Abstract
In this work, malachite green was degraded using different advanced oxidation processes (Fenton, photo-Fenton, sono-Fenton and electrochemical process). Malachite green is used as biocide in aquaculture and is usually discarded with the effluents. On higher pollutant concentration, all the Fenton-based reactions achieved excellent absorbance reduction up to 10 min. Classic Fenton was faster after 10 min of reaction and photo-Fenton acting faster before this point. The photocatalytic effect was better on the oxygen demand reduction (COD) with 86.91% against 79.19% of sono-Fenton and 62.72% of Fenton. All four methodologies had excellent absorbance reduction following the order: photo-Fenton (100% up to 30 min) > electrochemical (99.27%) > Fenton (98.11%) > sono-Fenton (73.99%). Despites the slowly initial degradation obtained for electrochemical process, the reaction achieved high capacity after 60 min. Toxicity tests, using Lactuca sativa seeds, indicated a significant reduction in the effluent toxicity following this sequence: sono-Fenton > photo-Fenton > Fenton > electrochemical. The results showed that all processes studied provided high levels of malachite green removal; however, the adequate use of each technique should be conduct with an accurate evaluation of the needed treatment considering the particularity of each method. Such techniques were successfully applied before to remove dye basic blue 99 and the hormone 17-α-methyltestosterone and corroborated by Lactuca sativa toxicity assays.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The fish farm is an alternative used to increase food amount; however, it has a high potential to environmental degradation. Beyond the pollution arising from feces and unconsumed feed, fish farming also launches chemicals waste, which are used in disinfection, pest and predator controls, disease treatments, hormones to induce reproduction, and sexual reversal (Eler and Millani 2007).
Among all, hormones and biocides deserve to be highlighted. Malachite green (MG) is a non-biodegradable compound, widely used in aquaculture because of its antiprotozoal and antifungal activity and relatively low operating cost (Sudova et al. 2007). MG also has other applications, such as dyeing cotton, silk, paper, and leather and in the manufacture of paints and printing inks (Alderman 1985); activities that generate a high effluent load and require proper treatment. The use of MG has been discussed because of its elevated and well-reported toxicity, since it intercalates with DNA causing carcinogenesis, mutagenesis, and teratogenicity, which can cause liver tumors (Srivastava et al. 2004). Besides problems with the immune and reproductive system (He and Tian 2016), having been detected in treated fish, because after being absorbed it is reduced through the metabolism to green leucomalachite (GLM) and accumulates in adipose tissues (Bergwerff and Scherpenisse 2003). Several studies reported the degradation of the MG using advanced oxidative processes (AOPs) as electrochemical oxidation, Fenton, photo-Fenton, and sono-Fenton (Chen et al. 2002b; Dutta et al. 2003; Hameed and Lee 2009; Moumeni et al. 2012; Sasidharan Pillai and Gupta 2016; Guo et al. 2017; Ansari and Nematollahi 2018; Jiang et al. 2018; Ghime et al. 2019).
The versatility of the advanced oxidative processes (AOPs) is already well reported by literature, regarding operational simplicity and the wide application in the degradation and mineralization of the most varied pollutants, even compounds resistant to conventional methods (Pignatello et al. 2006). AOPs are based on the generation of hydroxyl radicals (•OH), for the oxidation of organic molecules. Its efficiency is related to the high reactivity of these radicals, potent oxidants (E° = + 2.80 V vs ENH) and not selective. These oxidants can be generated through electrochemical processes and are thus called electrochemical advanced oxidation processes (EAOPs) (Brillas 2014).
The classical mechanism of Fenton process is a simple redox reaction, in which the Fe2+ ions are oxidized to Fe3+, and the H2O2 is reduced to a hydroxyl ion and a hydroxylradical (reaction 1).
In aqueous (pH ~ 3) reaction 1 is followed by the following steps:
Fenton reaction reduces the toxicity of contaminants, improves the biodegradability and odor of polluting species and removes the color of the effluent. The efficiency of the oxidation process by the Fenton reagent depends on the H2O2 ratio: organic carbon, organic matter content, pH, temperature and iron concentration, and different operating conditions can be used in order to achieve high efficiency in the degradation of organic pollutants, depending on the type of effluent to be treated. As reported, the efficiency of the Fenton reaction is related to the amount of (OH) radicals generated, factor initially linked to reaction 1, nevertheless, when Fe2+ is consumed, the reaction becomes dependent on the slow process of Fe3+ reduction, described in reaction 2 (Zanta et al. 2010; De Moura Gomes et al. 2014; da Silva Duarte et al. 2018, 2019; Gomes et al. 2019).
Photo-Fenton process (H2O2/Fe2+/UV) increases the formation of hydroxyl radicals (•OH), although it is slower when compared with Fenton (k1 > k4). UV irradiation can induce the reduction of Fe3+ to Fe+2 and decompose H2O2 molecules producing hydroxyl radicals (reaction 4) (Silva et al. 2007).
Ultrasonic reactions (US) receive relevant attention because they do not need the addition of reagents. In this way, through the bubbles that are generated, high pressure and temperature resulting from this process, organic pollutants can be directly degraded (reaction 5), especially volatile compounds. Besides, sonolysis of water generates radicals (reaction 6), contributing in the pollutant degradation (reaction 7) (Adityosulindro et al. 2017).
