Abstract
The environment has undergone significant change because of technological advancement. Industries are releasing pollutants directly into the environment. Water pollution is a growing issue for humanity. Methods for managing wastewater generated by biological and industrial wastes are being developed. Textile industries are risking the health of living beings by contaminating water with dyes. Azo dyes are the major constituent of wastewater from textile industries. This review article focuses on the photodegradation of Congo red, the most prominent Azo dye. For Congo red degradation, both biological (via microorganisms) and chemical (via nanoparticles) methods are being investigated. The biological method primarily employs bacterial and fungal species. Bacterial species such as Bacillus sp., Pseudomonas sp., and Staphylococcus lentus sp. efficiently degrade Congo red dye. The presence of functional groups on the cell wall of fungi, such as phosphates and hydroxyl, promotes efficient dye degradation. The use of nanoparticles for photodegradation of dyes is preferable because it does not result in polycyclic compounds after degradation. Many bimetallic catalysts, such as ZnO and TiO2, have shown promising photocatalytic properties due to their large band gap. The use of nanoparticles that can be easily separated after photodegradation is preferred. As Gd3+ doped cobalt ferrite nanoparticles have higher removed capabilities than undoped cobalt ferrite nanoparticles, doping improves the degrading capability of nanocatalysts.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The world’s growing population is constantly interacting with and interfering with natural resources in order to improve living standards (Khan et al., 2018). Water is a valuable natural resource that is significantly affected by anthropogenic activities (Nandhini et al., 2019). No one can deny the importance of colors, so humans use a large number of colored substances, either natural pigments or manmade dyes, to add color to fabrics (Blaszczyk, 2019), food, leather, and paper. Natural substances are obtained from plants and algae. The use of natural pigments as colorants for dyeing on a commercial scale has been limited due to their less thermo-stability, photo-stability (Konieczkowska et al., 2015), difficulties in obtaining them from biota, and their less capability to remain fixed with the fabrics after periodic washing (Maddhinni et al., 2013; Simon et al., 2017). To fulfill the requirement of these dyes, chemically synthesized dyes are common in the food, paper, and textile industries nowadays. Synthetic dyes are complex aromatic, poorly degraded colored compounds, mainly formed by using phenol and benzene (from petroleum) as starting materials (Mahapatra, 2016). There are different types of dyes; the most synthesized and used are azo dyes. Azo dyes contain –N = N– bonding and aromatics or heterocyclic compounds with recalcitrant structures (Khan et al., 2018; Tsuboy et al., 2007). The use of these compounds on a large scale and theoretical dumping of those color-loaded effluents without their degradation, into various natural compartments, antagonistically influences the harmony of these environmental frameworks (Khan et al., 2018). These dyes have the potential to absorb sunlight and reduce the photosynthetic activities of the aquatic flora (autotrophs) (Bianco Prevot et al., 2001). Their presence reduces the oxygen content in the water, thus causing the death of aerobes and ultimately disturbing the natural structure of freshwater bodies (Abe et al., 2017; Bianco Prevot et al., 2001; Zaini et al., 2014). Some of them are very toxic (Natarajan et al., 2011) and even cause a mutation in the DNA of aquatic life forms that leads to a malignant tumor formation in the brain (Fairuzi et al., 2018). Being genotoxic, mutagenic, and cancer-causing, these colors are very harmful to the soundness of biota (either oceanic or earthbound) (Kaushik & Malik, 2009b; Zaini et al., 2014). Textile industries are considered a big source of these contaminants as a huge amount of water used in the dying process. About 100 L of water is required for imparting color to 1 kg of pure cotton (Khan et al., 2016; Steingruber, 2000). A lot of these colors are impervious to debasement and remediation under normal conditions and through traditional strategies. These conditions have required the improvements of successful and effective wastewater treatment methodologies without disturbing nature and other living things (Krishnakumar et al., 2011; Rai et al., 2014). The removal of these water contaminants has gained much more attention over the last two decades. The purpose of this review is to assist researchers dealing with the degradation of Congo red (CR) dye by (i) assembling useful data on bacterial and fungal degradation; (ii) summarizing data on photodegradation of CR by nanoparticles (NPs); and (iii) discussing reaction mechanisms and intermediate products formed during degradation. Several reviews have been published that explain various methods for degrading dyes in textile wastewater. This review discusses the detailed mechanisms of biological degradation of CR, with a focus on bacterial and fungal degradation, as well as their shortcomings. A better method, photodegradation with nanocatalysts, has been thoroughly described, along with proposed mechanisms for efficiently removing CR from textile wastewater.
1.1 Origin of Dyes as Textile Colorants
Egyptians are considered the pioneers of fabric dyeing procedures. They are used to extract the colored pigments from naturally present entities. Chemical testing of several findings, for example, the red fabric from the ancient tomb of Egyptian King Tutankhamen, confirmed the existence of textile colorants (Yusuf et al., 2017). In the past, people used locally available colors for dyeing textile materials. These colorants were obtained from the plant’s berries, fruits, leaves, roots, stem, or animal skin and minerals with little processing. But the applicability of natural pigments was limited on a commercial scale due to their less photo-stability, poor fixation with the material being colored, and fadedness over time. Thus, there arose a need to synthesize dyes artificially from petroleum products. William Henry Perkin (1856) discovered the first synthetic dye (Mauveine) in an unanticipated effort (Couto, 2009; Travis & Culture, 1990). This discovery urged scientists to synthesize dyes and test their applicability on a massive scale. Discovery of Mauveine also paved the path for immunologists and chemotherapists to test the effects of dyes on healthy as well as malfunctioned cells. Paul Ehrlich (1891) found that certain cells (mostly cancerous) took up a specific dose of dyes that started their death without causing damage to healthy cells (Parascandola, 1981). The reason for dyes to be bright in colors is their absorbance in the visible region because those compounds appeared colored which absorbed radiation in the visible region (400–800 nm). Colored compounds contain specific functional groups (chromophores, auxochromes) that absorb a particular photon of light with a characteristic wavelength and thus give a specific color to each compound. According to Witt’s theory, auxochrome groups act as electron donors and play role in dyeing the materials while chromophore groups act as electron acceptors. This theory was further worked on by two other scientists who stated that both the auxochromes and chromophores are linked through a conjugated system. They formulated a base for a new chromogen system (Fairuzi et al., 2018; Gürses et al., 2016). According to electronic structure theory, the colors of dyes are due to excitations of delocalized electrons (Schaefer III et al., 1995).
1.2 Recalcitrant Substances
Substances that possess complex structures, resistant to natural degradation, remain in the ecosystem for longer durations, and are transported from one geological location to another are termed as recalcitrant substances or pollutants (Singh et al., 2015). Most recalcitrant compounds in our environment are the results of oil, polymer, pharmaceutical, and textile industries (Zhang et al., 2019c). They are still present in our environment due to the inefficient treatment of wastewater treatment plants (Duan et al., 2018; Yarahmadi et al., 2018). Biological processes are still used for the removal of such substances from wastewater, but the process is relatively slower than modern technologies, such as photocatalysis, implied for wastewater treatment (Crini & Lichtfouse, 2019a; Zhang et al., 2018, 2019a, b).
1.2.1 Textile Dyes as Recalcitrant Substances
The molecular sizes of dyes are usually large due to the presence of bigger aromatic rings and heavy substitutions; as a result, dyes resist natural degradation and pose serious harm to the environment (Kaushik & Malik, 2009b). In industries, structures of synthetic dyes are modified by incorporating certain biological, chemical, and physical agents. This enhances their photo-stability as well as their ability to resist fading over a long time (Shabir et al., 2017). The presence of these agents makes dyes defiant towards degradation. Synthetic dyes containing chromophores and auxochromes are highly reactive. When discharged into a natural environment, they react with several pollutants, for example, heavy metals and halogens, and form even more carcinogenic substances. Recalcitrant substances contribute significantly to the reduction of oxygen in water bodies, resulting in eutrophication; as a result, the availability of oxygen to aquatic biota decreases, limiting photosynthetic activities in these bodies and disrupting the food chain. (Jorfi et al., 2018; Kaushik & Malik, 2009b). Moreover, when such toxic entities enter the living bodies, they adversely affect the DNA of the cells as they tend to bind with the proteins. Many dyes have been proved to cause severe diseases such as cancer (from azo dyes). Recalcitrant substances equally affect aquatic and plant life. The synthetic color from the dyes also inhibits the natural photosynthetic procedure of aquatic plants (Mohapatra et al., 2020). For the removal of pollutants from the environment, various methods are being used over the past few years. Advanced oxidation processes (AOPs) used ozone gas along with hydrogen peroxide or ultraviolet light, H2O2/ultraviolet radiation, the photo-Fenton process, and the Fenton process for the removal of recalcitrant structures or to convert them into less harmful more biodegradable substances (Feuzer-Matos et al., 2021; Tahir et al., 2016). The most common dye present in the wastewaters is an azo dye.
1.2.2 Azo Dyes
Almost 9 lac tons of dyes are discharged into the aquatic environment annually, of which 70% are azo dyes (Balapure et al., 2015; Selvaraj et al., 2021). Azo dyes have been identified as known carcinogens for the human urinary bladder (Sen et al., 2016a). They are also known to cause liver and spleen cancers in humans (Solís et al., 2012a). More than 3000 azo dyes are being manufactured annually all over the world. Like many other dyes, azo dyes are also responsible to change the natural factors of water bodies such as color, oxygen demands, and pH. (Selvaraj et al., 2021). Common azo dyes and their applications are described below in Table 1.
1.2.3 Congo Red (CR)
Congo red (CR) is an important azo dye as it is used for dyeing of the cotton fabrics. They are known carcinogens (Selvaraj et al., 2021). The CR is an important sulfonated substituted benzidine diazo dye (Olivo-Alanis et al., 2018), which is considered a more recalcitrant compound than other mono and diazo dyes present in untreated wastewater. It can be used in leather, paper, plastic, printing, and textile industries as a coloring agent. Furthermore, its use as a pH indicator for the diagnosis of amyloidosis (Khurana et al., 2001; Puchtler et al., 1962) and in the detection of free HCl (hydrochloric acid) in gastric juice was reported (Shu et al., 2015). Due to its resistance to natural degradation, it causes spoilation of water bodies and the landmass (Dubé et al., 2008). UV–visible spectra of CR showed three distinct bands (Fig. 1) at 498 nm, 347 nm, and 235 nm attributed to the chromophore group, naphthalene, and benzoic ring respectively (Movahedi et al., 2009a). Table 2 describes some important features of CR.
2 Methods for the Degradation of Dyes
The removal of colored substances from the wastewater by conventional methods, such as adsorption (Banerjee et al., 2019), AOPs (Kanakaraju et al., 2018; Kraft et al., 2003; Tröster et al., 2002), coagulation (Hamoud et al., 2017; Sohrabi et al., 2017), Fenton process (Cruz-Rizo et al., 2017; Sohrabi et al., 2017), flocculation (Beluci et al., 2019; Sohrabi et al., 2017), ozonation (Mahmoodi, 2016; Manivel et al., 2015; Tapalad et al., 2008; Wu et al., 2015), and ultra-filtration (Korenak et al., 2018) (as shown in Fig. 2), was common in the last few decades, but all these methods were proved insufficient for the complete removal or transformation of toxic substances into eco-friendly substances because of high expense and high energy requirements (Chao et al., 2018). The application of such methods is thus prohibited due to additional energy requirements, high costs as compared to modern processes (Ekambaram et al., 2018), problems associated with the operation, production of a lot of sludge, and unsafe disposal, which can act as a secondary pollutant.
Many researchers have explored different methods, bioremediation (Mahmoud et al., 2017), phytoremediation (Ekambaram et al., 2018; Saraswathi et al., 2017), and degradation by using NPs (Lamba et al., 2015; Mahdiani et al., 2018; Qu et al., 2018) for the manipulation of dye-laden (Fig. 3). All these methods have their pros and cons.
2.1 Biological Methods for the Degradation of Dyes
AOPs require additional energy and space, high costs, and advanced instruments, for the treatment of wastewater pollutants as compared to photocatalytic degradation. They are also less efficient in terms of generating huge quantities of sludge, and the transformation of colorants into more harmful secondary pollutants (Pandey et al., 2007). The AOP has yet been successful in the past years in the mitigation of harmful impacts of dyes. AOPs bring physical changes in the chemical structure of pollutants (Alderete et al., 2021). On the other hand, biological methods are considered environment-friendly as they perform complete or nearly complete degradation of toxic effluents (Solís et al., 2012b). Microorganisms possessed the metabolic potential for the degradation of pollutants or xenobiotic compounds, due to the diversity of lysosomal enzymes (approximately 40 different types of enzymes, such as acid phosphatases, lipases, sulfatases). These enzymes are present in many species of algae, bacteria, fungi, and plants. Microbial decoloration by bacteria is considered an efficient method (Nigam et al., 1996). Bacteria that degrade synthetic pigments can easily be obtained from animal and human excreta, food, soil, and water contaminated with microbes. The degradation of dyes by using living organisms is a vital research area in environmental biology. The success of biological methods in treating effluents is basically due to the ability of biota to acclimatize themselves for toxic substances and development of resistance against these substances; as a result, they utilized toxic compounds and convert them into the more stable, less toxic form (Carliell et al., 1996). In the microbial degradation of dyes, there are two ways to convert dyes into less toxic substances: first is the adsorption of dyes onto the biomass of microbes, and second is the degradation of dyes intracellularly (Carliell et al., 1996). The fungal degradation of pollutants is associated with the extensive extracellular enzyme system (Lai et al., 2017). Among different cultures of fungi, white-rot fungi are considered best for degradation. Many species of marine algae were also investigated for their abilities to degrade pollutants. Phytoremediation is also another technique to handle pollutants (Saraswathi et al., 2017). Both of these techniques have their advantages as well as limitations; however, all these work efficiently only at optimum conditions like concentration of dye, energy sources for microbes, humidity, pH, structure of dye, temperature, and oxygen content available to biota (specific in discoloration of dyes) (Singh et al., 2012).
