Abstract
Creosote is a complex mixture containing mainly polycyclic aromatic hydrocarbons (PAHs). The remediation of creosote-contaminated sites becomes a challenge due to the numerous compounds and the specific soil properties. Treatability tests using advanced homogeneous (HM system) and heterogeneous (HT system) oxidative processes were applied with sandy soil artificially contaminated with creosote. The creosote was collected in a contaminated site in the state of São Paulo, Brazil. Sodium persulfate (SP) was the reaction oxidizing agent used. For the HM system, SP was activated by ferrous ions (Fe2+) chelated by citric acid (C6H8O7) and in the HT system, clay-based iron catalyst (CAT) was used for the SP activation. These two methods can be applied for in situ processes, without generating waste and effluents that need further treatment. Experimental designs were applied to determine the appropriate reagent concentrations to provide better removal efficiency for the total of 9 selected PAHs. As far as we know, this is the first study comparing homogeneous and heterogeneous systems while applying CAT to remediate PAH-contaminated soils. The results indicated that the HT system was more efficient than the HM system, with PAH removal of 97% and 61%, respectively. The treatability tests performed provide an efficient application of in situ chemical oxidation (ISCO) in tropical regions, such as Brazil, for the remediation of areas contaminated by PAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbon (PAH) compounds are one of the main contaminant groups found in São Paulo, Brazil, with 2719 contaminated sites (CETESB, 2019). The Superfund Remedy Report indicates that PAHs represent 47% of the contaminants found in soil at Superfund sites (US EPA, 2020). Due to the toxicity and complexity of these compounds, the remediation process is more difficult in sites where they are found (Duan et al., 2015). Creosote is an oil derived from the distillation of tar and consists of a complex mixture of different chemicals, formed mainly by PAHs, phenols, and heterocyclic compounds. This oil can be an example of a toxic and complex mixture that used to be consumed as a wood preservative, and can currently be found in the subsurface, polluting both soil and groundwater (Cargouët et al., 2018; Kueper et al., 2003). In addition, as creosote has a high viscosity and density greater than water, it presents a significant potential for migration. This behave can cause considerable impact on groundwater, which happens due to the partitioning of creosote’s components to the aqueous phase (Coll & Paschl, 2013). Thus, these substances are part of the main environmental problems of the last decades.
Studies developed mainly in countries such as Canada and USA or in Europe are not adapted to countries with tropical climate and their local conditions, like the climate, water, and soil characteristics. Tropical soils are deeper and warmer than temperate soils. The organic matter decomposition is faster, and plants in tropical soils absorb more water compared to temperate soils (Itthipoonthanakorn et al., 2019). Besides, in tropical regions, the soils are typically acidic, with low clay and organic matter contents and cation exchange capacity. Tropical soils present higher leaching than temperate soils (Payne & Edis, 2012), so the possibility of a contaminant reaching groundwater is greater. The Environmental Agency of the State of São Paulo (CETESB) published a document with 6285 contamination records (CETESB, 2019). In the State of Minas Gerais, 670 contaminated and rehabilitated sites were identified and registered (FEAM, 2019) and in the State of Rio de Janeiro, 328 contaminated and rehabilitated sites were reported (INEA, 2015). However, there are more than 23 states and the Federal District where no information was collected and published yet. Hence, in Brazil, for example, the number of potentially contaminated sites must be much greater. These statistics show the need for research and development of techniques for recovering contaminated sites in regions with tropical climate.
Oxidation processes are an efficient technique for eliminating a wide range of contaminants, such as in situ chemical oxidation (ISCO). ISCO consists in the injection of oxidants in the subsurface, in both saturated and unsaturated zones, to convert or mineralize organic pollutants into less harmful compounds (Ranc et al., 2016). Some advantages of ISCO are (1) reducing the contaminant mass in a relatively short period of time; (2) no generation of a large amount of waste; (3) the treatment can be implemented in a shorter period of time, comparing to other techniques; and (4) less rigorous monitoring is needed. Regarding the limitations, ISCO may be inefficient for determined contaminant and, depending on the amount of contaminant in the subsurface, a large amount of oxidant is required, then the cost–benefit ratio would be low (Pham et al., 2012). ISCO is also one of the remediation techniques applied in the State of São Paulo, with 364 cases registered (CETESB, 2019).
