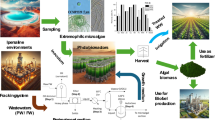
Abstract
Hydraulic fracturing is used to enhance oil and gas extraction from tight shale formations and generates millions of gallons of wastewater which needs to be cleaned up prior to disposal or reuse. The current technologies used for the management of this wastewater present technical, economic, and environmental challenges. Hence, the main objective of this study was to examine the potential of algal remediation of hydraulic fracturing wastewater (PW) as an alternative method. Considering that PW contains very low concentration of the nutrients needed for algae growth PW supplemented with animal wastewater (AW-PW) was also examined. Biomass production capacity, average biomass productivity, and specific growth rate of the microalgae strain used in the study, Picochlorum oklahomensis, were 1.87 g L−1, 268 mg L−1 day−1, and 0.35 day−1, respectively, when grown in PW. Complete nitrate, ammonia, and phosphate removal could be achieved by growing algae in PW. Supplementation of PW with animal wastewater enhanced biomass production (1.87–2.40 g L−1) and lipid content (15–25% wt) in the produced algal biomass. A mathematical model with a correlation coefficient of greater than 0.94 was developed to describe the growth kinetics of algae grown in AW-PW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydraulic fracturing, also known as fracking, is a process during which water containing various chemicals is injected at a high pressure into tight geologic formations holding oil and/or gas. The latter process induces fractures through which oil or natural gas can easily flow to the surface enhancing oil and gas recovery. The hydraulic fracturing process utilizes large volumes of freshwater depleting scarce resources, generating wastewater (WW), and creating environmental pollution. The amount of water used for each well varies between 10,000 and 236,620 m3 per well, depending on the well borehole configuration and target oil or gas reservoir characteristics (Gallegos et al. 2015). The liquid waste produced during the fracking operations is of great concern due to its potential adverse impact on ground and surface water and soil.
There are two main streams generated during hydraulic fracturing and oil and gas production. The injected fluid (fracking fluid) mixed with the formation water returns to the surface within the first few days following the initial fracturing of a shale (Lutzu and Dunford 2019a, 2019b; Li et al. 2020; Wang et al. 2020a). This stream is referred to as flowback water (FW). Then, more water comes to the surface mixed with oil and gas during the production period. The latter WW stream, which is known as produced water (PW), is the most concerning waste stream due to its larger production volume than FW and its potentially harmful chemical composition. PW may contain metals; radioactive compounds and other chemicals including sodium, potassium, chloride, bromide, calcium, barium, strontium, radium, and uranium; and organic chemicals such as solvents, biocides, and scale inhibitors (Maguire-Boyle and Barron 2014). Extremely high total dissolved solid (TDS) content in PW, in some cases exceeding 300,000 mg L−1, makes the handling and treatment of this stream very challenging (Estrada and Bhamidimarri 2016). PW can be stored in tanks and then transported and injected into deep wells approved by the local governments. Handling methods for the final disposal of frac water are limited to deep well injection and reuse and recycling for agricultural and industrial purposes including reuse in fracturing operations after treatment (Jackson et al. 2014; Kondash and Vengosh 2015; Notte et al. 2017). The most common WW management option, deep well injection, is troublesome due to its high cost and damaging environmental impacts (Shores et al. 2017; Acharya et al. 2011; Lester et al. 2014; Mauter et al. 2014).
Most of the research on algal WW remediation has focused on animal and municipal WW (Barrera Bernal et al. 2008, De Francisci et al. 2018). Although hydraulic fracturing WW is an important environmental problem, feasible technologies for its management are still not available (Wang et al. 2020a, 2020b). Only a few studies examined hydraulic fracturing WW as a potential growth medium for algal biomass production and contaminant removal (Lutzu and Dunford 2019a, 2019b; Li et al. 2018, 2020). For example, thirteen strains of microalgae isolated in southern Brazil are shown to grow in PW and remove pollutants (Mendes et al. 2010). Recent studies reported the effect of algae growth in FW and PW on residual water quality (Lutzu and Dunford 2019a, 2019b). Although microalgae could grow in FW, the biomass production was constrained by the limited nutrient (potassium and nitrogen) availability in the WW. Information on the algal treatment of PW is still limited (Racharaks et al. 2015; Wood et al. 2015).
