Abstract
Assisted phytoremediation using amendments is a cost-effective and environmentally friendly approach to control soil pollution. However, amendment type, combination and application rate can influence process effectiveness. In the present study, the effect of the association of red mud and carbon-based amendments on the physicochemical properties of a former mine soil as well as the growth and metal(loid) uptake of Trifolium repens was investigated. For this purpose, a mesocosm experiment was set up using a former mine technosol highly contaminated by As and Pb, amended with red mud combined with different carbon-based amendments, i.e., bamboo biochar, oak biochar, steam activated carbon and acidic activated carbon, and sown with Trifolium repens. The final goal was to determine which amendment combination allows soil metal(loid) immobilization and an efficient plant growth. Results showed that all the four different treatments improved soil characteristics by increasing pH and electrical conductivity and reducing redox potential. All the treatments were also effective in reducing soil pore water lead concentrations. Among the four treatments, the addition of red mud and acidic activated carbon in the soil showed better results regarding Trifolium repens growth. Finally, when grown on the soil amended with red mud and acidic activated carbon, Trifolium repens presented mainly a metal(loid) storage in roots, making it a right candidate for the establishment of a vegetation cover.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mining activities disturb the natural biogeochemical cycles of metal(loid)s and subsequently contaminate soil (Forjàn et al. 2016). Metal(loid)s are non-biodegradable, tend to persist in the environment, and pose permanent risk to ecosystems (Tang et al. 2015). Traditionally, negative effects of metal(loid) contamination in the environment have been mitigated with physicochemical methods such as controlled backfilling, soil fixation, or leaching. However, some of these traditional processes sometimes prove too costly and environmentally unfriendly. Consequently, cost-effective and greener biotechnologies, such as assisted phytoremediation, have gained considerable research attention in recent years (Vangrosveld et al. 2009). Assisted phytoremediation utilizes the combination of soil amendments and vegetation to immobilize, reduce, or remove metal(loid)s from the environment (Zubair et al. 2016). The combined use of mineral-based amendments and carbonaceous materials could be a promising choice to assist phytoremediation of contaminated soil by decreasing metal(loid) leaching and availability while improving soil fertility and plant growth in contaminated soils (Oustriere et al. 2017). Some mineral amendments are known to decrease the concentration of metal(loid) availability by enhancing adsorption, precipitation, and complexation reactions onto soils (Peng et al. 2009).
Red mud is a waste generated during caustic digestion of bauxite for aluminum production and could be used as a mineral amendment. It was shown that it has a great potential to remove pollutants (arsenic, cadmium, copper, and lead) from various environmental media (Taneez et al. 2015). Such reduction of metal(loid) mobility can be due to the high levels of Al and Fe oxides contained in red mud. These chemical compounds have active adsorption sites and thus could interact with metal(loid)s, reducing their mobility and availability (Lee et al. 2014; Zhou et al. 2017). In addition, red mud application to soil can rise soil pH, increasing the negative surface charge of the soil and consequently leading to the adsorption of metal(loid)s or their precipitation as metal hydroxides and carbonates (Hua et al. 2017; Lee et al. 2014). In the same way, pyrolyzed carbonaceous materials obtained from wood biomass or agricultural waste are low-cost and effective soil amendments to improve soil physicochemical characteristics (Hagemann et al. 2018; Nawab et al. 2016). This is the case for biochar and activated carbon which are both pyrogenic carbonaceous materials produced by thermochemical conversion of carbonaceous feedstock (pyrolysis and/or activation) (Hagemann et al. 2018; Qian et al. 2015). Such amendments have demonstrated positive effects on soil fertility (Liu et al. 2016; Lomaglio et al. 2017) and consequently on plant growth (Sabir et al. 2013; Wu et al. 2016). There are numerous ways in which biochar and activated carbon can improve soil quality. Biochar amendment can optimize water-holding capacity and cation exchange capacity as well as reduce the susceptibility of soil to erosion (Sarfraz et al. 2019). Furthermore, biochar can enhance the availability of essential nutrients such as nitrogen, carbon, and phosphorus and reduce the bioavailability of metal(loid)s as well as sequestrate carbon in soils (Sun et al. 2018). On the other hand, numerous studies demonstrated the positive effect that the activated carbon has on the soil fertility with the consequent increase in plant growth (Pallarés et al. 2018). Moreover, activated carbon and biochar are extremely porous materials with a large capacity to interact with metal(loid) ions and mobilize or immobilize them through the formation of more or less stable complexes (Alam et al. 2020; Hattab et al. 2014; Nawab et al. 2016). They also have functional groups on their surface, which can sorb metal(loid)s through ion exchange, surface complexation, precipitation… (Lebrun et al. 2018c). Additionally, similarly to red mud, biochar and activated carbon can increase soil pH, which leads to the precipitation of metal(loid)s. However, thermochemical conversion conditions and feedstock types are crucial determinants of biochar and activated carbon properties (Sun et al. 2014; Pallarés et al. 2018). It has been observed that both amendment application (Clapp 2007) and sowing of plant species (Curtis and Claassen 2009; Dunifon et al. 2011) promote revegetation of metal(loid) polluted soils. To achieve an overall and self-sustainable revegetation of metal(loid)-contaminated soils, it is essential to choose plants which have specific characteristics. Trifolium repens has characteristics which have led to it being selected for restoring metal(loid) polluted soils (Bidar et al. 2007). Trifolium repens has a high germination rate, a good resistance to environmental stress conditions, and its stoloniferous growth makes this plant able to colonize bare spaces in lawns. Besides, white clover enriches poor soils by fixing atmospheric nitrogen, which allows the development of other plant species (Nandillon et al. 2019a). Previous studies using Trifolium repens in contaminated zones have focused on metal uptake and soil remediation (Bidar et al. 2007, 2009; Lopareva-Pohu et al. 2011) and have shown that white clover mainly stores metal(loid)s in its roots (Bidar et al. 2009). Within this context, the specific objective of this study was to investigate the capacity of a red mud associated with two different types of biochar or two different activated carbons to stabilize metal(loid)s in a polluted soil revegetated with Trifolium repens.
For this purpose, (i) the effects of the amendment combination on the metal(loid)-contaminated soil characteristics, (ii) the effects of amendments on metal(loid) immobilization and white clover growth, and (iii) the capacity of white clover to store metal(loid)s in its roots when grown on such amended soil were evaluated.
2 Materials and Methods
2.1 Site Description
The study focused on a technosol derived from a former silver-lead extraction mine, located in Pontgibaud district (Auvergne-Rhône-Alpes, France). It has been disused since 1897, and, due to the intense mining activities, the site is highly contaminated by arsenic (1068 mg kg−1) and lead (23,387 mg kg−1) (Nandillon et al. 2019b). The technosol was sampled between 0 and 20 cm depth at the second settling pond (GPS coordinates 45° 47′ 27″ North and 2° 49′ 38″ East), located at Roure-les-Rosiers (Saint-Pierre-le-Chastel). The main physicochemical properties of the Pontgibaud technosol (PG) were determined in previous studies (Lebrun et al. 2019; Nandillon et al. 2019b) and are reported in Table 1.
