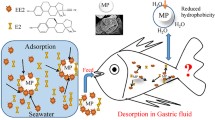
Abstract
Microplastics and endocrine-disrupting chemicals (EDCs) are two classes of emerging contaminants, and their compound combination effects pose a health threat to aquatic life. In this study, five types of microplastics with various characteristics, namely polyamide (PA), thermoplastic polyurethane (TPU), polyvinyl chloride (PVC), polyethylene (PE), and polystyrene (PS), were used to adsorb and desorb bisphenol A (BPA) as a closed loop. The adsorption capacity of BPA changed with the microplastic types following the sequence of PA > TPU > PS > PE > PVC, and the desorption capacity decreased in the order of TPU > PA > PS > PE > PVC. The desorption capacity of BPA on microplastics was positively correlated with the adsorption capacity. The sorption kinetic and sorption isotherms of BPA on five microplastics demonstrated that PA and TPU had the maximum adsorption capacities of 57.27 and 47.20 mg/g, which would be attributed to chemical and physical adsorption (chemical adsorption plays a major role), while the adsorption force of PE, PVC, and PS was mainly determined by physical adsorption. Furthermore, on account of the higher adsorption capacity of BPA on TPU and PA, both of them exhibited a higher desorption quantity compared with the three others. The final desorption capacities of TPU and PA in the three solutions were 0.33 ~ 0.36 mg/g and 0.30 ~ 0.36 mg/g. The desorption rates of BPA on microplastics in gastric and intestinal fluids were higher than those in deionized water, but no significant differences existed between them. This study reveals information crucial for understanding the ecological risks of coexisting microplastics and pollutants in the environment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microplastics are often defined as plastic particles < 5 mm, which originate from primary and secondary sources (Cole et al., 2011; Sun et al., 2022). Owing to their high surface area, strong chemical durability, and mobility, microplastics could absorb and accumulate pollutants from surrounding water, such as carcinogens, estrogens, antibiotics, pesticides, and heavy metals (Abbas et al., 2022; Fok et al., 2020; Yu et al., 2020). The multi-pollutants are released into the environment, causing severe long-term hazards after their accumulation in the aquatic system. When they are accumulated in foods or mistaken by environmental organisms ranging from protozoans to high mammals (Coffin et al., 2019a), those harmful substances might be released into their body, whose metabolism, growth, function, and sexual development could be affected. The nutrient diverts of microplastics indicate that those effects could be extended to food webs and affect organisms at higher trophic levels (Su et al., 2018; Tauqeer et al., 2022). The previous studies show that the desorption rates are faster in intestinal gastric juice compared with those in water, with a furtherly substantial increase under simulating warm-blooded biotic conditions (Coffin et al., 2019a; Razanajatovo et al., 2018). It is suggested that both sorbent and adsorbate have significant influence on the adsorption and desorption behaviors. For example, the properties particle size, surface structure, degree of crystallinity, and abundance of rubbery of microplastic have great effects on the sorption capacity of pollutants (Chen et al., 2019; Li et al., 2018). Besides, microplastic types and the different conditions (e.g., pH, ion concentration, and temperature) may impact the desorption of persistent pollutants from microplastic (Liu et al., 2020; Wu et al., 2020).
Today, the presence of endocrine-disrupting chemicals (EDCs) in the aquatic environment is a growing potential ecological environment problem. Bisphenol A (BPA) is an example of a critical environmental EDC, which has attracted widespread attention, because it is widely distributed in almost all water surroundings, including wastewater, groundwater, and surface water, even drinking water (Mao et al., 2020; Tizaoui et al., 2017). BPA is a monomer used in the manufacture of materials such as polycarbonate plastics, epoxy resins, polysulfone, and aromatic polyester, which might be released during the fragmentation of plastics. Microplastics were demonstrated to be both a sink and a source of BPA in the marine environment (Liu et al., 2019a). In virtue of their physicochemical properties (e.g., solubility [SW] and octanol/water partition coefficient [Kow]), only around 68–87% of BPA is removed by conventional processes in sewage treatment plants (Shehab et al., 2020). The interaction between BPA and microplastics finally forms co-contaminants, which can be transported to aquatic animals and soil animals. In the USA and Europe, regulating bodies have determined that 50 μg/kg/day of BPA exposure is the current tolerable daily intake (TDI) for humans, based largely on rodent multigenerational, subchronic, and oral toxicity studies, measuring endpoints such as body weight and developmental malformations (Rochester, 2013). Coffin et al. (2019a) found that both seabird and fish gut conditions significantly enhanced the biological estrogenicity releasing from plastic products. However, estrogen desorption under simulated conditions of artificial gastroenteric fluid is uncertain. Therefore, an intensive study should be carried out to fully understand the adsorption and desorption behavior of BPA on microplastics under different conditions.
