
3 Results and Discussion
3.1 Woolen Sludge Characterization
The moisture and organic matter contents, as well as mineralogy and elemental composition of the woolen sludge, are shown in Table 1. The matrix proposed as sorbent was characterized by 20% of humidity, 15% of organic matter in dry weight, and a high presence of microorganisms. The low moisture content is expected since the sludge was sampled from open air-drying piles located in an area with arid weather, where length of daily sunshine is about 10 h in summer and 4–5 h in winter, average annual precipitation below 200 mm, and strong winds (EMC, 2021). Regarding the relative low total organic content, it may be attributed to the fact that the industrial pond was characterized by generating an anaerobic liquid, which implies a null phytoplankton development in surface water, and this was consistent with the low content of pigments in the sludge. Among the three components of organic matter analyzed, the lipid content was approximately three times greater than protein and carbohydrates. This could be associated with the important presence of wool wax and wool grease, carried away during the scouring (Starovoytova & Namango, 2014). In particular, it was observed that wool wax is hydrolyzed forming sterols that are accumulated in the sludge and free fatty acids that are degraded (Gutiérrez et al., 1999).
The grain size was characterized by a broad predominance of sand (67% coarse, 12% medium, 6% fine) and 15% of silt clay (Table 1). X-ray diffraction patterns showed that the major crystalline phases were plagioclase, quartz, phyllosilicates, and, to a lesser extent, K-feldspar and calcite (Table 1). Other minerals were identified in contributions less than 1% (such as magnetite, hematite, and pyrite). Regarding the elemental composition obtained by SEM–EDS, oxygen was the most abundant element (43.7%) indicating likely the presence of silicates and oxides of different types, followed by carbon (26.9%) and silicon (18.8%). Besides the contribution of calcite, the relative high percentage of total C reveals the significant presence of the organic component; meanwhile, Si might indicate the sandy character of the sludge. These three elements represent almost 90% of the total elemental composition of the wool sludge. In the remaining 10%, several elements such as Al, Fe, K, Na, and Ca were also found although in lower concentrations (3.88–0.91%); meanwhile, Mg, Mo, S, Ti, and Cl were present in trace amounts (< 0.5%).
Regarding the initial content of the 4 studied metals in the raw sludge, low concentrations were found, which reflect no significant contribution from the industrial process (Table 1). Those values were below the maximum admissible limits in soils for agricultural uses established by the Secretary of Sustainable Development and Environmental Policy (Res. 97/2001).
The FTIR spectra are used to determine the vibrational frequency, allowing to identify the functional groups present in the sorbent (Lasheen et al., 2012). Figure 2a shows the FTIR spectra pattern, where the following functional groups in the woolen sludge were present: stretching of the hydroxyl groups and the amine group of polymeric compounds (OH- and –NH symmetric and antisymmetric; peak in 3409 cm−1); alkalyl group (–CH2; peaks in 2921 and 2852 cm−1), and carboxylic acid groups (-COOH; a peak around 1726 cm−1 and in 1242 cm−1). The absorption band at 1641 cm−1 can be assigned to the stretch of C = O in polysaccharides, peptides, and proteins (Kowalski et al., 2018). The band at 1542 cm−1 would correspond to the stretching vibrations of –CN and the deformation vibrations of –NH. The absorption band at 1423 cm−1 is attributed to the phenolic or alcoholic –OH groups and to the stretching of the C = O of the carboxylate group. The strongest band at 1033 cm−1 can be assigned to the vibration of the –C–O–C and –OH groups of polysaccharides. The signal between 1100 and 1160 cm−1 is attributed to the deformation of sulfonates (–SO2) present in functionalized polysaccharides. The band at 795 cm−1 would indicate the presence of sulfur compounds, while the bands between 460 and 520 cm−1 would indicate the presence of organophosphates aliphatic compounds with aromatic rings. The woolen sludge presented an important variety of functional groups, which have been described in other sorbents with active participation in the sorption of metals (Kiruba et al., 2014).
Result of scanning electron microscope (SEM) of the surface morphology of woolen sludge is given in Fig. 2b. The irregular surface and different porosity levels of sludge can facilitate the retention of metal ions.