2.2 Amendment Origin and Characterization
Five amendments were used: a red mud (R), a biochar obtained from bamboo biomass (BA), a biochar produced from the bark and sapwood of oak (BS2), a steam activated carbon (EK5), and an acidic activated carbon (L27).
The red mud was provided by Alteo Environment (Gardanne, France), and it was obtained from Bauxaline neutralized by gypsum addition (CaSO4·2H2O).
La Carbonerie (Crissey, France) provided the two biochars used. Both biochars were obtained by the slow pyrolysis of dry biomass (bark and sapwood from Quercus sp. for BS2 and bamboo for BA) at a temperature of 450 °C with a heating rate of 2.5 °C·min−1 and a 24-h residence time. The two different activated carbons were supplied by Jacobi Carbons (Paris, France).
EK5 was obtained by a physical activation with steam water at a temperature above 800 °C in a controlled atmosphere.
L27 was chemically activated by a treatment with phosphoric acid at a temperature between 400 and 600 °C.
The main physicochemical properties of the five amendments used are presented in Table 1. pH, electrical conductivity (EC), and redox potential (Eh) were determined in a previous study (Lebrun et al. 2020a). BET measurements, to assess specific surface area, pore volume, and pore diameter were realized using a BELSORP Mini II (MicrotracBEL) (LMI, Villeurbanne, France). The content of carbon, hydrogen, and nitrogen were determined by elemental analyzer (Flash 2000 (Thermo)).
2.3 Experimental Design
The five amendments were chosen based on a previous study (unpublished data) in which several red muds, biochars, and activated carbons were tested. Later, it was decided to use the red mud in combination with the other four amendments because Pontgibaud soil is contaminated with both arsenic and lead. Red mud is rich in iron and aluminum oxides and hydroxides which can interact with arsenic (Fresno et al. 2018), while biochar and activated carbon are inefficient or even negative for anions such as arsenic (Li et al. 2017; Liu et al. 2012) but show good potential for cationic metals such as lead (Rivera-Utrilla et al. 2001; Sun et al. 2018). In total, five different treatments were tested (Table 2): (i) non-amended Pontgibaud technosol (PG), (ii) Pontgibaud technosol amended with 1% (w/w) of red mud and 2% (w/w) of bamboo biochar (RBA), (iii) Pontgibaud technosol amended with 1% (w/w) of red mud and 2% (w/w) of oak biochar (RBS2), (iv) Pontgibaud technosol amended with 1% (w/w) of red mud and 2% (w/w) of steam activated carbon (REK5), and (v) Pontgibaud technosol amended with 1% (w/w) of red mud and 2% (w/w) of acidic activated carbon (RL27). Four replicates of the different mixtures were placed in 0.5 L pots, and 100 seeds of Trifolium repens were sown per pot. The experiment was carried out in a controlled growth chamber under the following conditions: day/night temperatures (22 °C/16 °C), 16 h of light/8 h of darkness, and with a light intensity of 400 W. The pots were watered each day with tap water. The experiment lasted 40 days.
2.4 Soil Analysis
Soil water holding capacity (WHC) for the five studied treatments was measured before sowing the clover seeds (T0) according to the protocol described in Lebrun et al. (2018a). At the end of the greenhouse experiment (T40) (40 days), soils were sampled in each pot, and the phytoavailable fractions of arsenic and lead for the five treatments were obtained as described by Qasim et al. (2015), using 0.01 M CaCl2 extractant (solid/liquid ratio 1:10). Metal(loid) concentrations were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (ULTIMA 2, HORIBA, Labcompare, San Francisco, USA).
2.5 Soil Pore Water (SPW) Sampling and Analysis
Soil pore waters were collected in each pot, before sowing the clover seeds (T0) and at the end of the experiment (T40) (40 days), using Rhizon® (Rhizosphere Research Product, Wageningen, the Netherlands) according to Lebrun et al. (2018a): Rhizon® were placed in the pot at an angle of 40°. Pots were watered and left to equilibrate for 4 h before sampling. Soil pore water samples (20 mL) were used directly to measure pH, electrical conductivity (EC), and redox potential (Eh) (Mettler Toledo, Serveur Excellence). Arsenic (As) and lead (Pb) concentrations were determined by ICP-AES after acidification, according to Bart et al. (2016).
2.6 Plant Analysis
At the end of the growing period (40 days), the entire plants of Trifolium repens were harvested from the pots. The roots were washed thoroughly with tap water and rinsed with distilled water to remove soil particles attached to the roots. Next, leaves, stems, roots, and root nodules were collected separately only for the plants grown in the RL27 treatment (Pontgibaud technosol amended with 1% (w/w) of red mud and 2% (w/w) of acidic activated carbon). This subdivision was only possible for the RL27 treatment in which the plants had sufficient growth to distinguish between the different plant organs. For all the other four treatments, the small size of the clover plant did not allow the separation between the different plant organs, and the measures were done on one sample bringing together roots (with their nodules), leaves, and stems. Samples were dried in an oven at 60 °C (until constant weight), and the dry weight (DW) was determined. Dry samples were finely ground (propeller mill, IKA, Staufen, Germany) before mineralization, and metal(loid) concentrations were measured by ICP-AES, according to the protocol described in Bart et al. (2016). In order to assess the As and Pb uptake and intake in Trifolium repens, the translocation factor (TF) and the total and organ-specific (root, stem, and leaf) bioconcentration factor (BCF) were then calculated for the plants grown in the RL27 treatment. The TF was calculated as the ratio of As or Pb concentration in aboveground (stems and leaves) to the concentrations in the belowground (roots) plant compartments. The BCF was calculated as the ratio of As or Pb concentrations in plant/organ to the concentrations in the soil.
2.7 Statistical Analysis
All statistical analyses were performed with the R software version 3.4.3 (R Development Core Team 2017). Normality and homoscedasticity of the data were assessed with the Shapiro and Bartlett tests, respectively. The mean values were compared using the parametric ANOVA test for normal data or the non-parametric Kruskal test for the non-normal data. Following, a post hoc test (Tukey HSD or pairwise Wilcox tests, respectively) was performed. Moreover, for soil pore water data, the difference between the different times of sampling was evaluated in each treatment, using the same procedure but using the parametric Paired Student test for mean comparison.
3 Results and Discussions
3.1 Effect of Amendments on Soil
Only the combination of the red mud amendment with biochar (RBA and RBS2) induced an increase in soil water-holding capacity (WHC) with respect to Pontgibaud soil (PG), averaging 33% for the treatment with bamboo biochar (RBA) and 11% for oak biochar (RBS2) (Table 3). Several studies reported a decrease in hydraulic conductivity of soil with biochar amendment rate (Molnàr et al. 2016). Furthermore, Barnes et al. (2014) strongly supported that biochar addition increases the water-holding capacity in soils, likely improving plant water availability. Biochar can increase soil WHC due to its porous structure that can retain water (Lebrun et al. 2018a). However, such effect is not always observed. For instance, only the three coarser biochars, and added at 5%, increased soil WHC of a mining soil, while the finest one had no effect, which was related to the pore structure properties of the biochars (Lebrun et al. 2018a).