Based on the mentioned discussion above, the objectives of this study are as follows: (1) to determine the adsorption behavior of BPA on five different microplastics: polyethylene (PE), polyvinyl chloride (PVC), polystyrene (PS), polyamide (PA), and thermoplastic polyurethane (TPU); the first four types have recently been found to be the most commonly detected microplastics in the aquatic environment, and the last has been discovered in some important watersheds; (2) to determine the desorption behavior of BPA on five different microplastics under simulated intestinal, gastric fluid conditions and in deionized water; and (3) to expound how the chemical structure and physical properties of microplastics affect the adsorption and desorption ability of BPA in different microplastics. This study delves into the adsorption and desorption behaviors of BPA and microplastics, which could produce novel dates to assess the ecological risks of microplastics in aquatic ecosystem.
2 Materials and Methods
2.1 Materials
The BPA standard sample was obtained from J&K Scientific Ltd. (Peking, China). Its physical and chemical properties are shown in Table 1. Thermoplastic polyurethane (TPU), polyamide (PA), polyvinyl chloride (PVC), polyethylene (PE), and polystyrene (PS) (80 mesh) were purchased as powders from China Petrochemical Corporation Ltd. (Beijing China). The physicochemical properties of the 5 types of microplastics are shown in Table 2. Pepsin A (1:10,000) and trypsin (1:250) were obtained from Guangzhou Saiguo Biotech Ltd. (Guangzhou, China). Methanol and acetonitrile used in high-performance liquid chromatography were purchased from Fisher Company (Fisher Scientific Ltd., Waltham, MA, USA). BPA stock was prepared using MeOH (0.5 g/L) and preserved under dark conditions at 20°C. The BPA aqueous solutions of the different concentrations were obtained by diluting the stock solution with a background solution that contained 200 mg/L NaN3 as bioinhibitor. All microplastics were washed with ethanol and ultrapure water, and then vacuum-dried before weighed. Blank control samples were conducted with the same initial BPA concentration and without MPs to ensure the loss of BPA caused by adsorption of MPs.
2.2 Analytical Methods and Instruments
The high-performance liquid chromatography, with a C18 separator column and UV detector, was used to separate and quantify the target pollutants. The measurement conditions were set as follows: moving phase acetonitrile/water 45/55 (v/v), flow rate of 0.7 mL/min, 10 µL injection volume, 35 ℃ oven temperature, and 205 nm detector wavelength. The retention time for BPA was approximately 9.2 min (Hu et al., 2018). The surface characteristics of microscopic polymers were scrutinized by a scanning electron microscope (SEM) (S-4800, Hitachi, Japan). The BET specific surface area was tested by Micromeritics ASAP 2460 BET, combined with the TriStar 3000 V4.0 data acquisition program. X-ray photoelectron spectroscopy (XPS) analysis was applied to Thermo Scientific K-Alpha. The contact angles were tested by LAUDA Scientific GmbH LSA-100, and the test droplet was an aqueous solution with a volume of 10 μL. In addition, an ultra-pure water apparatus, air bath thermostatic oscillator, vacuum filtration system, oven, and other conventional experimental equipment were used in this research.
2.3 Adsorption and Desorption Experiments
All of the experiments were conducted in duplicate. Batch adsorption experiments were carried out for the following conditions: 0.1 g MPs and 100 mL BPA solution of 4 mg \(\cdot\) L−1 in a glass conical flask. Samples were sealed and oscillated on an air bath thermostatic oscillator (200 rpm at 30℃) with adsorption assessed at 0, 0.08, 0.17, 0.33, 0.5, 1, 2, 3, 4, 6, 12, 18, 24, 36, 48, and 72 h. Adsorption isotherms for BPA and MPs were conducted with the concentration ranges of 0.5 − 14 mg \(\cdot\) L−1 of BPA using batch adsorption approach as described above. All samples were filtered through a 0.22-μm syringe filter to remove microplastics before measured.
The desorption experiments were performed after the adsorption experiments, while the microplastic dosage and BPA solution volume in the adsorption experiment were raised by 2.5 times in comparison to the adsorption kinetics experiment in order to reduce the experimental error. The microplastics were recovered by a vacuum filter after adsorption experiment and the desorption experiments were carried out immediately under the following 3 conditions: (a) deionized condition; (b) simulated digest conditions; (c) simulated intestinal conditions. The conical flask was shaken for 72 h, considering the typical fish intestinal residence time: vegetarian fish 3–10 h, predatory fish 10–158 h (Clements, 1997). According to the body temperature of internationally important commercial fish species, the digest temperature of the vertebrate body was set at 24℃ (Coffin et al., 2019a).
Simulated intestinal juice: Trypsin was added to deionized water for a final concentration of 1 g/L. The pH was adjusted to 8 by dropwise addition of 0.1 M NaOH and HCl (Cui et al., 2022).
Simulated digestive juice: Pepsin A was added to deionized water for a final concentration of 2 g/L. Then, on the basis of the methods standardized by the Association of Official Analytical Chemists and the optimal pH for pepsin activity (Coffin et al., 2019b), the pH was adjusted to 1.5 by adding 1 M HCl and NaOH dropwise.