The US process has two important drawbacks, low mineralization factor and high energy consumption (Liang et al. 2007), thus, arises the need for combining with other methodology, for high rates of mineralization in a smaller reaction time. Association of other processes with US may be motivated by the regeneration of ferric to ferrous ions, explained by reaction 8.
Electrochemical processes are very effective; the reactions depend on the application of a potential that can oxidize or reduce metallic ions, cyanide, organochlorines, and aromatic and aliphatic hydrocarbons. In an aqueous medium, the electrooxidation of organic compounds often occurs on high potential (De Moura Gomes et al. 2014). The dimensionally stable anodes (DSA®-Ti/Ru0.3Ti0.7O2) are a type of electrodes that can efficiently oxidize organic compounds. These electrodes consist primarily of Ti, stabilized in TiO2, with noble metal oxides exhibiting a rutile structure. DSA®-containing RuO2 and IrO2 is widely used in electrochemical processes because they are mechanically resistant and easy to scale up for industrial purposes (Duarte et al. 2013); these characteristics provide a wide applicability of the DSA® in the treatment of waste water, besides having a high active area due to its known morphology of cracked clay, which allows direct oxidation of the substrate on the surface of the electrode (reaction 11), as well as oxidation through of the electrogenerating oxidant species (De Moura Gomes et al. 2014). In a first step (reaction 9), H2O (acid medium) or -OH (basic medium) is oxidized on the surface of the electrodes forming the hydroxyl radical adsorbed. Reaction 10 describes the second stage, known as oxygen evolution reaction (OER) (Comninellis and De Battisti 1996).
In this context, the aim of this study is to compare classical Fenton, photo, sono-Fenton, and electrochemical process, using DSA® (Ti/Ru0.33Ti0.67O2) electrode, for malachite green degradation. Chemical oxygen demand (COD), kinetic parameters based on the reductions in absorbance and toxicity, using Lactuca sativa, were analyzed.
2 Materials and Methods
2.1 Chemicals and Materials
All the reagents were commercially available. Malachite green (C23H25ClN2) was purchase from Synth, and its spectrum and chemical structure are presented in Fig. 1. Hydrogen peroxide (H2O2, 50% m/m), ferrous sulfate (FeSO4.7H2O), sulfuric acid were purchased from Vetec. Sodium sulfate (Na2SO4) was acquired from ChemCruz. All the reagents were analytical grade and were used without purification. Deionized water was employed to prepare the different solutions of this study. All the experiments were conducted in duplicate.
2.2 Fenton Reaction
The effluent containing the model substrate MG (at of 0.002 g L−1 or 0.2 g L−1) was treated in a batch reactor containing 0.2 L of the reaction solution. A continuous magnetic stirring system was employed, and the solution pH was maintained at 3.0 ± 0.1. The Fenton reagent concentrations were as follows: 0.1, 0.3, 0.5 mM of Fe2+, and 0.1, 10, 50, 100 mM of H2O2. The reaction time was 60 min (De Moura Gomes et al. 2014; da Silva Duarte et al. 2018; Gomes et al. 2019).
2.3 Photo and Sono Fenton Reactions
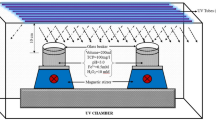
Concentrations of MG (0.002 g L−1 or 0.2 g L−1) were used and the Fenton reagents were set at 0.1; 0.5 mM Fe2+ and 1; 10 mM H2O2. In photo-Fenton, a Philips HPL-N 80 W lamp was used as radiation source. In the sono-Fenton studies, ultrasonic stimulation was performed using an Ultralonic Cleaner model USC-2850A, frequency 25 kHz and consumption of 800VA, replacing the magnetic stirrer used in other reactions.
2.4 Electrochemical Process
In this step, 0.002 g L−1 of VM was used. DSA® (Ti/Ru0.33Ti0.67O2), 16-cm2 area, was used as the anode and cathode by applying a current density of 10 mA cm−2. The electrolyte was Na2SO4 (50 and 100 mM).
2.5 Instrumentation and Analyses
Samples were collected at regular times along the experiments; the absorbance and process efficiency were determined by spectrophotometric analysis. The maximum MG absorbance at λ = 617 nm, which is directly related to the concentration of malachite green, a spectrophotometer Shimadzu MultiSpec 1501 Series was used. Degradation of the organic compounds was monitored through measurement of COD on a photometer from PoliControl. COD was analyzed by the standard APHA/AWWA method (APHA, AWWA, WEF 2012).
2.6 Toxicity Tests
Toxicity tests were performed using the twinning method of Lactuca sativa (lettuce). This assay was based on protocol 850.4200 (USEPA 1996; Tavares et al. 2016; Duarte et al. 2019). The seeds were commercially obtained and stored under refrigeration to ensure greater longevity. Each Petri dish had 10 seeds placed on filter paper soaked with 5 mL of treated effluent sample; the same procedure was performed with the effluent without treatment. The negative control was constituted using distilled water. The assays were made in duplicate and were considered valid replicates where the negative control had germination equal to or greater than 90%. The plates were sealed and placed in a styrofoam box for 5 days. After this period, the numbers of germinated seeds were observed in each plate, and the root length was measured. The results were expressed in the form of relative growth index (RGI), and germination index (GI), calculated through the following Eqs. 1 and 2.