3.2 Optimal Conditions of the Sorption Process
3.2.1 pH and Sorbent Mass Effects
The pH effect on removal of the 4 metals onto woolen sludge was evaluated with pH values of 2, 3, 4, 5, and 6 with an initial concentration of each metal of 50 mg L−1 (Fig. 3a). During the metal sorption process, the pH in the solution is a relevant aspect in the sorption efficiency. It determines the competition for the active sites of the sorbent between the protons and the metals present in the solution (Hammaini et al., 2007), influencing on the surface properties of the sorbent (Garg et al., 2007) and the metal speciation (Reddy & Lee, 2014). For Pb, Cd, and Ni, an increase in the removal efficiency was observed with the increasing pH values. The highest efficiency was registered for Pb at pH 6 (78.2%), with the minimum for this element at pH 2 (8.06%). For Cd, the maximum efficiency was obtained at pH values of 5 and 6 (average 33.07%), being less than 10% at the other pH values. For Ni, the mean removal was of 15.64% at pH values of 4, 5, and 6, while it was practically negligible at lower pH values. Greater sorption removal efficiencies have been described at pH values between 5 and 6, associated with the presence of negative charges at the sorbent surface, favoring the bond between sorbent and metal cations (Das et al., 2012; Dehghani et al., 2019). The opposite effect occurs at low pH, where the sorbent walls would be charged with protons, which exerts repulsive forces on metal cations (Hammaini et al., 2007). Chromium presented a different behavior, reaching an efficiency of 3.5% in the lowest pH values (2 and 3) and being null in the others. This is in accordance with the anionic form of this metal (HCrO4−), requiring the generation of positive charges on sorbent surfaces at low pH. It must be observed that maximum removal obtained in this study was lower than those reported for a wide variety of sorbents under similar pH conditions (Bansal et al., 2008; Garg et al., 2007; Khan et al., 2016; Zhang et al., 2017). This could be explained by the fact that generation of positive charges also depends on the pKa of organic functional groups and on the average point zero of charge of the mineral phases present in the sorbent. The low Cr removal at pH 2 in our study could be associated with a low ratio of sorbent mass/solution volume (0.1 g of sorbent in 100 mL of solution).
The influence of sorbent mass on sorption is direct and related with increased availability of active sites, improving the percentage of removal (Das et al., 2012). This pattern was observed for Pb, Cd, and Ni, which had the maximum removal with 1 g of sorbent mass (Fig. 3b). The removal efficiency for Pb was higher than 94% with masses above 0.25 g and of 60% with 0.1 g of sludge. While for Ni and Cd, there was a more attenuated progression with 0.25 g of sorbent, the removals were 59.68% and 32.07%, respectively; and with 1 g of sorbent, the removal efficiency was maximum, being 76.7% (Ni) and 95.5% (Cd). Some studies registered greater metal removal efficiency by increasing sorbent mass (Khan et al., 2016; Kiruba et al., 2014). A different behavior was obtained for Cr, with no removal efficiency for any masses tested. This may be directly related to the fact that the experiments were carried out with a pH of 4, which does not favor the Cr sorption (Cui et al., 2019).
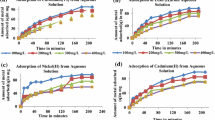
The removal efficiency presented the following decreasing affinity pattern Pb > Cd > Ni > Cr (Fig. 4). Regarding Cd, removal efficiencies were greater than 90% after 90 min of contact time. In the first 90 min, for the initial concentration of 100 mg L−1, the removal efficiency was lower than for the other concentrations, although after 15 min the removal reached 80.02% (Fig. 4a). The removal of Cr was lower than the other metals, reaching a maximum of 36.69% at the lowest initial concentration (10 mg L−1). Removal efficiencies between 14.05 and 20.42% were found for the other initial concentrations (Fig. 4b). Nickel sorption was characterized by a gradual increase in removal with the progress of the experiment, reaching at 240 min the following removals: 89.03% (10 mg L−1), 90.79% (25 mg L−1), 86.02% (50 mg L−1), and 68.06% (100 mg L−1) (Fig. 4c). The sorption mechanism of Pb was the fastest and the most effective even for the highest initial concentration (100 mg L−1), achieving a removal of 94.04% after 15 min (Fig. 4d). In general, there were no differences among the removals for the different initial concentrations of Pb, reaching values between 96 and 98%.
The fast sorption rate is directly related to the fact that at the beginning, the presence of available active sorbent sites is greater in proportion to the amount of metal in solution. The increase in the initial concentration of the metal causes a saturation of the active sites of the sorbent, requiring more contact time to bind the metal with an unoccupied site and achieve maximum efficiency. It is a rapid two-phase process: initially the driving force is higher, and binding sites with higher affinity are occupied very fast. The remaining less affinity sites are slowly occupied until equilibrium is reached (Choi & Yun, 2006; Remenárová et al., 2012).