2.4 Data Analysis
The adsorption capacity of BPA in microplastics at different times (Qt) and equilibrium conditions (Qe) was calculated by Eqs. (1) and (2), respectively:
where Qt (mg·g−1) and Qe (mg·g−1) are the amounts of BPA at any time t (h) and under equilibrium conditions, respectively; C0 (mg·L−1) and Ce (mg·L−1) are initial and counterpoise solution concentrations, respectively; Ct (mg·L−1) is the liquid-phase concentration of BPA at time t; the volume of the solution is V (L); and the quantity of microplastics is m (g).
The data of adsorption kinetics were fitted by a pseudo-first-order model (Eq. (3)), and pseudo-second-order model (Eq. (4)).
where k1 (min−1) and k2 [g·(mg·min)−1] are the first-order and second-order equilibrium rate constants, respectively; t is the contact time (min); Qt (mg·g−1) and Qe (mg·g−1) are the quantity of BPA adsorbed per unit mass of microplastics at time t and equilibrium, respectively.
The adsorption isotherm data of BPA were fitted using the Freundlich, Langmuir, and Dubinin-Radushkevich (D-R) isotherm models. The Freundlich model is given by:
where KF (mg·g−1)/(mg·L−1)n is the Freundlich adsorption coefficient, representing the adsorption capacity; n is the Freundlich isotherm index used to determine the non-linearity. The 1/n value is generally between 0 and 1, indicating the influence of concentration on adsorption. The smaller the 1/n value is, the better the adsorption performance is. The Langmuir isotherm model is expressed as follows:
The maximum adsorption capacity is expressed by Qmax (mg·g−1). KL (L·mg−1) is an equilibrium adsorption constant, which is proportional to the affinity between plastic particles and BPA. When KL is more than 1 or KL is less than 0, it demonstrates that it was difficult to adsorb. The D-R isotherm model is expressed as follows:
Qmax (mg·g−1) is the maximum adsorption capacity, Qe (mg·g−1) is the amount of adsorbate adsorbed per unit mass of adsorbentate equilibrium, KDR (mol2·kJ−2) is a constant related to the adsorption energy, ε (kJ·mol−1) is the adsorption potential based on Polanyi’s potential theory, R (8.314 \(\times\) 103 kJ·mol−1) is a universal gas constant, T (K) is the absolute temperature, Cs (mg·L−1) is the solubility of adsorbates, and Ce (mg·L−1) is the concentration of adsorbate at equilibrium. E0 (kJ \(\bullet\) mol−1) is the adsorption free energy, representing the energy consumption of 1 mol ion transferred from the infinite liquid interface to the adsorbent surface. If the value of E0 is less than 8 kJ \(\bullet\) mol−1, the adsorption process is mainly physical adsorption, while if the value of E0 is between 8 and 16 kJ \(\bullet\) mol−1, the adsorption process is mainly ionic exchange. When the value of E0 is more than 16 kJ \(\bullet\) mol−1, the adsorption process is dominated by chemical adsorption (Agboola & Benson, 2021).
To evaluate the fitness of the models, the correlation coefficient (R2) and the Akaike information criterion correction (AIC) were calculated. The sum of the squared residuals was used to calculate R2, which does not take the number of fitting parameters into account, unlike AIC. The AIC is based on information theory and was used to compare the fit of the different models of sorption to tire materials and their major components based on probabilities and evidence ratio. The desorption capacity of each treatment was evaluated by one-way analysis of variance (ANOVA) (P < 0.05). All statistical tests and model fits were performed using R Studio and Origin software.
3 Results and Discussion
3.1 Characterization of Microplastics
The adsorption behavior of microplastics on pollutants is complicated. The different adsorption characteristics of microplastics are related to their structural properties, hydrophobicity, functional groups, rubbery or glassy phases, particle sizes, and specific surface areas (Liu et al., 2019b). As shown in Table 2, the specific surface area of the five microplastics was 0.39–1.70 m2 g−1, the total pore volume was 0.0005–0.0052 cm3 g−1, and the average pore diameter was 5.02–20.55 nm. The water contact angle was 86.8–129.08°. PE, PA, and PVC were hydrophobic, while PS and TPU had weak hydrophilicity. As shown in Fig. 1, the five microplastic particles varied in morphology. Macroscopically, the surfaces of the PS, TPU, and PE particles were relatively flat, exhibiting numerous tiny folds and anomalous bulges on the surface. The surface of the PVC microplastic was smooth, but several spherical protrusions were observed. The surfaces of PA were extremely uneven, with the morphology of corrugation and laminated structure, which may provide a considerable number of adsorption sites (Li et al., 2018). As mentioned in the literature, the five types of microplastics were thermoplastic microplastics, and their spatial structures were linear or chain structures (Gokel, 2004). At a given environmental temperature, the microplastics were divided into two categories: rubbery plastics (PA, TPU, and PE) and glassy plastics (PVC and PS) (Gokel, 2004). Glassy polymers possess rigid and compact structures, whereas rubbery polymers possess flexible structures and large amounts of cavities between molecules (Chen et al., 2019). The chemical polarity of the five microplastics followed the order of TPU > PA > PVC > PS > PE (Gokel, 2004).
