Abstract
Stormwater harvesting and reuse is an attractive option to lower the demand placed on other sources of water supply. However, it contains a wide range of pollutants that need to be removed before it can be reused or even discharged to the waterways and receiving waters. An experimental protocol to estimate the efficiency of a soil-based-filter medium for the treatment of stormwater pollutants from 1 to 3 years rainfall experienced in the field was developed using a laboratory column-set-up over short-term duration. The filter removed substantial amounts of PO4-P and NH4-N for up to 8 h at a flow velocity of 100 mm/h which is a 1-year time-equivalent of rainfall at a locality in Sydney, Australia. An addition of 10% zeolite to the soil-based filter extended the column saturation period to 24 h. The breakthrough data for PO4-P and NH4-N were satisfactorily described by the Thomas model. The majority of the nine heavy metals tested were removed by more than 50% for up to 4 h in the soil-based filter. This level of removal increased to 16 h when 10% zeolite was added to the filter. The column with the soil-based filter + 10% zeolite had higher affinity for Pb, Cu, Zn, and As than Ni, with Pb having the highest percentage removal. Soil-based filter + 10% zeolite removed considerable amounts of 3 polycyclic aromatic hydrocarbons (PAHs) (30–50%), while soil-based filter + 10% zeolite + 0.3% granular activated carbon removed 65 to > 99% of the PAHs at 24-h operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water scarcity due to increasing human population, industries, and intensive agriculture, and persistent drought is driving many countries around the world to explore alternative freshwater resources. This has encouraged the harvesting and reusing of stormwater to lower the demand placed on municipal water supplies. If the stormwater is not harvested for reuse, it can freely runoff and increase pollution of natural waterways. However, stormwater contains a wide range of pollutants (e.g., suspended solids, nutrients, heavy metals, polycyclic aromatic hydrocarbons (PAHs) that need to be removed before the water can be reused or discharged to the waterways.
Biofiltration is a comparatively recent technique to treat stormwater and remove a wide range of pollutants. It is popular in field applications due to its simplicity and cost effectiveness (Hatt et al. 2009a, b; Henderson et al. 2007; Hsieh and Davis 2005). Biofiltration systems are configured as vegetated filtration trenches or basins with an underlying porous collection pipe and are designed to remove fine suspended solids and dissolved pollutants. Many studies have been carried out on vegetated biofiltration systems for removal of nutrients (Chen et al. 2013; Kim et al. 2003), and heavy metals (Li and Davis 2008). However, pollutant removal capacities of the filter medium below the vegetation level are poorly understood. Treatment reliability is an important issue highlighted by the number of studies that report significant variations in pollutant removal rates. Pollution retention through bio-filter systems occurs mainly by evaporation, filtration, adsorption to the medium, precipitation and biological intakes by plants and microbes. However, the short contact time between pollutants, and vegetation may reduce the biological uptake of nutrients and heavy metals from the stormwater (Denman et al. 2006). Hence, it is important to improve the filter medium of the biofiltration system to enhance pollutant removal by adsorption or precipitation when stormwater percolates through the medium.
The present study evaluated the performance of a soil medium (R165) proposed for use as a filter below the vegetation in biofiltration basins in Blacktown, New South Wales, Australia, to remove targeted pollutants. Apart from the performance of R165, other filter media such as zeolite and granular activated carbon (GAC) were mixed in with R165 to evaluate if pollutant removal could be further improved. Zeolite is well known for removing specific types of heavy metals (Nguyen et al. 2015) and ammonium (Cooney et al. 1999a, b) by cation exchange mechanism (Wang and Peng 2010). GAC is one of the most abundant adsorbents commonly used in water treatment and very effective in removing a wide range of organic pollutants including polycyclic aromatic hydrocarbons (PAH) and heavy metals through hydrophobic and electrostatic interactions, hydrogen bonding and chelation mechanisms (Eeshwarasinghe et al. 2018, 2019; Sounthararajah et al. 2016; Valderrama et al. 2008). The results of this study provide an in-depth knowledge on the application of zeolite and GAC as filter media for stormwater harvesting and reuse. The originality of this study is the development of a novel experimental protocol to estimate the efficiency of a filter medium for the treatment of stormwater pollutants from 1 to 3 years rainfall experienced in the field using a laboratory column-set-up over short-term duration. The pollutants’ removals by the filter medium were estimated by incorporating three adsorbents of contrasting properties which is another novelty of the study.