3.3 Sorption Kinetics
With the aim of better understand the sorption mechanism, it is important to determine the model that governs the sorption kinetics of a particular sorbent and to predict the controlling step in the whole process. Models of pseudo-first-order, pseudo-second-order, and intra-particle diffusion were used in order to assess best fitting of experimental data, showing the corresponding correlation coefficients (R2) and kinetic parameters in Table 2. When pseudo-second-order model is applicable, the plot t/qt versus t exhibits a linear relationship. The data of all metals fitted much better to the pseudo-second-order model (Table 2, Fig. 5), indicating that the kinetics of the sorption process onto woolen industrial sludge was characterized by a predominance of chemisorption phenomenon and it is the rate-determining step over other processes. The k2 values for all elements diminished with increasing initial concentrations, which could evidence an enhancement in the competition between metal cations themselves and with protons for the same active sites (Bohli et al., 2013). Considering the same initial concentration, k2 values found for each element decreased in the following order: Pb > Cd > Ni > Cr, which shows the affinity of woolen sludge for each metal ion. With the lowest Cr concentration, the three models presented similarly high R2 values.
3.4 Sorption Isotherms
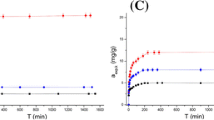
Equilibrium sorption studies were performed to determine the maximum metal adsorption capacities of woolen sludge. Sorption isotherm represents graphically the relationship between the quantity of metal adsorbed onto sorbent (qe) and the remainder concentration in the solution (Ce), at the equilibrium stage. Isotherms were evaluated using the widely used Langmuir and Freundlich models.
The constants and R2 values for both models are shown in Table 3. The isotherm data for Cd and Pb adjusted significantly to both Langmuir and Freundlich models, with R2 greater than 0.95. This implies that the sorption mechanism is the result of a combination of mono and multilayers on the surface of the wool sludge. The sorption of Ni was better explained by the Langmuir model (R2 = 0.97), occurring in a monolayer of active sites arranged homogeneously in the sorbent. Meanwhile, Cr fitted better to Freundlich model (R2 = 0.89), which indicates that the sorption would take place in a heterogeneously distributed multilayer. The sorption processes of the four metals onto woolen sludge presented values of 1/n between 0.1 and 1.0, which displays a favorable sorption, according to Bazargan-Lari et al. (2014). The sorbent showed the following efficiency gradient: Cd > Ni≈Pb > > Cr.
Sorption capacity of wooden sludge was compared through maximum metal uptake capacity (qm) with other unmodified sorbents under similar experimental conditions. Table 4 highlights the great variability among matrices and the complexity of making comparisons. Regardless of the metal, woolen sludge presented greater or similar sorption capacity than some agro-industry residues, microalgae biomass, and some activated sludge. However, it had lower efficiency than that registered by dairy sludge, vegetable biomass, peat and activated sludge.
3.5 Cationic Exchange in Metal Sorption Experiences
The ion exchange process occurs when heavy metal ions in the solution are exchanged with cations of other metals and/or protons present in the sorbent. The concentration of the soft metals K+, Mg2+, Ca2+, and Na+ is evaluated in solution and is displayed in Fig. 6. The same cation-releasing pattern (Na > Ca > K≈Mg) was observed for Pb, Cd, and Ni adsorption experiences. In a similar study, using mango peel waste for removal of Pb and Cd, it was found the following order in the cation exchange: Ca2+ > K+ > Mg2+ > Na+ (Iqbal et al., 2009). According to the high concentration of Ca2+ (9.5 meq Ca L−1) at the end of the assay with Cr, it is possible to highlight the significant role of Ca2+ in the ion exchange mechanism. The latter would be associated with the fact that the experiment with Cr was carried out at pH 2. Therefore, the exchange would be more efficient with protons than with Cr, as cell wall ligands would be closely associated to H3O+. The access of metal ions to ligands would be restricted because of repulsive forces (Hammaini et al., 2007), explaining the low efficiency in chromium removal. Functional groups participate in metal ion binding, being the sorption process strongly pH dependent. According to the experimental conditions, carboxyl groups would be deprotonated in the Cr experiment, while carboxyl groups and a part of phosphate groups would be deprotonated with the other elements (Chojnacka et al., 2005). This behavior contrasts with the cations distribution in the sludge, since the lowest concentration corresponded to Na+ and the highest to K+, presenting an inverse predisposition to its release. Since K+ exhibits a lower electronegativity and greater ionic radius, its diffusion is likely to be slower than Na+ (Malamis & Katsou, 2013).