2.1.1 Bacterial Degradation
Our environment is largely dependent upon the nutrient cycles taking place in our surroundings involving bacteria at various steps. Still, several areas, which may prove helpful in innovative ways, are yet to be explored or are on the verge of recognition by scientists (Telke et al., 2010b). Bacteria initiate the degradation of dyes by cleaving azo bond/s either in the presence or absence of oxygen due to the action of their enzymes as shown in Fig. 4. The culture of Bacillus subtilis was first isolated and reported as the dye decolorizing in the 1970s (Horitsu et al., 1977). Isolation of dye decolorizing cultures of bacteria seemed to be a difficult task. Many aerobic and anaerobic bacterial strains have been found to decolorize the azo and other nitrogenous dyes efficiently. Azoreductases and laccases are two major families of enzymes present in all these strains that possessed the potential to metabolize many industrial dyes. Azoreductases need NADPH, FADH, and NADH as reducing equivalents to reduce the dyes completely into colorless amines (Sarkar et al., 2017). Many species of bacteria, yeast, filamentous fungi, and plants have been reported that contain genes in their DNA for coding oxidative enzymes like lignin peroxidase and manganese peroxidase, capable to degrade dyes. Fungal cultures have proved their rapid efficiency in the degradation of dyes because they possess many ligninolytic enzymes. Fungi may perform the decoration by the adsorption process or enzymatic degradation or it may occur as a collective procedure of both (Sen et al., 2016b). Instead of individual bacterial cultures which are rather time-consuming to extract, mixed bacterial cultures are preferred but the results are not as promising as they would be with the individual cultures of aerobic, anaerobic, and anoxic bacterial conditions (Jamee et al., 2019).
2.1.2 Fungal Degradation
Fungi have also been used for biodegradation purposes. Their application in the decolorization of wastewater is numerous (Blais, 2006; Kaushik & Malik, 2009a). Fungi tend to degrade textile dyes by secreting extracellular enzymes oxidases and reductases as shown in Fig. 5. This is a factor that provides an advantage of fungal degradation over bacterial degradation. The large size of fungi as compared to bacteria also favors fungal degradation (Oliveira et al., 2007). Sometimes, enzymes are isolated from the fungi and used independently (Cristóvão et al., 2009). Moreover, fungi are capable of adapting themselves according to the environment unlike most bacteria (Ahmad et al., 2015; Rahimnejad et al., 2015). Some of the most important factors that control the degradation of textile dyes via fungi include temperature, pH, pretreatment, and the presence of some nutrient compounds (Arıca & Bayramoğlu, 2007; Kapdan & Kargi, 2002; Neoh et al., 2015). Spores of fungi have also been reported to remove pollutants from water (Hoque & Fritscher, 2019; Li et al., 2019).
2.2 Photodegradation
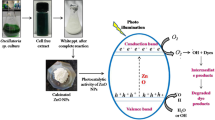
Photocatalytic degradation of dyes has several advantages over other methods. They involve the use of heterogeneous semiconductors that upon absorption of light degrade the toxic dyes (Rafiq et al., 2021). The photodegradation of dyes is controlled by several parameters including dye concentration, pH, and temperature (Ikram et al., 2020). Comparative to biological and other conventional methods which require a certain type of pretreatments to be done before treating organic pollutants (Fernández et al., 2010), photocatalysis may be a better choice. Photocatalytic degradation involving heterogeneous catalysts proves to be an efficient way to treat persistent organic pollutants (Singh et al., 2016). Photocatalysis possesses the ability to degrade poisonous organic pollutant into their least harmful form for example carbon dioxide using some sort of nanocatalyst (Meephon et al., 2019); photocatalysts on absorbing UV or visible light (depending upon their nature) generate high energy photons which further reduce the organic pollutants (Aslam et al., 2022). Photocatalysts generally involve semiconductor catalysts; they may be monometallic (consisting of one atom), bimetallic (consisting of two atoms), or tri-metallic (consisting of three atoms) (Rajendran et al., 2021a). To improve the efficiency of a photocatalyst, charge carriers are increased. The more the charge carriers are, the more efficient is the degradation. To improve the degradation capacity, semiconductors are often doped with other metals (Terna et al., 2021a). To understand the principle of photodegradation, we first need to understand the structure of a semiconductor. Semiconductor consists of two bands: a conduction band (CB) and a valence band (VB). There is a gap between these two bands, as shown in Fig. 6, called the band gap. When electrons present in the VB absorb energy equal to or greater than the band gap, they are promoted to the CB generating a hole (h+) in the VB and an electron (eˉ) in CB. These two, a hole (h+) and an excited electron (eˉ), are the main cause behind photocatalysis. Photo-generated eˉ in the CB and h+ in the VB act as oxidizing and reducing agents respectively (Bhat et al., 2020a; Pattnaik et al., 2018a; Vidya et al., 2017a). The efficiency of photocatalysts can be improved by various methods including doping and metallization. They tend to increase the charge separation of photocatalysts (Arkhipov et al., 2005a).
3 Degradation of Congo Red by Bacteria
Microorganisms could be acclimatized by providing different nutrients initially, and then exposing them to different concentrations of dyes (ranging from low to high). As soon as microorganisms become acclimatized, they start using dyes as their energy source as a result of recalcitrant substances degraded (Gopinath et al., 2009). The degradation of dyes by bacterial culture in the presence of sufficient nutrients could produce colorless intermediates and final products which may prove even more toxic than parent compounds. Thus, decoloration of dyes cannot be considered a single parameter to interpret percentage degradation. Proposed mechanism of biodegradation of CR is shown in Fig. 7.
The proposed mechanism of degradation of CR by microbes (Telke et al., 2010a)
K. P. Gopinath et al. reported nutrient-free degradation of CR by a Bacillus sp. Dye samples were pretreated with sonication and results were compared with non-pretreated samples. Sonication of dye samples with low-energy ultrasound created oxidants by the homolytic division of bonds present in water containing dye. Bacillus sp. decolorized a non-pretreated 100–300 mgL−1 sample of CR within 24–27 h of incubation, giving 100% degradation efficacy, 97% reduction in COD, at 7 pH, and 37 °C temperature. While with pretreated samples the incubation period was reduced to 6–7 h, in such case, the other parameters remained the same (Gopinath et al., 2009). One of the important findings by Amar et al. showed 97% degradation of 100 mgL−1 CR by a bacterium Pseudomonas sp. Su-EBT in 12 h with a 50% decrease in COD at 8 pH and 40 °C temperature (Telke et al., 2010b). It was reported that the degradation rate is maximum in static conditions although the bacterial growth rate is more in shaking conditions (Telke et al., 2009). The slow rate with less degradation efficacy in shaking conditions was attributed to the suppressed activity of laccase (one of the oxidases responsible for degradation). The time for degradation increased as the concentration of CR dye increased and the maximum degradation of CR is 97% within 60 h at a 1gL−1 concentration (the maximum tolerable amount of CR by Pseudomonas sp. Su-EBT) (Telke et al., 2010b). Dyes with fewer sulfonate groups decolorized rapidly than dyes with more sulfonate groups (Pearce et al., 2003). The degradation of dyes by bacteria is attributed to the adsorption of dyes to microbial cells. The degradation of CR has been described by Kamel Cheib et al. using a bacterium, Staphylococcus lentus. S. lentus decolorized a 100mgL−1 of CR (97.3% degradation efficacy) when incubated for 24 h, in the presence of medium containing mineral salts, yeast extract 0.10% w/v, and glucose (7 mM) at 7.2 pH and 37 °C temperature (Chaieb et al., 2016). The inoculum size used 2.2 × 106 colony-forming units mL−1 of liquid, in shaking conditions, contradictory to the results reported by Amar et al. The FT-IR spectrum of degraded CR confirmed the degradation as the position of peaks was found different than the non-degraded solution of CR. Moreover, phototoxicity test showed that the non-degraded solution of CR inhibits the germination of plumule and radicle of Triticum aestivum and sorghum bicolor, while degraded CR increases germination efficacy (Chaieb et al., 2016). The degradation of dyes in nutrient-rich medium containing microbial cells in static as well as shaking conditions results in loss of biomass (Bedekar et al., 2014; Girish, 2019). Alternatively, bioreactors (continuous or batch) are used for the degradation of dyes in which either a pure culture of bacteria or consortia of bacteria is used. Single cultures of bacteria mineralized only a few dyes specific to them, but are unable to mineralize higher molecular weight substituted, versatile dyes (Bedekar et al., 2014). In addition, consortia of bacteria were found more resistant towards diverse changing conditions and parameters such as concentration of dyes, pH, and temperature (Jalandoni-Buan et al., 2015). In such cases, degraded products of a dye by one kind of bacteria might act as nutrients for other kinds of bacteria, resulting in a maximum reduction in toxicity (Lade et al., 2015). In bioreactors, microbial cultures are immobilized on a carrier such as polyurethane, which efficiently holds the culture fixed and enhances the chances to reuse the same culture (Lade et al., 2015). A biocatalyst laccase has shown some promising results in wastewater treatment (mechanism shown in Fig. 8). But it only works if it has been immobilized and sustained its pH and thermal stability. Georgieva et al. reported that immobilization of laccase over the membrane of polypropylene enhanced the catalytic performance to a great extent (Zhou et al., 2021). Table 3 describes the biodegradation of CR by different bacteria at different conditions.
The proposed mechanism for the degradation of CR by laccase of Dietzia sp. (Babu et al., 2015a)
4 The Degradation of Congo Red by Fungi
Due to the extensive and efficient extracellular enzyme system of fungal culture, mycoremediation of industrial wastewater has received a lot of attention (Pundalik et al., (n.d); Noman et al., 2020). Different fungal species and their degradation efficiencies have been shown in Table 4. Dye degradation by fungal mycelia is associated with the adsorption of dye to the cell wall of fungi (Chakraborty et al., 2013). Functional groups present in the cell walls of fungi help the dyes to bind through electrostatic forces of interactions (Skanda et al., 2021). Reactive functional groups such as carboxyl, hydroxyl, and phosphate are present in hetero-polysaccharide and a hydrophilic lipid portion of the fungal cell wall is responsible for attractive forces, leading to biosorption of dye solution with the cell wall; thus, the color intensity of dye is reduced, indicating conversion of dye into less toxic colorless metabolites (Chakraborty et al., 2013; Singh et al., 2020). In mycoremediation of industrial effluents, several parameters like aeration, agitation speed, fungal species used, initial dye concentration, incubation time, nutrition sources, pH, presence of heavy metals, and temperature all affect degradation rates. Different substrates have been used by many researchers, to check the effect of nutrition sources on the degradation rate. Inorganic media containing glucose were reported as the best carbon source for fungal growth and maximum decoloration efficacy of CR. Shaking speed plays an important role in controlling the degradation rate (Asses et al., 2018). The degradation of azo dyes is greatly enhanced if the nutrient substrate contains carbon and nitrogen. Carbon and nitrogen act as the donors of electrons which are then accepted by the azo dyes which help in the degradation of dyes by the generation of reducing equivalents (Kapoor et al., 2021). Shaking promotes the growth of fungal culture by increasing the mixing of oxygen with biomass; as a result, degradation efficacy of dyes increases. Shaking speed from 0 to 250 rpm was reported by different authors. Aspergillus sp. showed the maximum degradation rate at an optimum shaking speed of 150 rpm for the degradation of CR; it was attributed to the maximum enzymatic activity of manganese peroxidase (MnP) and lignin peroxidase (LiP) (Asses et al., 2018). Optimum temperature is another factor that contributes to degradation. It was reported that extracellular enzymes of fungi like MnP and LiP showed maximum activities in temperature ranges between 25 and 35 °C (Parshetti et al., 2007). Enzymes are sensitive to high and low temperatures resulting in denaturation and inactivation, respectively. As the initial concentration of dye increased, the rate of degradation decreased; this could be attributed to the saturation of active sites of enzymes. Catalytic proteins are found sensitive to pH; thus, most of them work in a narrow range of pH (5–8) due to their structural stability in acidic conditions (Iark et al., 2019).