2 Materials and Methods
The laboratory testing mimics the 1–3-year rainfall conditions experienced in Blacktown, Sydney, Australia, using a high flow rate of water continuously through a column containing a soil medium so that the total water input to the column for 8 hours is equivalent to 1 year of rainfall falling over the field site (calculations shown in Methods section). Column studies were carried out to assess the performance of the R165 soil media, which is proposed for use in Blacktown’s biofiltration basins. The improvements in pollutant removal by incorporating small amounts of GAC (0.1-1%) and Zeolite (1-20%) with R165 were also evaluated.
2.1 Adsorbents
Zeolite
The zeolite used in the study is a locally available material which exhibits good. adsorption capacity towards heavy metals ((Nguyen et al. 2015). The zeolite was sourced from a natural deposit at Werris Creek, New South Wales and supplied by Zeolite Australia Pty Ltd., Australia. The Brunauer–Emmett–Teller (BET) surface area of zeolite was 15.4 g/m2 (Nguyen et al. 2015). The zero point of charge (ZPC, the pH at which the net surface charge is zero) of zeolite was 2.2 (Nguyen et al. 2015), suggesting that at the pH of most stormwaters (which is 6-7) the net surface charge on this material is negative and favourable for the adsorption of positively charged metals and ammonium. The zeta potential value of zeolite is -17 mv at pH 6.5 (Nguyen et al. 2015).
GAC
GAC (particle sizes of between 0.3 and 2.4 mm) was purchased from James Cummins P/L, Australia. A particle size range of 0.4–2 mm was collected by sieving the original material and used in the experiments. The BET surface area, pore volume, and average pore diameter of the GAC were 1000 m2/g, 0.69 cm3/g, and 2.7 nm, respectively The scanning electron micrographs of the GAC revealed the presence of large numbers of micropores and mesopores, which results in GAC having a high surface area (Eeshwarasinghe et al. 2018).
R165
The characteristics of this biofiltration media are presented in Table 1. It shows that the media is loamy sand containing 95% sand.
2.2 Synthetic Stormwater
The characteristics of stormwater were determined from field samplings of stormwater during several rainfall events that occurred in Blacktown. This information was used here to prepare a synthetic stormwater solution containing the pollutants at concentrations that closely match field conditions in Blacktown.
Synthetic stormwater was used for all experiments. The use of synthetic stormwater circumvents the need to collect large qualities of stormwater in the field in Blacktown, store, and transport to the laboratory for testing. The concentrations of the constituents of interest in the synthetic stormwater were adjusted to the expected range that occurs in Blacktown. The concentrations remain the same throughout all tests providing an easy comparison of performance of different media used in the various column studies. This would not be possible if actual stormwater that was collected in the field were used. The composition of synthetic stormwater is shown in Table 2.
Analytical grade nitrate salts of heavy metals were used. Polycyclic aromatic hydrocarbons (PAHs) employed in the adsorption experiments were acenaphthene, phenanthrene, and pyrene. All chemicals were obtained from Sigma-Aldrich (USA).
3 Methods
3.1 Column Experiment
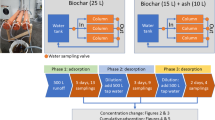
Column with dimensions 18.5 cm (diameter) × 18.5 cm (height) was used in the experiments. The R165 media was packed to a depth of 50 mm in the column. Coarse sand layers and gravels were placed above and below R165 as shown in Fig. 1. A layer of glass beads was placed on the top surface of the column for the added water to flow evenly through the column. The stormwater was passed through the column at a flow velocity of 100 mm/h for most experiments and at 300 mm/h for a few experiments. Downflow mode of operation was employed and effluent was collected at the bottom of the column at various times during the experiment. Pollutant concentrations in the influent solution (C0) and in the effluent solution at various times (t) (C) were measured and the breakthrough curves (C0/C vs time) were plotted. The breakthrough curves show the time-wise progress in pollutants removal by the column. The experimental set-up is illustrated in Fig. 1.