4 Conclusions
Unmodified sludge generated in a wastewater treatment system of the wool industry of Trelew city (Patagonia, Argentina) was examined as potential sorbent for the uptake of Cd, Cr, Ni, and Pb. Initial pH, sorbent mass, and the initial metal concentration influenced the sorption efficiency of the 4 studied metals. The sorption capacity was highest at pH 5–6 for Pb, Cd, and Ni and at pH 2 for Cr. Under the optimal conditions of pH and sorbent mass, the sorbent showed the following decreasing metal affinity pattern: Pb = Cd > Ni > Cr. The sorption efficiencies for 100 mg L−1 and at 240 min were of 96.09%, 93.91%, 68.06%, and 15.03%, respectively.
The experimental data showed a greater fit to the pseudo-second-order kinetic model, suggesting chemisorption as the dominating mechanism of sorption. The application of isotherm models varied according to the metal. Lead and Cd fitted to both Langmuir and Freundlich models, so the sorption would take place in a mix of homogeneous monolayer and heterogeneously distributed multilayer, while the equilibrium data for Ni fitted to Langmuir model, and Cr sorption isotherm to Freundlich model. The highest sorption capacities were 29.33 mg g−1 for Cd, 15.77 mg g−1 for Ni, 14.16 mg g−1 for Pb, and 1.1 mg g−1 for Cr. The presence of cations, mainly Na+ and Ca2+, after experiments evidences that cation exchange was involved in the mechanism of sorption. The woolen sludge showed a different adsorption capacity according to the study metals, implying that its potential use as a sorbent for a certain effluent will depend on the metal to be removed, its concentration, and the limit level established by authorities for its discharge.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed, M., Matsumoto, M., Ozaki, A., Van Thinh, N., & Kurosawa, K. (2019). Heavy metal contamination of irrigation water, soil, and vegetables and the difference between dry and wet seasons near a multi-industry zone in Bangladesh. Water, 11, 583. https://doi.org/10.3390/w11030583
AjayKumar, A. V., Darwish, N. A., & Hilal, N. (2009) Study of various parameters in the biosorption of heavy metals on activated sludge. World Applied Sciences Journal 5(Special Issue for Environment), 32–40.
American Sheep Industry Association. (2018). Wool trust report. Period, 2017–2018, 42. https://www.ams.usda.gov/sites/default/files/media/201718WoolTrustReport.pdf
AWF (Argentine Wool Federation). (2019). Argentine wool statistics (pp. 1–9). Retrieved July 21, 2021, from https://www.flasite.com/images/pdf/estadisticas/anuales/EL-728.pdf
Baird, R. B., Eaton, A. D., Rice, E. W. (2017) Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association, American Water Works Association, Water Environment Federation. Washington.
Bansal, M., Singh, D., Garg, V. K., & Rose, P. (2008). Mechanisms of Cr(VI) removal from synthetic wastewater by low cost adsorbents. Journal of Environmental Research and Development, 3, 228–243. Corpus ID 54972975.
Bartczak, P., Norman, M., Klapiszewski, L., Karwańska, N., Kawalec, M., Baczyńska, M., Wysokowski, M., Zdarta, J., Ciesielczyk, F., & Jesionowski, T. (2018). Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arabian Journal of Chemistry, 11(8), 1209–1222. https://doi.org/10.1016/j.arabjc.2015.07.018
Bazargan-Lari, R., Zafarani, H. R., Bahrololoom, M. E., & Nemati, A. (2014). Removal of Cu(II) ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. Journal of the Taiwan Institute of Chemical Engineers, 45(4), 1642–1648. https://doi.org/10.1016/j.jtice.2013.11.009
Beni, A. A., & Esmaeili, A. (2020). Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environmental Technology & Innovation, 7, 100503. https://doi.org/10.1016/j.eti.2019.100503
Bligh, E. G., & Dyer, W. (1959). A rapid method for total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917. https://doi.org/10.1139/o59-099