3.2 Adsorption Kinetics
As shown in Fig. 2, the adsorption capacities of the five microplastics to BPA changed with time. After the BPA solution was added to PE, PVC, and PS, it was rapidly adsorbed within 10 min. The adsorption capacity accounted for 52–85% of the initial BPA amount. Then, the adsorption capacity increased slightly in fluctuation, reached its highest adsorption capacity at 24 h, and decreased slightly before finally achieving equilibrium. The adsorption capacity of PA and TPU to BPA increased rapidly in the first 2 h, and then slowed down gradually until the sorption equilibrium was achieved. Combined with the data in Table 3, it can be concluded that the adsorption rates of microplastics to BPA were in the order of PS > PVC > PE > PA > TPU.
Figure 2 and Table 3 show the details of the adsorption kinetics fitted by the pseudo-first-order and pseudo-second-order models. These results imply that the pseudo-first-order model can better fit the adsorption process of BPA on PVC, PS, and PE based on the values of R2 and AIC. The pseudo-first-order model describes when an adsorption process is mainly controlled by physical diffusion in a solid–liquid system (Zhang et al., 2020); this suggests that physical diffusion is the primary adsorption mechanism. The physical interaction forces around the boundary layer of microplastics are Van der Waals forces (such as the interaction force of a dipole), electrostatic forces, and π track interaction force (Seichi et al., 2006). These forces are referred to as reversible adsorption and are easily desorbed when affected by external conditions (e.g., pH, ion concentration, and temperature) (Seichi et al., 2006). PS and BPA have aromatic rings that can offer π-π bond, prompting the PS to capture BPA from water favorably. Studies have shown several possible adsorption mechanisms of BPA on PVC microplastics, including hydrophobic interactions, electrostatic forces, and noncovalent interactions (Wu et al., 2020), while aliphatic polymer PE can offer hydrophobic interactions and non-specific Vander Waal forces.
The simulated adsorption capacities of PA and TPU obtained by the pseudo-second-order model agreed with the experimental values, according to the R2 and AIC values. The pseudo-secondary model assumes that the adsorption capacity is controlled by the chemical adsorption mechanism (Plazinski et al., 2013). This indicated that chemical adsorption played a major role in the adsorption of BPA on TPU and PA, which can be attributed to the specific functional groups (i.e., the amide group of PA and the carbamate group of TPU). Hydrogen bonding can be formed between the proton donor group (i.e., the amide and carbamate groups) and the carbonyl group (the proton acceptor group of BPA). Liu et al., (2019a, b) found that the BPA sorption capacity (Qe) of PA or TPU through H-bond interactions was significantly greater than that of the other MPs (Liu et al., 2019a).
3.3 Adsorption Isotherms
The sorption isotherms of BPA on the microplastics are shown in Fig. 3. The Langmuir, Freundlich, and D-R isotherm models were used to fit the isotherm data (Table 4). The sorption isotherm data of PE, PS, and PVC were well fitted by the Langmuir model, whereas PA and TPU were more suitable for the Freundlich and D-R models. The Langmuir adsorption model postulated by Langmuir describes the sorption relating to homogeneous surfaces, on which the micro-pollutants will express high sorption affinity and form monolayers with specific sorption sites of the MNPs (Agboola & Benson, 2021; Vieira et al., 2021). The results indicated that the partition of BPA between PE, PS, and PVC was monolayer adsorption. Wang et al. (2020) found that the partitioning of BPA between PE, PS, PVC, and the solution dominated the sorption process (Wang et al., 2020). The Freundlich model is an empirical adsorption model that can be used to explore the adsorption or equilibrium data on heterogeneous surfaces, which applies to multilayer sorption (Agboola & Benson, 2021; Vieira et al., 2021; Yang, 1998). The D-R isotherm model is widely used to explain single-solute adsorption and to account for the effect of the porosity of the heterogeneous adsorbent surface (Hu & Zhang, 2019). It indicates that the interaction between BPA and PA/TPU microplastics was multilayer adsorption on nonuniform surfaces affected by physical and chemical sorption processes. In addition, the separation coefficient (KL) of the Langmuir model, the adsorption exponent (n) in the Freundlich model, and the E0 of the D-R model are crucial parameters for describing the adsorption behavior. As shown in Table 4, all values of n were > 1, and all KL values were ≤ 1, indicating that BPA was the preferred adsorption for microplastics (Chen et al., 2019). The E0 value of PE and PS was < 8 kJ/mol, indicating that the adsorption processes were mainly physical adsorption. The E0 value of PVC, PA, and TPU was > 8 kJ/mol, indicating that ionic exchange occurred during the adsorption stages. According to the Qmax value, the sorption capacity of BPA varied with the type of microplastics in the following order: PA > TPU > PS > PE > PVC.