Animal wastewater (AW) is a rich source of nutrients needed for algae cell growth. Hence, it can be used to supplement hydraulic fracturing WW to enhance algae growth.
The main objective of this study was to examine the potential of using AW as an additive to improve the algal biomass production in hydraulic fracturing wastewater (HFWW). Considering that the chemical composition of FW and PW is significantly different (Ferrer and Thurman 2015), and the volume of the PW generated during oil and gas production is much larger than that of the FW, PW was chosen as the algae growth medium to be examined in this study. An Oklahoma native microalgae strain, Picochlorum oklahomensis, was grown in PW, AW, and their mixtures. The effects of the WW source on the algal biomass growth profile, contaminant removal efficiency, and the biomass characteristics were investigated. A mathematical model was successfully developed to describe the microalgae growth kinetics.
2 Material and Methods
2.1 Inoculum and Culture Medium Preparation
The algae strain Picochlorum oklahomensis (CCMP2329), thereafter referred as PO, was obtained from the culture collection of the National Center for Marine Algae and Microbiota (NCMA), Boothbay, Maine (2016). The detailed chemical composition of the culture media used to maintain algae can be found on the UTEX (2012) and NCMA official websites (NCMA 2016). All the chemicals used in this study were reagent grade unless otherwise stated.
2.2 Wastewater Collection and Pre-Treatment
The PW used in this study was collected from the wells operating in southeast, OK, USA, and stored at room temperature until further use. The WW samples were filtered using a filter paper disk (#1, Whatman, UK) and then sterilized at 121 °C, 0.1 MPa for 20 min in an autoclave (Hirayama, HVE-50, Ramsey, MN, USA) prior to microalgae cultivation. The AW which was collected from a swine farm at the Oklahoma State University, Stillwater, OK, was first filtered through a sand filter in order to remove suspended solids that could interfere with light availability to the algal cells. Then, the filtered WW was autoclaved at 121 °c for 20 min.
2.3 Algae Cultivation
The algae were cultivated in 2 L (1.2 L working medium and at an initial cell concentration of 0.1 g L−1) photobioreactors (PBR), sealed with a GL45 3-port cap equipped with tube adapters (CP Lab Safety, Novato, CA). The gas (air and CO2 mixture) was bubbled through a sterile syringe filter (Argos Technologies, Elgin, IL). A polypropylene check valve (VWR Science, Bristol, CT) allowed air leaving the PBR and prevented the reverse flow. The PBR were kept in a closed growth chamber with inner dimensions of 118.8 × 58.4 × 76.9 cm (length × height × width). The chamber was maintained at 23 ± 4 °C. Twelve 23 W cool white fluorescent bulbs (Osram Sylvania, Wilmington, MA), installed on the ceiling of the growth chamber, were the light source providing a PPF of 85 ± 4 μmol m−2 s−1 measured by a quantum meter (model QMSW-SS, Apogee Instruments Inc., Logan, UT). During a 12:12-h cycle, the lights in the growth chamber and the gas flow through the reactors were on for 12 h while the next 12 h was dark without gas flow. The gas flow rate was 20 mL min−1, and the concentration of CO2 (industrial carbon dioxide, Airgas, Stillwater, OK) in the air (Grade D breathing air, Airgas, Stillwater, OK) was 2% v/v. The initial cell concentration of the culture medium was 0.1 g L−1 (based on algal dry weight). After one month of cell growth, the cultures were centrifuged at 9722 g/RCF for 10 min (Sorval RC-5C Plus, Ramsey, MN). The liquid phase was separated and used for wastewater analysis (as described in section 2.7). The algal biomass was collected and dried as is without further treatment according to the procedure described in Section 2.4. More details on the PBR and the cultivation technique can be found in a previous publication (Lutzu and Dunford 2019a).