Boateng, K. S., Agyei-Baffour, P., Boateng, D., Rockson, G. N. K., Kofi Mensah, K. A., & Edusei, A. K. (2019). Household willingness-to-pay for improved solid waste management services in four major metropolitan cities in Ghana. Journal of Environmental and Public Health, Article ID, 5468381, 9. https://doi.org/10.1155/2019/5468381
Bohli, T., Villaescusa, I., & Ouederni, A. (2013). Comparative study of bivalent cationic metals adsorption Pb (II), Cd (II), Ni (II) and Cu (II) on olive stones chemically activated carbon. Journal of Chemical Engineering & Process Technology, 4(4), 1–7. https://doi.org/10.4172/2157-7048.1000158
Carolin, C. F., Kumara, P. S., Saravanana, A., Joshibaa, G. J., & Naushad, M. (2017). Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering, 5(3), 2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chai, W. S., Cheun, J. Y., Senthil Kumar, P., Mubashir, M., Majeed, Z., Banat, F., Ho, S. H., & Show, P. L. (2021). A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. Journal of Cleaner Production, 296, 126589. https://doi.org/10.1016/j.jclepro.2021.126589
Chen, H., Dou, J., & Xu, H. (2017). Removal of Cr(VI) ions by sewage sludge compost biomass from aqueous solutions: Reduction to Cr(III) and biosorption. Applied Surface Science, 425, 728–735. https://doi.org/10.1016/j.apsusc.2017.07.053
Choi, S. B., & Yun, Y. S. (2006). Biosorption of cadmium by various types of dried sludge: An equilibrium study and investigation of mechanisms. Journal of Hazardous Materials, B138, 378–383. https://doi.org/10.1016/j.jhazmat.2006.05.059
Chojnacka, K., Chojnacki, A., & Górecka, H. (2005). Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere, 59, 75–84. https://doi.org/10.1016/j.chemosphere.2004.10.005
Coggins, L. X., Crosbie, N. D., & Ghadouani, A. (2019). The small, the big, and the beautiful: Emerging challenges and opportunities for waste stabilization ponds in Australia. Wires Water, 6(6), 1–18. https://doi.org/10.1002/wat2.1383
Cui, Y., Masud, A., Aich, N., & Atkinson, J. D. (2019). Phenol and Cr(VI) removal using materials derived from harmful algal bloom biomass: Characterization and performance assessment for a biosorbent, a porous carbon, and Fe/C composites. Journal of Hazardous Materials, 368, 477–486. https://doi.org/10.1016/j.jhazmat.2019.01.075
Das, D., Basak, G., Lakshmi, V., & Das, N. (2012). Kinetics and equilibrium studies on removal of zinc (II) by untreated and anionic surfactant treated dead biomass of yeast: Batch and column mode. Biochemical Engineering Journal, 64, 30–47. https://doi.org/10.1016/j.bej.2012.03.001
Dehghani, M. H., Sarmadi, M., Alipour, M. R., Sanaei, D., Abdolmaleki, H., Agarwal, S., & Gupta, V. K. (2019). Investigating the equilibrium and adsorption kinetics for the removal of Ni (II) ions from aqueous solutions using adsorbents prepared from the modified waste newspapers: A low-cost and available adsorbent. Microchemical Journal, 146, 1043–1053. https://doi.org/10.1016/j.microc.2019.02.042
Demey, H., Vincent, T., & Guibal, E. (2018). A novel algal-based sorbent for heavy metal removal. Chemical Engineering Journal, 332, 582–595. https://doi.org/10.1016/j.cej.2017.09.083
Deshmukh, P. D., Khadse, G. K., Shinde, V. M., & Labhasetwar, P. (2017). Cadmium removal from aqueous solutions using dried banana peels as an adsorbent: Kinetics and equilibrium modeling. Journal of Bioremediation and Biodegradation, 8, 395. https://doi.org/10.4172/2155-6199.1000395
Dönmez, G. Ç., Aksu, Z., Öztürk, A., & Kutsal, T. (1999). A comparative study on heavy metal biosorption characteristics of some algae. Process Biochemistry, 34(9), 885–892. https://doi.org/10.1016/S0032-9592(99)00005-9
Dubis, B., Jankowski, K. J., Zaluski, D., & Sokólski, M. (2020). The effect of sewage sludge fertilization on the biomass yield of giant miscanthus and the energy balance of the production process. Energy, 206, 118189. https://doi.org/10.1016/j.energy.2020.118189
EMC (Estación Meteorológica Cenpat) (2021) Retrieved July 15, 2021, from http://repositorio.cenpat-conicet.gob.ar:8081/xmlui/603handle/123456789/400.