Beside other techniques, such as pump-and-treat and multiphase extraction reaction, ISCO proves to be a potentially viable technology to remediate creosote-contaminated sites (Forsey, 2004). Laboratory-scale treatability studies are used to predict the effectiveness of the remediation technique. The main oxidants applied are hydrogen peroxide, permanganates, persulfates, and ozone (Bendouz et al., 2017; Peluffo et al., 2016; Yen et al., 2011). Chemical oxidation with persulfate (S2O8−2) has been widely applied for the degradation of organic contaminants such as PAHs, trichlorethylene, BTEX, and 1,2,4-trimethylbenzene (Killian et al., 2007; Lemaire et al., 2013; Liang et al., 2006). Persulfate presents greater persistence in the subsurface, when compared to ozone and hydrogen peroxide, and can be transported for greater distances beside remaining longer in the reaction medium. In addition, it has less affinity for the soil natural organic compounds when compared to permanganate. This characteristic is important once the oxidation process is nonspecific, i.e., the oxidant in the medium can be consumed not only by the target pollutants, but also by the organic matter (Forsey, 2004; Oliveira et al., 2016; Ranc et al., 2016; Usman et al., 2012).
To enhance chemical oxidation efficiency, persulfate can be activated in different ways, such as the use of transition metals, for example, ferrous ions (Fe2+), heat, or alkaline activation (Matzek, 2016). Fe2+ activation is widely used, mainly for in situ applications, due to its natural abundance in porous media and benign nature. Depending on the activation method, the reaction will be homogeneous or heterogeneous, and both types have been discussed in several studies (Pham et al., 2009; Usman et al., 2012b; Pu et al., 2017; Silva-Rackov et al., 2017; Adityosulindro et al., 2018; Zhou et al., 2019; Magalhães et al., 2020). Possible mechanisms involve free radicals formed by Fe2+ in solution for homogeneous route or iron species on the surface of a catalyst for heterogeneous reactions (Bastidas et al., 2018; Magalhães et al., 2020; Pham et al., 2009).
In homogeneous routes, Fe2+ will remain in solution under acid conditions, which can negatively impact the natural media, disturbing the microbial community and plants growth (Peluffo et al., 2016; Ranc et al., 2016; Tsitonaki et al., 2010). The use of chelating agents keeps the iron species in solution at neutral conditions and allows the continuous release of the catalyst in the reaction medium (Yan and Lo, 2013; Ranc et al., 2016). Citric acid is an example of the chelating agents applied in oxidation reactions and was chosen to be used in this study for the homogeneous reactions due to its high efficiency (Killian et al., 2007; Liang et al., 2004; Zhao et al., 2013).
During heterogeneous reactions, the persulfate decomposition can occur via two mechanisms: 2 and 3 valent iron ions in solution (Fe2+ or Fe3+) and/or by iron species on the catalyst surface. When pH values are close to neutrality, the activation reactions will predominantly involve the iron stabilized on the catalyst surface, since the iron leaching to Fe2+ or Fe3+ form only occurs under acid conditions (Magalhães et al., 2020; Pham et al., 2009). Magalhães et al. (2020) evaluated the best synthesis conditions of a clay-based iron catalyst (CAT) to remediate phenanthrene-contaminated soil. The authors tested the catalyst to activate persulfate in the oxidation reaction and achieved about 80% of phenanthrene removal. The CAT will be applied here to treat creosote-contaminated soil in the heterogeneous reactions.
There is a wide variety of treatments for contaminated soil and groundwater in the literature. However, studies that assess the effectiveness of eliminating or reducing contaminants of complex mixtures in these media are still needed. To our knowledge, this is the first work that compares both homogeneous and heterogeneous systems applying CAT to remediate PAH-contaminated soils. These PAHs can be found in creosote, which is considered a complex mixture. So, this work aims at the application of homogeneous and heterogeneous systems in sandy soil artificially contaminated with creosote. Persulfate activation in the homogeneous reactions was carried out with ferrous ions (Fe2+) complexed by citrate ion. Clay-based iron catalyst (CAT) was applied in the heterogeneous reactions. The tests were performed based on Central Composite Designs.
2 Materials and Methods
2.1 Soil Characterization and Contamination
The clean soil used for the experiments was collected in São Paulo/SP, Brazil, at 4.6 m depth using a Shelby sampler. Granulometric characterization for the clean soil was performed through sedimentation process, beside pH in water and total organic carbon (TOC-L, SSM-5000A, Shimadzu) analyses, according to EMBRAPA Brazilian methods (2017).
The creosote used in this study was obtained by recovering this oil in a contaminated area which a wood treatment plant operated for 20 years. The creosote chemical characterization was performed by gas chromatography coupled with a mass spectrometer (GC–MS) after dilution in dichloromethane (DCM, 1:1000 v/v).