2.4 Characterization of Microalgae Growth Pattern
Cell growth was monitored by measuring the absorbance (ABS) of the culture at 680 nm for 30 consecutive days. A regression equation describing the relationship between dried biomass concentration and ABS was generated and used to monitor biomass concentration during cell growth. Actual dry biomass concentrations were determined as follows: (a) about 5 ml of culture (V) was drawn from the reactors, (b) the sample was passed through a pre-dried and weighted (W1) glass microfiber filter (GF/C™ 55-mm diameter, Whatman, UK), and (c) the biomass retained on the filter was dried to a constant weight (W2) at 105 °C. The biomass amount was reported as the weight difference, W1−W2. The cell concentration (dry weight V−1), Xdw (g L−1), average biomass productivity (Px), specific growth rate (μ), and doubling time (td) calculations were described in detail elsewhere (Zhou and Dunford 2017a, 2013). The pH of culture suspensions was measured by a pH meter (model AR20, Fisher Scientific, Waltham, MA).
2.5 Algal Biomass Characterization
The algal biomass was characterized by using a Thermogravimetric Analysis (TGA) method (García et al. 2013; Ross et al. 2008). In summary, the sample was heated from 25 to 110 °C at a heating rate of 20 °C min−1 under a nitrogen atmosphere (flow rate 50 mL min−1) and held at 110 °C for 6 min. The weight loss at this stage corresponded to the moisture (M) content of the sample. After the isothermal period, temperature was increased to 575 °C at a rate of 80 °C min−1 and held for 10 min; during which, the weight loss was determined as the volatile matter (VM). Then, the atmosphere was switched from nitrogen to air (50 mL min−1), and the temperature was increased to 800 °C at a heating rate of 80 °C min−1 and then maintained at this temperature until a constant sample weight was obtained. The weight loss at this stage was accounted as fixed carbon (FC), and the remaining weight was ash (A). The higher heating value (HHV), MJ kg−1 (dry basis), was calculated as described elsewhere (Zhou and Dunford 2017a, 2017b).
2.6 Lipid Content Determination
The lipids were extracted from PO biomass following the procedure described in Zhou and Dunford (2017a). Briefly, a small amount of dried biomass (120 mg) was first suspended in 5-mL phosphate buffer (pH 7.4) and treated in a bead-beater (model HBB908, Hamilton Beach Richmond, VA) which was half-filled with 1-mm glass beads for 1 min. The obtained suspension was then transferred to a separatory funnel where a 30 mL of chloroform-methanol (2:1 v v−1) solution was added to allow the lipid extraction. The liquid mixture was then shaken vigorously for 20 min with an orbital shaker (model S500, VWR, Radnor, PA), left to stand for 30 min to achieve a clear phase separation, and the bottom layer (corresponding to the organic phase containing lipid) was decanted and saved. The upper layer (aqueous layer containing residual cells) was extracted with 20 mL solvent The combined organic phases were washed with 20 mL of 5% (w v−1) sodium chloride solution in the separatory funnel, and the final organic layer (bottom layer) was decanted and transferred into a pre-weighed beaker, which was then placed in a RapidVap (Labconco Corp., Kansas City, KS) to evaporate the solvent at 40 °C until a constant weight was obtained. Finally, the lipid content in the algal biomass was determined by the weight increase of the beaker divided by the dry weight of the algae sample.
2.7 Wastewater Quality
The standard analytical water quality methods were used for testing (APHA 2005). At the end of the algae growth period, the culture was centrifuged at 9722 g/RCF for 10 min, and the supernatant was filtered through a glass microfiber filter (GF/C™, Whatman, UK) prior to the chemical testing. The chemical composition of the PW and AW was analyzed before and after microalgae cultivation. All the relevant ions and chemicals such as sodium, calcium, magnesium, sulfate, chloride, nitrate, carbonate, and bicarbonate of the water samples were analyzed by either an ICP (inductively coupled plasma) emission spectrometer or flow injection autoanalyzer (FIA). Phosphorous content was analyzed by inductively coupled argon plasma (ICAP) emission spectrophotometer. The chemical oxygen demand (COD) of the WW samples was determined according to the USEPA method (1980).
2.8 Data Analysis
All algae growth experiments and the analytical tests were carried out at least in duplicate with the mean values being reported. Means were compared by Tukey’s HSD test at a 95% confidence interval. Statistical analyses of the data were performed using SAS 9.3 and SAS 9.2 (SAS Institute Inc., Cary, NC). The regression equations for dry biomass concentration vs. ABS, specific growth rate, doubling time, and proximate analysis by TGA were calculated using Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, WA).