At the end of the experiment (T40), soil phytoavailable arsenic (As) concentration decreased in all amended soils with respect to Pontgibaud soil (PG) except for RL27 treatment (Table 3). The RBA, RBS2, and REK5 treatments induced a soil phytoavailable As concentration decrease of 49%, 51%, and 54%, respectively, compared to non-amended Pontgibaud technosol. Several studies showed that the addition of biochar into soil increased As leaching since biochar has a low sorption capacity for metal(loid) anions (Hartley et al. 2009; Li et al. 2017). The negatively charged functional groups (e.g., —COO—, —COH, and —OH) on biochars promote the As mobilization into soil solution through charge repulsion (Li et al. 2017). Therefore, the soil phytoavailable As concentration decrease showed in our study was probably due to the combination of the red mud with the BA, BS2, and EK5 amendments. The addition of red mud to PG soil could have affected As mobility directly since one of the significant components of this amendment is iron oxides. The mobility of As is greatly influenced by the biogeochemical interactions with soil minerals such as iron oxides, as the negatively charged As ions (As(III) and As(V)) are strongly adsorbed on iron oxides in soils (Fresno et al. 2018), and as a result As solubility and availability is reduced through the formation of amorphous iron oxides. However, the presence of red mud in the RL27 treatment was not sufficient to induce a decrease in soil phytoavailable As concentration. L27 was activated by phosphoric acid, so the competition between phosphate and arsenate could explain why the RL27 treatment led to a non-significant soil phytoavailable As concentration increase of 27% compared to non-amended Pontgibaud technosol. Indeed, phosphate-based materials may enhance the leaching of As since arsenic oxyanions and phosphate compete for the same adsorption site in soils and around plant roots (Zeng et al. 2017).
All the treatments induced a soil phytoavailable lead (Pb) concentration decrease, by 70% for RBA, 47% for RBS2, 52% for REK5, and 95% for RL27 with respect to Pontgibaud soil (Table 3). Several studies showed that the addition of biochar and activated carbon into soil decreased Pb leaching since these amendments have a high sorption capacity for metal cations (Li et al. 2017; Liu et al. 2012; Sun et al. 2018). This property could be responsible for the reductions of soil phytoavailable Pb concentration in all the four amended soils. When mixed with Pontgibaud technosol, biochar and activated carbon with negatively charged functional groups (e.g., —COO—, —COH, and —OH) can complex the cationic metals such as Pb2+ and so promote the Pb immobilization into the soil (Li et al. 2017; Liu et al. 2012). Other studies showed that also the addition of red mud into soil decreased phytoavailable Pb concentration (Gautam et al. 2017; Taneez et al. 2015). Red mud has been shown to have a great potential to remove Pb from soil. Indeed, low phytoavailable concentration of Pb may be ascribed to tectosilicate structures, e.g., cancrinite and hematite, the two principal phases of red mud, which provide a high metal adsorption capacity (Santona et al. 2006). Moreover, in soil, phytoavailable metal concentrations are influenced by several factors such as pH, organic carbon content, and soil texture (Sherene 2010). Addition of amendments in Pontgibaud soil raised the pH under the different treatments (Table 4) which could elicit the reduction of phytoavailable Pb concentration. Under neutral to alkaline soil condition, soluble and phytoavailable metal concentrations decrease due to precipitation followed by adsorption on amendments (Lombi et al. 2003).
The highest decrease of the soil phytoavailable Pb concentration was observed in the RL27 treatment. Contrary to what is reported for arsenic, it was proved that phosphate-based materials are beneficial and attractive for the stabilization of lead in soils (Zeng et al. 2017). In the presence of phosphate, Pb could be immobilized into a mineral phase like pyromorphite, which is presented as Pb5(PO4)3X [X = F (fluorine), Cl (chlorine), Br (bromine), and OH (hydroxy)], and once the Pb in contaminated soils is converted into Pb-phosphate, it will be immobilized, and its concentration will be reduced (Hafsteinsdóttir et al. 2015; Weber et al. 2015).
3.2 Effect of Amendments on SPW Characteristics
At the beginning of the experiment (T0), the non-amended Pontgibaud technosol (PG) was acidic (pH 4.5, Table 4). The application of amendments increased soil pore water (SPW) pH, with 2.6, 2.3, 2.9, and 2.2 units’ rise for RBA, RBS2, REK5, and RL27, respectively (Table 4). The highest SPW pH increase observed in the REK5 treatment was due to the highest pH of the steam activated carbon (EK5) with respect to the other amendments (Table 1). In agreement with several studies, soil and SPW pH improvement following red mud (Hurel et al. 2017; Taneez et al. 2015), biochar (Lebrun et al. 2018b), and activated carbon (Lebrun et al. 2020b) application can be attributed to (i) the alkalinity of used amendments (R, BA, BS2, and EK5) which induced a liming effect and (ii) the presence of functional groups on biochar and activated carbon surfaces, like carbonyls, phenols, carboxyls, and pyrones. The carboxyl and phenolic hydroxyl of amendments can react with protons: they can accept protons which increases pH and provide protons which decreases pH. Therefore, adding soil with such amendments can increase soil pH (Marks et al. 2014). Similarly, at the end of the experiment (T40), SPW pH was higher in the amended soils with respect to PG with an average increase of 3 units (Table 4). In three treatments (PG, RBS2, and REK5), a SPW pH decrease was observed between the two different time sampling. There are several factors that influence soil pH, such as plant growth and organic matter content.
In soil, plants take up water and nutrients, undergo root elongation and expansion, release exudates, and respire (Turpault et al. 2007). Through some of these biological processes, plant roots have the ability to induce pH changes in the soil either by releasing protons (H+) or hydroxyl ions (OH−) to maintain ion balance, depending on the nutritional status of the plants (Hinsinger et al. 2003). Therefore, soil pH could increase or decrease depending on the prevailing process and types of ions released. Plant root-induced soil pH change is controlled by specific processes and factors such as (i) ion uptake coupled with the release of inorganic ions that maintain electroneutrality, (ii) the excretion of organic acid anions, (iii) root exudation and respiration, (iv) redox-coupled processes, and (v) microbial production of acids after the assimilation of released root carbon (Neina 2019). Plants release amounts of carbon dioxide (CO2) from the root respiration. Carbon dioxide forms carbonic acid (H2CO3) when mixed with water in the soil, and the production of carbonic acid can cause a decrease in soil pH (Mohd-Aizat et al. 2014). Trifolium repens growth could explain the SPW pH decrease observed between the beginning and the end of the experiment.