The breakthrough data for NH4+ and PO43- were simulated using Thomas model (Thomas 1944; Nur et al. 2015; Eeshwarasinghe et al. 2018). Thomas model equation is given below.
where, kT is the Thomas rate constant (mL/min.mg), q0 is the maximum solid-phase concentration of the solute (mg/g), mC is the mass of adsorbent in the column (g), Q is the volumetric flow rate (mL/min), Co (mg P/L) is the inlet phosphate concentration; C (mg P/L) is the outlet phosphate concentration at time t (min). The values for kT and q0 were determined from the slope and intercept of a linear plot of ln (C0/C -1) vs t.
Rainfall Intensity
Laboratory testing was conducted to mimic rainfall that occurs in Blacktown. The annual rainfall in Blacktown is 800 mm. The water flow through the column in experiments where the flow velocity is 100 mm/h (0.1 m/h) over an 8-h duration is equivalent to 1 year of rainfall (800 mm/year). The rate of stormwater inflow to column at the rainfall intensity of 0.1 m/h (superficial velocity 100 mm/h, interstitial velocity 100 mm/h/porosity (0.3) = 333 mm/h) and 0.3 m/h (superficial velocity 300 mm/h, interstitial velocity 1000 mm/h) used in the study were 45 ml/min and 135 ml/min, respectively as shown below. These interstitial velocities are much higher than those reported for field soils (0-100 cm/day or 0-42 mm/h, Schneider et al. 2019). The higher velocities used in this study is to mimic 1-3 years of rainfall in the field equivalent to 8-24 h of continuous water flow in the laboratory column set-up.
The surface area of the column is 22/7 × 0.185/2 × 0.185/2 m2 = 0.0269 m2
For the rainfall intensity of 0.1 m/h, the rate of stormwater inflow to the column is 0.1 m/h × 0.0269 m2 = 0.00269 × 106 cm3/h = 2690 mL/h = 2690/60 mL/min or 45 mL/min
Similarly, for a rainfall intensity of 0.3 m/h, the rate would be 135 mL/min
The experiments were conducted until the media was exhausted for up to 25–30 h (run intermittently for 8 h/day) representing intermittent rainfall.
GAC, Zeolite Additives to Enhance Biofilter Performances
Experiments were initially conducted using two separate columns, one with a mix of zeolite and R165 (1% Zeolite+R165) and the other with GAC and R165 (1% GAC+R165) at a flow velocity of 100 mm/h to evaluate any improvement to the performance of the bio-retention R165 media. Based on the outcome of these two experiments, further column experiments were carried out to test the improvement with varying amounts of zeolite (5%, 10%, 20%) and 0.3% GAC mixed with R165. Experiments conducted are summarized in Table 3. The percentage of GAC was reduced from that used in the initial experiments to reduce the cost of a filter that would be installed in the field.
3.2 Measured Chemical Parameters
Concentrations of dissolved nutrients (total N (TN), NH4+, total Kjeldahl N (TKN), PO43-, total P (TP)), and metals (Na+, K+, Ca2+, Mg2+, Pb2+, Cu2+, Zn2+, AsO43-, Fe3+, Mn2+, Al3+, Ni2+) in the influent and effluent were measured. Polycyclic aromatic hydrocarbons (acenaphthene, phenanthrene and pyrene) were measured only 2 times per experiment, once at the start of the experiment and secondly at the end, to avoid the expensive analytical costs. Other parameters (pH, turbidity, DO, EC, and DOC) were measured on an hourly basis. Analytical methods used in this study are presented in Table 4. The variation of flow rate over time and the degree of clogging of the filter medium were monitored continuously.