The soil was spiked with creosote dissolved in DCM to achieve a target concentration of 4500 mg of creosote per kg of soil (mg kg−1). The soil was mechanically mixed (15 rpm) for 24 h and maintained in a closed container for 2 days. Then, the container was kept open in the lab hood for about 90 min to allow DCM evaporation. Finally, it was sealed and stored under refrigerated conditions for the oxidation tests. The initial contaminant mass in the soil was analyzed in triplicate.
2.2 Oxidation Tests
Homogeneous (HM system) and heterogeneous (HT system) reactions were performed during the experiments and sodium persulfate (SP) was used as oxidant agent in both cases. The SP activation occurred with transition metal ions, such as ferrous ions (Fe2+). In the HM system, citric acid (C6H8O7) was used as chelating agent in attempt to provide the adequate proportion of Fe2+ in solution during the oxidation process. For the HT system, a clay-based iron catalyst (CAT) produced in previous work (Magalhães et al., 2020) was applied.
Experimental design was employed to evaluate the effects of independent variables on the selected response. In this study, we evaluated the creosote removal, focusing on the total mass of certain PAHs. The tests were based on a 2 k Central Composite Design (CCD) with 3 central points (CP), where k represents the number of independent variables studied. For the HM system, k = 3, so three independent variables were selected: sodium persulfate concentration ([SP], x1), ferrous sulfate concentration ([FeSO4], x2), and citric acid concentration ([C6H8O7], x3), resulting in 11 oxidation reactions (23 + 3CP). For the HT system, k = 2, so the independent variables were sodium persulfate concentration ([SP], x1) and catalyst mass (mCAT, x2), totaling 22 + 3CP = 7 oxidation reactions. Table 1 summarizes the reaction conditions and codified levels for each case. The reactions occurred for 72 h at 25 °C, without stirring, to simulate an approximate subsurface condition in Brazil.
For the tests, a soil to liquid ratio of 1:2 was maintained for both HM and HT systems. Besides, the range of iron concentration applied in each system was the same. Thus, in the HM system, 3.75 g of contaminated soil was added to a 20-mL crimped glass vial with 6.00 mL of sodium persulfate solution, 1.00 mL of ferrous sulfate solution, and 0.5 mL of citric acid solution (Killian et al., 2007; Zhao et al., 2013). For the HT system, we added 3.75 g of contaminated soil, 7.5 mL of sodium persulfate solution, and the catalyst mass according to the experimental design. In terms of the number of moles of SP, it varied between 9.45 × 10−4 mol (Level − 1) and 6.30 × 10−3 mol (Level + 1) for both systems. The respective concentrations and mass applied in the experimental design are shown in Table 1.
2.3 Chemical Analyses
At the end of the experiments, solid and liquid phases were separated by decantation. Solid–liquid and liquid–liquid extractions were performed according to 3550C and 3510C EPA Methods, respectively (US EPA, 1996, 2007), to analyze the residual concentration of certain PAHs in both phases. Glass fiber and sodium sulfate were used to filter the extract, which resulted in a volume of approximately 45 mL that was concentrated in the Kuderna-Danish evaporative concentrator coupled with a thermostatic bath (50 °C).
All the samples were analyzed by GC–MS and the analytical conditions were as follows: DB5MS 30 m × 0.25 mm capillary column; injection temperature of 280 °C and inlet pressure of 100 kPa; oven temperature varying from 40 to 320 °C at 10 °C min−1; 2 μL samples injected in splitless mode and helium as the carrier gas at constant flow rate (linear velocity 48.1 cm s−1 and column flow 1.78 mL min−1), with a total run time of 34 min. The following PAHs were chosen to be evaluated in this study because they were the most representative compounds in the creosote sample used: Naphthalene (NAP), 2-Methylnaphthalene (MNAP), Acenaphthene (ACE), Fluorene (FLU), Phenanthrene (PHE), Anthracene (ANT), Fluoranthene (FLT), Pyrene (PYR), and Benz[a]anthracene (BAA).
3 Results and Discussion
3.1 Soil Characterization and Contamination
The soil was classified as a sandy soil, based on the results of the granulometric distribution analysis: 93% of sand, > 5% of silt, and > 3% of clay (USDA, 1993). The total organic carbon (TOC) analysis resulted in a concentration of 0.13 mg kg−1, which is considered a low value, as expected for sandy soils. The soil pH in water was 5.7.
Table 2 shows the initial respective masses and concentrations in each process for the nine PAHs chosen to be evaluated after the oxidation reactions applied in this study. PHE presented the highest mass in the contaminated soil for both cases, followed by NAP, MNAP, ACE, and PYR. The coefficient of variation (CV) is within the 20% acceptable for soil studies, considering the matrix’s heterogeneity. The creosote used for the artificial contamination was characterized by Aranha et al. (2020).