5 Biological Methods and Their Drawbacks
Microorganisms and adsorption/sorption techniques are the most commonly used biological methods for dye removal (Bal & Thakur, 2021). Although biological methods are often regarded as environmentally friendly, they did not always meet our expectations when it came to wastewater treatment. The use of enzymes, for example, is not always successful because the process requires optimal conditions to be met and they do not completely remove the dyes from wastewater. Even minor changes in the conditions or parameters result in a complete loss of enzyme activity (Bento et al., 2020; Carolin et al., 2021). Biodegradation is also sensitive to certain experimental conditions such as accumulation of ammonia inside the reactor (Garcia et al., 2020). Biosorption also causes biomass accumulation, which prevents the optimum process from establishing (M-Ridha et al. 2020; Sosa-Martínez et al., 2020). Another factor that contributes towards the ineffectiveness of biological and other conventional treatment methodologies is the chemical instability in the different organic mediums (Ali, 2010). When azo dyes were treated with bacterial cultures, they frequently produced toxic amines, resulting in secondary pollution (Brüschweiler & Merlot, 2017). Table 5 describes some advantages and disadvantages of photodegradation and biodegradation.
6 Nanoparticles as a Preferred Mode for Degrading
Nano-photocatalysts have long been used in wastewater treatment. Several nanocatalysts have demonstrated outstanding performance in degrading textile dyes from water. The most important feature of nanomaterials is their use of energy-efficient pathways. Furthermore, several structural changes enable greater light absorption and less recombination (Molinari et al., 2020). According to Ch-Th et al., 2021, the techniques used for the modifications are nanoscale designing and doping (Ch-Th et al., 2021). Tofa et al., 2019) and Uheida et al., 2021) degraded LDPE and microplastics using zinc nanorods with improved visible light photocatalytic efficiency modified from ZnO seed layers (Tofa et al., 2019; Uheida et al., 2021). Several studies show that nano-photocatalysts are more efficient than biological methods. Green synthesis allows nanoparticles to be synthesized with less environmental impact and provides nanoparticles with unique properties such as nontoxicity, high stability, and cost effectiveness (Chauhan et al., 2020). Biosynthesized ZnO nanoparticles demonstrated excellent photodegradation activities towards dyes such as CR (Weldegebrieal, 2020) and other organic compounds (Chauhan et al., 2020). Furthermore, we can summarize the advantages of photodegradation as follows (Khan et al., 2020b).
-
Use of a nanocatalyst limits the need of pre- and post-treatments of the organic compounds to be degraded.
-
Nanocatalysts are liable to recovery and can be reused.
-
High chemical stability in various organic environments.
-
No external arrangements are required to carry on photodegradation such as temperature and pressure.
-
Variety of organic compounds can be degraded by nanocatalysts.
7 Photodegradation of Congo Red by Nanoparticles
One of the important green chemistry technologies is the photodegradation of organic dyes. Photocatalysts (PCs) are required for the degradation of dye. In photocatalysis, dyes are degraded under the influence of UV–visible radiations. This method is beneficial as compared to the conventional methods of pigment degradation because it produces no polycyclic products (Nagajyothi et al., 2020). Photodegradation by nanoparticles (NPs) bears many advantages; the most important is the complete degradation of dyes (Banerjee et al., 2019). Due to the active sites, catalytic potential, high surface area, high chemical stability, low cost, nontoxicity, and small size (Gunjakar et al., 2011), nanomaterials have proved themselves as efficient adsorbents (Raval et al., 2016). Photocatalytic degradation happens by using heterogeneous catalysts such as ZnO, Fe2O3, TiO2, CdS, and ZnS under the influence of solar energy (Merah, 2020). ZnO NPs exhibit worthwhile properties regarding the degradation of organic dyes due to their wide band gap ranges and act as a promising semiconductor in photocatalytic activity (Nguyen et al., 2021). ZnO semiconductor falls under the category of N-type, and when it is irradiated with solar light and UV radiations, it produces electrons and holes which proves to be very helpful in the degradation of dyes completely (Debnath et al., 2020). NiO NPs are extremely efficient in dye degradation activity because of the super antioxidant properties that they are going to be used to provide clean water for drinking purposes without any pollutants and dyes present in the industrial wastewater (Bhat et al., 2020b). The concentration of the catalyst NiO has directly affected the antioxidant activity which in turn enhances the reduction of the dyes (Khan et al., 2021b). Antioxidant activity is the free radical scavenging activity that corresponds to the antioxidant potential due to the negatively charged NP surfaces. These free radicals then attract the hydroxyl ions of the dye (Bhat et al., 2020b). TiO2 possesses promising photocatalytic activity, it exists in three types of modifications, and studies illustrate that brookite and rutile perform the photocatalytic activity to a lesser extent than anatase (Dontsova et al., 2020).
Semiconductor oxide such as TiO2 and SnO2 possesses a broad band gap and utilizes only 5% solar light in the UV region. Therefore, there is a need to prepare PCs, which would be capable to utilize a major portion of solar light in the visible region (Pattnaik et al., 2018b). Researchers found a problem with the separation of NPs while using them as photocatalysts. The separation of NPs after catalysis proved an expensive, hard, and time-consuming task (Konstantinou & Albanis, 2004; Patil et al., 2019). To overcome these problems, researchers focused on preparing magnetic NPs that can be separated by using an external magnet and utilizing solar light effectively. The NPs made up of Au (Ayati et al., 2011; Liu et al., 2016; Oros-Ruiz et al., 2011), Ag (Mo et al., 2015; Namratha et al., 2013; Xiong et al., 2011), Fe2O3 (Jiang et al., 2013; Roushenas et al., 2016), Fe (Kozma et al., 2016; Zelmanov & Semiat, 2015), Ni(OH)2 (El Hassani et al., 2019; Jayakumar et al., 2017; Nagajyothi et al., 2019; Yousefi et al., 2016), SiO2 (Qin et al., 2018), SnO (Bhattacharjee et al., 2016; Elango et al., 2015, 2016; Kumar et al., 2018), TiO2 (Barbosa et al., 2015; Raliya et al., 2017; Sahoo et al., 2012), ZnS (Yang et al., 2015), ZrO2 (Bansal et al., 2015), CuO (Katwal et al., 2015; Mehr et al., 2018), CoFe2O4 (Kalam et al., 2018; Sundararajan et al., 2017), anatase (TiO2)n (Gautam et al., 2016), and CdS (Darwish et al., 2016) are commonly used for water treatment. Among the salts of transition metals, Cr2O3 NPs show better results in the photocatalytic activity of dyes and pigments. AgCrO4 NPs were prepared by using various methods including direct precipitation, electro-synthesis, and hydrothermal methods. In the visible region, these NPs show maximum results as PCs (Sarani et al., 2021). Fe2O3 NPs have been found the most excellent PCs due to their higher adsorption capacity and ease in separation after catalysis (Giri et al., 2011; Vasantharaj et al., 2019). Moreover, nanomaterials have found applications in pollutant sensing, antibacterial activity, remediation of industrial effluents, and so on. The production of nanomaterials has been increased since 2011–2020 from 4000 to 58,000 tons, due to their diverse utilization in various fields (Sharma, 2009). CuO NPs show excellent results in photocatalytic degradation of CR dye by using cyanobacterium. It proved to be very less toxic and cheap method of dye degradation (Alsamhary et al., 2021). Azar et al. prepared CuO NPs having three different morphologies (nanosheets, nanorods, nanoleaves). This was associated with many hydroxyl ions, produced due to oxygen vacancies. These hydroxyl radicals act as scavengers and degrade the dye into inorganic smaller molecules (Sadollahkhani et al., 2014). Zhang et al. used 1gL−1 magnetic Sr5(PO4)3(OH)/Fe3O4 NPs for 89% degradation of CR (Zhang et al., 2016). Titanate-supported intercalated CdS NPs showed complete color removal within 15 min (Sehati et al., 2016). Electron mediators like monometallic Ru NPs caused electrons to shift from the reducing agent to the characteristic bond (Azo bond) of CR; it caused an increase in degradation rate (Gupta et al., 2013). NPs which are a combination of two or three metals make an extraordinary catalyst for the breakdown of many azo compounds (Eskandarinezhad et al., 2021). Gd3+ doped cobalt ferrite NPs possessed greater removal ability (16 mg g−1) than un-doped cobalt ferrite NPs (131 mg g−1) (Zhao et al., 2014). Doping increases the absorption capabilities of cobalt ferrite NPs by generating unpaired electrons (Masunga et al., 2019). Similarly, ZnO shows better results in dye degradation when it is blended with polyvinyl pyrrolidone because the surface area and pore size of this composite are larger than the simple unmodified ZnO PC (Khan et al., 2020a). Silver NPs synthesized by using leaf extract of Chinese spinach (Amaranthus gangeticus Linn) were found to exhibit high catalytic potential (more than 50%) for decoloring CR (Kolya et al., 2015). The high pH value increased the production of OHˉ ions which react to h+ and form OH˙ radicals. Thus, the higher number of OH˙ radicals increased the photodegradation activity (Khairnar et al., 2018). At lower pH, an increased number of holes act as major oxidizing agents; as a result, photodegradation increased (Adam et al., 2018; Nezamzadeh-Ejhieh et al., 2015). Some dyes can be degraded by the use of UV irradiation only (Zhao et al., 2005). Kinetics studies illustrate that degradation of CR dye is followed by pseudo-1st-order reaction mechanism and CR dye degeneration shows maximum value of rate constant under the influence of UV light (Tama et al., 2020). Movahedi et al. showed that both PCs and UV irradiation are necessary for the effective degradation of CR. The pH of the solution containing dye and PCs greatly affects photocatalytic activity, because most semiconductor PCs show amphoteric behavior due to zero-point charge (ZPC) (6 for TiO2) (Lizama et al., 2002; Zhao et al., 1998). Anionic dyes such as CR degraded effectively with PCs having low ZPC as at a high ZPC, the negative surface of the PCs repels sulfonated ions (Swarnalatha & Anjaneyulu, 2004). Besides, molecules of the dyes tend to aggregate in acidic or highly acidic media and form tautomer (Fig. 9) due to which photocatalytic activity was not completed (Movahedi et al., 2009b; Vidya et al., 2017b). The optimum concentration of catalyst increased the photocatalytic activity. The higher the number of catalysts caused the solution to become turbid, which results in light scattering phenomena (Adam et al., 2018; Ajoudanian & Nezamzadeh-Ejhieh, 2015; Ramaswamy et al., 2008; Ullah et al., 2018). As the concentration of dye increased by keeping the catalyst amount constant, photodegradation activity decreased due to saturation of active sites by the given catalyst (Khairnar et al., 2018). At a higher concentration of dye, the contact time increased and photocatalytic activity decreased due to light hindering property of dye molecules (Bansal & Sud, 2011). For maximum degradation, there is an optimum duration of time above; this time degradation rate remains constant due to saturation of active sites of the catalyst by dye molecules (Bansal & Sud, 2011; Khairnar et al., 2018; Ullah et al., 2018). One of the important green chemistry technologies is the photodegradation of organic dyes. PCs are required for the degradation of dye. Photodegradation by NPs bears many advantages; the most important is the complete degradation of dyes (Banerjee et al., 2019). Due to the high surface area, active sites, small size, nontoxicity, high chemical stability (Gunjakar et al., 2011), low cost, and high catalytic potential, nanomaterials have proved themselves as efficient adsorbents (Raval et al., 2016).
Protonation of CR in the acidic medium (Movahedi et al., 2009b)
The catalytic activity of bismuth ferrite (BFO) NPs was found to increase as preparation temperature increased from 350 to 500 °C and then decreased as temperature increased (Pattnaik et al., 2018b). The green synthesis of NPs using plants and microorganisms is the other choice for researchers as it proves less hazardous. However, biosynthesis is a very slow process, which is considered the major limitation of this method (Gadd et al., 1989; Saifuddin et al., 2009). Plant extract acts as a green reducing agent that helps to form metal nanoparticles (MNPs) via the conversion of metal ions into atoms and finally into MNPs by coagulation (Maryami et al., 2016). S.A Moon et al. prepared NPs of CuO, Ag, and MnO2 by using the extract of plant Kalopanax pictus; photocatalytic potential for CR of these NPs showed that MnO2 is more effective in the removal of CR from the aqueous media than that of CuO and Ag NPs (Moon et al., 2018).