2.9 Quantitative Evaluation of Algae Growth Using a Logistic Model
The following equation, based on a logistic growth model, was used for the model development:
where Xmax (g L−1) represents the maximum algae concentration achieved during the growth and μmax (day−1) is the maximum specific growth rate while ti (day) is the so-called time of inflection, i.e., the time when the instantaneous growth rate starts to decrease. It should be noted that the logistic model has been widely applied in the literature to capture the growth behavior of microalgae strains (Xu and Boeing 2014; Bhola et al. 2016; Banks et al. 2017; Stemkovski et al. 2016). In order to identify the model parameter values permitting to achieve a good agreement with experimental data, the following strategy was adopted. The value of Xmax was set to the maximum concentration of algae detected during the experiments. The maximum growth rate was evaluated by linearly fitting the experimental data related to the early days of cultivation when the absence of growth controlling factors could be reasonably assumed. Subsequently, the time evolution data were fitted through the logistic model by using the previously obtained values of Xmax and μmax while tuning the value of the time of inflection ti that minimizes the residual sum of squares. Therefore, only one parameter was adjusted to fit the data.
3 Results and Discussion
3.1 Initial Wastewater Composition
Two PW samples examined in this study (Table 1) were collected from the wells operating in Oklahoma, USA. According to the Frac Focus Chemical Disclosure Registry (FFCDR 2018), the well, from which PW-1 was collected, was fractured in April 2013. The hydraulic fracking fluid used in this well contained proppant (crystalline silica), friction reducer (trade name FR-66), biocide (glutaraldehyde, trade name Bactron K-176), scale inhibitor (methanol and nonylphenol ethoxylate, trade name Gyptron T-390), breaker (sodium perborate tetrahydrate, trade name Optikleen), and a number of other non-MSDS (material safety data sheet) ingredients. The well was producing gas at the time of sampling. Sodium (8956 mg L−1) and chloride (13,492 mg L−1) were the major ions in PW-1. It also contained substantial amount of bicarbonate (868 mg L−1). Potassium, a nutrient needed for algae growth, was the next most dominant cation (179 mg L−1), followed by calcium (101 mg L−1). High calcium levels are common in PW and contribute to scaling and mineral/ash precipitation. The TDS content of PW-1, 25,014 mg L−1 (Table 1), was within the range reported for PW generated in other states (Benko and Drewes 2008). However, significant differences between PW and FW were observed. For example, TDS of the FW collected from the same region and used for algae growth in an earlier study (Lutzu and Dunford 2019a) and the FW from a well in the Denver-Julesburg basin in Colorado were higher (Lester et al. 2014; Racharaks et al. 2015), while TDS of the water from a well in Texas was significantly lower than that of PW-1 (Arthur et al. 2017). The PW-1 was alkaline, pH 8.5, and had a very high concentration of chloride, 13,492 mg L−1, and lower sodium and other mineral contents compared with those reported in PW from different regions in the USA (Cluff et al. 2014). The concentrations of nutrients needed for algae growth, nitrogen (N) and phosphorous (P), were quite low which is typical for the WW generated during hydraulic fracturing. Iron, copper, manganese, and zinc are considered micronutrients required by all algae for their growth. The latter compounds were not detected in PW-1.
PW-2 was collected from another well which was in the same region as the well from which PW-1 was collected. The well was fractured in March 2016. Fracturing fluid contained proppant (silica, quartz), biocide (trade name AQUCAR GA 50 and B-445-50), friction reducer (trade name FRW-200), polymer breaker (Trade name CS POLYBREAK 210W), and a number of other compounds. The complete fracturing fluid composition can be found on the Frac Focus web site (2018). The PW-2 sample used in this study was collected when the well was producing oil. The composition of PW-2 (Table 1) was similar to that of PW-1, except for a higher content of sulfate (1065 mg L−1) and nitrate (231 mg L−1) and a lower content of TDS (15,642 mg L−1), chloride (6836 mg L−1), and ammonium (17 mg L−1). According to a National Alliance for Advanced Biofuels and Bioproducts (NAABB) report (2014), the TDS of the PW collected from wells in southwestern USA range from 7744 to 38,000 mg L−1 (NAABB 2014; Lammers et al. 2017). Therefore, the TDS in both PW-1 and PW-2 were within the range reported by the NAABB. However, it is important to point out the significant difference in TDS in PW-1 and PW-2. Small quantities of micronutrients, zinc, copper, and iron were detected in PW-2.