4 Results and Discussion
4.1 Nutrients Removal
PO4-P Removal
The removal rates of PO4-P during four columns test run at 100 mm/h are shown in Fig. 2a. The results show that the proportion of PO4-P removed decreased with time. The R165 media was exhausted within 15 h (equivalent to less than 2 years rainfall) in terms of removing PO4-P. The addition of zeolite in larger amounts (or higher percentages) improved PO4-P removal rates extending the life of the R165 media up to more than 40 h (equivalent to 5 years rainfall) depending on the amount of zeolite used. More than 50% of the PO4 was removed by R165 + 10 % zeolite media for up to 20 h (equivalent to 2.5 years rainfall).
NH4-N
The removal of NH4-N during four columns test runs at 100 mm/h is shown in Fig. 2b. As in the case of PO4-P removal, the tests show that the R165 media was exhausted within 15 h. The addition of zeolite at increasing percentages improved NH4-N removal rates. The life of the media in the case of a 20% zeolite addition to R165 was extended to over 40 h (equivalent to 5 years rainfall).
Previous research concluded that NH4+ and PO43- were simultaneously removed from an aqueous solution using zeolites (Sun et al. 2011; Wild et al. 1996). NH4+ was removed by a cation exchange reaction on the zeolite, whereas the Ca2+ in zeolite contributed to the removal of PO43- due to the formation of the surface precipitate of Ca3(PO4)2. Another explanation for the enhanced adsorption of PO43- is bridge provided by the positively charged Ca (Ca2+) between the negatively charged zeolite and PO43- (Zhan et al. 2017). Cooney et al. (1999a, b) investigated the capability of using Australian natural zeolite to remove NH4+ employing a fixed-bed ion-exchange process and achieved high removal efficiencies from wastewater. Furthermore, they observed a reduction of adsorption capacity of NH4+ in the presence of Ca2+, Mg2+, and K+ because these cations competed with NH4+ for adsorption.
Nutrients (NH4-N and PO4-P) removal by the 10% Zeolite+R165 media at a flow velocity of 300 mm/h was also investigated (Fig. 3). According to the breakthrough curve, the removal capacity decreased over the time and the column became saturated within 5 h. This means that the media would only be sufficient to remove a 1-2 years equivalent pollution load under this flow velocity. This is because at higher flow rate, a larger amount of nutrients enters the column per unit time and the media’s capacity to adsorb nutrients becomes more quickly exhausted.
The results of the nutrients adsorption study showed that the column with R165 alone is not satisfactorily for removing both NH4+ and PO43- for water discharge into a freshwater body to comply with the recommended Australian guidelines (ANZEC 2000). In contrast, the R165 column mixed with at least 10% zeolite is capable of sufficiently removing the nutrients for safe water discharge into a freshwater body (Supplementary Data I).
The Thomas model satisfactorily described the NH4+ and PO43- breakthrough at the two flow velocities tested (Figs. 2 and 3) as shown by the high coefficient of determinations (R2) (0.72–0.99, Tables 5 and 6). Increased percentage of zeolite in the filter medium increased the Thomas adsorption capacity for both NH4+ and PO43- at the flow velocity of 100 mm/h (Table 5). When the flow velocity was increased, the adsorption capacity decreased for the 10% zeolite column (Tables 5 and 6). This is probably because at higher flow velocity the nutrients had less time to interact with zeolite and get adsorbed (Nur et al. 2015).
Previous researchers have observed effective removal of total P (TP) by bio-filter media in laboratory scale experiments (Davis et al. 2003; Fletcher et al. 2007; Henderson et al. 2007). However, they reported high variability in total N (TN) removal from the media. Hatt et al. (2009b) conducted a field scale experiment on bio-filtration filter media (soil based) and while observing effective removal of TP, reported significant variability of TN concentrations in the effluent. Furthermore, they emphasized the need to use media containing low concentrations of P to prevent possible leaching of P from the medium. In the present study, R165 contained considerable amount of P (101 mg/kg) (Table 1); however, TP was not detected in the leachate when R165 was leached with distilled water. TN and NO3-N removals by R165 were not significant and highly variable. This remained so even with the addition of zeolite to R165 (Supplementary Data II).