RLS is the radical length in the sample, RLC is the radical length in the negative control, GSS is the number of germinated seeds of the sample, and GSC is the number of germinated seeds in the negative control (Young et al. 2012).
3 Results and Discussion
3.1 Fenton Reaction
3.1.1 Effect of [Fe2+]
A study of the oxidation kinetics was carried out to evaluate the reducing of UV-Vis absorption band (Fig. 2) and COD values (Table 1) using 0.2 g L−1 of MG.
The increase on concentration of ferrous ions indicated a greater efficiency in the MG oxidation. When the concentration was elevated from 0.1 to 0.3 mM, an increase from 28.85 to 89.50% of MG removal of MG was observed for the first 10 min (Table 1). This was corrobotared by the apparent constant of pseudo-first order, kobs (min−1), where in 0.3 mM Fe2+, the reaction rate was approximately 6.6 times faster than in 0.1 mM Fe2+. When working with 0.5 mM Fe2+, a slight increase in MG oxidation efficiency was observed in Fig. 2, with consequent increase in reaction kinetics, see insert Fig. 2. Similar behavior has already been observed by Zanta et al. (2010) and Tavares et al. (2016).
Two different kinetic regions are shown in Fig. 2. The first region with t < 10 min has quite high reaction kinetics and reduces the MG concentration around 90%, similar behavior was observed by Bañuelos et al. (2016) in the MG degradation using carbon felted and Boron-doped diamond (BDD) in the presence of ferrous ions. The DDB electrode has a high generation of OH radicals, since carbon electrodes are capable of produce H2O2 with efficiency when oxygen is added to the medium. Therefore, this reaction model allowed the production of hydroxyl radicals on two fronts, DDB and electro-Fenton. After 10 min of reaction, the observed rate constant becomes approximately 26 times lower for the higher concentration of ferrous ions, indicating that the initial concentration of malachite green had a strong influence on the reaction rate.
Figure 3a relates the kobs values with the percentages of MG absorbance reduction in the first 10 min of the reaction, highlighting the great efficiency increase between 0.1 and 0.3 mM Fe2+. However, between 0.3 and 0.5 mM Fe2+, the improvement was discreet. When the absorbance reduction is compared with COD reduction (Fig. 3b), after 60 min, the rise in the concentration caused a slight increase in the total absorbance reduction from 97.42 to 98.33% but resulted in the COD reduction from 66.86 to 62.72% (Table 1). Similar results were reported by De Moura Gomes et al. (2014), in COD reduction by Fenton reactions applied to the coconut industry effluent and El-Ghenymy et al. (2013) in the degradation of the antibiotic sulfanilamide. The initial velocity increment by 0.5 mM Fe2+ and the almost total degradation of malachite green in a few minutes triggered a greater consumption of hydroxyl radicals in t < 10 min, consequently a lower concentration of oxidant species to act on the mineralization of degradation byproducts. As described previously, the Fenton reaction has a very fast initial stage (reaction 1); however, its continuity is dependent on a very slow second stage (reactions 2 and 3), which explains the difference between the values of absorbance reduction and COD.
3.1.2 Effect of [H2O2]
Figure 4 shows an effective reduction of the absorbance at all studied H2O2 concentrations (10, 50, and 100 mM), for [Fe2+] = 0.3 mM. However, the increase of the hydrogen peroxide concentration reduced the reaction yield. Similar results were observed by Mandal et al. (2004) in catechol removal, Sun et al. (2007) in the degradation of p-nitroaniline, Zanta et al. (2010) in surfactant degradation and De Moura Gomes et al. (2014) in the effluent treatment of the coconut processing industry.
Total reductions of 98.25%, 98.32%, and 97.27% were obtained for 10, 50, and 100 mM H2O2, respectively. Despite the similar final reduction for the three concentrations studied, using 100 mM, the reaction was slower obtaining only 41% of MG removal in the first 10 min. While at 10 and 50 mM, more than 90% of MG degradation was observed at the same time. The higher amount of H2O2 cause the reaction became dependent on [H2O2]. Moreover, for the lowers concentrations of hydrogen peroxide, it is possible to see two different kinetics zones; the first one (t < 20 min), a rapidly reduction of absorbance, can be seen. The second zone, after 20 min, the reaction assumed a kinetic dawdling, possible due to low amount of MG.