7.1 Mechanism of Photocatalysis
Semiconductors are mostly used as PCs. They are considered highly stable and environment-friendly (Rajendran et al., 2021b). Efficiencies of various photocatalysts have been mentioned in Table 6. The process of catalysis is carried out with the help of charge carriers as described in Fig. 10. The number of charge carriers can be increased with the help of doping (Terna et al., 2021b). The photo-generated hole/electron has possibilities of recombining again or may combine with the adsorbate (dye). Photo-generated electron in the CB and h+ in the VB served as potential oxidizing and reducing agents respectively. As a result of oxidation–reduction reactions, adsorbate (dye) is converted into small nontoxic inorganic substances (Bhat et al., 2020b; Pattnaik et al., 2018b; Vidya et al., 2017b). The separation of the charge carrier is an important factor that contributes to the efficiency of PCs. This separation can be achieved by different methods including metallization and doping (Arkhipov et al., 2005b). Hydroxyl radical OH˙ is the main active agent for the photodegradation of organic substances, although the process mechanism is different in the case of different dyes and NPs. In the photodegradation process as described in Fig. 11, aromatic rings were attacked by free OH˙ radicals, resulting in hydroxylated intermediates and finally release of CO2 by a series of photo-Kolbe reactions. Gaseous nitrogen is released in the photocatalytic degradation of azo dyes, which is an ideal condition for the mineralization of nitrogen-bearing pollutants (Movahedi et al., 2009b). It is reported that heteroatoms such as sulfur are converted into nontoxic sulfate SO4−2 ions. It is reported that heteroatoms such as sulfur are converted into nontoxic sulfate SO4−2 ions (Lachheb et al., 2002).
Mechanism of photodegradation of CR (Hairom et al., 2015)
8 Conclusion
Synthetic dyes have replaced natural pigments due to their extensive photostable and thermostable properties. Various conventional methods for removing dyes from wastewater, such as coagulation, ozonation, ultra-filtration, and AOP, are now prohibited due to their high cost, high energy consumption, and high sludge production. Different useful methods for the conversion of recalcitrant CR dye into less toxic degraded products have been explicated. CR dye is an azo dye that is a complex aromatic structure attached with –N = N– bonding. Because of its large aromatic structure, it is resistant to natural degradation and has a harmful effect on the ecosystem by reducing the photosynthetic activities of aquatic flora, causing eutrophication. Auxochromes and chromophores attached to dyes are carcinogenic when they react with heavy metals. The preceding discussion aims to collect data on the degradation of CR dye using fungi and bacterial species, as well as the photodegradation method using NPs. The presence of various lysosomal enzymes in several bacterial species has helped in the discovery of dye degradation in a less toxic way. The extraction of bacterial cultures is easily done from the soil, water, or contaminated food. The dye pretreated with sonication by using ultrasounds reduced the degradation time by Bacillus-Aspergillus than the non-pretreated dye sample. Anaerobic, anoxic, and aerobic are different conditions required by bacterial species to degrade dyes. Fungal degradation of dyes is becoming a crucial method due to the presence of extracellular enzyme activity in fungi. Among fungi, white-rot fungi and many marine fungal species are proved to be very efficient in the degradation process of dyes. Experiments have been done to check the effect of nutrient sources in fungal culture. Inorganic media are known as the best source of carbon for fungal growth resulting in the efficient decoloration of CR dye. Aspergillus sp. showed efficient degradation of dye at optimum shaking speed. At high shaking speed, the enzymatic activity of manganese peroxidase (MnP) and lignin peroxidase (LiP) in fungi is maximum which results in efficient degradation of CR dye. In like manner, Aspergillus sp. showed maximum extracellular activity of MnP and LiP at optimum temperature range. One of the best and most prominent methods is photocatalytic degradation of dyes by using NPs. It is becoming a vital method because of its capability to degrade the dye completely, high stability, nontoxic nature, low cost, high surface area, active sites, and high catalytic potential. Iron oxide NPs are found to be the most efficient PCs due to their high maximum adsorption capacity. Experiments have shown that CuO nanorods are more efficient in CR photodegradation due to the presence of hydroxyl ions, which act as scavengers and break the dye into small inorganic ions. Similarly, silver nanoparticles have a high catalytic potential for degrading pigment. PCs and ultraviolet (UV) irradiation have both been shown in studies to be very effective in CR degradation.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Abe, F. R., Mendonça, J. N., Moraes, L. A., De Oliveira, G. A., Gravato, C., Soares, A. M., & De Oliveira, D. P. (2017). Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere, 178, 282–290.
Adam, R. E., Pozina, G., Willander, M., & Nur, O. (2018). Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. In: Photonics and Nanostructures-Fundamentals, Applications, 32, 11–18.
Ahmad, A., Mohd-Setapar, S. H., Chuong, C. S., Khatoon, A., Wani, W. A., Kumar, R., & Rafatullah, M. (2015). Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Advances, 5(39), 30801–30818.
Aithal, P. S., & Aithal, S. (2022). Opportunities and challenges for green and eco-friendly nanotechnology in twenty-first century. Sustainable nanotechnology: Strategies, products, and applications. 31–50
Ajoudanian, N., & Nezamzadeh-Ejhieh, A. (2015). Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Materials Science in Semiconductor Processing, 36, 162–169.
Alderete, B. L., Da Silva, J., Godoi, R., Da Silva, F. R., Taffarel, S. R., Da Silva, L. P., Garcia, A. L. H., Júnior, H. M., De Amorim, H. L. N., & Picada, J. N. (2021). Evaluation of Toxicity and Mutagenicity of a Synthetic Effluent Containing Azo Dye after Advanced Oxidation Process Treatment. Chemosphere, 263, 128291.
Ali, H. (2010). Biodegradation of synthetic dyes—A review. Water, Air, & Soil Pollution, 213(1), 251–273.
Alsamhary, K., Al-Enazi, N. M., Alhomaidi, E., & Alwakeel, S. (2022). Spirulina platensis mediated biosynthesis of Cuo Nps and photocatalytic degradation of toxic azo dye Congo red and kinetic studies. Environmental Research, 207, 112172.
Arıca, M. Y., & Bayramoğlu, G. (2007). Biosorption of reactive red-120 dye from aqueous solution by native and modified fungus biomass preparations of Lentinus sajor-caju. Journal of Hazardous Materials, 1492, 499–507.
Arkhipov, V., Heremans, P., Emelianova, E., & Baessler, H. (2005). Effect of doping on the density-of-states distribution and carrier hopping in disordered organic semiconductors. Physical Review B, 71(4), 045214.
Arkhipov, V. I., Heremans, P., Emelianova, E. V., & Baessler, H. (2005). Effect of Doping on the Density-of-States Distribution and Carrier Hopping in Disordered Organic Semiconductors. Physical Review B, 71(4), 045214.
Aslam, M., Fazal, D. B., Ahmad, F., Fazal, A. B., Abdullah, A. Z., Ahmed, M., Qamar, M., & Rafatullah, M. (2022). Photocatalytic degradation of recalcitrant pollutants of greywater. Catalysts, 12(5), 557.
Asses, N., Ayed, L., Hkiri, N., & Hamdi, M. (2018). Congo red decolorization and detoxification by aspergillus niger: removal mechanisms and dye degradation pathway. BioMed Research International, 2018
Ayati, A., Ahmadpour, A., Bamoharram, F. F., Heravi, M. M., & Rashidi, H. (2011). Photocatalytic Synthesis of Gold Nanoparticles Using Preyssler Acid and Their Photocatalytic Activity. Chinese Journal of Catalysis, 32(6–8), 978–982.
Babu, S. S., Mohandass, C., Vijayaraj, A., & Dhale, M. A. (2015). Detoxification and color removal of Congo red by a novel Dietzia sp. (DTS26)–A microcosm approach. Ecotoxicology and Environmental Safety, 114, 52–60.
Babu, S. S., Mohandass, C., Vijayaraj, A., & Dhale, M. A. (2015). Detoxification and color removal of Congo red by a novel Dietzia sp. (DTS26)–A microcosm approach. Ecotoxicology and Environmental Safety, 114, 52–60.
Bal, G., & Thakur, A. (2022). Distinct approaches of removal of dyes from wastewater: A review. Materials Today: Proceedings, 50, 1575–1579.
Balapure, K., Bhatt, N., & Madamwar, D. (2015). Mineralization of reactive azo dyes present in simulated textile waste water using down flow microaerophilic fixed film bioreactor. Bioresource Technology, 175, 1–7.
Banerjee, S., Dubey, S., Gautam, R. K., Chattopadhyaya, M., & Sharma, Y. C. (2019). Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye Orange G from Aqueous Solutions. Arabian Journal of Chemistry, 12(8), 5339–5354.
Bansal, P., & Sud, D. (2011). Photodegradation of commercial dye, Procion Blue HERD from real textile wastewater using nanocatalysts. Desalination, 267(2–3), 244–249.
Bansal, P., Chaudhary, G. R., & Mehta, S. K. (2015). Comparative study of catalytic activity of ZrO2 nanoparticles for sonocatalytic and photocatalytic degradation of cationic and anionic dyes. Chemical Engineering Journal, 280, 475–485.
Barbosa, L. V., Marçal, L., Nassar, E. J., Calefi, P. S., Vicente, M. A., Trujillano, R., Rives, V., Gil, A., Korili, S. A., & Ciuffi, K. J. (2015). Kaolinite-Titanium Oxide Nanocomposites Prepared via Sol-Gel as Heterogeneous Photocatalysts for Dyes Degradation. Catalysis Today, 246, 133–142.
Bedekar, P. A., Saratale, R. G., Saratale, G. D., & Govindwar, S. P. (2014). Development of low cost upflow column bioreactor for degradation and detoxification of Blue HERD and textile effluent by Lysinibacillus sp RGS Immobilized on Loofa. International Biodeterioration and Biodegradation, 96, 112–120.
Bento, R. M., Almeida, M. R., Bharmoria, P., Freire, M. G., & Tavares, A. P. (2020). Improvements in the enzymatic degradation of textile dyes using ionic-liquid-based surfactants. Separation and Purification Technology, 235, 116191.
Bhat, S. A., Zafar, F., Mondal, A. H., Kareem, A., Mirza, A. U., Khan, S., Mohammad, A., Haq, Q. M., & Nishat, N. (2020). Photocatalytic degradation of carcinogenic Congo red dye in aqueous solution, antioxidant activity and bactericidal effect of NiO nanoparticles. Journal of the Iranian Chemical Society, 17(1), 215–227.
Bhat, S. A., Zafar, F., Mondal, A. H., Kareem, A., Mirza, A. U., Khan, S., Mohammad, A., Haq, Q. M. R., & Nishat, N. (2020). Photocatalytic degradation of carcinogenic Congo red dye in aqueous solution, antioxidant activity and bactericidal effect of NiO nanoparticles. Journal of the Iranian Chemical Society, 17(1), 215–227.
Bhattacharjee, A., Ahmaruzzaman, M., Devi, T. B., & Nath, J. (2016). Photodegradation of methyl violet 6B and methylene blue using tin-oxide nanoparticles (synthesized via a green route). Journal of Photochemistry and Photobiology A: Chemistry, 325, 116–124.
Bianco Prevot, A., Baiocchi, C., Brussino, M. C., Pramauro, E., Savarino, P., Augugliaro, V., Marci, G., & Palmisano, L. (2001). Photocatalytic degradation of acid blue 80 in aqueous solutions containing TiO2 suspensions. Environmental Science & Technology, 35(5), 971–976.
Blais, K. M. J. (2006). TM Satinder Kaur Brar b. Mausam Verma. Imprint, 150(419), 1501
Blaszczyk, R. L. (2019). Colors in Fashion. Textile History, Taylor & Francis, 50(1), 113–115.
Brüschweiler, B. J., & Merlot, C. (2017). Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regulatory Toxicology and Pharmacology, 88, 214–226.
Carliell, C., Barclay, S., & Buckley, C. J. (1996). Treatment of exhausted reactive dyebath effluent using anaerobic digestion: laboratory and full-scale trials. Water SA, 22(3), 225–233.
Carolin, C. F., Kumar, P. S., & Joshiba, G. J. (2021). Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technologies and Environmental Policy, 23(1), 173–181.
Chaieb, K., Hagar, M., & Radwan, N. R. E. (2016). Biodegradation and decolorization of azo dyes by adherent Staphylococcus lentus strain. Applied Biological Chemistry, 59(3), 405–413.
Chakraborty, S., Basak, B., Dutta, S., Bhunia, B., & Dey, A. (2013). Decolorization and biodegradation of Congo red dye by a novel white rot fungus Alternaria alternata CMERI F6. Bioresource Technology, 147, 662–666.
Chantarasiri, A., & Boontanom, P. (2017). Decolorization of synthetic dyes by ligninolytic Lysinibacillus sphaericus JD1103 isolated from Thai wetland ecosystems. Aquaculture, Aquarium, Conservation & Legislation, 10(4), 814–819.
Chao, C., Guan, H., Zhang, J., Liu, Y., Zhao, Y., & Zhang, B. (2018). Immobilization of laccase onto porous polyvinyl alcohol/halloysite hybrid beads for dye removal. Water Science and Technology, 77(3), 809–818.
Chauhan, A., Verma, R., Kumari, S., Sharma, A., Shandilya, P., Li, X., Batoo, K. M., Imran, A., Kulshrestha, S., & Kumar, R. (2020). Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Scientific Reports, 10(1), 1–16.
Chen, C., Wen, Z., Wang, Y., Zhang, W., & Zhang, T. (2022). Multi-objective optimization of technology solutions in municipal solid waste treatment system coupled with pollutants cross-media metabolism issues. Science of the Total Environment, 807, 150664.