Ferro Orozco, A. M., Contreras, E. M., & Zaritzky, N. E. (2008). Modelling Cr(VI) removal by a combined carbon-activated sludge system. Journal of Hazardous Materials, 150, 46–52. https://doi.org/10.1016/j.jhazmat.2007.04.043
Garg, U. K., Kaura, M. P., Garg, V. K., & Sud, D. (2007). Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. Journal of Hazardous Materials, 140(1–2), 60–68. https://doi.org/10.1016/j.jhazmat.2006.06.056
Gerchakov, S. M., & Hatcher, P. G. (1972). Improved technique for analysis of carbohydrates in sediments. Limnology and Oceanography, 17(6), 938–943. https://doi.org/10.4319/lo.1972.17.6.0938
Giarratano, E., Faleschini, M., Bruni, C., Olivera, N. L., & Gil, M. N. (2019). Metal removal from wastewater using sludge from a natural stabilization pond as biosorbent. International Journal of Environmental Research, 13, 581–595. https://doi.org/10.1007/s41742-019-00196-7
Gil, M. N., Giarratano, E., Barros, V., Bortolus, A., Codignotto, J. O., Delfino Schenke, R., Góngora, M. E., Lovrich, G., Monti, A. J., Pascual, M., Rivas, A. L., & Tagliorette, A. (2019) Southern Argentina. The Patagonian continental shelf. In C. Sheppard (Ed.) World Seas: An Environmental Evaluation, Volume I: Europe, The Americas and West Africa (2nd ed., pp. 783–811). Academic Press. ISBN: 9780128050682. https://doi.org/10.1016/B978-0-12-805068-2.00040-1
Gulnaz, O., Saygideger, S., & Kusvuran, E. (2005). Study of Cu(II) biosorption by dried activated sludge: Effect of physico-chemical environment and kinetics study. Journal of Hazardous Materials, B120, 193–200. https://doi.org/10.1016/j.jhazmat.2005.01.003
Gupta, P., Rani, R., Chandra, A., & Kumar, V. (2018). Potential applications of Pseudomonas sp. (strain CPSB21) to ameliorate Cr6+ stress and phytoremediation of tannery effluent contaminated agricultural soils. Scientific Reports, 8, 4860. https://doi.org/10.1038/s41598-018-23322-5
Gutiérrez, S., Hernández, A., & Viñas, M. (1999). Mechanism of degradation of wool wax in the anaerobic treatment of woolscouring wastewater. Water Science and Technology, 40(8), 17–23. https://doi.org/10.1016/S0273-1223(99)00604-6
Hammaini, A., González, F., Ballester, A., Blázquez, M. L., & Muñoz, J. A. (2007). Biosorption of heavy metals by activated sludge and their desorption characteristics. Journal of Environmental Management, 84, 419–426. https://doi.org/10.1016/j.jenvman.2006.06.015
Huang, D., Li, B., Ou, J., Xue, W., Li, J., Li, Z., Li, T., Chen, S., Deng, R., & Guo, W. (2020). Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: preparation, final disposal, mechanism and influencing factors. Journal of Environmental Management, 261, 109879. https://doi.org/10.1016/j.jenvman.2019.109879
Idaszkin, Y. L., Carol, E. S., Barcia-Piedras, J. M., Bouza, P. J., & Mateos-Naranjo, E. (2020). Trace metal concentrations in soil-plant complex in rocky shore salt marshes of Central Patagonia. Continental Shelf Research, 211, 104280. https://doi.org/10.1016/j.csr.2020.104280
Iqbal, M., Saeed, A., & Kalim, I. (2009). Characterization of adsorptive capacity and investigation of mechanism of Cu2+, Ni2+, and Zn2+ adsorption on mango peel waste from constituted metal solution and genuine electroplating effluent. Separation Science and Technology, 44, 3770–3791. https://doi.org/10.1080/01496390903182305
Khan, S. U., Khan, F. U., Khan, I. U., Muhammad, N., Badshah, S., Khan, A., Ullah, A., Khan, A. S., Bilal, H., & Nasrullah, A. (2016) Biosorption of nickel (II) and copper (II) ions from aqueous solution using novel biomass derived from Nannorrhops ritchiana (Mazri Palm). Desalination and Water Treatment, 1–11. https://doi.org/10.1080/19443994.2014.989268
Kiruba, U. P., Kumar, P. S., Prabhakaran, C., & Aditya, V. (2014). Characteristics of thermodynamic, isotherm, kinetic, mechanism and design equations for the analysis of adsorption in Cd(II) ions-surface modified Eucalyptus seeds system. Journal of the Taiwan Institute of Chemical Engineers, 45(6), 2957–2968. https://doi.org/10.1016/j.jtice.2014.08.016
Kowalski, M., Kowalska, K., Wiszniowski, J., & Turek-Szytow, J. (2018). Qualitative analysis of activated sludge using FT-IR technique. Chemical Papers, 72, 2699–2706. https://doi.org/10.1007/s11696-018-0514-7
Kumar, J., & Oommen, C. (2012). Removal of heavy metals by biosorption using freshwater alga Spirogyra hyalina. Journal of Environmental Biology, 33(1), 27–31. ISSN 0254-8704.