3.1.3 Photo and Sono-Fenton
Ultrasonic and photocatalysis effects were tested combined with Fenton reagents on oxidation of [MG] = 0.2 g L−1. [Fe2+] concentration was maintained at 0.5 mM, and the [H2O2] was fixed at 10 mM. Figure 5 and Table 2 show the behavior among the three methodologies tested, total absorbance reductions values were quite similar, 98.33%, 99.07%, and 98.46 for Fenton, photo-Fenton, and sono-Fenton. The reactions showed pseudo-first order behavior with two distinct terminating regions (Fig. 5a), with the classic Fenton reaction exhibiting higher kobs (min−1) in the first region (t < 10 min), following Fenton > photo-Fenton > sono-Fenton (Fig. 5b). However, in the second region (t < 60 min), the photo-Fenton reaction was faster, photo-Fenton > sono-Fenton > Fenton. This behavior can be attributed to two factors, the low concentration of MG after t = 10 min, as well as the reduction of ferric to ferrous ions, characteristics of photocatalytic, and ultrasonic effects, which can be better observed in Fig. 5c with the percentage values of reduction of COD.
The existence of toxic by-products, such as leucomalaquita green (LMG), originated from the metabolism of MG in fishes (Bergwerff and Scherpenisse 2003), leads to the need for efficient removal of MG and its by-products. In spite of the three applied methodologies reaching absorbance reductions nearby 100%, after 60 min (Table 2), when the COD reduction can be observed, where the remaining organic load is disposed. The reduction of COD values corroborates the kobs kinetic data (min−1), where the Fenton reaction was more effective at t < 10 min, when the concentration of malachite green was maximal, and in the second kinetic region (Fig. 5a), with t > 10 min and t < 60 min, the behavior was different.
The reduction in absorbance is not enough to justify the difference in yield between the two kinetic regions, since [MG] after 10 min has already been reduced above 90%. On the other hand, the final reduction of absorbance (Table 2) was more effective with the addition of photo and sonocatalytic effects. Light and ultrasound added 24.19% and 16.47% of COD removal, respectively, relative to the classical Fenton reaction, the effect described by reactions 4 and 8, thus the Fenton reaction was highly efficient in the degradation of MG. Photo and sono-Fenton reactions were more effective in the removal of their by-products from initial degradation. Fuentealba et al. obtained similar differences between Abs. and COD reductions, using photo-Fenton in the degradation of MG. Authors reached total discoloration of the solution in 120 min and reduction of 70% of COD in 180 min, a minimum change in the reduction of COD between 60 and 120 min, possibly by the formation of recalcitrant compounds already reported as degradation products of malachite green by •OH radicals (Fuentealba et al. 2016), such as N,N-dimethylaniline, 4-dimethylaminophenol, 4-methylamino benzophenone, 4-dimethylaminobenzophenone, benzeneacetic acids, and 4-hydroxybenzoic acid (Oturan et al. 2008; Singh et al. 2014).
Oturan et al. (2008) used electro-Fenton in the MG degradation and observed the formation of N-dimethylated aromatic amines. Oxidative process starts at the central molecule carbon, located between and connected to the aromatic rings of MG, forming the colorless base of malachite green carbinol via electron transfer between hydroxyl radical and MG (Chen et al. 2002a; Oturan et al. 2008). This step is fast and causes a sudden reduction of the absorbance of the solution, as occurred in the first 10 min of reaction, Figs. 2, 4, and 5a.
3.2 Electrochemical Reactions
In order to study the effect of the [MG], as well as the proximity to the real effluent, it was decided to work with 2 mg L−1 in this step, since in aquaculture the concentrations are between 0.0001 and 0.002 g L−1 of MG (Rocha et al. 1994; Klein et al. 2004; Carneiro et al. 2005). The electrochemical degradation of MG was classified in kinetic terms as pseudo-first order (Fig. 6a) with R2 = (0.994 and 0.968), reaching oxidation of up to 64.74% in the first 10 min of reaction, and 99.27% of absorbance reduction after 1 h (Table 3). Similar kinetic behavior has been reported, where the MG was completely removed after 56 min of electrolysis, following a pseudo-first-order kinetics (Oturan et al. 2008). Figure 6b shows the relationship between the constant Kobs (min−1) and the percentage of absorbance reduction as a function of the electrolyte concentration, which shows that when the Na2SO4 amount doubles, the constant value goes from 0.0447 to 0.0721; however, even with a difference in reaction rate, after 60 min, similar absorbance reductions of 94.02 and 99.27% were achieved for 50 and 100 mM of sodium sulfate, respectively.
The results of the electrochemical reactions expressed in Table 3 indicate good efficiency in the MG degradation, with advantage for the reaction with a higher electrolytic concentration. On the other hand, the high electrolytic concentration resulted in higher energy consumption, which could be occurred due to possible ionic congestion between the electrodes, increasing the voltage of the reaction. The volume of treated effluent, due to the reduction of MG in the solution, was calculated in Brazilian currency costing R$ 3.10 and R$ 3.96, respectively, for reactions with 50 and 100 mM of sodium sulfate.