Ch-Th, T., Manisekaran, R., Santoyo-Salazar, J., Schoefs, B., Velumani, S., Castaneda, H., & Jantrania, A. (2021). Graphene oxide decorated TiO2 and BiVO4 nanocatalysts for enhanced visible-light-driven photocatalytic bacterial inactivation. Journal of Photochemistry and Photobiology a: Chemistry, 418, 113374.
Crini, G., & Lichtfouse, E. (2019). Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 17(1), 145–155.
Crini, G., & Lichtfouse, E. (2019). Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 17(1), 145–155.
Cristóvão, R. O., Tavares, A. P., Ferreira, L. A., Loureiro, J. M., Boaventura, R. A., & Macedo, E. A. (2009). Modeling the discoloration of a mixture of reactive textile dyes by commercial laccase. Bioresource Technology, 100(3), 1094–1099.
Cruz-Rizo, A., Gutiérrez-Granados, S., Salazar, R., Peralta-Hernández, J. M. J. S., & Technology, P. (2017). Application of electro-fenton/bdd process for treating tannery wastewaters with industrial dyes. Separation and Purification Technology, 172, 296–302.
Daniel, A. J., Enzo, E. R., Juliana, M. S., Stefanie, B. C-G., Analia, A., Claudia, S. B., & Marta, A. P. (2022). The current approach to soil remediation: A review of physicochemical and biological technologies, and the potential of their strategic combination. Journal of Environmental Chemical Engineering, 10(2), 107141.
Darwish, M., Mohammadi, A., & Assi, N. (2016). Microwave-assisted polyol synthesis and characterization of pvp-capped cds nanoparticles for the photocatalytic degradation of tartrazine. Materials Research Bulletin, 74, 387–396.
Das, A., Bhattacharya, S., Panchanan, G., Navya, B., & Nambiar, P. (2016). Production, characterization and Congo red dye decolourizing efficiency of a laccase from Pleurotus ostreatus MTCC 142 cultivated on co-substrates of paddy straw and corn husk. Journal of Genetic Engineering and Biotechnology, 14(2), 281–288.
de Beluci, N. C. L., Mateus, G. A. P., Miyashiro, C. S., Homem, N. C., Gomes, R. G., Fagundes-Klen, M. R., Bergamasco, R., & Vieira, A. M. S. (2019). Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TIO2-modified membranes to improve the removal of reactive black 5 dye. Science of The Total Environment, 664, 222–229.
Debnath, P., & Mondal, N. K. (2020). Effective removal of Congo red dye from aqueous solution using biosynthesized zinc oxide nanoparticles. Environmental Nanotechnology, Monitoring and Management, 14, 100320.
Domínguez-Cuevas, P., González-Pastor, J.-E., Marqués, S., Ramos, J.-L., & De Lorenzo, V. (2006). Transcriptional tradeoff between metabolic and stress-response programs in Pseudomonas putida KT2440 cells exposed to toluene. Journal of Biological Chemistry, 281(17), 11981–11991.
Dontsova, T. A., Kutuzova, A. S., Bila, K. O., Kyrii, S. O., Kosogina, I. V., & Nechyporuk, D. O. (2020). Enhanced photocatalytic activity of TiO2/SnO2 binary nanocomposites. Journal of Nanomaterials, 2020
Duan, L., Zhang, Y., Wang, B., Deng, S., Huang, J., Wang, Y., & Yu, G. (2018). Occurrence, elimination, enantiomeric distribution and intra-day variations of chiral pharmaceuticals in major wastewater treatment plants in Beijing, China. Environmental Pollution, 239, 473–482.
Dubé, E., Shareck, F., Hurtubise, Y., Beauregard, M., & Daneault, C. (2008). Decolourization of recalcitrant dyes with a laccase from Streptomyces coelicolor under alkaline conditions. Journal of Industrial Microbiology and Biotechnology, 35(10), 1123–1129.
Ekambaram, S. P., Perumal, S. S., Rajendran, D., Samivel, D., Khan, M. N. (2018). Toxicity and biodegradation testing: Springer 241–267
El Hassani, K., Kalnina, D., Turks, M., Beakou, B. H., & Anouar, A. (2019). Enhanced degradation of an azo dye by catalytic ozonation over ni-containing layered double hydroxide nanocatalyst. Separation and Purification Technology, 210, 764–774.
Elango, G., & Roopan, S. M. (2016). Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. Journal of Photochemistry and Photobiology B: Biology, 155, 34–38.
Elango, G., Kumaran, S. M., Kumar, S. S., Muthuraja, S., & Roopan, S. M. (2015). Green synthesis of SnO2 nanoparticles and its photocatalytic activity of phenolsulfonphthalein dye. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 145, 176–180.
El-Salamony, R. A., Amdeha, E., Badawy, N. A., Ghoneim, S. A., & Al-Sabagh, A. M. (2018). Visible light sensitive activated carbon-metal oxide (TiO2, WO3, NiO, and SnO) nano-catalysts for photo-degradation of methylene blue: A comparative study. Toxicological & Environmental Chemistry, 100(2), 143–156.
Eskandarinezhad, S., Khosravi, R., Amarzadeh, M., Mondal, P., & Correa Magalhaes Filho, F. J. (2021). Application of different Nanocatalysts in industrial effluent treatment: A review. Journal of Composites and Compounds, 3(6), 43–56.
Fairuzi, A., Bonnia, N., Akhir, R., Abrani, M., Akil, H. (2018). Degradation of methylene blue using silver nanoparticles synthesized from Imperata cylindrica aqueous extract. IOP Publishing, IOP Conference Series: Earth and Environmental Science, 012018
Fernández, C., Larrechi, M. S., & Callao, M. P. (2010). An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends in Analytical Chemistry, 29(10), 1202–1211.
Feuzer-Matos, A. J., Testolin, R. C., Cotelle, S., Sanches-Simões, E., Pimentel-Almeida, W., Niero, G., Walz, G. C., Ariente-Neto, R., Somensi, C. A., & Radetski, C. M. (2021). Degradation of recalcitrant textile azo-dyes by fenton-based process followed by biochar polishing. Journal of Environmental Science and Health, Part A, 56(9), 1019–1029.
Gadd, G. M., Laurence, O. S., Briscoe, P. A., & Trevors, J. T. (1989). Silver accumulation in Pseudomonas stutzeri AG259. Biology of Metals, 2(3), 168–173.
Gao, Y., Wu, Y., Xiao, J., & Lu, D. (2018). An experimental research on the machinability of a high temperature titanium alloy BTi-6431S in turning process. Manufacturing Review, 5, 12.
Garcia, B. B., Lourinho, G., Romano, P., & Brito, P. (2020). Photocatalytic degradation of swine wastewater on aqueous TiO2 suspensions: Optimization and modeling via Box-Behnken design. Heliyon, 6(1), e03293.
Gautam, A., Kshirsagar, A., Biswas, R., Banerjee, S., & Khanna, P. K. (2016). Photodegradation of organic dyes based on anatase and rutile TiO 2 nanoparticles. RSC Advances, 6(4), 2746–2759.
Giri, S., Das, N., & Pradhan, G. C. (2011). Synthesis and characterization of magnetite nanoparticles using waste iron ore tailings for adsorptive removal of dyes from aqueous solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 389(1–3), 43–49.
Girish, K. (2019). Chapter-3 Microbial decolourization of textile dyes and biodegradation of textile industry effluent. Advances In, 37
Gopinath, K. P., Sahib, H. A. M., Muthukumar, K., & Velan, M. (2009). Improved biodegradation of Congored by using Bacillus sp. Bioresource technology, 100(2), 670–675.
Gunjakar, J. L., Kim, T. W., Kim, H. N., Kim, I. Y., & Hwang, S. J. (2011). Mesoporous layer-by-layer ordered nanohybrids of layered double hydroxide and layered metal oxide: Highly active visible light photocatalysts with improved chemical stability. Journal of the American Chemical Society, 133(38), 14998–15007.
Gupta, S., Giordano, C., Gradzielski, M., & Mehta, S. K. (2013). Microwave-assisted synthesis of small ru nanoparticles and their role in degradation of congo red. Journal of Colloid and Interface Science, 411, 173–181.
Gürses, A., Açıkyıldız, M., Güneş, K., & Gürses, M. S. (2016). Dyes and Pigments. Gürses, A., Açıkyıldız, M., Güneş, K. and Gürses, M.S. (eds). Cham: Springer International Publishing, 13–29.
Hairom, N. H. H., Mohammad, A. W., Ng, L. Y., & Kadhum, A. (2015). Utilization of self-synthesized ZnO nanoparticles in MPR for industrial dye wastewater treatment using NF and UF membrane. Desalination and Water Treatment, 54(4–5), 944–955.
Hamoud, H. I., Finqueneisel, G., & Azambre, B. (2017). Removal of binary dyes mixtures with opposite and similar charges by adsorption, coagulation/flocculation and catalytic oxidation in the presence of CeO2/H2O2 Fenton-like system. Journal of Environmental Management, 195, 195–207.
Hazarika, A., Yadav, M., Yadav, D. K., & Yadav, H. S. (2022). An overview of the role of nanoparticles in sustainable agriculture. Biocatalysis and Agricultural Biotechnology, 102399
He, Q.-B., Hu, Z., & Ge, M. (2021). Research progress on photo-degradation of antibiotics in water by BiOX (X= Cl, Br, I) composite photocatalytic materials. Chinese Journal of Applied Chemistry, 38(7), 754.
Hoque, E., & Fritscher, J. (2019). Multimetal bioremediation and biomining by a combination of new aquatic strains of Mucor hiemalis. Scientific Reports, 9(1), 1–16.
Horitsu, H., Takada, M., Idaka, E., Tomoyeda, M., & Ogawa, T. (1977). Degradation of p-Aminoazobenzene byBacillus subtilis. European Journal of Applied Microbiology and Biotechnology, 4(3), 217–224.
Iark, D., Dos Reis, Buzzo A J., Garcia, J. A. A., Côrrea, V. G., Helm, C. V., Corrêa, R. C. G., Peralta, R. A., Moreira, R. D. F. P. M., Bracht, A., & Peralta, R. M. (2019). Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresource Technology, 289, 121655.
Ikram, M., Hassan, J., Raza, A., Haider, A., Naz, S., Ul-Hamid, A., Haider, J., Shahzadi, I., Qamar, U., & Ali, S. (2020). Photocatalytic and bactericidal properties and molecular docking analysis of TiO 2 nanoparticles conjugated with Zr for environmental remediation. RSC Advances, 10(50), 30007–30024.
Jalandoni-Buan, A. C., Decena-Soliven, A. L. A., Cao, E. P., Barraquio, V. L., & Barraquio, W. L. (2015). Microbial Degradation of Synthetic Dyes in Wastewaters. Singh, S.N. (ed). Cham: Springer International Publishing, 135–148.
Jamee, R., & Siddique, R. (2019). Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. European Journal of Microbiology and Immunology, 9(4), 114–118.
Jayakumar, G., Irudayaraj, A. A., & Raj, A. D. (2017). Photocatalytic degradation of methylene blue by nickel oxide nanoparticles. Materials Today: Proceedings, 4(11), 11690–11695.
Jiang, W., Pelaez, M., Dionysiou, D. D., Entezari, M. H., Tsoutsou, D., & O’shea, K. (2013). Chromium (VI) removal by maghemite nanoparticles. Chemical Engineering Journal, 222, 527–533.
Jorfi, S., Pourfadakari, S., & Kakavandi, B. (2018). A new approach in sono-photocatalytic degradation of recalcitrant textile wastewater using MgO@ Zeolite nanostructure under UVA irradiation. Chemical Engineering Journal, 343, 95–107.
Kalam, A., Al-Sehemi, A. G., Assiri, M., Du, G., Ahmad, T., Ahmad, I., & Pannipara, M. (2018). Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results in Physics, 8, 1046–1053.
Kanakaraju, D., Glass, B. D., & Oelgemöller, M. (2018). Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. Journal of Environmental Management, 219, 189–207.
Kapdan, I. K., & Kargi, F. (2002). Biological decolorization of textile dyestuff containing wastewater by Coriolus versicolor in a rotating biological contactor. Enzyme and Microbial Technology, 30(2), 195–199.
Kapoor, R. T., Danish, M., Singh, R. S., Rafatullah, M., & Hps, A. K. (2021). Exploiting microbial biomass in treating azo dyes contaminated wastewater: mechanism of degradation and factors affecting microbial efficiency. Journal of Water Process Engineering, 43, 102255.
Katwal, R., Kaur, H., Sharma, G., Naushad, M., Pathania, D., & Chemistry, E. (2015). Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. Journal of Industrial and Engineering Chemistry, 31, 173–184.
Kaushik, P., & Malik, A. (2009). Fungal dye decolourization: Recent advances and future potential. Environment International, 35(1), 127–141.