At time T0, SPW electrical conductivity (EC) increased on average sixfold in all the four amended soils (RBA, RBS2, REK5, and RL27) with respect to PG (Table 4), and this SPW EC rise could be due to the high amendment EC itself (Table 1). At the end of the experiment (T40), a significant SPW EC increase was only observed in the RL27 condition (7239 μS cm−1) with respect to non-amended soil (2210 μS cm−1). Besides, SPW EC increased significantly between the beginning (T0) and the end (T40) of the experiment in PG and RL27 (with a fourfold and a threefold increase, respectively). This EC increase in PG was coherent with the SPW pH decrease because these two parameters are inversely proportional (Mohd-Aizat et al. 2014). In RL27, the EC rise could be due to mineral leaching from the surface of the acidic activated carbon used in this treatment.
At the beginning of the experiment (T0), while the amendment addition to PG soil increased SPW pH, it decreased the redox potential (Eh). SPW Eh decreased by 25%, 21%, 29%, and 22% for RBA, RBS2, REK5, and RL27 treatment, respectively. Similarly, at T40, SPW Eh values were also lower in all the four amended soils with respect to non-amended Pontgibaud technosol. The amended soils induced a SPW Eh decrease, averaging 19% for RBA, 14% for RBS2, 21% for REK5, and 25% for RL27 compared to PG. In addition, between the two times of sampling, SPW Eh increased in RBA, RBS2, and REK5 (Table 4), and this was coherent with the SPW pH decrease because these two parameters are negatively correlated in soils (Bohrerova et al. 2004; Husson 2013).
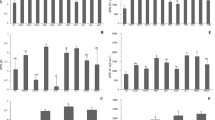
At both T0 and T40, a SPW As concentration increase was observed only in the RL27 treatment with an increment of 33% and 178%, respectively (Fig. 1). Biochar and activated carbon are not able to effectively immobilize arsenic on their surface due to the presence of negatively charged groups that cause a charge repulsion with arsenic anions. Moreover, the observed SPW As concentration increase could be related to the SPW pH increase explained above in the current paragraph. Indeed, As mobility in soil raises with the pH increase (Zheng et al. 2019). However, in RBA, RBS2, and REK5 treatments, the combination of these amendments with the red med limits arsenic mobility. The major components of the red mud are iron and aluminum oxides, and it is known that arsenic retention in soils is influenced by the presence of these oxides which are able to immobilize arsenic in soils (Talukder et al. 2015). As shown for the soil phytoavailable As concentration (Table 3), the presence of the red mud in the RL27 treatment was not sufficient to induce a decrease in soil As mobility. Indeed, the high SPW As concentration in RL27 was probably due to the competition between phosphate and arsenate for the adsorption sites in the soil (Sun et al. 2019).
Soil pore water arsenic concentration ([As] mg L−1) determined in Pontgibaud technosol (PG); PG + 1% red mud + 2% bamboo biochar (RBA); PG + 1% red mud + 2% oak biochar (RBS2); PG + 1% red mud + 2% steam activated carbon (REK5); PG + 1% red mud + 2% acidic activated carbon (RL27). SPWs were sampled at the beginning of the experiment (T0—light gray box) and after 40 days of Trifolium repens plant growth (T40—dark gray box). The “treatment effect” for each sampling time is represented by case letters for T0 and capital letters for T40 (p < 0.05) (n = 4 ± SE). No difference was observed between T0 and T40
At T0, all four treatments (RBA, RBS2, REK5, and RL27) induced a SPW Pb concentration decrease with an average decrease of 11-folds with respect to Pontgibaud technosol (13.05 mg L−1). A decrease in SPW Pb concentration was also observed at T40 in all treatments compared to PG (7.64 mg L−1): the lowest SPW Pb concentrations were measured in RBA (2.22 mg L−1) and RL27 (1.00 mg L−1). Both biochar and activated carbon have high surface areas, a microporous structure, and functional groups on their surface (Liu et al. 2012; Sun et al. 2018). These properties could be responsible for the reductions of SPW Pb concentration in all the four amended soils. When mixed with Pontgibaud technosol, biochar and activated carbon with negative charges can tightly adsorb the cationic metal(loid)s such as Pb2+. For Pb cations, the physical adsorption, surface (co)precipitation, and surface/inner complexation with functional groups are considered as the major mechanism for the immobilization of Pb by biochar and activated carbon (Alpaslan and Yukselen 2002). Moreover, in our study, the red mud-, biochar-, and activated carbon-induced changes in soils such as the increase of soil pH (Table 4) could further lower the solubility of Pb. Under neutral to alkaline soil condition, soluble and mobile Pb concentrations also decrease due to precipitation followed by adsorption onto charged colloids of red mud (Lombi et al. 2003). Low SPW Pb concentration may also be ascribed to tectosilicate structures, e.g., cancrinite and hematite, the two principal phases of red mud, which provide a high Pb adsorption capacity (Santona et al. 2006). In cancrinite and hematite, Pb cations are incorporated in a cage and the channels of the negatively charged lattice and adsorbed on the mineral surface resulting in reduced mobility of Pb (Castaldi et al. 2009; Gray et al. 2006). In addition, at the end of the experiment (T40), the treatment with the red mud and acidic activated carbon combination (RL27) appeared to be the treatment that decreased the SPW Pb concentration the most significantly (Fig. 2), suggesting that the phosphoric acid for the activation of L27 contributed to reducing the lead mobility in the soil. Zeng et al. (2017) reported that phosphate amendments which could transfer Pb from unstable fraction to stable fraction are commonly used to immobilize Pb in soils and that the principal mechanism responsible for P-induced Pb immobilization is the precipitation of Pb-phosphates (including direct precipitation, ion-exchange (or substitution) effect, and liming effect). Regarding the time effect, between the beginning and the end of the experiment, the SPW Pb concentration decreased in PG while it increased in RBS2 (Fig. 2). In the PG soil, this decrease was probably due to Trifolium repens growth. Indeed, plants have the ability to decrease the mobility or/and concentration of a metal(loid) by certain mechanisms including adsorption by roots, precipitation, and complexation in the root zone (Erakhrumen 2007). In the RBS2 treatment, probably, the small specific surface area and the high mean pore diameter of BS2 biochar compared to the other amendments (Table 1) could have been unable to further immobilize lead over time, leading to an increase in SPW Pb concentration.