Kwon, E. E., Kim, S., Jeon, Y. J., & Yi, H. (2012). Biodiesel production from sewage sludge: New paradigm for mining energy from municipal hazardous material. Environmental Science & Technology, 46, 10222–10228. https://doi.org/10.1021/es3019435
Lasheen, M. R., Ammar, N. S., & Ibrahim, H. S. (2012). Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sciences, 14(2), 202–210. https://doi.org/10.1016/j.solidstatesciences.2011.11.029
Liu, X., Zhu, F., Zhang, R., Zhao, L., & Qi, J. (2021). Recent progress on biodiesel production from municipal sewage sludge. Renewable and Sustainable Energy Reviews, 135, 110260. https://doi.org/10.1016/j.rser.2020.110260
Lorenzen, C. J., & Jeffrey, S. W. (1980). Determination of chlorophyll in seawater. UNESCO Technical Papers in Marine Sciences, 35, 1–20.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin-Phenol reagents. Journal of Biological Chemistry, 193(1), 265–275.
Malamis, S., & Katsou, E. (2013). A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. Journal of Hazardous Materials, 252–253, 428–461. https://doi.org/10.1016/j.jhazmat.2013.03.024
Marinho, C. H., Giarratano, E., Esteves, J. L., Narvarte, M. A., & Gil, M. N. (2017). Hazardous metal pollution in a protected coastal area from Northern Patagonia (Argentina). Environmental Science and Pollution Research, 24(7), 6724–6735. https://doi.org/10.1007/s11356-017-8393-y
Martin, J. P. (1950). Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Science, 69(3), 215–232. https://doi.org/10.1097/00010694-195003000-00006
Mondal, M. K. (2010). Removal of Pb(II) from aqueous solution by adsorption using activated tea waste. Korean Journal of Chemical Engineering, 27(1), 144–151. https://doi.org/10.1007/s11814-009-0304-6
Noormohamadi, H. R., Fat’hi, M. R., Ghaedi, M., & Ghezelbash, G. R. (2019). Potentiality of white-rot fungi in biosorption of nickel and cadmium: Modeling optimization and kinetics study. Chemosphere, 216, 124–130. https://doi.org/10.1016/j.chemosphere.2018.10.113
Nunes, N., Ragonezi, C., Gouveia, C. S. S., & Pinheiro de Carvalho, M. A. A. (2021). Review of sewage sludge as a soil amendment in relation to current international guidelines: A heavy metal perspective. Sustainability, 13(4), 2317. https://doi.org/10.3390/su13042317
Ong, S. A., Toorisaka, E., Hirata, M., & Hano, T. (2010). Adsorption and toxicity of heavy metals on activated sludge. ScienceAsia, 36, 204–209. https://doi.org/10.2306/scienceasia1513-1874.2010.36.204
Pagnanelli, F., Mainelli, S., Bornoroni, L., Dionisi, D., & Toro, L. (2009). Mechanisms of heavy-metal removal by activated sludge. Chemosphere, 75, 1028–1034. https://doi.org/10.1016/j.chemosphere.2009.01.043
Peláez, H., Gutiérrez, S., Castro, G., Hernández, A., & Viñas, M. (2001). An integrated anaerobic - physico-chemical treatment concept for wool scouring wastewater. Water Science & Technology, 44(4), 41–47. https://doi.org/10.2166/wst.2001.0173
Pradhan, D., Sukla, L. B., Mishra, B. B., & Devi, N. (2019). Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. Journal of Cleaner Production, 209, 617–629. https://doi.org/10.1016/j.jclepro.2018.10.288
Rathinam, A., Maharshi, B., Janardhanan, S. K., Jonnalagadda, R., & Nair, B. U. (2010). Biosorption of cadmium metal ion from simulated wastewater using Hypnea valentiae biomass: A kinetic and thermodynamic study. Bioresource Technology, 101, 1466–1470. https://doi.org/10.1016/j.biortech.2009.08.008
Reddy, D. H. K., & Lee, S. (2014). Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 454(1), 96–103. https://doi.org/10.1016/j.colsurfa.2014.03.105
Remenárová, L., Pipíška, M., Horník, M., Rozložník, M., Augustín, J., & Lesný, J. (2012). Biosorption of cadmium and zinc by activated sludge from single and binary solutions: Mechanism, equilibrium and experimental design study. Journal of the Taiwan Institute of Chemical Engineers, 43(3), 433–443. https://doi.org/10.1016/j.jtice.2011.12.004
Rulkens, W. (2008). Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy & Fuels, 22, 9–15. https://doi.org/10.1021/ef700267m
Sari, A., & Tuzen, M. (2008). Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials, 160(2–3), 349–355. https://doi.org/10.1016/j.jhazmat.2008.03.005
Sassi, M., Bestani, B., Hadj Said, A., Benderdouche, N., & Guibal, E. (2010). Removal of heavy metal ions from aqueous solutions by a local dairy sludge as a biosorbant. Desalination, 262, 243–250. https://doi.org/10.1016/j.desal.2010.06.022
Scaglia, B., Tambone, F., Corno, L., Orzi, V., Lazzarini, Y., Garuti, G., & Adani, F. (2018). Potential agronomic and environmental properties of thermophilic anaerobically digested municipal sewage sludge measured by an unsupervised and a supervised chemometric approach. Science of the Total Environment, 637–638, 791–802. https://doi.org/10.1016/j.scitotenv.2018.04.426
Simpson, W. S. (2002) Wool production and fibre marketing. In W. S. Simpson & G. H. Crawshaw (Eds.), Wool Science and Technology (pp. 1–20). Cambridge, UK, Woodhead. ISBN 978–1–85573–574–3
Singh, R., & Yadav, Y. (2014). Effluents quality of woolen industrial units and efficiency of wastewater treatment plant at Jorbir, Bikaner, Rajasthan (India). Oriental Journal of Chemistry, 30(1), 49–56. https://doi.org/10.13005/ojc/300106
Starovoytova, D. M., & Namango, S. S. (2014). Wool grease recovery from scouring effluent at textile mill. Journal of Agriculture, Pure and Applied Science and Technology, 10, 1–9. ISSN 2073-8749.