Kaushik, P., & Malik, A. J. E. I. (2009). Fungal Dye Decolourization: Recent Advances and Future Potential. Environment International, 35(1), 127–141.
Khairnar, S. D., Patil, M. R., & Shrivastava, V. S. (2018). Hydrothermally synthesized nanocrystalline Nb2O5 and its visible-light photocatalytic activity for the degradation of Congo red and methylene blue. Iranian Journal of Catalysis, 8(2), 143–150.
Khan, R., Adnan, A., Pervaiz, M., Raza, M., Sagir, M., & Naz, M. J. R. J. O. P. C. B. (2016). Biodegradation of h acid by bacillus subtilis and rp-hplc method development for percent degradation estimation Russian. Journal of Physical Chemistry B, 10(3), 517–523.
Khan, A., Naeem, A., & Mahmood, T. (2020). Kinetic studies of methyl orange and Congo red adsorption and photocatalytic degradation onto PVP-functionalized ZnO. Kinetics and Catalysis, 61(5), 730–739.
Khan, I., Saeed, K., Ali, N., Khan, I., Zhang, B., & Sadiq, M. (2020). Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. Journal of Environmental Chemical Engineering, 8(5), 104364.
Khan, S., Naushad, M., Al-Gheethi, A., & Iqbal, J. (2021). Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. Journal of Environmental Chemical Engineering, 9(5), 106160.
Khan, Z. U. H., Khan, A., Shah, N. S., Din, I. U., Salam, M. A., Iqbal, J., Muhammad, N., Imran, M., Ali, M., & Sayed, M. (2021). Photocatalytic and biomedical investigation of green synthesized NiONPs: Toxicities and degradation pathways of Congo red dye. Surfaces and Interfaces, 23, 100944.
Khan, R. R. M., Saeed, S., & Adnan, A. (2018). Toxicity and Biodegradation Testing. Bidoia, E.D. and Montagnolli, R.N. (eds). New York, NY: Springer New York, 269–280.
Khaniabadi, Y. O., Mohammadi, M. J., Shegerd, M., Sadeghi, S., Saeedi, S., & Basiri, H. (2017). Removal of congo red dye from aqueous solutions by a low-cost adsorbent: activated carbon prepared from aloe vera leaves shell. Environmental Health Engineering and Management Journal, 4(1), 29–35.
Khehra, M. S., Saini, H. S., Sharma, D. K., Chadha, B. S., & Chimni, S. S. (2005). Decolorization of various azo dyes by bacterial consortium. Dyes and Pigments, 67(1), 55–61.
Khurana, R., Uversky, V. N., Nielsen, L., & Fink, A. L. (2001). Is Congo red an amyloid-specific dye? Journal of Biological Chemistry, 276(25), 22715–22721.
Kolya, H., Maiti, P., Pandey, A., & Tripathy, T. (2015). Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. Journal of Analytical Science and Technology, 6(1), 33.
Konieczkowska, J., Wojtowicz, M., Sobolewska, A., Noga, J., Jarczyk-Jedryka, A., Kozanecka-Szmigiel, A., & Schab-Balcerzak, E. (2015). Thermal, optical and photoinduced properties of a series of homo and co-polyimides with two kinds of covalently bonded azo-dyes and their supramolecular counterparts. Optical Materials, 48, 139–149.
Konstantinou, I. K., & Albanis, T. A. (2004). TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: a review. Applied Catalysis B: Environmental, 49(1), 1–14.
Korenak, J., Ploder, J., Trček, J., Hélix-Nielsen, C., & Petrinic, I. (2018). Decolourisations and biodegradations of model azo dye solutions using a sequence batch reactor, followed by ultrafiltration. International Journal of Environmental Science and Technology, 15(3), 483–492.
Kozma, G., Rónavári, A., Kónya, Z., & Kukovecz, A. (2016). Environmentally benign synthesis methods of zero-valent iron nanoparticles. ACS Sustainable Chemistry & Engineering, 4(1), 291–297.
Kraft, A., Stadelmann, M., & Blaschke, M. (2003). Anodic oxidation with doped diamond electrodes: a new advanced oxidation process. Journal of Hazardous Materials, 103(3), 247–261.
Krishnakumar, B., & Swaminathan, M. (2011). Influence of operational parameters on photocatalytic degradation of a genotoxic azo dye Acid Violet 7 in aqueous ZnO suspensions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 81(1), 739–744.
Kumar, M., Mehta, A., Mishra, A., Singh, J., Rawat, M., & Basu, S. (2018). Biosynthesis of tin oxide nanoparticles using psidium guajava leave extract for photocatalytic dye degradation under sunlight. Materials Letters, 215, 121–124.
Kuppusamy, S., Sethurajan, M., Kadarkarai, M., & Aruliah, R. (2017). Biodecolourization of textile dyes by novel, indigenous Pseudomonas stutzeri MN1 and Acinetobacter baumannii MN3. Journal of Environmental Chemical Engineering, 5(1), 716–724.
Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., & Herrmann, J.-M. (2002). Photocatalytic degradation of various types of dyes (alizarin S, Crocein Orange G, methyl red, Congo red, methylene blue) in water by UV-irradiated titania. Applied Catalysis B: Environmental, 39(1), 75–90.
Lade, H., Govindwar, S., & Paul, D. (2015). Mineralization and detoxification of the carcinogenic azo dye congo red and real textile effluent by a polyurethane foam immobilized microbial consortium in an upflow column bioreactor. International Journal of Environmental Research and Public Health, 12(6), 6894–6918.
Lai, C.-Y., Wu, C.-H., Meng, C.-T., & Lin, C. W. (2017). Decolorization of azo dye and generation of electricity by microbial fuel cell with laccase-producing white-rot fungus on cathode. Applied Energy, 188, 392–398.
Lamba, R., Umar, A., Mehta, S., & Kansal, S. K. (2015). ZnO doped SnO2 nanoparticles heterojunction photo-catalyst for environmental remediation. Journal of Alloys and Compounds, 653, 327–333.
Li, S., Huang, J., Mao, J., Zhang, L., He, C., Chen, G., Parkin, I. P., & Lai, Y. (2019). In vivo and in vitro efficient textile wastewater remediation by Aspergillus niger biosorbent. Nanoscale Advances, 1(1), 168–176.
Li, X., Li, W., Wang, M., & Liao, Z. (2021). Magnetic nanoparticles for cancer theranostics: Advances and prospects. Journal of Controlled Release, 335, 437–448.
Liu, H., Hao, H., Xing, J., Dong, J., Zhang, Z., Zheng, Z., & Zhao, K. (2016). Enhanced Photocatalytic Capability of Zinc Ferrite Nanotube Arrays Decorated with Gold Nanoparticles for Visible Light-Driven Photodegradation of Rhodamine b. Journal of Materials Science, 51(12), 5872–5879.
Lizama, C., Freer, J., Baeza, J., & Mansilla, H. D. (2002). Optimized photodegradation of reactive blue 19 on TiO2 and ZnO suspensions. Catalysis Today, 76(2–4), 235–246.
Maddhinni, V. L., Vurimindi, H. B., & Yerramilli, A. (2013). Degradation of azo dye with horse radish peroxidase (hrp). Journal of the Indian Institute of Science, 86(5), 507.
Mahapatra, N. (2016). Introduction to textile dyes. Textile Dyes (pp. 17-30). WPI Publishing.
Mahdiani, M., Soofivand, F., Ansari, F., & Salavati-Niasari, M. (2018). Grafting of CuFe12O19 nanoparticles on CNT and graphene: Eco-friendly synthesis, characterization and photocatalytic activity. Journal of Cleaner Production, 176, 1185–1197.
Mahmoodi, N. M. (2016). Photocatalytic degradation of textile dyes using ozonation and magnetic nickel ferrite nanoparticle. Progress in Color, Colorants and Coatings, 9, 161–172.
Mahmoud, M. S., Mostafa, M. K., Mohamed, S. A., Sobhy, N. A., & Nasr, M. (2017). Bioremediation of red azo dye from aqueous solutions by aspergillus niger strain isolated from textile wastewater. Journal of Environmental Chemical Engineering, 5(1), 547–554.
Manivel, A., Lee, G.-J., Chen, C.-Y., Chen, J.-H., Ma, S.-H., Horng, T.-L., & Wu, J. J. (2015). Synthesis of MoO3 nanoparticles for azo dye degradation by catalytic ozonation. Materials Research Bulletin, 62, 184–191.
Maryami, M., Nasrollahzadeh, M., Mehdipour, E., & Sajadi, S. M. J. I. J. O. H. E. (2016). Preparation of the ag/rgo nanocomposite by use of abutilon hirtum leaf extract: a recoverable catalyst for the reduction of organic dyes in aqueous medium at room temperature. International Journal of Hydrogen Energy, 41(46), 21236–21245.
Masunga, N., Mmelesi, O. K., Kefeni, K. K., & Mamba, B. B. (2019). Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment. Journal of Environmental Chemical Engineering, 7(3), 103179.
Meephon, S., Rungrotmongkol, T., Puttamat, S., Praserthdam, S., & Pavarajarn, V. (2019). Heterogeneous photocatalytic degradation of diuron on zinc oxide: Influence of surface-dependent adsorption on kinetics, degradation pathway, and toxicity of intermediates. Journal of Environmental Sciences, 84, 97–111.
Mehr, E. S., Sorbiun, M., Ramazani, A., & Fardood, S. (2018). Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using Ferulago angulata (Schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. Journal of Materials Science: Materials in Electronics, 29(2), 1333–1340.
Meng, D., Liu, X., Xie, Y., Du, Y., Yang, Y., & Xiao, C. (2019). Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping. Advances in Materials Science and Engineering, 2019, 1–8.
Merah, C. (2020). Electrosynthesis of silver oxide deposited onto hot spring mud with enhanced degradation of Congo red. Malaysian Journal of Analytical Sciences, 24(2), 266–275.
Mo, Y.-Y., Tang, Y.-K., Wang, S.-Y., Lin, J.-M., Zhang, H.-B., & Luo, D.-Y. (2015). Green synthesis of silver nanoparticles using eucalyptus leaf extract. Materials Letters, 144, 165–167.
Mohamed, A., Karima, S., & Nadia, O. (2022). The use of medicinal plants against cancer: An ethnobotanical study in the Beni Mellal-Khenifra Region in Morocco. European Journal of Integrative Medicine, 52, 102137.
Mohapatra, R. K., Behera, S. S., Patra, J. K., Thatoi, H., & Parhi, P. K. (2020). New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms. Yadav, M.K. and Singh, B.P. (eds): Elsevier, 267–281.
Molinari, R., Lavorato, C., & Argurio, P. (2020). Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts, 10(11), 1334.
Moon, S. A., Salunke, B. K., Saha, P., Deshmukh, A. R., & Kim, B. S. (2018). Comparison of dye degradation potential of biosynthesized copper oxide, manganese dioxide, and silver nanoparticles using Kalopanax pictus plant extract. Korean Journal of Chemical Engineering, 35(3), 702–708.
Moosvi, S., Keharia, H., & Madamwar, D. (2005). Decolourization of textile dye Reactive Violet 5 by a newly isolated bacterial consortium RVM 11.1. World Journal of Microbiology and Biotechnology, 21(5), 667–672.
Movahedi, M., Mahjoub, A., & Janitabar-Darzi, S. (2009). Photodegradation of Congo red in aqueous solution on ZnO as an alternative catalyst to TiO2. Journal of the Iranian Chemical Society, 6(3), 570–577.
Movahedi, M., Mahjoub, A., & Janitabar-Darzi, S. (2009). Photodegradation of Congo red in aqueous solution on ZnO as an alternative catalyst to TiO 2. Journal of the Iranian Chemical Society, 6(3), 570–577.
M-Ridha, M. J., Hussein, S. I., Alismaeel, Z. T., Atiya, M. A., & Aziz, G. M. (2020). Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alexandria Engineering Journal, 59(5), 3551–3563.
Musa, M. A., & Idrus, S. (2021). Physical and biological treatment technologies of slaughterhouse wastewater: a review. Sustainability, 13(9), 4656.
Nagajyothi, P., Deyarayapalli, K., Tettey, C., Vattikuti, S. P., & Shim, J. (2019). Eco-friendly green synthesis: catalytic activity of nickel hydroxide nanoparticles. Materials Research Express, 6(5), 055036.
Nagajyothi, P., Prabhakar Vattikuti, S., Devarayapalli, K., Yoo, K., Shim, J., & Sreekanth, T. V. M. (2020). Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Critical Reviews in Environmental Science and Technology, 50(24), 2617–2723.
Namratha, N., & Monica, P. V. (2013). Synthesis of silver nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. Asian Journal of Pharmacy and Technolog, 3(4), 170–174.
Nandhini, N. T., Rajeshkumar, S., & Mythili, S. (2019). The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatalysis and Agricultural Biotechnology, 19, 101138.
Natarajan, T. S., Thomas, M., Natarajan, K., Bajaj, H. C., & Tayade, R. J. (2011). Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chemical Engineering Journal, 169(1–3), 126–134.