Soil pore water lead concentration ([Pb] mg L−1) determined in: Pontgibaud technosol (PG); PG + 1% red mud + 2% bamboo biochar (RBA); PG + 1% red mud + 2% oak biochar (RBS2); PG + 1% red mud + 2% steam activated carbon (REK5); PG + 1% red mud + 2% acidic activated carbon (RL27). SPWs were sampled at the beginning of the experiment (T0—light gray box) and after 40 days of Trifolium repens plant growth (T40—dark gray box). The “treatment effect” for each sampling time is represented by case letters for T0 and capital letters for T40 (p < 0.05) (n = 4 ± SE). “Time effect” for each treatment is shown by: **(p < 0.01), ns (non-significant)
3.3 Effect of Amendments on Trifolium repens
After 40 days of growth, Trifolium repens dry weight (DW) was low in PG (0.08 g), while it increased in RBA (0.24 g) and reaching the highest value in RL27 (1.16 g) (Fig. 3). These results are probably related to the improvements that RBA and RL27 treatments had on the characteristics of the Pontgibaud-contaminated soil. The stability of pH values (Table 4), a reasonable right amount of nutrients provided by bamboo biochar (BA) and acidic activated carbon (L27), and containment of lead toxicity (Table 3 and Fig. 2) could support the good Trifolium repens growth observed in the two treatments.
Total dry weight (g) (leaves, stems, roots, and root nodules) of Trifolium repens grown for 40 days on the five studied treatments: Pontgibaud technosol (PG); PG + 1% red mud + 2% bamboo biochar (RBA); PG + 1% red mud + 2% oak biochar (RBS2); PG + 1% red mud + 2% steam activated carbon (REK5); PG + 1% red mud + 2% acidic activated carbon (RL27). The “treatment effect” is represented by letters (p < 0.05) (n = 4 ± SE)
The total concentration of arsenic in plants grown on RBA, RBS2, REK5, and RL27 was significantly lower by 80%, 66%, 56%, and 74%, respectively, compared to plants grown on non-amended Pontgibaud technosol (Fig. 4). The three treatments RBA, RBS2, and REK5 had stabilized arsenic in the soil: soil phytoavailable As concentrations were low in all these treatments (Table 3). Therefore, Trifolium repens plants grown in these soils have not accumulated high concentrations of the metalloid. On the contrary, the RL27 treatment had increased the phytoavailability of arsenic (Table 3 and Fig. 1) making this metalloid more available for plant uptake, but, despite this, arsenic concentration in plants did not increase. As already discussed, arsenic concentrations in this soil increased due to competition with phosphate (Zeng et al. 2017) introduced by the addition of phosphoric acid activated carbon (L27). Due to a chemical similarity, phosphate and arsenate share the same transport pathway in plants, with the transporters having a higher affinity for phosphate than arsenate (Talukder et al. 2015; Ye et al. 2019). This could explain how the phosphate-based amendment (L27) increased arsenic leaching, whereas decreased the metalloid plant uptake from the soil.
Arsenic concentration ([As] mg kg−1) in Trifolium repens plants grown for 40 days on the five studied treatments: Pontgibaud technosol (PG); PG + 1% red mud + 2% bamboo biochar (RBA); PG + 1% red mud + 2% oak biochar (RBS2); PG + 1% red mud + 2% steam activated carbon (REK5); PG + 1% red mud + 2% acidic activated carbon (RL27). The “treatment effect” is represented by letters (p < 0.05) (n = 4 ± SE)
The total concentration of lead in plants grown on all four different amended soils was significantly lower than in plants grown on non-amended Pontgibaud technosol (13,559 mg kg−1) (Fig. 5). The lowest total lead concentrations were found in plants grown on RBA (3386 mg kg−1) and RL27 (2332 mg kg−1) soils. The concentrations of lead in Trifolium repens plants grown on RBS2 and REK5 were 5333 mg kg−1 and 7832 mg kg−1, respectively (Fig. 5). The four different amendment combinations had stabilized lead in the soil, with low soil phytoavailable and soil pore water Pb concentrations in these soils, with RBA and RL27 leading to the largest decreases (Table 3 and Fig. 2). This could explain why the plants grown on the RBA and RL27 treatments accumulated the lowest lead concentrations.
Lead concentration ([Pb] mg kg−1) in Trifolium repens plants grown for 40 days on the five studied treatments: Pontgibaud technosol (PG); PG + 1% red mud + 2% bamboo biochar (RBA); PG + 1% red mud + 2% oak biochar (RBS2); PG + 1% red mud + 2% steam activated carbon (REK5); PG + 1% red mud + 2% acidic activated carbon (RL27). The “treatment effect” is represented by letters (p < 0.05) (n = 4 ± SE)
As shown in Fig. 3, the best growth of Trifolium repens had taken place in the treatment involving the combination of red mud and acidic activated carbon (RL27). For the plants grown in this treatment, it was thus possible to divide the different organs (leaves, stems, roots, and root nodules). The dry weight (DW) and the metal(loid) concentration were then determined for each plant organ. These determinations were carried out to observe if the preferential organ of metal(loid) accumulation were the roots over the aerial part of Trifolium repens. The DW of the white clover aerial part (0.57 g for the leaves and 0.33 for the stems) was greater than that measured in the root part (0.21 for the roots and 0.04 for the root nodules) (Table 5). The white clover plants accumulated higher concentrations of arsenic and lead in roots (814 mg kg−1 for As and 7803 mg kg−1 for Pb) and root nodules (325 mg kg−1 for As and 3267 mg kg−1 for Pb) than the concentrations measured in leaves (144 mg kg−1 for As and 1283 mg kg−1 for Pb) and stems (169 mg kg−1 for As and 2107 mg kg−1 for Pb) (Table 5). In order to understand the ability of Trifolium repens to remove and accumulate the metal(loid) compounds from the soil and the transfer mechanism of them from roots to other organs, the bioconcentration factor (BCF) and the translocation factor (TF) were calculated, respectively. The calculation of accumulation indexes showed that, for both As and Pb, the specific BCF values were higher in roots (1.07 for As and 0.47 for Pb) than in stems (0.16 for As and 0.09 for Pb) and leaves (0.14 for As and 0.05 for Pb) (Table 6). Moreover, translocation factor (TF) values for As and Pb in Trifolium repens did not exceed one (TF < 1) (Table 6). Plants that have bioconcentration and translocation factors greater than 1 can be used as bioaccumulators (Usman et al. 2013). Plants can be used as phytostabilizers if they have bioconcentration factors higher than 1 and translocation factors less than 1 and as phytoextractors if they have bioconcentration factors less than 1 and translocation factors greater than 1 (Takarina and Pin 2017). A BCF value greater than 1 was found for As in Trifolium repens roots (1.07) indicating a higher accumulation in the roots than the concentration found in the soil. For both As and Pb, the above-mentioned values of BCF higher in the roots than in the aboveground plant organs indicated that Trifolium repens has stored the metal(loid)s in its roots without transferring to the stems and leaves. Indeed, the low TF values (0.28 for As and 0.31 for Pb) of Trifolium repens indicate that plants limited translocation of arsenic and lead from roots to shoots. This seems to indicate that roots have a greater ability to accumulate rather than translocate the studied metal(loid)s. In leaves, metal(loid)s like As and Pb are toxic, especially for the plant functions of photosynthesis and the synthesis of chlorophyll and antioxidant enzymes. For this reason, Trifolium repens can prevent the transport of non-essential metal(loid)s to the plant aboveground part by accumulating them in the roots (Yoon et al. 2006). Thus, Trifolium repens accumulated arsenic and lead at radical level with low metal(loid) translocation to the aerial part, namely, that roots were the preferential metal(loid) storage organs, suggesting thereby the usefulness of these plant species for the phytostabilization of metal(loid)s in polluted soils and amended with both red mud and acidic activated carbon. This is consistent with the results of Bidar et al. (2007, 2009), Lopareva-Pohu et al. (2011), and Nandillon et al. (2019a) for Trifolium repens growing on metal(loid) contaminated soil.