According to the data in Table 2, NAP, PHE, and BAA had initial concentrations higher than the screening levels (CETESB, 2016; US EPA, 2019). These compounds are important creosote and PAH tracers (Simarro et al., 2011) and many researchers select them to verify the impact creosote can have on groundwater quality (Kueper et al., 2003). NAP is the most available one and can partition to the groundwater and to the vapor phase (Aranha et al., 2020), and this behavior may cause risks to the human health due to water ingestion and vapor inhalation. In this case, the remediation of the area will have to be assessed.
3.2 Oxidation Tests
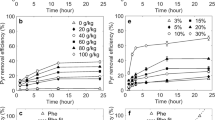
Table 3 shows the percentage of total PAH removal and the reaction conditions applied in each trial for the HM system. The total PAH removal percentage refers to the sum of the residual mass of the nine PAH studied compared to the initial mass in the soil. Trial 8 presented the best result for the total PAH removal (61.40% after 72 h), with the following reaction conditions: [SP] = 250 g L−1, [Fe2SO4] = 314.30 g L−1, and [C6H8O7] = 650 g L−1. Comparing trials 1 and 2, it is possible to note that higher [SP] increases the PAH removal in 18.10%. Besides, the importance of the chelating agent (C6H8O7) can be seen when analyzing trials 2 and 4, which shows that increasing [Fe2SO4] without changing the amount of chelating agent in the medium reduces PAH removal in about 18%. However, if the [C6H8O7] increases, PAH removal also increases in 36.40% (trials 4 and 8).
The regression coefficient obtained for the data (R2 = 0.84) presented a relatively good fit for experiments with soil, because of the complexity and heterogeneity of the soil matrix (Magalhães et al., 2020; Mendes et al., 2020). However, from the analysis of variance (ANOVA) in Table 4 and the F test, the empirical model was not significative at 95% confidence.
The results for the HT system are shown in Table 5. Trial 4 presented the best result, with 96.74% PAH removal. In this reaction, the independent variable conditions were [SP] = 200 g L−1 and mCAT = 3.00 g. For the same amount of CAT in the reaction, increasing [SP] resulted in an increase of 34% on the removal percentage, comparing trials 1 and 2. However, the variable mCAT has a greater influence on the response, since its increase promotes about 70% increase in PAH removal, comparing trials 1 and 3.
Considering that the catalyst is clay based, the adsorption properties of the clay could also be responsible for reducing the residual PAH concentration after the 72-h reaction. According to Magalhães et al. (2020) though, the specific surface area of CAT is equal to 25.59 m2 g−1, 82% lower than the raw clay used to produce it. Hence, the clay capacity to adsorb organic compounds was significantly reduced after the impregnation of iron oxides, which can activate the oxidant during the reaction.
The regression model obtained for the data in Table 5 also presented a regression coefficient of R2 = 0.84. The analysis of variance (ANOVA) and the F test shown in Table 6 indicate that the model was not significative at 95% confidence.
The experiment’s reproducibility was verified based on the results for the triplicate trials of the central point condition in both processes, and the variance coefficient was lower than 20% (Magalhães et al., 2020). According to the ANOVA, the lack of fit for the HT system was higher than for the HM system. However, the pure error was lower for the HT system. For both HM and HT systems, the best results were achieved with the highest levels of the independent variables (Table 1). In general, the HT system process presented better results for the PAH removal, with about 97% removal. Thus, the oxidation process with SP activation with the iron catalyst showed superior performance when compared to the homogeneous activation with chelated ferrous ions under similar experimental conditions. This conclusion was also obtained by Pulicharla et al. (2018), that studied the degradation of chlortetracycline.
After 72-h homogeneous reactions, the presence of bubbles was observed in the vials (Fig. 1). It is probably CO2, but not identified analytically. This behavior indicates the mineralization of the contaminants. The bubbles did not appear in the HT system experiments.
Despite the specific surface area of CAT being smaller than the raw clay employed in its production (Magalhães et al., 2020), the high removal percentages of HT system suggest that combined mechanisms of oxidation and sorption may be occurring. Luthy et al. (1997) and Rivas (2006) point out that PAHs can also interact with inorganic matrices. Thus, the double character exhibited by the modified clay (sorbent and catalyst) makes the CAT a promising material for use as a reactive medium in permeable reactive barriers (PRBs), aiming at the treatment of soils and groundwater contaminated with PAHs. Clay minerals are reported in the literature as efficient supports for heterogeneous catalysts in oxidation reactions, mainly for the treatment of contaminated groundwater (Bel Hadjltaief et al., 2014; Gao et al., 2016; Pouran et al., 2014; Wei et al., 2017; Zeng et al., 2017).