Sonstrom et al. (2002) reported that a bio-filtration system retained 49% total suspended solids, 74% total P, 44% total Kjeldahl-N (TKN), 45% total Zn, 29% total Cu, 2% total Pb on a mass basis, and 99% faecal coliform on a concentration basis in a field study conducted at a commercial parking lot in Connecticut, USA. In Victoria, Australia, Hatt et al. (2009a) conducted a field scale study on the removal of pollutants using bio-filters from urban run-off and stated that nutrient retention was variable, and ranged from consistent leaching to effective and reliable removal, depending on the bio-filter design. Of the pollutants, nitrogen was found to be more difficult to remove because it is highly soluble and strongly influenced by the variable wetting and drying regime that is inherent in bio-filter operation. However, they reported that average removal efficiencies of total TKN and TP were 9% and 12%, respectively. They suggested that soil-based bio-filters especially those which were low in P content were suitable in removing TP.
4.2 DOC Removal
The concentration of DOC in the feed was 8-9 mg/L. There was only a slight reduction in the concentration of DOC in the effluent and no significant removal was observed for DOC by R165. This remained so even with the addition of zeolite (Supplementary Data III). Even though GAC is known to remove DOC (Sounthararajah et al. 2016), there was hardly any improvement in DOC removal in the column with the addition of GAC to the media and likely because of the low GAC dosage (0.3%) used in the experiment.
4.3 Heavy Metal Removal
Heavy metal removal by R165 was good for up to 3 h (40–90% for all heavy metals) (Fig. 4a). After 16 h, the percentages of removal reduced to 45% for Zn, 30% for Cu, 20% for Pb and less than 10% for the remainder of the heavy metals. The addition of 10% zeolite to R165 significantly increased the removal rates of most heavy metals and the life of the R165 media (Fig. 4b). The removal percentages of Ni, Fe, and Mn remained the lowest even after the addition of zeolite.
Of the heavy metals, R165+10% zeolite had the highest removal percentages for Pb, Cu, Zn, Cd and As and the lowest for Ni, Mn and Fe (Fig. 4b). Pitcher et al. (2004) conducted batch experiments using two zeolites, a synthetic zeolite and a natural mordenite to test their ability to remove dissolved heavy metals from simulated and spiked motorway stormwaters. They reported up to 42-89% reduction of heavy metals from the stormwater by mordenite and, also stated the percentage reduction order was Pb > Cu > Zn and Cd. Removal of heavy metals mainly took place by ion exchange mechanism on the zeolite and through the impurities present in natural zeolites (Ćurković et al. 1997). The difference in the removal of heavy metals in percent terms depends on the concentrations of the heavy metals in the stormwater and the chemical characteristics of the heavy metals. The first hydrolysis constant of the metals (M) (MOH+ formation) and solubility product of the metal hydroxides control the adsorption affinity of the metals (Nguyen et al. 2015). The lower the first hydrolysis constant, the greater the proportion of MOH+ which has stronger adsorption than M2+ among the various metal species in solution. High solubility product favours precipitation of metals, especially on the surface of the adsorbent. Of the heavy metals tested, Pb has the lowest hydrolysis constant and the highest solubility product and therefore the highest removal percentage at 24 h (Fig. 4b), the longest filtration time that was tested. This agrees with the results of heavy metals adsorption on other adsorption media (Nguyen et al. 2015; Sounthararajah et al. 2015).
The results of the heavy metal removal study show that use of R165 without additives as filter medium can remove only a one-year heavy equivalent metal load from stormwater to comply with the water quality required for safe disposal to natural waters. An addition of 10% zeolite to the medium increased the capacity of the filter to remove heavy metals and produce safe water for a period longer than two years.
4.4 Turbidity and Conductivity Removals
Figure 5 shows the turbidity removal by the column experiments at 100 mm/h flow velocity. R165 reduced the turbidity by 68–78% whereas the column of R165 mixed with 10% zeolite removed turbidity by 83–86%. When the flow rate increased (300 mm/h) in the latter column, the removal percentage decreased to 65–75%. This is due to the entry of a larger quantity of turbidity into the column per unit time.
The column containing R165 without additives and that containing R165 mixed with 10% zeolite decreased conductivity only during the first 4 h at the infiltration rate of 100 mm/h, and thereafter the turbidity remained the same as the influent solution (Supplementary Data IV). However, the reduction in conductivity was much higher in the latter column.