As shown in Table 2 and Fig. 1, the adsorption properties of the five microplastics can be explained by their physicochemical properties. PA and TPU had the greatest adsorption capacity for BPA, which was one order of magnitude higher than that of the other types of microplastics. PA possessed the pore abundance and extremely uneven surface, which may provide the most adsorption sites. It has been learned that compounds with similar polarities have stronger adsorption properties. BPA is a polar compound, which is supposed to have a stronger adsorption capacity for polar microplastics by polar-polar interactions (Li et al., 2018). Polar PA and strongly polar TPU, with polar groups such as carbamate (-NHCOO-), amino (-NH-), and carbonyl (-CO-), will increase the accessibility of BPA on the material surface. Furthermore, H-bond interactions between BPA and PA, TPU were the primary reason for their highest adsorption. Then, the higher adsorption capacity of PS may be due to its benzene ring structure, greater total pore volume, and surface area. In theory, adsorbents with larger specific surface areas and pore volumes accumulate more pollutants (Zhou et al., 2020). PE has the greatest contact angle, which readily adsorbs hydrophobic compounds. Furthermore, the numerous crinkles and hierarchical structures on its surface enabled PE to have a greater number of adsorption sites. It was reported that rubbery plastic (i.e., PE) exhibited a higher adsorption capacity for organic pollutants than glassier plastics (Wang et al., 2015). For the adsorption capacity of PVC, hydrophobic interactions, electrostatic forces, and noncovalent interactions had a positive effect on the adsorption. However, the electrostatic repulsion was inhibited by its ionization in a neutral solution (Wu et al., 2019). Consequently, PVC had a comparatively limited adsorption capacity for BPA. Previous research has shown that PVC had a lower sorption capacity for POPs than other polymers (Agboola & Benson, 2021). In general, chemical factors had a greater influence on the absorption of BPA than physical properties.
3.4 The XPS Characterization of Microplastics Before and After Adsorption
XPS characterization technology can accurately measure the inner electron binding energy and chemical shift of atoms, which can provide molecular structure and valence state information (Bunker, 2010). In this study, XPS characterization technology was used to analyze the elemental content, functional group composition, and chemical valence state of the five microplastics before and after the adsorption of pollutants. The technology can enable a further understanding of the interaction between microplastics and pollutants. The fine spectrum, chemical elements, and functional composition of the five microplastics before and after adsorption are shown in Fig. 4 and Table 5. After adsorption, the O atom and the content of the C-O functional group in PVC increased according to the peak position and its corresponding area, indicating that BPA was adsorbed on PVC. The chemical changes of PS showed that a new functional group, C–OH, was present and the proportion of π-π bonds increased. The results were probably because of the new π-π bonds formed between PS and BPA. The element ratio (C/O) of PE increased, which was related to BPA adsorption. PE had a simple C-H structure, which indicated physical adsorption is the main adsorption mode of BPA. Thus, there was no significant change in the chemical functional groups before and after adsorption. The proportion of the C-N group in PA decreased, while the proportion of the C = O group increased, and a new C-O group appeared. Because the electronegativity of O was stronger than that of N, strong polar C-O and C = O groups were formed through electrostatic forces, which was more conducive to forming intermolecular hydrogen bonds. The proportion of C-O and N–C = O groups in TPU decreased, while C = O groups appeared. TPU has strong intermolecular hydrogen bonds; perhaps, the -OH ground had greater molecular polarity as BPA adsorption existed and it could break the intermolecular hydrogen bonds of TPU and release C = O groups. Moreover, the charge displacement of N–C = O was approximately 1.5 eV to the lower, indicating that the electrons were obtained. This was due to the electronegativity differences between different components, and there may be a strong electron effect between them, which is usually revealed by the binding energy shift in XPS (Wang et al., 2017). In summary, it was clear that PVC, PS, and PE were mainly physically adsorbed, while PA and TPU were chemically adsorbed.
3.5 Desorption of BPA from Microplastics
After the sorption experiment, the final concentrations of BPA sorption onto the TPU, PA, PVC, PE, and PS microplastics for all the replications were 2.43, 2.52, 0.91, 0.95, and 0.77 mg/g, respectively. As shown in Fig. 5, most of the adsorption of BPA on the five microplastics was reversible and quasi-reversible. The desorption processes of BPA on TPU and PA were as follows: fast desorption occurred in the first 8 h, and then slowed down and finally reached equilibration in fluctuations. The final desorption capacities of BPA on TPU and PA in the three solutions were 0.33–0.34 mg/g and 0.29–0.32 mg/g, respectively. The desorption capacity of BPA on PVC, PE, and PS increased gradually with time, and the final desorption capacities of BPA in the three solutions were 0.016–0.021, 0.013–0.023, and 0.022–0.026 mg/g, respectively. The desorption capacity decreased in the order of TPU > PA > PS > PE > PVC. At a confidence level of P < 0.05, there was a significant difference in the desorption rate of TPU and PA between PS, PVC, and PE.