Both trials 8 and 4 of the HM and HT systems, respectively, were performed with the maximum levels of the experimental design and achieved the highest percentage removal. Table 7 shows the individual percentage removal results for each one of the 9 PAHs in these trials. In the HM system, ACE and ANT presented more than 90% removal. For the HT system, MNAP, ACE, PHE, and PYR stand out with removal percentage greater than 90%.
The relationship between the oxidation efficiency and each PAH species can be explained by the PAH reactivity to the sulfate radicals formed during the reaction. Angular PAHs usually exhibit slightly higher kinetic stability than linear PAHs, which can hinder the degradation reaction (Wang et al., 2014). For example, ACE, ANT, and PYR are expected to be easier to degrade than PHE and BAA, which can be seen in Table 7 for the HM system (trial #8). Besides, for the HM system, a greater degradation was obtained for NAP and MNAP, that have linear configuration, in opposed to PHE, which has an angular configuration. For the HT system (trial #4) though, while PYR and ACE presented the highest percent removal, as expected, PHE also achieved a high percent removal. In this case, the sorption process may have played an important role, contributing to this result.
In the HM system, the concentrations of NAP, MNAP, PHE, and BAA were above the screening levels (Table 8). After the HM system in trial #8, only MNAP reached a value below the US EPA (2019) screening level, considering a residential scenario. In the HT system (Table 8), NAP and PHE presented concentrations above the screening levels. After the HT system in trial #4, only PHE reached a value below CETESB (2016) screening level, considering a residential scenario, and BAA was not detected. In both processes, the contaminants were not identified in the aqueous phase.
Even though the remediation goal has not been reached for all the 9 selected PAHs, the result was relevant, considering the high initial PAH concentration, the reaction time evaluated, and the fact that only chemical oxidation technique was applied. In order to obtain higher degradation values, we suggest increasing the experimental scale, because the 20 mL volume is a limiting factor when treating high concentrations of contaminants. In addition, increasing the reaction time and testing sequential oxidant addition to limit parallel reactions that can interfere with the contaminant degradation efficiency may improve the removal percentage (Killian et al., 2007). Finally, the authors recommend that for complex contaminants such as creosote, and considering large-scale in situ application, the chemical oxidation process described in this work should be applied with other remediation techniques, such as bioremediation and electrokinetics (Isosaari et al., 2007; Kulik et al., 2006; Valderrama et al., 2009).
4 Conclusions
Creosote obtained from a contaminated area in the state of São Paulo (Brazil) was used for artificial contamination of sandy soil. The efficiency of the advanced chemical oxidation through homogeneous (HM) and heterogeneous (HT) systems was evaluated in this study. Treatability tests were performed to assess the efficiency of in situ chemical oxidation (ISCO) in tropical soils for remediation of PAH-contaminated areas. For the best conditions studied, the HM system achieved approximately 61% removal of the selected PAHs. The HT system achieved 97% removal. In the HT systems, a clay-based iron catalyst (CAT) was used. The use of clay has advantages such as low cost; innocuous material; and little or no chemical alterations in the subsurface, when compared to conventional advanced oxidation methods.
The knowledge of the oxidative process efficiency for the specific conditions of a certain contaminated area is important to evaluate the process viability, mainly considering the tropical climate. In Brazil, for example, remediation techniques such as pumping and treatment, which generates waste and has long operating time, are still applied. ISCO can reduce the contaminant mass in the subsurface without generating waste in a shorter remediation period.