4.5 Polycyclic Aromatic Hydrocarbons (PAH) Removal
PAH removal by R165 at a flow velocity of 100 mm/h was 30–99% for acenaphthene, 50–99% for phenanthrene, and 50–92% for pyrene (Fig. 6a). The lowest removal for each PAH was, as expected, at the highest time of testing (20 h) at between 30 and 50% removal. The adsorbent medium was increasingly saturated with PAH as time progressed resulting in decrease in the available sites for further adsorption.
However, when 0.3% GAC and 10 % zeolite were mixed with R165, higher percentages of the PAHs were removed at both 100 and 300 mm/h flow velocities (Fig. 6b, c). The percentage removals at 20 h for the flow velocity of 100 mm/h ranged 65 to > 99% (Fig. 6b) compared to 30–50% in the column with only R165. Phenanthrene and pyrene were almost completely removed. The higher percentage removals at the lower flow rate compared to the higher velocity are due to a lower amount of PAH entering the filter per unit time. The higher removals of PAHs even with the addition of a very small amount of GAC to the filter media was due to the high affinity of PAHs to GAC (Eeshwarasinghe et al. 2018) and not likely to be due to adsorption onto zeolite in the filter. Preliminary batch experiments on adsorption of phenanthrene on R165, zeolite and GAC at an equilibrium phenanthrene concentration of 0.5 mg/L showed that their adsorption capacities were 0.05, 0.05, and 20 mg/g, respectively. This indicates that the adsorption capacity of GAC is nearly 400 times that of zeolite. Therefore, zeolite even with 33 times the weight of GAC (10% vs 0.3% GAC) in the filter media would not have made any significant contribution towards the removal of the PAHs. Huttenloch et al. (2001) also reported that the adsorption capacity of a natural zeolite, clinoptilolite for the PAH, naphthalene was almost zero. The inability of zeolite to adsorb PAHs is due to its low degree of hydrophobicity. To overcome this problem others have modified the surface of zeolite by grafting hydrophobic organic groups such as quaternary ammonium groups and surfactants which made the modified zeolite an attractive adsorbent for removing PAHs (Lemić et al. 2007; Wołowiec et al. 2017).
The removal percentage of acenaphthene was lower than that of phenanthrene and pyrene because the hydrophobicity of acenaphthene (log Kow 3.92) was lower than the other two PAHs (phenanthrene log Kow 4.46, pyrene log Kow 5.18) (Eeshwarasinghe et al. 2018). Acenaphthene appears to progress towards saturation of the three columns faster at both flow velocities than phenanthrene and pyrene, because of its lower adsorption capacity. Media containing 0.3 % GAC +10% Zeolite+ R165 at 100 mm/h resulted in nearly 100% removal of both phenanthrene and pyrene for an estimated period of three years equivalent rainfall.
5 Conclusions
A novel experimental protocol to estimate the efficiency of a filter medium for the treatment of stormwater pollutants from 1 to 3 years rainfall experienced in the field was developed using a laboratory column-set-up over short-term duration. The column with a soil-based filter medium (R165) is capable of removing PO4-P and NH4-N for up to 8 h at a flow velocity of 100 mm/h which is a time-equivalent of 1 year of rainfall at Blacktown, New South Wales, Australia. An addition of 10% of zeolite to R165 extended the column saturation period to 24 h. The breakthrough data for PO4-P and NH4-N were satisfactorily described by the Thomas model. However, the tested media were unable to remove TN and NO3-N even with the addition of 10% zeolite, with higher variability of these pollutants in effluent concentrations.
The majority of the nine heavy metals tested were removed by more than 50% for up to 4 h in the soil-based filter. This level of removal increased to 16 h (rainfall equivalent to 2 years) when 10% zeolite was added to the filter. The column with R165 + 10% zeolite showed higher affinity towards Pb, Cu, Zn, and As regardless of their initial concentrations and less affinity towards Ni.