Neoh, C. H., Lam, C. Y., Lim, C. K., Yahya, A., Bay, H. H., Ibrahim, Z., & Noor, Z. Z. (2015). Biodecolorization of recalcitrant dye as the sole sourceof nutrition using Curvularia clavata NZ2 and decolorization ability of its crude enzymes. Environmental Science and Pollution Research, 22(15), 11669–11678.
Nezamzadeh-Ejhieh, A., & Bahrami, M. (2015). Investigation of the photocatalytic activity of supported ZnO–TiO2 on clinoptilolite nano-particles towards photodegradation of wastewater-contained phenol. Desalination and Water Treatment, 55(4), 1096–1104.
Nguyen, D. T. C., Le, H. T., Nguyen, T. T., Nguyen, T. T. T., Bach, L. G., Nguyen, T. D., & Van Tran, T. (2021). Multifunctional ZnO nanoparticles bio-fabricated from Canna indica L. flowers for seed germination, adsorption, and photocatalytic degradation of organic dyes. Journal of Hazardous Materials, 420, 126586.
Niculescu, A-G., Chircov, C., & Grumezescu, A. M. (2022). Magnetite nanoparticles: Synthesis methods – A comparative review. Methods, 199, 16–27.
Nigam, P., Banat, I. M., Singh, D., & Marchant, R. (1996). Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochemistry, 31(5), 435–442.
Noman, E., Talip, B. A., Al-Gheethi, A., Mohamed, R., & Nagao, H. (2020). Decolourisation of dyes in greywater by mycoremediation and mycosorption process of fungi from peatland; primary study. Materials Today: Proceedings, 31, 23–30.
Oliveira, D. P., Carneiro, P. A., Sakagami, M. K., Zanoni, M. V. B., & Umbuzeiro, G. A. (2007). Chemical characterization of a dye processing plant effluent—identification of the mutagenic components. Mutation Research/genetic Toxicology and Environmental Mutagenesis, 626(1–2), 135–142.
Olivo-Alanis, D., Garcia-Reyes, R. B., Alvarez, L. H., & Garcia-Gonzalez, A. (2018). Mechanism of anaerobic bio-reduction of azo dye assisted with lawsone-immobilized activated carbon. Journal of Hazardous Materials, 347, 423–430.
Oros-Ruiz, S., Pedraza-Avella, J., Guzmán, C., Quintana, M., Moctezuma, E., Del Angel, G., Gómez, R., & Pérez, E. (2011). Effect of gold particle size and deposition method on the photodegradation of 4-chlorophenol by Au/TiO2. Topics in Catalysis, 54(8–9), 519–526.
Pandey, A., Singh, P., & Iyengar, L. (2007). Bacterial decolorization and degradation of azo dyes. International Biodeterioration & Biodegradation, 59(2), 73–84.
Parascandola, J. (1981). The theoretical basis of Paul Ehrlich’s chemotherapy. Journal of the History of Medicine and Allied Sciences, 36(1), 19–43.
Parshetti, G., Kalme, S., Gomare, S., & Govindwar, S. P. (2007). Biodegradation of reactive blue-25 by Aspergillus ochraceus NCIM-1146. Bioresource Technology, 98(18), 3638–3642.
Parveen, K., Banse, V., & Ledwani, L. (2016). Green synthesis of nanoparticles: Their advantages and disadvantages: AIP Publishing LLC, 020048 https://doi.org/10.1063/1.4945168.
Patil, S., Deshmukh, S., More, K., Shevale, V., Mullani, S., Dhodamani, A., & Delekar, S. D. (2019). Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Materials Chemistry and Physics, 225, 247–255.
Pattnaik, S. P., Behera, A., Martha, S., Acharya, R., & Parida, K. (2018). Synthesis, photoelectrochemical properties and solar light-induced photocatalytic activity of bismuth ferrite nanoparticles. Journal of Nanoparticle Research, 20(1), 1–15.
Pattnaik, S. P., Behera, A., Martha, S., Acharya, R., & Parida, K. (2018). Synthesis, photoelectrochemical properties and solar light-induced photocatalytic activity of bismuth ferrite nanoparticles. Journal of Nanoparticle Research, 20(1), 10.
Pearce C, Lloyd J, Guthrie J J D, Pigments 2003). The removal of colour from textile wastewater using whole bacterial cells: a review. 58(3): 179–196
Puchtler, H., Sweat, F., & Levine, M. (1962). On the binding of Congo red by amyloid. Journal of Histochemistry & Cytochemistry, 10(3), 355–364.
Pundalik, E., Salagare, A., & Tandon, G. (2019). Degradation of Azo Dyes Using Mycoremediation. International Journal of Pharmacy and Biological Sciences, 159–169.
Puvaneswari, N., Muthukrishnan, J., & Gunasekaran, P. (2006). Toxicity assessment and microbial degradation of azo dyes. CSIR, 44(08), 618–626.
Qin, P., Yang, Y., Zhang, X., Niu, J., Yang, H., Tian, S., Zhu, J., & Lu, M. (2018). Highly efficient, rapid, and simultaneous removal of cationic dyes from aqueous solution using monodispersed mesoporous silica nanoparticles as the adsorbent. Nanomaterials, 8(1), 4.
Qu, R., Zhang, W., Liu, N., Zhang, Q., Liu, Y., Li, X., Wei, Y., & Feng, L. (2018). Antioil Ag3PO4 nanoparticle/polydopamine/Al2O3 sandwich structure for complex wastewater treatment: Dynamic catalysis under natural light. ACS Sustainable Chemistry & Engineering, 6(6), 8019–8028.
Rafiq, A., Ikram, M., Ali, S., Niaz, F., Khan, M., Khan, Q., & Maqbool, M. (2021). Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. Journal of Industrial and Engineering Chemistry, 97, 111–128.
Rahimnejad, M., Adhami, A., Darvari, S., Zirepour, A., & Oh, S.-E. (2015). Microbial fuel cell as new technology for bioelectricity generation: A review. Alexandria Engineering Journal, 54(3), 745–756.
Rai, M. S., Bhat, P. R., Prajna, P., Jayadev, K., & Rao, P. V. (2014). Degradation of malachite green and congo red using aloe barabadensis mill extract. International Journal of Current Microbiology and Applied Sciences, 3(4), 330–340.
Rajendran, R., Vignesh, S., Sasireka, A., Priya, P., Suganthi, S., Raj, V., Sundar, J. K., Srinivasan, M., Shkir, M., & Alfaify, S. (2021). Investigation on novel Cu2O modified g-C3N4/ZnO heterostructures for efficient photocatalytic dye degradation performance under visible-light exposure. Colloid and Interface Science Communications, 44, 100480.
Rajendran, R., Vignesh, S., Sasireka, A., Priya, P., Suganthi, S., Raj, V., Sundar, J. K., Srinivasan, M., Shkir, M., & Alfaify, S. (2021). Investigation on novel Cu2O modified g-C3N4/ZnO heterostructures for efficient photocatalytic dye degradation performance under visible-light exposure. Colloid and Interface Science Communications, 44, 100480.
Raliya, R., Avery, C., Chakrabarti, S., & Biswas, P. (2017). Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Applied Nanoscience, 7(5), 253–259.
Ramaswamy, V., Jagtap, N., Vijayanand, S., Bhange, D., & Awati, P. S. (2008). Photocatalytic decomposition of methylene blue on nanocrystalline titania prepared by different methods. Materials Research Bulletin, 43(5), 1145–1152.
Rambabu, K., Banat, F., Pham, Q. M., Ho, S.-H., Ren, N.-Q., & Show, P. L. (2020). Biological remediation of acid mine drainage: Review of past trends and current outlook. Environmental Science and Ecotechnology, 2, 100024.
Ranga, P., Saharan, B. S., & Sharma, D. (2015). Bacterial degradation and decolorization of textile dyes by newly isolated Lysobacter sp. African Journal of Microbiology Research, 9(14), 979–987.
Raval, N. P., Shah, P. U., & Shah, N. K. (2016). Adsorptive amputation of hazardous azo dye congo red from wastewater: a critical review. Environmental Science and Pollution Research, 23(15), 14810–14853.
Remya, R., Julius, A., Suman, T., Mohanavel, V., Karthick, A., Pazhanimuthu, C., Samrot, & A. V., Muhibbullah, M. (2022). Role of Nanoparticles in Biodegradation and Their Importance in Environmental and Biomedical Applications. Journal of Nanomaterials, 2022.
Rodríguez Couto, S. (2009). Dye removal by immobilised fungi. Biotechnology Advances, 27(3), 227–235.
Roushenas, P., Yusop, Z., Majidnia, Z., & Nasrollahpour, R. (2016). Photocatalytic degradation of spilled oil in sea water using maghemite nanoparticles. Desalination and Water Treatment, 57(13), 5837–5841.
Sadollahkhani, A., Ibupoto, Z. H., Elhag, S., Nur, O., & Willander, M. (2014). Photocatalytic properties of different morphologies of cuo for the degradation of congo red organic dye. Ceramics International, 40(7), 11311–11317.
Sahoo, C., Gupta, A. K., & Sasidharan Pillai, I. M. (2012). Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile wastewater. Journal of Environmental Science and Health, Part A, 47(10), 1428–1438.
Saifuddin, N., Wong, C., & Yasumira, A. A. (2009). Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-journal of Chemistry, 6(1), 61–70.
Sajid, M., & Płotka-Wasylka, J. (2020). Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchemical Journal, 154, 104623.
Santoso, I., Purnomo, W., Oktavianto, H., Sjamsuridzal, W., Handayani, W., & Sunardi. (2016). Isolation and characterization of potential bacteria with the ability to degrade Congo Red dye: AIP Publishing LLC, 020072.
Sarani, M., Joshaghani, A. B., Najafidoust, A., Asl, E. A., Hakki, H. K., Bananifard, H., & Sillanpaa, M. (2021). Sun-light driven photo degradation of organic dyes from wastewater on precipitation Ag2CrO4 over SiO2-aerogel and nano silica. Inorganic Chemistry Communications, 133, 108877.
Saraswathi, V. S., Kamarudheen, N., Bhaskararao, K., & Santhakumar, K. (2017). Phytoremediation of dyes using lagerstroemia speciosa mediated silver nanoparticles and its biofilm activity against clinical strains pseudomonas aeruginosa. Journal of Photochemistry and Photobiology B: Biology, 168, 107–116.
Saravanan, A., Kumar, P. S., Karishma, S., Vo, D.-V.N., Jeevanantham, S., Yaashikaa, P., & George, C. S. (2021). A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere, 264, 128580.
Sarkar, S., Banerjee, A., Halder, U., Biswas, R., & Bandopadhyay, R. (2017). Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conservation Science and Engineering, 2(4), 121–131.
Schaefer III, H. F., Thomas, J. R., Yamaguchi, Y., DeLeeuw, B. J., & Vacek, G. (1995). The chemical applicability of standard methods in ab initio molecular quantum mechanics TiO. Modern Electronic Structure Theory (In 2 Parts)-Part 1, 2, 1. 2: 1
Sehati, S., & Entezari, M. H. (2016). Sono-intercalation of cds nanoparticles into the layers of titanate facilitates the sunlight degradation of congo red. Journal of Colloid and Interface Science, 462, 130–139.
Selvaraj, V., Karthika, T. S., Mansiya, C., & Alagar, M. (2021). An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. Journal of Molecular Structure, 1224, 129195.
Sen, S. K., Raut, S., Bandyopadhyay, P., & Raut, S. (2016). Fungal decolouration and degradation of azo dyes: A review. Fungal Biology Reviews, 30(3), 112–133.
Sen, S. K., Raut, S., Bandyopadhyay, P., & Raut, S. (2016). Fungal decolouration and degradation of azo dyes: a review. Fungal Biology Reviews, 30(3), 112–133.
Shabir, G., Saeed, A., Arshad, M., & Channar, P. A. (2017). Synthesis, Characterization and Applications of High Fastness Reactive Dyes on Cotton Fibers. Journal of the Chemical Society of Pakistan, 39(6).
Sharma, V. K. (2009). Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—a review. Journal of Environmental Science and Health Part A, 44(14), 1485–1495.
Shu, J., Wang, Z., Huang, Y., Huang, N., Ren, C., & Zhang, W. (2015). Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. Journal of Alloys and Compounds, 633, 338–346.
Simon, J. E., Decker, E. A., Ferruzzi, M. G., Giusti, M. M., Mejia, C. D., Goldschmidt, M., & Talcott, S. T. J. J. O. F. S. (2017). Establishing Standards on Colors from Natural Sources., 82(11), 2539–2553.
Singh, P., & Borthakur, A. (2018). A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. Journal of Cleaner Production, 196, 1669–1680.
Singh, G., & Dwivedi, S. K. (2020). Decolorization and degradation of direct blue-1 (azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environmental Technology & Innovation, 18, 100751.
Singh, R. L., Singh, P. K., & Singh, R. P. (2015). Enzymatic decolorization and degradation of azo dyes–A review. International Biodeterioration & Biodegradation, 104, 21–31.