4 Conclusion
A greenhouse experiment of assisted phytoremediation was performed in order to evaluate the effect of four different amendments (bamboo biochar, oak biochar, steam activated carbon, and acidic activated carbon) combined with red mud addition on (i) the physicochemical properties of a former mine soil, highly contaminated by arsenic and lead; (ii) the soil metal(loid) immobilization; and (iii) the growth and metal(loid) uptake of Trifolium repens. Soil physicochemical characteristics were improved (acidity and redox potential decrease, electrical conductivity increase) following amendment addition. Arsenic availability in the soil was decreased in all the amended soils except for the soil amended with red mud and acidic activated carbon in which arsenic leaching was raised. All the four treatments decreased lead mobility in the soil, especially when red mud was combined with bamboo biochar and acidic activated carbon. In the soil amended with red mud and acidic activated carbon, Trifolium repens plants grew well while accumulating high arsenic and lead concentrations and storing these metal(loid)s in their roots. Our results showed that the best treatment for lead stabilization in the soil using a Trifolium repens cover was the combination of red mud and acidic activated carbon. However, the use of these two soil amendments had no positive effect on arsenic stabilization in contaminated soil. Therefore, further studies are needed to evaluate (i) the use of a higher concentration of red mud (higher than the 1% concentration used in our study) to limit arsenic leaching and (ii) the field use of this treatment for arsenic and lead stabilization in contaminated soils using a cover with white clover.
References
Alam, M., Hussain, Z., Khan, A., Khan, M. A., Rab, A., Asif, M., Shah, M. A., & Muhammad, A. (2020). The effects of organic amendments on heavy metals bioavailability in mine impacted soil and associated human health risk. Scientia Horticulturae, 262, 109067.
Alpaslan, B., & Yukselen, M. A. (2002). Remediation of lead contaminated soils by stabilization/solidification. Water, Air, and Soil Pollution, 133, 253–263.
Barnes, R. T., Gallagher, M. E., Masiello, C. A., Liu, Z., & Dugan, B. (2014). Biochar-induced changes in soil hydraulic conductivity and dissolved nutrient fluxes constrained by laboratory experiments. PLoS One, 9(9), e108340. https://doi.org/10.1371/journal.pone.0108340.
Bart, S., Motelica-Heino, M., Miard, F., Joussein, E., Soubrand, M., Bourgerie, S., & Morabito, D. (2016). Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena, 136, 44–52.
Bidar, G., Garçon, G., Pruvot, C., Dewaele, D., Cazier, F., Douay, F., & Shirali, P. (2007). Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environmental Pollution, 147, 546–553.
Bidar, G., Pruvot, C., Garçon, G., Verdin, A., Shirali, P., & Douay, F. (2009). Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environmental Science and Pollution Research, 16, 42–53.
Bohrerova, Z., Stralkova, R., Podesvova, J., Bohrer, G., & Pokorny, E. (2004). The relationship between redox potential and nitrification under different sequences of crop rotations. Soil and Tillage Research, 77, 25–33.
Castaldi, P., Melis, P., Silvetti, M., Deiana, P., & Garau, G. (2009). Influence of pea and wheat growth on Pb, Cd, and Zn mobility and soil biological status in a polluted amended soil. Geoderma, 151, 241–248.
Clapp, E. C. (2007). Organic wastes in soils: Biogeochemical and environmental aspects. Soil Biology and Biochemistry, 39, 1239–1243.
Curtis, M. J., & Claassen, V. P. (2009). Regenerating topsoil functionality in four drastically disturbed soil types by compost incorporation. Restoration Ecology, 17, 24–32.
Dunifon, S. N., Evanylo, G. K., Maguire, R. O., & Goatley, J. M. (2011). Soil nutrient and fescue (Festuca spp.) responses to compost and hydroseed on a disturbed roadside. Compost Science & Utilization, 19, 147–151.
Erakhrumen, A. A. (2007). Phytoremediation: An environmentally sound technology for pollution prevention, control and remediation in developing countries. Educational Research Review, 2, 151–156.
Forjàn, R., Asensio, V., Rodrìguez-Vila, A., & Covelo, E. F. (2016). Contribution of waste and biochar amendment to the sorption of metals in a copper mine tailing. Catena, 137, 120–125.
Fresno, T., Moreno-Jimenez, E., Zornoza, P., & Peñalosa, J. M. (2018). Aided phytostabilisation of As- and Cu-contaminated soils using white lupin and combined iron and organic amendments. Journal of Environmental Management, 205, 142–150.
Gautam, M., Pandey, D., & Agrawal, M. (2017). Phytoremediation of metals using lemongrass (Cymbopogon citratus (D.C.) Stapf.) grown under different levels of red mud in soil amended with biowastes. International Journal of Phytoremediation, 19(6), 555–562.
Gray, C. W., Dunham, S. J., Dennis, P. G., Zhao, F. J., & McGrath, S. P. (2006). Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environmental Pollution, 142, 530–539.
Hafsteinsdóttir, E. G., Camenzuli, D., Rocavert, A. L., Walworth, J., & Gore, D. B. (2015). Chemical immobilization of metals and metalloids by phosphates. Applied Geochemistry, 59, 47–62.
Hagemann, N., Spokas, K., Schmidt, H. P., Kägi, R., Bholer, M. A., & Bucheli, T. D. (2018). Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water, 10, 182. https://doi.org/10.3390/w10020182.
Hartley, W., Dickinson, N. M., Riby, P., & Lepp, N. W. (2009). Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environmental Pollution, 157, 2654–2662.
Hattab, N., Soubrand, M., Guégan, R., Motelica-Heino, M., Bourrat, X., Faure, O., & Bouchardon, J. L. (2014). Effect of organic amendments on the mobility of trace elements in phytoremediated techno-soils: Role of the humic substances. Environmental Science and Pollution Research, 21, 10470–10480.
Hinsinger, P., Plassard, C., Tang, C., & Jaillard, B. (2003). Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil, 248, 43–59.
Hua, Y., Heal, K. V., & Friesl-Hanl, W. (2017). The use of red mud as an immobiliser for metal/metalloid-contaminated soil: a review. Journal of Hazardous Materials, 325, 17–30.
Hurel, C., Taneez, M., Volpi, Ghirardini, A., & Libralato, G. (2017). Effects of mineral amendments on trace elements leaching from pre-treated marine sediment after simulated rainfall events. Environmental Pollution, 220, 364–374.