The contaminant process on microplastics consists of desorption from the microplastic surface and the reverse reaction against adsorption, both of which determine the pollutants’ adsorption capacity. The physical interaction forces around the boundary layer of microplastics, such as the Van der Waals forces, electrostatic force, and π track interaction forces, are very weak at approximately 10−5 times the interaction force of hydrogen bonding (Seichi et al., 2006). These forces are referred to as reversible adsorption and are easily desorbed when affected by external conditions (e.g., pH, ion concentration, and temperature). In this study, TPU and PA exhibited the highest desorption rate compared with the other microplastics. The adsorption of BPA on PA and TPU combined chemical and physical adsorption processes affected by multilayer adsorption on heterogeneous surfaces, and the high desorption rate was due to multilayer physical adsorption. Besides, TPU and PA were rubbery polymers, and the high segmental flexibility and random chain orientation of rubbery domains can assist in the release of the adsorbed contaminants (Cui et al., 2022). In accordance with our findings, Zhang et al. (2017) found that the desorption rate of antibiotics on microplastics was positively correlated with the adsorption capacity (Zhang et al., 2017). Because the chemical adsorption capacity on BPA of PA was stronger than that of TPU, its desorption rate contrasted with the adsorption rate. Based on the higher surface adsorption, the interaction forces between BPA and PS were Van der Waals interactions and π-π interactions at the aromatic surface, which are very weak and can be interrupted. The adsorption of BPA on both PE and PVC was physical, and its adsorption on PE was more significant than that on PVC. Because of the reversibility of physical adsorption, the desorption rate of PE was higher than that of PVC. The low desorption rate of PVC can also be attributed to the glassy structure. In water, BPA can be entrapped in the dense and glassy structure of the PVC microplastics, which therefore plays a substantial role in giving rise to the irreversible desorption (Wu et al., 2020). During the adsorption process, the high-energy sites on the surface of microplastics were first occupied by BPA molecules, followed by low-energy sites. Hence, the BPA release from microplastics might occur at low-energy adsorption sites. Oleszczuk et al. (2010) reported that it was tough to desorb drugs from the high-energy adsorption sites of nanotubes (Oleszczuk et al., 2010). The release of BPA from microplastics may be mainly attributed to the electrostatic force and the discrete element method of fine particles in the presence of Van der Waals forces.
Referred to the Chinese Pharmacopoeia, pepsin A and trypsin were used to prepare for artificial gastric juice and artificial intestinal juice, respectively (Cui et al., 2022). As seen from Fig. 6, the average desorption amounts of BPA in deionized water, intestinal juice, and gastric juice were in the order of gastric juice > intestinal juice > deionized water. However, at a confidence level of P < 0.05, there was no significant difference between them. The BPA desorption on microplastics in gastric and intestinal fluids was higher than that in deionized water. The higher stripping ratio might be due to the effective ratio of pepsin A and trypsin components in aqueous solutions, which could dissolve BPA molecules. Furthermore, the hydrophobic residues (isoleucine, methionine, and leucine, etc.) and aromatic residues (histidine, phenylalanine, and tyrosine, etc.) on pepsin and trypsin could adsorb BPA from MPs (Matsuura et al., 2006). The formation of micelles increases the solubility of adsorbed hydrophobic compounds, thereby facilitating their migration from plastic (Bakir et al., 2014). The presence of digestible surfactants and dissolved organics increased the rate of solute diffusion from polymers. Moreover, pH had a substantial effect on desorption rates. Research has found that a lower pH might enhance the desorption of hydrophobic substances from microplastics (Bakir et al., 2014). This indicated that once contaminated microplastics were ingested, the desorption of sorbed contaminants would be greatly enhanced for warm-blooded animals. The increased desorption rates in artificial gastrointestinal fluid revealed that microplastics could be a potential health risk upon ingestion by organisms.
4 Conclusion
The adsorption and desorption behaviors of BPA on five microplastics (TPU, PA, PVC, PS, and PE) were investigated. Our results reveal that the adsorption and desorption capacities vary with the plastic type. The sorption kinetics, sorption isotherms, and desorption experiments of BPA on the five microplastics demonstrated that chemical adsorption plays a major role in the adsorption process of BPA on TPU and PA. The main adsorption mechanism of PVC, PS, and PE is physical adsorption. Because of the higher adsorption capacity of BPA on TPU and PA, their desorption quantities are higher than those of the three other microplastics. The desorption quantities of the five microplastics in the gastric and intestinal fluids are higher than that in deionized water. This indicates that PA and TPU put aquatic ecosystems at a higher risk. Due to the composition effect of microplastics and contaminants in water environments, the desorption mechanism and effects on organisms through estrogenic endocrine disruptor release must be explored in depth to refine the environmental risk of microplastics in the future.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbas, M. A., Iqbal, M., Tauqeer, H. M., Turan, V., & Farhad, M. (2022). Chapter 16 - Microcontaminants in wastewater. In M. Z. Hashmi, S. Wang, & Z. Ahmed (Eds.), Environmental Micropollutants (pp. 315–329). Elsevier.