Column with R165+10% zeolite removed considerable amounts of PAHs (30–50%) while a column with R165+10% zeolite+0.3% GAC removed 65- > 99% of PAHs from the influent at 20 h operation—phenanthrene and pyrene were almost completely removed. The removal percentage of acenaphthene was lower than phenanthrene and pyrene because the hydrophobicity of acenaphthene (log Kow 3.92) was lower than that of the other two PAHs (phenanthrene log Kow 4.46, pyrene log Kow 5.18).
Overall, the results indicated that modification of the soil-based filter medium with zeolite and GAC can increase the removal of nutrients (PO4-P, NH4+), heavy metals and PAHs from stormwater. This application will greatly facilitate the reduction of pollutant concentration in biofilter-treated stormwater in many stormwater harvesting projects which are currently experiencing difficulties in achieving clean reusable water.
References
ANZECC and ARMCANZ (2000). Australian and New Zealand guidelines for fresh and marine water quality, Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand. Canberra, 2000.
Chen, X., Peltier, E., Sturm, B. S., & Young, C. B. (2013). Nitrogen removal and nitrifying and denitrifying bacteria quantification in a stormwater bioretention system. Water Research, 47, 1691–1700.
Cooney, E. L., Booker, N. A., Shallcross, D. C., & Stevens, G. W. (1999a). Ammonia removal from wastewaters using natural Australian zeolite I. Characterization of the zeolite. Separation Science and Technology, 34, 2307–2327.
Cooney, E. L., Booker, N. A., Shallcross, D. C., & Stevens, G. W. (1999b). Ammonia removal from wastewaters using natural Australian zeolite II. Pilot-scale study using continuous packed column process. Separation Science and Technology, 34, 2741–2760.
Ćurković, L., Cerjan-Stefanović, Š., & Filipan, T. (1997). Metal ion exchange by natural and modified zeolites. Water Research, 31, 1379–1382.
Davis, A. P., Shokouhian, M., Sharma, H., Minami, C., & Winogradoff, D. (2003). Water quality improvement through bioretention: Lead, copper, and zinc removal. Water and Environmental Research, 75, 73–82.
Denman, L., May, P., & Breen, P. (2006). An investigation of the potential to use street trees and their root zone soils to remove nitrogen from urban stormwater. Australaian Journal of Water Resources, 10, 303–311.
Eeshwarasinghe, D., Loganathan, P., Kalaruban, M., Sounthararajah, D. P., Kandasamy, J., & Vigneswaran, S. (2018). Removing polycyclic aromatic hydrocarbons from water using granular activated carbon: kinetic and equilibrium adsorption studies. Environmental Science and Pollution Research, 25, 13511–13524.
Eeshwarasinghe, D., Loganathan, P., & Vigneswaran, S. (2019). Simultaneous removal of polycyclic aromatic hydrocarbons and heavy metals from water using granular activated carbon. Chemosphere, 223, 616–627.
Fletcher, T. D., Mitchell, V., Deletic, A., Ladson, T. R., & Seven, A. (2007). Is stormwater harvesting beneficial to urban waterway environmental flows? Water Science and Technology, 55, 265–272.
Hatt, B. E., Fletcher, T. D., & Deletic, A. (2009a). Hydrologic and pollutant removal performance of stormwater biofiltration systems at the field scale. Journal of Hydrology, 365, 310–321.
Hatt, B. E., Fletcher, T. D., & Deletic, A. (2009b). Pollutant removal performance of field-scale stormwater biofiltration systems. Water Science and Technology, 59, 1567–1576.
Henderson, C., Greenway, M., & Phillips, I. (2007). Removal of dissolved nitrogen, phosphorus and carbon from stormwater by biofiltration mesocosms. Water Science and Technology, 55, 183–191.
Hsieh, C. H., & Davis, A. P. (2005). Evaluation and optimization of bioretention media for treatment of urban storm water runoff. Journal of Environmental Engineering, 131, 1521–1531.
Huttenloch, P., Roehl, K. B., & Czurda, K. (2001). Sorption of nonpolar aromatic contaminants by chlorosilane surface modified natural minerals. Environmental Science and Technology, 35, 4260–4264.
Kim, H., Seagren, E. A., & Davis, A. P. (2003). Engineered bioretention for removal of nitrate from stormwater runoff. Water and Environmental Research, 75, 355–367.