The composition of the AW was significantly different than that of both PW-1 and PW-2 (Table 1). Much lower sodium, chloride, TDS, and COD and higher P contents in AW as compared with those in PW-1 and PW-2 make AW a good resource for blending PW and AW for enhancing algae growth.
3.2 Algae Growth in Produced Water
PO, CCMP2329, is a unicellular halotolerant green algae which belongs to the class of Trebouxiphyceae (Henley et al. 2002, 2004). CCMP2329 is characterized by having a spherical shape of approximately 2 μm in diameter, a nucleus, one lateral chloroplast, one mitochondrion, plasmalemma, and cell wall, but lacking flagella. This strain contains chlorophylls a and b, lutein, neoxanthin, and ß-carotene, as a typical feature for the members of Trebouxiophyceae, and it is known to reproduce by autosporulation (Annan 2008).
PO was originally isolated by the Salt Plains Microbial Observatory program in the area of the Great Salt Plains (GSP), northwestern Oklahoma (Kirkwood et al. 2008). This environment is considered extremely hard for most life forms, because of the wide temperature and salinity fluctuations. The variation in salinity can be greater than 5%, ranging from the condition typical of fresh water to salt saturation. The daily temperature variance can be over 15 °C (Kirkwood and Henley 2006). PO was selected for this study, because, algae living in extreme environments exhibit a greater genetic and metabolic adaptability compared with the organism acclimatized to milder aquatic habitats (Sheehan et al. 1998). Considering that WW treatment requires very large volume reactors, open algae ponds are more suitable for algal WW remediation than the closed systems (Soru et al. 2019). Hence, selecting a native algae strain to be grown in an open pond system may minimize the risk of introducing potentially harmful non-native organisms to the local ecosystems. Furthermore, the previous work carried out with the same strain (Zhu and Dunford 2013) demonstrated that PO produced more biomass than Dunaliella sp. and Nannochloropsis oculate grown under similar conditions. Since, scientific data on this strain is very limited, this study contributes to the knowledge in the field.
The specific growth rates (μ) of PO in PW-1 and PW-2 were lower than the growth rates achieved in regular media (MAS and f/2) for the same strain (Table 2) (Zhu and Dunford 2013; Mayers et al. 2018). The latter results are due to the limited nutrient availability, mainly low N and P in PW, and the fact that the chemical compositions of f/2 and MAS are optimized for algae growth. However, PO performed much better (1.87 g L−1 and 1.20 g L−1 in PW-1 and PW-2, respectively) than most of the strains examined by other research groups (Xmax in the range 0.29–1.40 g L−1) (Table 2), except for Nannochloropsis salina (2.78 g L−1).
Although, both, the PW-1 used in this study and the standard MAS medium had N and P in the form of NO3− and PO4−3 and the similar N/P ratio of 20 (Table 1), μ in MAS was slightly higher than that in PW-1 due to the differences in the composition of other growth medium components. The highest μ for PO, 0.84 day−1, was achieved in f/2 media which had a N/P ratio of 15 (Table 2) (Mayers et al. 2018).
The maximum biomass concentration, Xmax, in PW-1 was 1.87 g L−1 which was higher than Xmax achieved in PW-2 for PO (Table 2). Previously reported Xmax for PO grown in MAS medium under similar growth conditions used in the present study, 2.10 g L−1 (Zhou and Dunford 2017a), and the PW-1, 1.87 g L−1 (Table 2), were not significantly different (p > 0.05). It is important to note that although the strains used in both studies were the same, Zhu and Dunford’s study (2013) used cultures obtained from UTEX (UTEX B2795), while this study was conducted with the same strain obtained from NCMA, CCMP2329. At the time of this study, UTEX B2795 was not available from UTEX.