3.3 Comparison Between the AOPs
3.3.1 Degradation and Kinetics
At this stage of the work, Fenton reagents concentrations were 0.1 mM Fe2+ and 1 mM H2O2, the same for photo-Fenton and sono-Fenton. The four methodologies studied showed an efficient reduction of MG absorbance after 60 min of reaction, obtaining the order indicated in Fig. 7 and Table 4, Fenton (98.11%) > electrochemical (99.27%) > Fenton (73.99%), with the photocatalytic reaction reaching 100% degradation for 2 mg L−1 of MG solution in only 30 min. Figure 7a shows the reduction of the MG absorbance in function of the applied methodology. Similar behavior between Fenton and photo during the reaction is evidenced. The electrolytic reaction, despite having a kinetic constant 2.8 and 3.7 times smaller than the Fenton and photo-Fenton constants after 60 min of reaction, reached similar reduction of the MG absorbance. The efficiency of the degradation can be explained by the amount of •OH radicals produced. Fenton reactions have a high initial generation of these radicals, leading to the rapid degradation of the organic substrate in the first few minutes of experiment. However, excellent alternatives for compounds that generate recalcitrant degradation by-products are the photocatalytic effect and electrochemical process. The first has extra catalyst regeneration through light, and the second is a constant source of radicals (Zanta et al. 2010; Duarte et al. 2013; Zhang et al. 2014).
As described in the literature (Adityosulindro et al. 2017), the sonolysis is efficient in the degradation of the initial substrates, but it has low yields in the mineralization, being necessary the coupling of techniques, aiming the optimization of the reaction time, and minimizing the energy consumption. In this study, the sono-Fenton reaction presented the lowest yield among the applied methodologies, demonstrating that the catalyst regeneration is more pronounced in light presence. The kinetic constant of pseudo-first order was similar between the electrochemical reaction and sono-Fenton; on the other hand, the electrochemical experiment obtained 25.28% more absorbance reduction, which can be explained by the nature of the electrode used, which produces many intermediates. Besides, the oxidation process is dependent on the absorption of the pollutant in the active sites of the MOx + 1 electrode for direct oxidation. Part of the pollutant can also be oxidized indirectly by the electrogenerated radicals (Comninellis and Chen 2010; Duarte et al. 2013; De Moura Gomes et al. 2014). The kinetics as well the values of the kobs (min−1) demonstrate that the reactions were governed by the concentration of •OH, with the distinguish efficiencies, which can be explained by the different origins of the radicals produced.
3.3.2 Toxicity Tests
Toxicity tests are of utmost importance for the monitoring the quality of discarded effluents. The physico-chemical parameters are not enough to infer if the treated effluent will bring changes in the aquatic species life. Nevertheless, toxicity tests are not a substitute for physico-chemical analyzes, which identify and quantify contaminants, but rather assess their biological effects on receiving water bodies, so that they complement each other (Chaparro and Pires 2010).
For the root lengthening bioassays and seed germination rate, USEPA (1996) advises the use of Lactuca sativa seeds is economic and ecological relevant. Lettuce is a suitable organism for ecotoxicological analyzes because it represents a widely cultivated vegetable, used in large scale in food, human and animal, and vulnerable to contamination by irrigation containing wastewater (Giorgetti et al. 2011).
It can be observed in Table 5 that the MG has high toxicity, presenting a GI of 43% and an ICR of 0.55. The dilution tests are applied in a simulation of the effluent disposal rate depending on the body of the receiving water, even if diluted to 25%, the 2 mg L−1 solution still showed toxicity, corroborating the need for oxidation of the pollutant. The Fenton reaction can reduce effluent toxicity under optimized experimental conditions. On the other hand, the electrochemical processes have the potential deviation of the formation of toxic products, as well as the presence of the electrolyte, which contributes significantly to the toxicity of the treated effluent (Tavares et al. 2016).
The post-treatment toxicity evaluation revealed that all the reactions tested were able to reduce effluent toxicity, except the electrochemical reaction with 100 mM electrolyte. However, when comparing the results at 50 and 25% dilution, it is verified that the treated effluent showed less toxicity than the pre-treated, confirming the influence of the salinity and the reduction of the initial toxicity from the MG. The electrochemical reaction with 50 mM of electrolyte, when compared with the other methodologies, showed the lowest reduction of the acute toxicity of the effluent, yet when compared with the values obtained from the pre-treatment tests, it showed reduction of toxicity in all the dilutions tested (Table 5).
The Fenton, photo-Fenton, and sono-Fenton reactions showed an efficient reduction of toxicity, and for all dilutions, the reduction was quite significant reaching values interpreted as growth stimuli (Young et al. 2012). Other toxicity tests should be applied to understand the possible effects on different species in the food chain. On the other hand, lettuce seeds are widely used and can be a relevant indication of water reuse for agricultural propose without harmful effects to the plants or the soil used to produce the vegetables.
Similar results were obtained by Borba et al. in the treatment of effluents from the leather industry, and by Luna et al. in effluent contaminated with dyes, via photo-Fenton (Borba et al. 2013; de Luna et al. 2014), demonstrating that Fenton’s reactions are capable of promoting reduction of acute toxicity, depending on the experimental conditions and mainly on the nature of the pollutant, in what concerns the formation or not of more toxic by-products in case the applied processes are not sufficient to promote the complete mineralization.