Agboola, O. D., & Benson, N. U. (2021). Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: A review. Frontiers in Environmental Science, 9, 1–27.
Bakir, A., Rowland, S. J., & Thompson, R. C. (2014). Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environmental Pollution, 185, 16–23.
Bunker, G. (2010). Introduction to XAFS: A practical guide to X-ray absorption fine structure spectroscopy. Cambridge University Press.
Chen, S., Tan, Z., Qi, Y., & Ouyang, C. (2019). Sorption of tri-n-butyl phosphate and tris(2-chloroethyl) phosphate on polyethylene and polyvinyl chloride microplastics in seawater. Marine Pollution Bulletin, 149, 110490.
Clements, K. D. (1997). Fermentation and gastrointestinal microorganisms in fishes. Gastrointestinal Ecosystems and FermentationsIn R. I. Mackie & B. A. White (Eds.), Gastrointestinal microbiology (Vol. 1, pp. 156–198). Springer.
Coffin, S., Huang, G. Y., Lee, I., & Schlenk, D. (2019a). Fish and seabird gut conditions enhance desorption of estrogenic chemicals from commonly-ingested plastic items. Environmental Science and Technology, 53, 4588–4599.
Coffin, S., Lee, I., Gan, J., & Schlenk, D. (2019b). Simulated digestion of polystyrene foam enhances desorption of diethylhexyl phthalate (DEHP) and In vitro estrogenic activity in a size-dependent manner. Environmental Pollution, 246, 452–462.
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Mar Pollut Bull, 62, 2588–2597.
Cui, R., Jong, M. C., You, L., Mao, F., Yao, D., Gin, K. Y., & He, Y. (2022). Size-dependent adsorption of waterborne benzophenone-3 on microplastics and its desorption under simulated gastrointestinal conditions. Chemosphere, 286, 131735.
Fok, L., Lam, T. W. L., Li, H.-X., & Xu, X.-R. (2020). A meta-analysis of methodologies adopted by microplastic studies in China. Science of the Total Environment, 718, 135371.
Gokel, G.W. (2004) Dean’s handbook of organic chemistry 2nd Ed. Mcgraw Hill Handbooks
Hu, Q., & Zhang, Z. (2019). Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. Journal of Molecular Liquids, 277, 646–648.
Hu, Y., Yang, Q., Sun, J., Chen, Q., Fan, J., & Mei, X. (2018). Adsorption and desorption behaviors of four endocrine disrupting chemicals in soils from the water-level fluctuation zone of the Three Gorges Reservoir, China. Sustainability, 10, 2531.
Li, J., Zhang, K., & Zhang, H. (2018). Adsorption of antibiotics on microplastics. Environmental Pollution, 237, 460–467.
Liu, X., Shi, H., Xie, B., Dionysiou, D. D., & Zhao, Y. (2019a). Microplastics as both a sink and a source of bisphenol A in the marine environment. Environmental Science and Technology, 53, 10188–10196.
Liu, X., Xu, J., Zhao, Y., Shi, H., & Huang, C. H. (2019b). Hydrophobic sorption behaviors of 17beta-estradiol on environmental microplastics. Chemosphere, 226, 726–735.
Liu, P., Wu, X., Liu, H., Wang, H., Lu, K., & Gao, S. (2020). Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. Journal of Hazardous Materials, 392, 122346.
Mao, Y., Li, H., Gu, W., Yang, G., Liu, Y., & He, Q. (2020). Distribution and characteristics of microplastics in the Yulin River, China: Role of environmental and spatial factors. Environmental Pollution, 265, 115033.
Matsuura, K., Saito, T., Okazaki, T., Ohshima, S., Yumura, M., & Iijima, S. (2006). Selectivity of water-soluble proteins in single-walled carbon nanotube dispersions. Chemical Physics Letters, 429, 497–502.
Oleszczuk, P., Pan, B., & Xing, B. S. (2010). Adsorption and desorption of oxytetracycline and carbamazepine by multiwalled carbon nanotubes. Environmental Science & Technology, 44, 4830–4830.
Plazinski, W., Dziuba, J., & Rudzinski, W. (2013). Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption, 19, 1055–1064.
Razanajatovo, R. M., Ding, J., Zhang, S., Jiang, H., & Zou, H. (2018). Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Marine Pollution Bulletin, 136, 516–523.
Rochester, J. R. (2013). Bisphenol A and human health: A review of the literature. Reproductive Toxicology, 42, 132–155.
Seichi, K., Tatsuo, I., & Ikuo, Y. (2006). Adsorption science (2nd ed., pp. 134–148). Chemical Industry Press.
Shehab, Z. N., Jamil, N. R., & Aris, A. Z. (2020). Modelling the fate and transport of colloidal particles in association with BPA in river water. Journal of Environmental Management, 274, 111141.