There are studies indicating that some algae strains are capable of metabolizing petroleum hydrocarbons. For example, Prototheca zopfi was shown to degrade n-alkanes and isoalkanes as well as aromatic hydrocarbons (de Bashan and Bashan 2010), while some Chlorella strains metabolized benzopyrene (Takáčová et al. 2014). Further research is needed to ascertain the effect of petroleum hydrocarbons present in PW on PO growth.
3.3 Biomass Characteristics
Chemical composition of biomass produced by algae is a critical factor in choosing a strain for commercial cultivation. Hence, the moisture (M), volatile matter (VM), fixed carbon (FC), and ash contents of the biomass obtained in PW-1 were examined (Table 3). The reason for choosing algal biomass grown in PW-1 for the characterization work is that PO had a higher Xmax and HHV than that grown in PW-2 providing more biomass to be used for testing.
The VM content of the algal biomass generated in PW-1 was 61.7% which was similar to the range reported in the literature for other algae strains (Table 3) (Li et al. 2010; Maddi et al. 2011; Hu et al. 2015; Soria-Verdugo et al. 2017). The biomass generated by PO grown in PW had a FC (14.6%) similar to that of Chlorella vulgaris (López-González et al. 2014; Soria-Verdugo et al. 2017; Haugstad 2017) and Chlorococcum humicola (Kirtania and Bhattacharya 2013). High VM and FC are desirable for feedstock to be used for bio-oil production via pyrolysis.
The ash content of the PO grown in PW was 17.5%. A similar ash content in algal biomass was also observed when microalgae were grown in FW (Lutzu and Dunford 2019a). High ash content in the biomass results in poor combustion and limits biomass disposal options leading to high processing costs, consequently reducing its value for most conversion processes. Morphology of the algae strain and the growth media constituents may have significant effects on the chemical composition of the algal biomass harvested. The chemicals present in the medium may stick to the surface and/or get entrapped in the biomass matrix. For instance, the strains PO and Lyngbya sp. (Table 3) are green algae and filamentous cyanobacteria, respectively. The filamentous biomass matrix produced by the non-motile strain Lyngbya sp. is conducive to entrapment of salt in the large colonies and/or filaments formed. Considering that PO is grown in a medium with very high salt concentration (Table 1), it is likely that a significant portion of the ash in the biomass could be due to the salt attached on the cell surface rather than internalized. Indeed, the initial ash content of the biomass produced by PO grown in MAS medium, about 42.1%, was reduced to 5.0% by washing the intact cells with fresh water (Zhu and Dunford 2013). The latter result clearly demonstrates that a significant portion of the ash was on the biomass surface, not internalized by the cells. However, washing the biomass might not be a practical approach for commercial operations since it requires fresh water increasing the pressure on scarce fresh water supplies and adds an extra unit operation to the production process potentially adversely affecting the economic viability of the entire operation. Considering that the biomass harvesting technique used would have a significant effect on the residual growth medium in the biomass, washing the biomass to remove excess salt might not be necessary for every application.
In general, the biomass produced by PO in regular medium (Zhu and Dunford 2013) had lower ash content than that in PW-1 and FW (Lutzu and Dunford 2019a). The latter result can be explained by the higher salt content in PW and FW as compared with the regular media.
The HHV of PO (15.7 MJ kg−1) is within the range recommended for biomass to be considered for biofuel production (Table 3) (Orosz and Forney 2008). Table 3 also shows the adverse effect of high ash content on the HHV of algal biomass.
3.4 Algae Growth in Animal Wastewater and Produced Water Mixtures
A previous study reported that PO produced significantly lower Xmax in FW (Lutzu and Dunford 2019a) as compared with that in PW-1 and PW-2. It was also shown that algae growth in HFWW can be significantly improved by nutrient supplementation (Zhou and Dunford 2017a). To further test our hypothesis that PO can grow in PW and nutrient supplementation of PW enhances algal cell growth, the same strain was cultivated in PW-2 supplemented with growth nutrients by mixing it with AW at the ratios of 1:1, 1:2, and 1:5 by volume (Table 4). PW-2 rather than PW-1 was selected for the AW supplementation study because of the lower TDS and Xmax obtained in PW-2 as compared with those in PW-1.