Based on the premise that all reactions were able to reduce effluent toxicity following the efficiency order of sono-Fenton > photo-Fenton > Fenton > electrochemistry, it can be understood that the difference between the germination and growth indices was governed by nature and amount of the oxidant species present in the reaction medium. The salinity of the electrochemical reactions, as well as the concentration difference of the main oxidizing species in the Fenton base reactions, the •OH radicals, has already been commented. The concentration of hydroxyl radicals during the reaction, which influenced the better oxidative yield of the photo-Fenton reaction against the MG, was a major factor in the final toxicity, since this behavior can be considered as a reflection of the residual concentration of these reactive species. In contrast, the reaction that obtained lower oxidative efficiency, as it has a lower capacity to promote radicals •OH in the medium, consequently showed less toxicity after treatment.
4 Conclusions
The results indicated an effective degradation of the malachite green in the studied concentrations. The increase in the concentration of the ferrous catalyst in the Fenton reaction demonstrated an increase in the reaction efficiency, the opposite occurring with the increase of the concentration of the oxidant H2O2. Photo- and sono-Fenton reactions exhibited behavior similar to classical Fenton, with effective removal of the MG in the first 10 min of reaction and photo-Fenton obtained the highest absorbance and COD reductions, 99.07 and 86.91%, respectively, followed by Fenton and sono-Fenton.
The variation of the electrolyte concentration increased the efficiency of the electrochemical reaction, significantly increasing the observed kinetic constant; however, there was an elevation in the energy consumption, and the reduction of the absorbance after 60 min was very similar (94.02 and 99.27%), not influencing in practice the reflection of the double use of the electrolytic concentration.
The comparison between the four applied methodologies confirmed photo-Fenton as more effective in removing the MG, with a 100% reduction in the absorbance of the solution in 30 min. The reactions showed pseudo-first order kinetics, strongly dependent on the concentration of •OH radicals. The electrochemical reaction is slower, depending on the different reaction mechanism, depending on the absorption stage of the pollutant on the electrode surface.
Tests with Lactuca sativa indicated that all reactions were able to reduce the initial toxicity of the effluent; on the other hand, the salinity of the electrochemical reactions showed toxicity proved by the dilution results. Reactions based on the Fenton reagents were able to reduce the total toxicity of the solution, and the sono-Fenton reaction presented the best results.
References
Adityosulindro, S., Barthe, L., González-Labrada, K., Jáuregui Haza, U. J., Delmas, H., & Julcour, C. (2017). Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrasonics Sonochemistry. https://doi.org/10.1016/j.ultsonch.2017.06.008.
Alderman, D. J. (1985). Malachite green: a review. Journal of Fish Diseases. https://doi.org/10.1111/j.1365-2761.1985.tb00945.x.
Ansari, A., & Nematollahi, D. (2018). A comprehensive study on the electrocatalytic degradation, electrochemical behavior and degradation mechanism of malachite green using electrodeposited nanostructured Β-PbO2 electrodes. Water Research. https://doi.org/10.1016/j.watres.2018.07.056.
APHA, AWWA, WEF. (2012). Standard methods for examination of water and wastewater. Washington: American Public Health Association.
Bañuelos, J. A., García-Rodríguez, O., El-Ghenymy, A., Rodríguez-Valadez, F. J., Godínez, L. A., & Brillas, E. (2016). Advanced oxidation treatment of malachite green dye using a low-cost carbon-felt air-diffusion cathode. Journal of Environmental Chemical Engineering. https://doi.org/10.1016/j.jece.2016.03.012.
Bergwerff, A. A., & Scherpenisse, P. (2003). Determination of residues of malachite green in aquatic animals. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. https://doi.org/10.1016/S1570-0232(03)00042-4.
Borba, F. H., Módenes, A. N., Espinoza-Quiñones, F. R., Manenti, D. R., Bergamasco, R., & Mora, N. D. (2013). Toxicity assessment of tannery effluent treated by an optimized photo-Fenton process. Environmental Technology (United Kingdom). https://doi.org/10.1080/09593330.2012.710407.
Brillas, E. (2014). A review on the degradation of organic pollutants in waters by UV photoelectro-Fenton and solar photoelectro-Fenton. Journal of the Brazilian Chemical Society. https://doi.org/10.5935/0103-5053.20130257.
Carneiro, P. C. F., Schorer, M., & Mikos, J. D. (2005). Conventional therapeutic treatments on the control of the parasite Ichthyophtirius multifiliis in Rhamdia quelen. Pesquisa Agropecuária Brasileira. https://doi.org/10.1590/S0100-204X2005000100015.
Chaparro, T. R., & Pires, E. C. (2010). Toxicity evaluation as a tool to asses the performance of an anaerobic immobilized biomass reactor. DYNA (Colombia).
Chen, F., He, J., Zhao, J., & Yu, J. C. (2002a). Photo-Fenton degradation of malachite green catalyzed by aromatic compounds under visible light irradiation. New Journal of Chemistry. https://doi.org/10.1039/b107404k.
Chen, F., Ma, W., He, J., & Zhao, J. (2002b). Fenton degradation of malachite green catalyzed by aromatic additives. The Journal of Physical Chemistry. A. https://doi.org/10.1021/jp0144350.
Comninellis, C., & Chen, G. (2010). Electrochemistry for the environment. Electrochemistry for the Environment. https://doi.org/10.1007/978-0-387-68318-8.