Su, L., Cai, H. W., Kolandhasamy, P., Wu, C. X., Rochman, C. M., & Shi, H. H. (2018). Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environmental Pollution, 234, 347–355.
Sun, J., Zheng, H., Xiang, H., Fan, J., & Jiang, H. (2022). The surface degradation and release of microplastics from plastic films studied by UV radiation and mechanical abrasion. Science of the Total Environment, 838, 156369.
Tauqeer, H. M., Turan, V., Farhad, M., Iqbal, M. (2022) Sustainable agriculture and plant production by virtue of biochar in the era of climate change, Managing Plant Production Under Changing Environment, pp. 21–42
Tizaoui, C., Fredj, S. B., & Monser, L. (2017). Polyamide-6 for the removal and recovery of the estrogenic endocrine disruptors estrone, 17β-estradiol, 17α-ethinylestradiol and the oxidation product 2-hydroxyestradiol in water. Chemical Engineering Journal, 328, 98–105.
Vieira, Y., Lima, E. C., Foletto, E. L., & Dotto, G. L. (2021). Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Science of the Total Environment, 753, 141981.
Wang, F., He, P., Li, Y., Shifa, T. A., Deng, Y., Liu, K., Wang, Q., Wang, F., Wen, Y., Wang, Z., Zhan, X., Sun, L., & He, J. (2017). Interface engineered WxC@WS2 nanostructure for enhanced hydrogen evolution catalysis. Advanced Functional Materials, 27, 1605802.
Wang, F., Shih, K. M., Li, X. Y. (2015). The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere, 119, 841–847.
Wang, T., Yu, C., Chu, Q., Wang, F., Lan, T., & Wang, J. (2020). Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere, 244, 125491.
Wu, P., Cai, Z., Jin, H., & Tang, Y. (2019). Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Science of the Total Environment, 650, 671–678.
Wu, P., Tang, Y., Jin, H., Song, Y., Liu, Y., & Cai, Z. (2020). Consequential fate of bisphenol-attached PVC microplastics in water and simulated intestinal fluids. Environmental Science and Ecotechnology, 2, 100027.
Yang, C. (1998). Statistical mechanical study on the Freundlich isotherm equation. Journal of Colloid and Interface Science, 208, 379–387.
Yu, F., Li, Y., Huang, G., Yang, C., Chen, C., Zhou, T., Zhao, Y., Ma, J. (2020) Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 260.
Zhang, K., Li, J., Li, X., & Zhang, H. (2017). Mechanisms and kinetics of oxytetracycline adsorption-desorption onto microplastics. Environmental Chemistry, 36, 2531–2540.
Zhang, J., Chen, H., He, H., Cheng, X., Ma, T., Hu, J., Yang, S., Li, S., & Zhang, L. (2020). Adsorption behavior and mechanism of 9-nitroanthracene on typical microplastics in aqueous solutions. Chemosphere, 245, 125628.
Zhou, Y., Yang, Y., Liu, G., He, G., & Liu, W. (2020). Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Research, 184, 116209.
Acknowledgements
The authors express special thanks to the Basic Science and Frontier Technology Research Program of Chongqing Science and Technology Commission for providing experimental conditions, and the financial support from Basic Science and Frontier Technology Research Program of Chongqing Science and Technology Commission, and Chongqing 2021 Urban Management Scientific Science Project.
Funding
Access funding is provided by the Basic Science and Frontier Technology Research Program of Chongqing Science and Technology Commission (Grant No. cstc2018jcyjAX0322), the Primary and Secondary School Innovative Talent Training Project of Chongqing (CY210704), and the Chongqing 2021 Urban Management Scientific Science Project (CGKZ2021-04).
Author information
Authors and Affiliations
Contributions
Jiang Hui: made substantial contributions to the conception or design of the work, contributed to the revision and translation of manuscripts.
Li Qiao-Ying: participated in experiments and data analysis, first draft writing, and translation.
Yu-feng Mao: contributed significantly to analysis and manuscript preparation.
Xin Liu: performed the data analyses and wrote the manuscript.
Sisi Que, Weiwei Yu: helped perform the analysis with constructive discussions.
Yi-sen Kan: participated in experiments and data analysis, and manuscript submission.
Corresponding author
Ethics declarations
Ethics Approval
We would like to state that the study does not involve human subjects.
Consent to Participate
The authors declare that the study does not involve human materials.
Consent for Publication
All authors have consented for the manuscript content for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Five types of microplastics were used to adsorb and desorb bisphenol A (BPA) as a closed loop to research the ecological risks.
• Sorption/desorption capacities differed from the physical and chemical properties of microplastics.
• Hyclone and pepsin A were used to modify the gastrointestinal conditions.
• Desorption of BPA from five microplastics was enhanced in simulated gastrointestinal conditions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, H., Li, Qy., Sun, Jx. et al. The Adsorption and Desorption Behavior of Bisphenol A on Five Microplastics Under Simulated Gastrointestinal Conditions. Water Air Soil Pollut 234, 106 (2023). https://doi.org/10.1007/s11270-023-06105-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06105-1