Future studies can evaluate the process optimization, distinguishing the results between sorption and degradation phenomena, beside simulating the process in a column and/or pilot scale and evaluating the best configuration for reagents injection in the subsurface.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Adityosulindro, S., Julcour, C., & Barthe, L. (2018). Heterogeneous Fenton oxidation using Fe-ZSM5 catalyst for removal of ibuprofen in wastewater. Journal of Environmental Chemical Engineering. https://doi.org/10.1016/j.jece.2018.09.007
Aranha, R. M., Magalhães, V. M. A., Mendes, G. P., Soares, L. C. R., Barbosa, A. M., Nascimento, C. A. O., Vianna, M. M. G. R., & Chiavone-Filho, O. (2020). Characterization and partitioning behavior of creosote in different matrices: Soil, water and air. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-020-04772-y
Bastidas, K. G., Sierra, C. A., & Ramirez, H. R. Z. (2018). Heterogeneous Fenton oxidation of Orange II using iron nanoparticles supported on natural and functionalized fique fiber. Journal of Environmental Chemical Engineering. https://doi.org/10.1016/j.jece.2018.06.001
Bel Hadjltaief, H., Costa, P., Beaunier, P., Gálvez, M. E., & Ben Zina, M. (2014). Fe-clay-plate as a heterogeneous catalyst in photo-Fenton oxidation of phenol as probe molecule for water treatment. Applied Clay Science. https://doi.org/10.1016/j.clay.2014.01.02
Bendouz, M., Dionne, J., Tran, L. H., Coudert, L., Mercier, G., & Blaiz, J. F. (2017). Polycyclic aromatic hydrocarbon oxidation from concentrates issued from an attrition process of polluted soil using the Fenton reagent and permanganate. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-017-3292-x
Cargouët, M., Jeannee, N., Vidart, B., & Gregori, P. (2018). Polycyclic aromatic hydrocarbon (PAH) levels in environmental media potentially impacted by reused or stored creosote-treated railway ties. Environmental Science and Pollution Research 25.https://doi.org/10.1007/s11356-018-1910-9
CETESB, (2016). Valores Orientadores para Solos e Águas Subterrâneas no Estado de São Paulo. São Paulo, Brasil. Retrieved April 28, 2021, from https://cetesb.sp.gov.br/aguas-subterraneas/wp-content/uploads/sites/13/2013/11/tabela_vos_2016_site.pdf
CETESB, (2019). Relatório de áreas contaminadas e reabilitadas no Estado de São Paulo. Companhia Ambiental do Estado de São Paulo, Brasil. Retrieved April 23, 2021, from https://cetesb.sp.gov.br/areas-contaminadas/wp-content/uploads/sites/17/2020/02/TEXTO-EXPLICATIVO-2019-12.02.20.pdf.
Coll, F. R., & Paschl, K. P. (2013). Creosote DNAPL recovery-well design for mass removal. Remediation Journal. https://doi.org/10.1002/rem.21346
Duan, L., Naidu, R., Thavamani, P., Meaklim, J., & Megharaj, M. (2015). Managing long-term polycyclic aromatic hydrocarbon contaminated soils: A risk-based approach. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-013-2270-0
EMBRAPA - Centro Nacional de Pesquisa de Solos. (2017). Manual de Métodos de Análise de Solo. 3 Ed. Centro Nacional de Pesquisa de Solos. Empresa Brasileira de Pesquisa Agropecuária Rio de Janeiro, Brasil.
FEAM (2019) Inventário de áreas contaminadas de minas gerais. Fundação Estadual do Meio Ambiente. Belo Horizonte, Brasil. Retrieved April 23, 2021, from http://www.feam.br/images/stories/2019/GEST%C3%83O_AREAS_CONTAMINADAS/Invent%C3%A1rio_de_%C3%A1reas_contaminadas_2019.pdf
Forsey, S. (2004). In situ chemical oxidation of creosote/coal tar residuals: Experimental and numerical investigation. UW Space. Retrieved April 28, 2021, from http://hdl.handle.net/10012/1275
Gao, Y., Zhang, Z., Li, S., Liu, J., Yao, L., Li, Y., & Zhang, H. (2016). Insights into the mechanism of heterogeneous activation of persulfate with a clay/iron-based catalyst under visible LED light irradiation. Applied Catalysis b: Environmental. https://doi.org/10.1016/j.apcatb.2015.12.002
INEA. (2015). Cadastro de áreas contaminadas no Estado do Rio de Janeiro - 3ª edição. Instituto Estadual do Ambiente. Rio de Janeiro, Brasil. Retrieved April 23, 2021, from http://www.inea.rj.gov.br/Portal/Agendas/LicenciamentoAmbiental/Licenciamento-saiba-mais/GestaodeRiscoAmbientalTec/AvaliacaodeAreasContaminadas/index.htm
Isosaari, P., Piskonen, R., Ojala, P., Voipio, S., Eilola, K., Lehmus, E., & Itävaara, M. (2007). Integration of electrokinetics and chemical oxidation for the remediation of creosote-contaminated clay. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2006.10.068
Itthipoonthanakorn, T., Dann, S. E., Crout, N. M. J., & Shaw, G. (2019). Nuclear weapons fallout 137Cs in temperate and tropical pine forest soils, 50 years post-deposition. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.01.073
Killian, P. F., Bruell, C. J., Liang, C., & Marley, M. C. (2007). Iron (II) activated persulfate oxidation of MGP contaminated soil. Soil & Sediment Contamination. https://doi.org/10.1080/15320380701623206
Kueper, B. H., Lerner, D. N., Wealthall, G. P., Smith, J. W. N., & Leharne, S. A. (2003). An illustrated handbook of DNAPL transport and fate in the subsurface. Research Report. Environment Agency, Bristol, UK.