Husson, O. (2013). Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant and Soil, 362, 389–417.
Lebrun, M., Miard, F., Nandillon, R., Hattab-Hambli, N., Scippa, G. S., Bourgerie, S., & Morabito, D. (2018a). Eco-restoration of a mine technosol according to biochar particle size and dose application: study of soil physico-chemical properties and phytostabilization capacities of Salix viminalis. Journal of Soils and Sediments, 18, 2188–2202.
Lebrun, M., Miard, F., Nandillon, R., Léger, J. C., Hattab-Hambli, N., Scippa, G. S., Bourgerie, S., & Morabito, D. (2018b). Assisted phytostabilization of a multicontaminated mine technosol using biochar amendment: early stage evaluation of biochar feedstock and particle size effects on as and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere, 194, 316–326.
Lebrun, M., Miard, F., Renouard, S., Nandillon, R., Scippa, G. S., Morabito, D., & Bourgerie, S. (2018c). Effect of Fe-functionalized biochar on toxicity of a technosol contaminated by Pb and As: sorption and phytotoxicity tests. Environmental Science and Pollution Research, 25(33), 33678–33690.
Lebrun, M., De Zio, E., Miard, F., Scippa, G. S., Renzone, G., Scaloni, A., Bourgerie, S., Morabito, D., & Trupiano, D. (2019). Amending an As/Pb contaminated soil with biochar, compost and iron grit: effect on Salix viminalis growth, root proteome profiles and metal(loid) accumulation indexes. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125397.
Lebrun, M., Miard, F., Scippa, G. S., Hano, C., Morabito, D., & Bourgerie, S. (2020a). Effect of biochar and red mud amendment combinations on Salix triandra growth, metal(loid) accumulation and oxidative stress response. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2020.110466.
Lebrun, M., Van Poucke, R., Miard, F., Scippa, G. S., Bourgerie, S., Morabito, D., & Tack, F. M. (2020b). Effects of carbon‐based materials and redmuds on metal (loid) immobilization and growth of Salix dasyclados Wimm. on a former mine technosol contaminated by arsenic and lead. Land Degradation & Development. https://doi.org/10.1002/ldr.3726
Lee, S. H., Ji, W., Lee, W. S., Koo, N., Koh, I. H., Kim, M. S., & Park, J. S. (2014). Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. Journal of Environmental Management, 139, 15–21.
Li, H., Dong, X., da Silva, E. B., de Oliveira, L. M., Chen, Y., & Ma, L. Q. (2017). Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere, 178, 466–478.
Liu, Z. T., Zhang, G. Y., & Hu, Z. S. (2012). Immobilization of cadmium and lead in contaminated soils by different amendments. Advanced Materials and Processes, 415, 1662–1666.
Liu, Z., Dugan, B., Masiello, C., Barnes, R., Gallagher, M., & Gonnermann, H. (2016). Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. Journal of Hydrology, 533, 461–472.
Lomaglio, T., Hattab-Hambli, N., Bret, A., Miard, F., Trupiano, D., Scippa, G. S., Motelica-Heino, M., Bourgerie, S., & Morabito, D. (2017). Effect of biochar amendments on the mobility and (bio) availability of As, Sb and Pb in a contaminated mine technosol. Journal of Geochemical Exploration, 182, 138–148.
Lombi, E., Hamon, R. E., McGrath, S. P., & McLaughlin, M. J. (2003). Lability of Cd, Cu, and Zn in polluted soils treated with lime, beringite, and red mud and identification of a non-labile colloidal fraction of metals using isotopic techniques. Environmental Science & Technology, 37, 979–984.
Lopareva-Pohu, A., Verdin, A., Garçon, G., Sahraoui, A. L., Pourrut, B., Debiane, D., Waterlot, C., Laruelle, F., Bidar, G., Douay, F., & Shirali, P. (2011). Influence of fly ash aided phytostabilisation of Pb, Cd and Zn highly contaminated soils on Lolium perenne and Trifolium repens metal transfer and physiological stress. Environmental Pollution, 159, 1721–1729.
Marks, E., Alcaniz, J., & Domene, X. (2014). Unintended effects of biochars on short term plant growth in a calcareous soil. Plant and Soil, 385(1–2), 87–105.
Mohd-Aizat, A., Mohamad-Roslan, M. K., Sulaiman, W. N. A., & Karam, D. S. (2014). The relationship between soil pH and selected soil properties in 48 years logged-over forest. International Journal of Environmental Sciences, 4(6), 1129–1140.
Molnàr, M., Vaszita, E., Farkas, E., Ujaczki, E., Fekete-Kertész, I., Tolner, H., Kleberez, O., Kirchkeszner, C., Gruiz, K., Uzinger, N., & Feigl, V. (2016). Acidic sandy soil improvement with biochar – a microcosm study. The Science of the Total Environment, 563–564, 855–865.
Nandillon, R., Lahwegue, O., Miard, F., Lebrun, M., Gaillard, M., Sabatier, S., Battaglia-Brunet, F., Morabito, D., & Bourgerie, S. (2019a). Potential use of biochar, compost and iron grit associated with Trifolium repens to stabilize Pb and As on a multi-contaminated technosol. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2019.109432.
Nandillon, R., Lebrun, M., Miard, F., Gaillard, M., Sabatier, S., Morabito, D., & Bourgerie, S. (2019b). Contrasted tolerance of Agrostis capillaris metallicolous and non-metallicolous ecotypes in the context of a mining technosol amended by biochar, compost and iron sulphate. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00447-8.
Nawab, J., Khan, S., Aamir, M., Shamshad, I., Qamar, Z., Din, I., & Huang, Q. (2016). Organic amendments impact the availability of heavy metal(loid)s in mine-impacted soil and their phytoremediation by Penisitum americanum and Sorghum bicolor. Environmental Science and Pollution Research, 23, 2381–2390.
Neina, D. (2019). The role of soil pH in plant nutrition and soil remediation. Applied and Environmental Soil Science. https://doi.org/10.1155/2019/5794869.
Oustriere, N., Marchand, L., Rosette, G., Friesl-Hanl, W., & Mench, M. (2017). Wood-derived-biochar combined with compost or iron grit for in situ stabilization of Cd, Pb, and Zn in a contaminated soil. Environmental Science and Pollution Research, 24, 7468–7481.
Pallarés, J., González-Cencerrado, A., & Arauzo, I. (2018). Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass and Bioenergy, 115, 64–73.
Peng, J.-F., Song, Y.-H., Yuan, P., Cui, X.-Y., & Qiu, G.-L. (2009). The remediation of heavy metals contaminated sediment. Journal of Hazardous Materials, 161, 633–640.
Qasim, B., Motelica-Heino, M., Joussein, E., Soubrand, M., & Gauthier, A. (2015). Potentially toxic element phytoavailability assessment in Technosols from former smelting and mining areas. Environmental Science and Pollution Research, 22, 5961–5974.