Starovoytova, D. M. (2012) Analysis of liquid effluents from wool processing textile mill. Journal of Agriculture, Pure and Applied Science and Technology, 13, 1–9. ISSN 2073–8749
Torab-Mostaedi, M., Asadollahzadeh, M., Hemmati, A., & Khosravi, A. (2013). Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. Journal of the Taiwan Institute of Chemical Engineers, 44(2), 295–302. https://doi.org/10.1016/j.jtice.2012.11.001
USEPA (2007) Method 3051A: microwave assisted acid digestion of sediments, sludges, soils and oils. Test methods for evaluating solid waste (3rd ed.). US Environmental Protection Agency, Washington, DC, USA.
Verbyla, M., von Sperling, M., & Maiga, Y. (2017) Waste stabilization ponds. In J.B. Rose & B. Jiménez Cisneros (Eds.), Global Water Pathogens Project. http://www.waterpathogens.org/book/waste-stabilization-ponds Michigan State University, E. Lansing, MI, UNESCO. https://doi.org/10.14321/waterpathogens.65.
Wang, G., Zhang, S., Yao, P., Chen, Y., Xu, X., Li, T., & Gong, G. (2015). Removal of Pb(II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arabian Journal of Chemistry, 11, 99–110. https://doi.org/10.1016/j.arabjc.2015.06.011
Wollum, A. G., II. (1982). Cultural methods for soil microorganisms. In A. L. Page, R. H. Miller, & D. R. Keeney (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties (pp. 781–802). American Society of Agronomy.
Zhai, Y., Wei, X., Zeng, G., Zhang, D., & Chu, K. (2004). Study of adsorbent derived from sewage sludge for the removal of Cd2+, Ni2+ in aqueous solutions. Separation and Purification Technology, 38(2), 191–196. https://doi.org/10.1016/j.seppur.2003.11.007
Zhang, X., Zhang, X., & Chen, Z. (2017). Biosorption of Cr(VI) from aqueous solution by biochar derived from the leaf of Leersia hexandra Swartz. Environmental Earth Sciences, 76(2), 67. https://doi.org/10.1007/s12665-016-6336-4
Zhou, Q., Yang, N., Li, Y., Ren, B., Ding, X., Bian, H., & Yao, X. (2020). Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Global Ecology and Conservation, 22, e00925. https://doi.org/10.1016/j.gecco.2020.e00925
Acknowledgements
This work was funded by the National Agency for Science and Technology Promotion of Argentina (ANPCyT) through the grant PICT Nº 2013-2144. Authors would like to thank Dr. Nelda Olivera for microbiological analysis and the anonymous reviewer for the valuable comments.
Funding
This work was funded by the National Agency for Science and Technology Promotion of Argentina (ANPCyT) through the grant PICT Nº 2013–2144.
Author information
Authors and Affiliations
Contributions
Dr. Mauricio Faleschini: Writing-original draft, Formal analysis, Visualization. Dr. Erica Giarratano: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing-review and editing, Funding acquisition. Dr. Mónica Gil: Conceptualization, Methodology, Resources, Writing-review and editing, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent for Publication
All authors declare all data and materials support the published claims and comply with field standards. All authors agree with the content, and all give explicit consent to submit the article, as well as the responsible authorities at the institute where the work has been carried out.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faleschini, M., Giarratano, E. & Gil, M.N. Sorption of Cd(II), Cr(VI), Ni(II), and Pb(II) onto unmodified sludge from a pond treating woolen processing industry wastewater. Water Air Soil Pollut 234, 143 (2023). https://doi.org/10.1007/s11270-023-06166-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06166-2