Kulik, N., Goi, A., Trapido, M., & Tuhkanen, T. (2006). Degradation of polycyclic aromatic hydrocarbons by combined chemical pre-oxidation and bioremediation in creosote contaminated soil. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2005.05.005
Lemaire, J., Buès, M., Kabeche, T., Hanna, K., & Simonnot, M. O. (2013). Oxidant selection to treat an aged PAH contaminated soil by in situ chemical oxidation. Journal of Environmental Chemical Engineering. https://doi.org/10.1016/j.jece.2013.09.018
Liang, C., Bruell, C. J., Marley, M. C., & Sperry, K. L. (2004). Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere. https://doi.org/10.1016/j.chemosphere.2004.01.030
Liang, C., Wang, Z. S., & Mohanty, N. (2006). Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 °C. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2006.08.028
Luthy, R. G., Aiken, G. R., Brusseau, M. L., Cunningham, S. D., Gschwend, P. M., Pignatello, J. J., Reinhard, M., Traina, S. J., Weber, W. J., & Westall, J. C. (1997). Sequestration of hydrophobic organic contaminants by geosorbents. Environmental Science and Technology. https://doi.org/10.1021/es970512m
Magalhães, V. M. A., Mendes, G. P., Costa-Filho, J. D. B., Cohen, R., Partiti, C. S., Vianna, M. M., & Chiavone-Filho, O. (2020). Clay-based catalyst synthesized for chemical oxidation of phenanthrene contaminated soil using hydrogen peroxide and persulfate. Journal of Environmental Chemical Engineering.https://doi.org/10.1016/j.jece.2019.103568
Matzek, L. W., & Carter, K. E. (2018). Activated persulfate for organic chemical degradation: A review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2016.02.055
Mendes, G. P., Magalhães, V. M. A., Soares, L. C. R., Aranha, R. M., Nascimento, C. A. O., Vianna, M. M. G. R., & Chiavone-Filho, O. (2020). Treatability studies of naphthalene in soil, water and air with persulfate activated by iron(II). Journal of Environmental Sciences. https://doi.org/10.1016/j.jes.2019.11.015
Oliveira, F. C., Freitas, J. G., Furquim, S. A. C., Rollo, R. M., Thomnson, N. R., Alleoni, L. R. F., & Nascimento, C. A. O. (2016). Persulfate interaction with tropical soils. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-016-3000-2
Payne, T. E., & Edis, R. (2012). Chapter 3 - Mobility of radionuclides in tropical soils and groundwater, Editor(s): John R. Twining, Radioactivity in the environment, Elsevier, Volume 18. Pages 93–120, ISSN 1569–4860, ISBN 9780080450162. https://doi.org/10.1016/B978-0-08-045016-2.00003-5.
Peluffo, M., Pardo, F., Santos, A., & Romero, A. (2016). Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2015.09.034
Pham, A. L. T., Lee, C., Doyle, F. M., & Sedlak, D. L. (2009). A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environmental Science and Technology. https://doi.org/10.1021/es902296k
Pham, A. L., Doyle, F. M., & Sedlak, D. L. (2012). Inhibitory effect of dissolved silica on H2O2 decomposition by iron(III) and manganese(IV) oxides: Implications for H2O2 -based in situ chemical oxidation. Environmental Science & Technology. https://doi.org/10.1021/es203612d
Pouran, S. R., Raman, A. A. A., & Daud, W. M. A. (2014). Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2013.09.013
Pu, M., Ma, Y., Wan, J., Wang, Y., Wang, J., & Brusseau, M. L. (2017). Activation performance and mechanism of a novel heterogeneous persulfate catalyst: Metal-organic framework MIL-53(Fe) with FeII/FeIII mixed-valence coordinatively unsaturated iron center. Catalysis Science and Technology. https://doi.org/10.1039/C6CY02355J
Pulicharla, R., Drouinaud, R., Brar, S. K., Drogui, P., Proulx, F., Verma, M., & Surampalli, R. Y. (2018). Activation of persulfate by homogeneous and heterogeneous iron catalyst to degrade chlortetracycline in aqueous solution. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.05.134
Ranc, B., Faure, P., Croze, V., & Simonnot, M. O. (2016). Selection of oxidant doses for in situ chemical oxidation of soils contaminated by polycyclic aromatic hydrocarbons (PAHs): A review. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2016.03.068
Rivas, F. J. (2006). Polycyclic aromatic hydrocarbons sorbed on soils: A short review of chemical oxidation based treatments. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2006.07.048
Silva-Rackov, C. K. O., Aguiar, L. G., Souza, A. R., Silva, S. S. O., Câmara, A. G., Vianna, M. M. G. R., Foletto, E. L. F., Nascimento, C. A. O., & Chiavone-Filho, O. (2017). Remediation of phenanthrene-contaminated soil by persulfate activated with Fe-modified diatomite: Kinetic and statistical approaches. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-017-3456-8