Qian, K. Z., Kumar, A., Zhang, H. L., Bellmer, D., & Huhnke, R. (2015). Recent advances in utilization of biochar. Renewable and Sustainable Energy Reviews, 42, 1055–1064.
R Development Core Team. (2017). R: a language and environment for statistical computing. Vienne: R foundation for statistical Computing.
Rivera-Utrilla, J., Bautista-Toledo, I., Ferro-García, M. A., & Moreno-Castilla, C. (2001). Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. Journal of Chemical Technology and Biotechnology, 76(12), 1209–1215.
Sabir, M., Hanafi, M. M., Aziz, T., Ahmad, H. R., Zia-Ur-Rehman, M., Saifullah, G. M., & Hakeem, K. R. (2013). Comparative effect of activated carbon, pressmud and poultry manure on immobilization and concentration of metals in maize (Zea mays) grown on contaminated soil. International Journal of Agriculture and Biology, 15, 559–564.
Santona, L., Castaldi, P., & Melis, P. (2006). Evaluation of the interaction mechanisms between red muds and heavy metals. Journal of Hazardous Materials, 136, 324–329.
Sarfraz, R., Li, S., Yang, W., Zhou, B., & Xing, S. (2019). Assessment of physicochemical and nutritional characteristics of waste mushroom substrate biochar under various pyrolysis temperatures and times. Sustainability, 11, 277. https://doi.org/10.3390/su11010277.
Sherene, T. (2010). Mobility and transport of heavy metals in polluted soil environment. Biological Forum-An International Journal, 2, 112–121.
Sun, L., Li, L., Chen, Z., Wang, J., & Xiong, Z. (2014). Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil Science & Plant Nutrition, 60, 254–265.
Sun, W., Zhang, S., & Su, C. (2018). Impact of biochar on the bioremediation and phytoremediation of heavy metal(loid)s in soil (Chapter 9, advances in bioremediation and phytoremediation ed.pp. 149–168). Rijeka: InTech.
Sun, Q., Ding, S., Zhang, L., Chen, X., Liu, Q., Chen, M., & Wang, Y. (2019). Effect of phosphorus competition on arsenic bioavailability in dry and flooded soils: comparative study using diffusive gradients in thin films and chemical extraction methods. Journal of Soils and Sediments, 19, 1830–1838.
Takarina, N. D., & Pin, T. G. (2017). Bioconcentration factor (BCF) and translocation factor (TF) of heavy metals in mangrove trees of Blanakan fish farm. Makara Journal of Science, 21, 77–81.
Talukder, A. H., Mahmud, S., Shaon, S. M., Tanvir, R. Z., Saha, M. K., Imran, A. A., & Islam, M. S. (2015). Arsenic detoxification by phytoremediation. International Journal of Basic & Clinical Pharmacology, 4(5), 822–846.
Taneez, M., Hurel, C., & Marmier, N. (2015). Ex-situ evaluation of bauxite residues as amendment for trace elements stabilization in dredged sediment from Mediterranean Sea: a case study. Marine Pollution Bulletin, 98, 229–234.
Tang, Z., Zhang, L., Huang, Q., Yang, Y., Nie, Z., Yang, J., Wang, Y., & Chai, M. (2015). Contamination and risk of heavy metals in soils and sediments from a typical plastic waste recycling area in North China. Ecotoxicology and Environmental Safety, 122, 343–351.
Turpault, M. P., Gobran, G. R., & Bonnaud, P. (2007). Temporal variations of rhizosphere and bulk soil chemistry in a Douglas fir stand. Geoderma, 137, 490–496.
Usman, A. R. A., Alkredaa, R. S., & Al-Wabel, M. I. (2013). Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia sp. marina as potential metal bioaccumulator. Ecotoxicology and Environmental Safety, 97, 263–270.
Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriaensen, K., Ruttens, A., Thewys, T., Vassilev, A., Meers, E., Nehnevajova, E., van der Lie, D., & Mench, M. (2009). Phytoremediation of contaminated soils and groundwater: lessons from the field. Environmental Science and Pollution Research, 16, 765–794.
Weber, J. S., Goyne, K. W., Luxton, T. P., & Thompson, A. L. (2015). Phosphate treatment of lead-contaminated soil: Effects on water quality, plant uptake, and lead speciation. Journal of Environmental Quality, 44, 1127–1136.
Wu, B., Cheng, G., Jiao, K., Shi, W., Wang, C., & Xu, H. (2016). Mycoextraction by Clitocybe maxima combined with metal immobilization by biochar and activated carbon in an aged soil. Science of the Total Environment, 562, 732–739.
Ye, S., Zeng, G., Wu, H., Liang, J., Zhang, C., Dai, J., Xiong, W., Song, B., Wu, S., & Yu, J. (2019). The effects of activated biochar addition on remediation efficiency of cocomposting with contaminated wetland soil. Resources, Conservation & Recycling, 140, 278–285.
Yoon, J., Xinde, C., Qixing, Z., & Ma, L. Q. (2006). Accumulation of Pb, Cu and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment, 368(2-3), 456–464.
Zeng, G., Wan, J., Huang, D., Hu, L., Huang, C., Cheng, M., Xue, W., Gong, X., Wang, R., & Danni Jiang, D. (2017). Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils-a review. Journal of Hazardous Materials, 339, 354–367.
Zheng, C., Wang, X., Liu, J., Ji, X., & Huang, B. (2019). Biochar-assisted phytoextraction of arsenic in soil using Pteris vittata L. Environmental Science and Pollution Research, 26, 36688–36697.
Zhou, R., Liu, X., Luo, L., Zhou, Y., Wei, J., Chen, A., Tang, L., Wu, H., Deng, Y., Zhang, F., & Wang, Y. (2017). Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. International Biodeterioration & Biodegradation, 118, 73–81.
Zubair, M., Shakir, M., Ali, Q., Rani, N., Fatima, N., Farooq, S., Shafiq, S., Kanwal, N., Ali, F., & Nasir, I. A. (2016). Rhizobacteria and phytoremediation of heavy metals. Environmental Technology Reviews, 5, 112–119.
Acknowledgments
The authors wish to thank La Carbonerie, Jacobi Carbons, and Alteo Environment for providing the amendments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Assisted phytoremediation of a soil polluted by As and Pb was investigated.
• Combining red mud and biochar or activated carbon improved soil characteristics.
• Acidic activated carbon use improved soil Pb stabilization but not As immobilization.
• Red mud-acidic activated carbon association improved plant growth.
• Trifolium repens was a successful phytostabilizer for an As- and Pb-contaminated soil.
Rights and permissions
About this article
Cite this article
Simiele, M., Lebrun, M., Del Cioppo, G. et al. Evaluation of Different Amendment Combinations Associated with Trifolium repens to Stabilize Pb and As in a Mine-Contaminated Soil. Water Air Soil Pollut 231, 539 (2020). https://doi.org/10.1007/s11270-020-04908-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04908-0