Singh, P., Ojha, A., Borthakur, A., Singh, R., Lahiry, D., Tiwary, D., & Mishra, P. K. (2016). Emerging trends in photodegradation of petrochemical wastes: A review. Environmental Science and Pollution Research, 23(22), 22340–22364.
Singh, A., Pal, D. B., Mohammad, A., Alhazmi, A., Haque, S., Yoon, T., Srivastava, N., & Gupta, V. K. (2022). Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresource Technology, 343, 126154.
Singh, P., Iyengar, L., & Pandey, A. (2012). Bacterial decolorization and degradation of azo dyes. Microbial degradation of xenobiotics (pp. 101-133). Springer, Berlin, Heidelberg.
Skanda, S., Bharadwaj, P., Kar, S., Sai Muthukumar, V., & Vijayakumar, B. (2021). Bioremoval capacity of recalcitrant azo dye Congo red by soil fungus Aspergillus arcoverdensis SSSIHL-01. Bioremediation Journal, 1–12.
Sohrabi, M. R., Khavaran, A., Shariati, S., & Shariati, S. (2017). Removal of carmoisine edible dye by fenton and photo fenton processes using taguchi orthogonal array design. Arabian Journal of Chemistry, 10, S3523–S3531.
Solís, M., Solís, A., Pérez, H. I., Manjarrez, N., & Flores, M. (2012). Microbial decolouration of azo dyes: A review. Process Biochemistry, 47(12), 1723–1748.
Solís, M., Solís, A., Pérez, H. I., Manjarrez, N., & Flores, M. (2012). Microbial decolouration of azo dyes: a review. Process Biochemistry, 47(12), 1723–1748.
Sosa-Martínez, J. D., Balagurusamy, N., Montañez, J., Peralta, R. A., Moreira, R. D. F. P. M., Bracht, A., Peralta, R. M., & Morales-Oyervides, L. (2020). Synthetic dyes biodegradation by fungal ligninolytic enzymes: Process optimization, metabolites evaluation and toxicity assessment. Journal of Hazardous Materials, 400, 123254.
Steingruber, E. (2000). Indigo and indigo colorants. Ullmann's Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a14_149.
Sudha, M., Saranya, A., Selvakumar, G., & Sivakumar, N. (2014). Microbial degradation of azo dyes: A review. International Journal of Current Microbiology and Applied Sciences, 3(2), 670–690.
Sundararajan, M., Sailaja, V., Kennedy, L. J., & Vijaya, J. J. (2017). Photocatalytic degradation of rhodamine b under visible light using nanostructured zinc doped cobalt ferrite: kinetics and mechanism. Ceramics International, 43(1), 540–548.
Swarnalatha, B., & Anjaneyulu, Y. (2004). Studies on the heterogeneous photocatalytic oxidation of 2, 6-dinitrophenol in aqueous TiO2 suspension. Journal of Molecular Catalysis A: Chemical, 223(1–2), 161–165.
Tahir, U., Yasmin, A., & Khan, U. H. (2016). Phytoremediation: Potential flora for synthetic dyestuff metabolism. Journal of King Saud University-Science, 28(2), 119–130.
Tama, J., Riyani, K., & Setyaningtyas, T. (2020). Effect of ultraviolet and visible lights on degradation of congo red dye using Fe2+/H2O2: Journal of Physics: Conference Series (Vol. 1494, No. 1, p. 012029). IOP Publishing.
Tapalad, T., Neramittagapong, A., Neramittagapong, S., & Boonmee, M. (2008). Degradation of congo red dye by ozonation. Chiang Mai Journal of Science, 35(1), 63–68.
Telke, A. A., Kalyani, D. C., Dawkar, V. V., & Govindwar, S. P. (2009). Influence of organic and inorganic compounds on oxidoreductive decolorization of sulfonated azo dye CI Reactive Orange 16. Journal of Hazardous Materials, 172(1), 298–309.
Telke, A. A., Joshi, S. M., Jadhav, S. U., Tamboli, D. P., & Govindwar, S. P. (2010). Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp SU-EBT. Biodegradation, 21(2), 283–296.
Telke, A. A., Joshi, S. M., Jadhav, S. U., Tamboli, D. P., & Govindwar, S. P. (2010). Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation, 21(2), 283–296.
Terna, A. D., Elemike, E. E., Mbonu, J. I., Osafile, O. E., & Ezeani, R. O. (2021). The future of semiconductors nanoparticles: Synthesis, properties and applications. Materials Science and Engineering: B, 272, 115363.
Terna, A. D., Elemike, E. E., Mbonu, J. I., Osafile, O. E., & Ezeani, R. O. (2021). The future of semiconductors nanoparticles: Synthesis, properties and applications. Materials Science and Engineering: B, 272, 115363.
Tofa, T. S., Kunjali, K. L., Paul, S., & Dutta, J. (2019). Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environmental Chemistry Letters, 17(3), 1341–1346.
Travis, A. S. (1990). Perkin’s mauve: Ancestor of the organic chemical industry. Technology and Culture, 31(1), 51–82.
Tröster, I., Fryda, M., Herrmann, D., Schäfer, L., Hänni, W., Perret, A., Blaschke, M., Kraft, A., & Stadelmann, M. (2002). Electrochemical advanced oxidation process for water treatment using DiaChem® electrodes. Diamond and Related Materials, 11(3–6), 640–645.
Tsuboy, M., Angeli, J., Mantovani, M., Knasmüller, S., Umbuzeiro, G., & Ribeiro, L. R. (2007). Genotoxic, mutagenic and cytotoxic effects of the commercial dye CI Disperse Blue 291 in the human hepatic cell line HepG2. Toxicology in vitro, 21(8), 1650–1655.
Uheida, A., Mejía, H. G., Abdel-Rehim, M., Hamd, W., & Dutta, J. (2021). Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. Journal of Hazardous Materials, 406, 124299.
Ullah, I., Haider, A., Khalid, N., Ali, S., Ahmed, S., Khan, Y., Ahmed, N., & Zubair, M. (2018). Tuning the band gap of TiO2 by tungsten doping for efficient UV and visible photodegradation of Congo red dye. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 204, 150–157.
Varshney, G., Kanel, S. R., Kempisty, D. M., Varshney, V., Agrawal, A., Sahle-Demessie, E., Varma, R. S., & Nadagouda, M. N. (2016). Nanoscale TiO2 films and their application in remediation of organic pollutants. Coordination Chemistry Reviews, 306, 43–64.
Vasantharaj, S., Sathiyavimal, S., Senthilkumar, P., Lewisoscar, F., & Pugazhendhi, A. (2019). Biosynthesis of iron oxide nanoparticles using leaf extract of ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. Journal of Photochemistry and Photobiology B: Biology, 192, 74–82.
Vidya, C., Manjunatha, C., Chandraprabha, M., Rajshekar, M., & Mal, A. R. (2017). Hazard free green synthesis of ZnO nano-photo-catalyst using Artocarpus heterophyllus leaf extract for the degradation of Congo red dye in water treatment applications. Journal of Environmental Chemical Engineering, 5(4), 3172–3180.
Vidya, C., Manjunatha, C., Chandraprabha, M., Rajshekar, M., & Mal, A. R. (2017). Hazard free green synthesis of zno nano-photo-catalyst using artocarpus heterophyllus leaf extract for the degradation of congo red dye in water treatment applications. Journal of Environmental Chemical Engineering, 5(4), 3172–3180.
Wang, N., Chu, Y., Zhao, Z., & Xu, X. (2017). Decolorization and degradation of congo red by a newly isolated white rot fungus ceriporia lacerata, from decayed mulberry branches. International Biodeterioration & Biodegradation, 117, 236–244.
Weldegebrieal, G. K. (2020). Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorganic Chemistry Communications, 120, 108140.
Wu, J., Gao, H., Yao, S., Chen, L., Gao, Y., & Zhang, H. (2015). Degradation of crystal violet by catalytic ozonation using fe/activated carbon catalyst. Separation and Purification Technology, 147, 179–185.
Xiong, Z., Ma, J., Ng, W. J., Waite, T. D., & Zhao, X. S. (2011). Silver-modified mesoporous TiO2 photocatalyst for water purification. Water Research, 45(5), 2095–2103.
Yang, W., Liu, X., Li, D., Fan, L., & Li, Y. (2015). Aggregation-induced preparation of ultrastable zinc sulfide colloidal nanospheres and their photocatalytic degradation of multiple organic dyes. Physical Chemistry Chemical Physics, 17(22), 14532–14541.
Yarahmadi, H., Duy, S. V., Hachad, M., Dorner, S., Sauvé, S., & Prévost, M. (2018). Seasonal variations of steroid hormones released by wastewater treatment plants to river water and sediments: Distribution between particulate and dissolved phases. Science of the Total Environment, 635, 144–155.
Yehia, R., & Rodriguez-Couto, S. (2017). Discoloration of the azo dye Congo red by manganese-dependent peroxidase from Pleurotus sajor caju. Applied Biochemistry and Microbiology, 53(2), 222–229.
Yousefi, S. R., Ghanbari, D., Salavati-Niasari, M., & Hassanpour, M. (2016). Photo-degradation of organic dyes: Simple chemical synthesis of Ni (OH) 2 nanoparticles, Ni/Ni (OH) 2 and Ni/NiO magnetic nanocomposites. Journal of Materials Science: Materials in Electronics, 27(2), 1244–1253.
Yusuf, M., Shabbir, M., & Mohammad, F. (2017). Natural colorants: Historical, processing and sustainable prospects. Natural Products and Bioprospecting, 7(1), 123–145.
Zabed, H. M., Akter, S., Yun, J., Zhang, G., Awad, F. N., Qi, X., & Sahu, J. (2019). Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renewable and Sustainable Energy Reviews, 105, 105–128.
Zabed, H., Sultana, S., Sahu, J. N., & Qi, X. (2018). An overview on the application of ligninolytic microorganisms and enzymes for pretreatment of lignocellulosic biomass. Recent advancements in biofuels and bioenergy utilization. 53–72.
Zaini, M. A. A., Cher, T. Y., Zakaria, M., Kamaruddin, M. J., Mohd Setapar, S. H., & Che Yunus, M. A. (2014). Palm oil mill effluent sludge ash as adsorbent for methylene blue dye removal. Desalination and Water Treatment, 52(19–21), 3654–3662.
Zelmanov, G., & Semiat, R. (2015). The influence of competitive inorganic ions on phosphate removal from water by adsorption on iron (Fe+ 3) oxide/hydroxide nanoparticles-based agglomerates. Journal of Water Process Engineering, 5, 143–152.
Zhang, F., Yin, X., & Zhang, W. (2016). Development of magnetic Sr5 (PO4) 3 (OH)/Fe3O4 nanorod for adsorption of Congo red from solution. Journal of Alloys and Compounds, 657, 809–817.
Zhang, C., Li, Y., Shuai, D., Zhang, W., Niu, L., Wang, L., & Zhang, H. (2018). Visible-light-driven, water-surface-floating antimicrobials developed from graphitic carbon nitride and expanded perlite for water disinfection. Chemosphere, 208, 84–92.
Zhang, C., Li, Y., Shuai, D., Shen, Y., & Wang, D. (2019). Progress and challenges in photocatalytic disinfection of waterborne viruses: A review to fill current knowledge gaps. Chemical Engineering Journal, 355, 399–415.
Zhang, C., Zhang, M., Li, Y., & Shuai, D. (2019). Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Applied Catalysis b: Environmental, 248, 11–21.
Zhang, T., Lowry, G. V., Capiro, N. L., Chen, J., Chen, W., Chen, Y., Dionysiou, D. D., Elliott, D. W., Ghoshal, S., & Hofmann, T. (2019). In situ remediation of subsurface contamination: Opportunities and challenges for nanotechnology and advanced materials. Environmental Science: Nano, 6(5), 1283–1302.
Zhao, J., Wu, T., Wu, K., Oikawa, K., Hidaka, H., & Serpone, N. (1998). Photoassisted degradation of dye pollutants. 3. Degradation of the cationic dye rhodamine B in aqueous anionic surfactant/TiO2 dispersions under visible light irradiation: Evidence for the need of substrate adsorption on TiO2 particles. Environmental Science & Technology, 32(16), 2394–2400.
Zhao, W., Wu, Z., Shi, H., & Wang, D. (2005). UV photodegradation of azo dye Diacryl Red X-GRL. Journal of Photochemistry and Photobiology A: Chemistry, 171(2), 97–106.
Zhao, X., Wang, W., Zhang, Y., Wu, S., Li, F., & Liu, J. P. (2014). Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for congo red. Chemical Engineering Journal, 250, 164–174.
Zhou, W., Zhang, W., & Cai, Y. (2021). Laccase immobilization for water purification: a comprehensive review. Chemical Engineering Journal, 403, 126272.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, R.R.M., Qamar, H., Hameed, A. et al. Biological and Photocatalytic Degradation of Congo Red, a Diazo Sulfonated Substituted Dye: a Review. Water Air Soil Pollut 233, 468 (2022). https://doi.org/10.1007/s11270-022-05935-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05935-9