Simarro, R., González, N., Bautista, L. F., Sanz, R., & Molina, M. C. (2011). Optimisation of key abiotic factors of PAH (naphthalene, phenanthrene and anthracene) biodegradation process by a bacterial consortium. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-010-0593-8
Tsitonaki, A., Petri, B., Crimi, M., Mosbæk, H., Siegrist, R. L., & Bjerg, P. L. (2010). In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Critical Reviews in Environmental Science and Technology. https://doi.org/10.1080/10643380802039303
US EPA - United States Environmental Protection Agency. (1996). Method 3510C - Separatory funnel liquid-liquid extraction. Retrieved May 15, 2021, from https://www.epa.gov/sites/production/files/2015-12/documents/3510c.pdf
US EPA - United States Environmental Protection Agency. (2019). Regional Screening Level (RSL) Summary Table (TR=1E-06, HQ=1). Retrieved May 16, 2021, from https://semspub.epa.gov/work/HQ/199628.pdf
US EPA- United States Environmental Protection Agency. (2007). Method 3550C - Ultrasonic extraction. Retrieved March 25, 2021, from https://www.epa.gov/sites/production/files/2015-12/documents/3550c.pdf. Accessed 22 May 2021
US EPA - United States Environmental Protection Agency. (2020). Superfund Remedy Report. 16th Edition. Retrieved March 25, 2021, from https://www.epa.gov/sites/production/files/2020-07/documents/100002509.pdf
USDA - United States Department of Agriculture. Soil Survey Division Staff. (1993). Clarification of soil texture class boundaries. Retrieved March 05, 2021, from https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_031477.pdf
Usman, M., Faure, P., Hanna, K., Abdelmoula, M., & Ruby, C. (2012). Application of magnetite catalyzed chemical oxidation (Fenton-like and persulfate) for the remediation of oil hydrocarbon contamination. Fuel. https://doi.org/10.1016/j.fuel.2012.01.017
Valderrama, C., Alessandri, R., Aunola, T., Cortina, J. L., Gamisans, X., & Tuhkanen, T. (2009). Oxidation by Fenton’s reagent combined with biological treatment applied to a creosote-comtaminated soil. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2008.11.108
Wang, Y., Zhang, W., Fan, R., Sheng, G., & Fu, J. (2014). Biological monitoring of environmental exposure to polycyclic aromatic hydrocarbons in subjects living in the area of recycling electronic garbage, in Southern China. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-014-2869-9
Wei, X., Wu, H., & Sun, F. (2017). Magnetite/Fe-Al-montmorillonite as a Fenton catalyst with efficient degradation of phenol. Journal of Colloid and Interface Science. https://doi.org/10.1016/j.jcis.2017.05.110
Yan, D. Y. S., & Lo, I. M. C. (2013). Removal effectiveness and mechanisms of naphthalene and heavy metals from artificially contaminated soil by iron chelate-activated persulfate. Environmental Pollution. https://doi.org/10.1016/j.envpol.2013.02.030
Yen, C., Chen, K., Kao, C., Liang, S., & Chen, T. (2011). Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2010.12.129
Zeng, Q., Dong, H., Wang, X., Yu, T., & Cui, W. (2017). Degradation of 1, 4-dioxane by hydroxyl radicals produced from clay minerals. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2017.01.040
Zhao, D., Liao, X., Yan, X., Hulling, S. G., Chai, T., & Tao, H. (2013). Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2013.03.056
Zhou, Z., Liu, X., Sun, K., Lin, C., Ma, J., He, M., & Ouyang, W. (2019). Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chemical Engineering Journal. https://doi.org/10.1016/j.cej.2019.04.213
Acknowledgements
The authors would like to express their gratitude to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Project PROCAD No. 88887.124192/2014-00) and to the Technological Research Institute (IPT) and its foundation (FIPT), through the Novos Talentos Program.
Funding
This research was supported by the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES – Project PROCAD-CAPES No. 88881.068433/2014–01) and the Technological Research Institute (IPT) and its foundation (FIPT), through the Novos Talentos Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors agree to participate in this publication.
Consent for Publication
All authors agree with the publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magalhães, V.M.A., Aranha, R.M., Mendes, G.P. et al. Homogeneous and Heterogeneous Advanced Oxidation Processes: Treatability Studies on Artificially Contaminated Soils with Creosote. Water Air Soil Pollut 233, 29 (2022). https://doi.org/10.1007/s11270-022-05498-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05498-9