Comninellis, C., & De Battisti, A. (1996). Electrocatalysis in anodic oxidation of organics with simultaneous oxygen evolution. Journal de Chimie Physique et de Physico-Chimie Biologique. https://doi.org/10.1051/jcp/1996930673.
da Silva Duarte, J. L., Solano, A. M. S., Arguelho, M. L. P. M., Tonholo, J., Martínez-Huitle, C. A., & de P. e. S Zanta, C. L. (2018). Evaluation of treatment of effluents contaminated with rifampicin by Fenton, electrochemical and associated processes. Journal of Water Process Engineering. https://doi.org/10.1016/j.jwpe.2018.02.012.
da Silva Duarte, J. L., Meili, L., de M Gomez, L., Soletti, J. I., & de P. e. S Zanta, C. L. (2019). Electrochemical process and Fenton reaction followed by lamellar settler to oil/surfactant effluent degradation. Journal of Water Process Engineering. https://doi.org/10.1016/j.jwpe.2019.100841.
de Luna, L. A. V., da Silva, T. H. G., Nogueira, R. F. P., Kummrow, F., & Umbuzeiro, G. A. (2014). Aquatic toxicity of dyes before and after photo-Fenton treatment. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2014.05.047.
De Moura Gomes, L., Da Silva Duarte, J. L., Pereira, N. M., Martínez-Huitle, C. A., Tonholo, J., & De Zanta, C. L. P. E. S. (2014). Development of a system for treatment of coconut industry wastewater using electrochemical processes followed by Fenton reaction. Water Science and Technology. https://doi.org/10.2166/wst.2014.129.
Duarte, J. L. S., Soares, W. M. G., Gomes, L. M., Tonholo, J., & Zanta, C. L. P. S. (2013). Electrochemical oxidation of Safrole using Ti/RuXTi(1 - X)O2 system: preparation, characterization, and role of electrode composition. Electrocatalysis. https://doi.org/10.1007/s12678-013-0153-2.
Duarte, J. L. S., Meili, L., Gomes, L. M., Melo, J. M. O., Ferro, A. B., Tavares, M. G., et al. (2019). Electrochemical degradation of 17-α-Methyltestosterone over DSA® electrodes. Chemical Engineering and Processing Process Intensification, 107548. https://doi.org/10.1016/j.cep.2019.107548.
Dutta, K., Bhattacharjee, S., Chaudhuri, B., & Mukhopadhyay, S. (2003). Oxidative degradation of malachite green by Fenton generated hydroxyl radicals in aqueous acidic media. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering. https://doi.org/10.1081/ESE-120021128.
Eler, M. N., & Millani, T. J. (2007). Métodos de estudos de sustentabilidade aplicados a aquicultura TT - Sustainable development in aquiculture: methodology and strategies. Revista Brasileira de Zootecnia. https://doi.org/10.1590/S1516-35982007001000004.
El-Ghenymy, A., Oturan, N., Oturan, M. A., Garrido, J. A., Cabot, P. L., Centellas, F., et al. (2013). Comparative electro-Fenton and UVA photoelectro-Fenton degradation of the antibiotic sulfanilamide using a stirred BDD/air-diffusion tank reactor. Chemical Engineering Journal. https://doi.org/10.1016/j.cej.2013.08.080.
EPA (1996). Ecological effects test guidelines OPPTS (850.4200): seed germination/root elongation toxicity test. United States Environmental Protection Agency. EPA 712-C-96-154.
Fuentealba, D., Venegas, C., Morales, M., & Waissbluth, O. (2016). Comparing photo-fenton degradation of malachite green using FeII and FeIII salts under UVA light irradiation. Journal of the Brazilian Chemical Society. https://doi.org/10.5935/0103-5053.20150258.
Ghime, D., Goru, P., Ojha, S., & Ghosh, P. (2019). Oxidative decolorization of a malachite green oxalate dye through the photochemical advanced oxidation processes. Global Nest Journal. https://doi.org/10.30955/gnj.003000.
Giorgetti, L., Talouizte, H., Merzouki, M., Caltavuturo, L., Geri, C., & Frassinetti, S. (2011). Genotoxicity evaluation of effluents from textile industries of the region Fez-Boulmane, Morocco: a case study. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2011.08.002.
Gomes, L. M., Silva, J. M., Duarte, J. L. S., Tavares, M. G., Santos, E. L., Machado, S. S., et al. (2019). Ecotoxicological evaluation of a fish farming effluent treated by Fenton oxidation and coagulation process. Separation Science and Technology. https://doi.org/10.1080/01496395.2019.1662808.
Guo, X. C., Cao, X., Wang, H. H., Yuan, M., Chen, X. J., Kang, W. Y., & Zhou, W. H. (2017). Graphene-gold nanoparticles nanohybrids for electrochemical detection of malachite green. International Journal of Electrochemical Science. https://doi.org/10.20964/2017.08.49.
Hameed, B. H., & Lee, T. W. (2009). Degradation of malachite green in aqueous solution by Fenton process. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2008.08.018.
He, H. Y., & Tian, C. Y. (2016). Rapid photo- and photo-Fenton-like catalytic removals of malachite green in aqueous solution on undoped and doped TiO2 nanotubes. Desalination and Water Treatment. https://doi.org/10.1080/19443994.2015.1064033.