Lemić, J., Tomašević-Čanović, M., Adamović, M., Kovačević, D., & Milićević, S. (2007). Competitive adsorption of polycyclic aromatic hydrocarbons on organo-zeolites. Microporous and Mesoporous Materials, 105, 317–323.
Li, H., & Davis, A. P. (2008). Urban particle capture in bioretention media. I: Laboratory and field studies. Journal of Environmental Engineering, 134, 409–418.
Nguyen, T. C., Loganathan, P., Nguyen, T. V., Vigneswaran, S., Kandasamy, J., & Naidu, R. (2015). Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chemical Engineering Journal, 270, 393–404.
Nur, C. T., Shim, W. G., Loganathan, P., Vigneswaran, S., & Kandasamy, J. (2015). Nitrate removal using Purolite A520E ion exchange resin: Batch and fixed-bed column adsorption modelling. International Journal of Environmental Science and Technology, 12, 1311–1320.
Pitcher, S., Slade, R., & Ward, N. (2004). Heavy metal removal from motorway stormwater using zeolites. The Science of the Total Environment, 334, 161–166.
Schneider, H. A., Jackson, W. A., Rainwater, K., Reible, D., Morse, S., Hatzinger, P. B., & Garza-Rubalcava, U. (2019). Estimation of interstitial velocity using a direct drive high-resolution passive profiler. Ground Water, 57, 915–924.
Sonstrom, R. S., Clausen, J. C., & Askew, D. R. (2002). Treatment of parking lot stormwater using a StormTreat system. Environmental Science and Technology, 36, 4441–4446.
Sounthararajah, D. P., Loganathan, P., Kandasamy, J., & Vigneswaran, S. (2015). Adsorptive removal of heavy metals from water using sodium titanate nanofibers loaded onto GAC in fixed-bed columns. Journal of Hazardous Materials, 287, 306–316.
Sounthararajah, D. P., Loganathan, P., Kandasamy, J., & Vigneswaran, S. (2016). Column studies on the removal of dissolved organic carbon, turbidity and heavy metals from stormwater using granular activated carbon. Desalination and Water Treatment, 57, 5045–5055.
Sun, S., Wang, L., Huang, S., Tu, T., & Sun, H. (2011). The effect of capping with natural and modified zeolites on the release of phosphorus and organic contaminants from river sediment. Frontiers in Chemical Science and Engineering, 5, 308–313.
Thomas, H. C. (1944). Heterogeneous ion exchange in a flowing system. Journal of American Chemical Society, 66, 664–1666.
Valderrama, C., Gamisans, X., De las Heras, X., Farran, A., & Cortina, J. (2008). Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: intraparticle diffusion coefficients. Journal of Hazardous Materials, 157, 386–396.
Wang, S., & Peng, Y. (2010). Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal, 156, 11–24.
Wild, D., Kisliakova, A., & Siegrist, H. (1996). P-fixation by Mg, Ca and zeolite A during stabilization of excess sludge from enhanced biological P-removal. Water Science and Technology, 34, 391–398.
Wołowiec, M., Muir, B., Zięba, K., Bajda, T., Kowalik, M., & Franus, W. (2017). Experimental study on the removal of VOCs and PAHs by zeolites and surfactant-modified zeolites. Energy and Fuels, 31, 8803–8812.
Zhan, Y., Zhang, H., Lin, J., Zhang, Z., & Gao, J. (2017). Role of zeolite's exchangeable cations in phosphate adsorption onto zirconium-modified zeolite. Journal of Molecular Liquids, 243, 624–637.
Acknowledgements
We thank Craig Bush of Blacktown City Council, Blacktown, NSW, Australia for providing the biofilter media and offering suggestions for the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Ekanayake, D., Loganathan, P., Johir, M.A.H. et al. Enhanced Removal of Nutrients, Heavy Metals, and PAH from Synthetic Stormwater by Incorporating Different Adsorbents into a Filter Media. Water Air Soil Pollut 232, 96 (2021). https://doi.org/10.1007/s11270-021-05059-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05059-6