Jiang, D. B., Liu, X., Xu, X., & Zhang, Y. X. (2018). Double-shell Fe 2 O 3 hollow box-like structure for enhanced photo-Fenton degradation of malachite green dye. Journal of Physics and Chemistry of Solids. https://doi.org/10.1016/j.jpcs.2017.09.033.
Klein, S., Feiden, A., Boscolo, W. R., et al. (2004). Chemical products for Ichthyophthirius multifiliis, Fouquet (1876) controll in Surubim do Iguaçu Steindachneridion sp., Garavello (1991) fingerlings. Semin Ciências Agrárias, 25, 51–58.
Liang, J., Komarov, S., Hayashi, N., & Kasai, E. (2007). Improvement in sonochemical degradation of 4-chlorophenol by combined use of Fenton-like reagents. Ultrasonics Sonochemistry. https://doi.org/10.1016/j.ultsonch.2006.05.002.
Mandal, A., Ojha, K., De, A. K., & Bhattacharjee, S. (2004). Removal of catechol from aqueous solution by advanced photo-oxidation process. Chemical Engineering Journal. https://doi.org/10.1016/j.cej.2004.05.007.
Moumeni, O., Hamdaoui, O., & Pétrier, C. (2012). Sonochemical degradation of malachite green in water. Chemical Engineering and Processing: Process Intensification. https://doi.org/10.1016/j.cep.2012.09.011.
Oturan, M. A., Guivarch, E., Oturan, N., & Sirés, I. (2008). Oxidation pathways of malachite green by Fe3+−catalyzed electro-Fenton process. Applied Catalysis B: Environmental. https://doi.org/10.1016/j.apcatb.2008.01.016.
Pignatello, J. J., Oliveros, E., & MacKay, A. (2006). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical Reviews in Environmental Science and Technology. https://doi.org/10.1080/10643380500326564.
Rocha, A., Ceccarelli, R. C. G., Neto, P. S. S., et al. (1994). Eficácia de diferentes produtos químicos no controle de Ichthyophthirius multifiliis Fouquet (1876), em alevinos de pacu Piaractus mesopotamicus Holmberg (1887). Bol técnico do CEPTA, 7, 2–5.
Sasidharan Pillai, I. M., & Gupta, A. K. (2016). Electrochemical degradation of malachite green: multivariate optimization, pathway identification and toxicity analysis. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering. https://doi.org/10.1080/10934529.2016.1199640.
Silva, M. R. A., Trovó, A. G., & Nogueira, R. F. P. (2007). Degradation of the herbicide tebuthiuron using solar photo-Fenton process and ferric citrate complex at circumneutral pH. Journal of Photochemistry and Photobiology, A: Chemistry. https://doi.org/10.1016/j.jphotochem.2007.04.022.
Singh, P., Raizada, P., Kumari, S., Kumar, A., Pathania, D., & Thakur, P. (2014). Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Applied Catalysis A: General. https://doi.org/10.1016/j.apcata.2014.02.009.
Srivastava, S., Sinha, R., & Roy, D. (2004). Toxicological effects of malachite green. Aquatic Toxicology. https://doi.org/10.1016/j.aquatox.2003.09.008.
Sudova, E., Machova, J., Svobodova, Z., & Vesely, T. (2007). Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Veterinarni Medicina. https://doi.org/10.17221/2027-VETMED.
Sun, J. H., Sun, S. P., Fan, M. H., Guo, H. Q., Qiao, L. P., & Sun, R. X. (2007). A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2007.02.022.
Tavares, M. G., Da Silva Santos, D. H., Albuquerque Torres, S. J., Oliveira Pimentel, W. R., Tonholo, J., & De Paiva E Silva Zanta, C. L. (2016). Efficiency and toxicity: comparison between the Fenton and electrochemical processes. Water Science and Technology. https://doi.org/10.2166/wst.2016.278.
Young, B. J., Riera, N. I., Beily, M. E., Bres, P. A., Crespo, D. C., & Ronco, A. E. (2012). Toxicity of the effluent from an anaerobic bioreactor treating cereal residues on Lactuca sativa. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2011.09.019.
Zanta, C. L. P. S., Friedrich, L. C., Machulek, A., Higa, K. M., & Quina, F. H. (2010). Surfactant degradation by a catechol-driven Fenton reaction. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2010.01.071.
Zhang, F., Feng, C., Li, W., & Cui, J. (2014). Indirect electrochemical oxidation of dye wastewater containing acid orange 7 using Ti/ruo2-Pt electrode. International Journal of Electrochemical Science.
Funding
Jéssica M. O. Melo and José L.S. Duarte gratefully acknowledge the CNPq fellowship PROJETO: 870220/2000-4 -Processo: 161798/2014-4. Carmem L.P.S. Zanta thanks the financial support provided by FAPEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melo, J.M.O., Duarte, J.L.S., Ferro, A.B. et al. Comparing Electrochemical and Fenton-Based Processes for Aquaculture Biocide Degradation. Water Air Soil Pollut 231, 79 (2020). https://doi.org/10.1007/s11270-020-4454-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4454-9