Although the μ of PO in AW, PW-2, and their mixtures were similar, doubling time (td), Xmax, and average biomass productivity (Px) were significantly higher in AW as compared with the other water samples examined in this study (Table 4). Supplementation of PW-2 with AW significantly improved algal biomass production. The optimum AW/PW-2 mixture ratio was 1:1 by volume. Under the latter conditions, Xmax doubled as compared with that obtained in PW-2 only. At higher mixture ratios, Xmax decreased significantly. A similar trend was reported for two marine strains, Nannochloropsis oculata (Arriada and Abreu 2014; Ammar et al. 2018) and Isochrysis galbana (Ammar et al. 2018).
AW may introduce a significant amount of organic substrate to the culture medium and promote bacteria proliferation. Ma et al. (2014) reported that algae competed with bacteria more efficiently for nutrient absorption when a high initial algae inoculum amount was used in the culture and more algal biomass was produced. In our study, a relatively high inoculum amount, 0.1 g L−1, was used, and WW samples were autoclaved prior to algae inoculation under sterile conditions. Hence, under the experimental conditions used in this study, the amount of bacteria in the produced biomass is expected to be not significant.
The current study established that microalgae can grow in PW and the chemicals present in PW do not have a significant inhibitory effect on cell growth. Hence, PW could be a potential WW source for algal biomass production.
3.5 Algae Growth Kinetics
The time evolution of cell concentration in the culture is shown in Fig. 1 along with the corresponding model fittings. The shadowed areas on the figure represent the 95% confidence intervals for the fit. It can be observed that the experimental data are well described by the logistic model. As explained in Section 2, the parameters Xmax and μmax were set equal to those shown in Table 4 while the value of the time of inflection ti was suitably tuned through a proper fitting procedure to match the experimental results. The obtained values of ti are shown in Table 5 along with the main statistics describing the fitting performance. It can be seen that, except for the data obtained when using the PW-2 medium, a correlation coefficient always greater than 0.94 was obtained thus demonstrating the reliability of the proposed model. Fig. 2 shows a clear trend where ti decreases and the PW-2 content of the medium increases. Since the inflection time is the time at which the growth rate starts to decrease, it also represents the time when the culture is controlled by a limiting nutrient. Therefore, it can be inferred that the medium PW-2 has at least one critical nutrient whose concentration is so low that growth can be inhibited after a relatively short time. From Table 6, it can be observed that this nutrient could be nitrate, ammonia, or P. However, Table 6 shows that algae continue to grow even when ammonium and nitrate are totally exhausted in the AW medium indicating that it is likely that cells are using internal N reserves. Therefore, the only nutrient that is almost totally consumed in PW-2 but not in the other growth media is P. Thus, P represents probably the controlling nutrient in PW-2, and P addition to the growth medium may greatly increase the growth of microalgae.
3.6 Lipid Content of Microalgae
In order to evaluate the possibility to exploit the produced microalgal biomass as a valuable product for energy or other commercial applications, the final lipid content in algal biomass grown in WW was assessed (Fig. 3). When considering the algae growth in AW, the lipids accounted for about 15% wt of the total biomass weight. When the AW/PW-2 mixtures are considered, by starting from the very low lipid content of the algal biomass grown in AW/PW-2 mixtures, i.e., about 3% wt, a significant increase to about 25% wt can be observed as the PW-2 fraction in the water mixture increased from 1:1 to 1:5, respectively. A similar value, i.e., about 25% wt, for the biomass lipid content was obtained when PW-2 was used in the growth experiments. Therefore, it can be concluded that by increasing the frac water content in the water mixture significantly increases the lipid content in the biomass. This is probably due just to the fact that microalgae tend to accumulate lipids under stress, specifically under low nutrient availability. Considering that PW-2 nutrient content (likely P but even N) is low, starvation phenomenon controls the biomass chemical composition. In fact, it is well-established that starvation is capable to trigger lipid biosynthesis in the cells as a measure to store the photosynthetically assimilated carbon in the absence of N and P resources for protein synthesis (Concas et al. 2013, 2016a, 2016b).
The latter findings are very important indicating the flexibility of AW/PW mixtures as algae growth medium to control the biomass chemical composition for a given application. Furthermore, this study clearly demonstrates the need for an optimization study to determine the algae growth conditions for technical and economic feasibility and biomass composition required for specific applications.
3.7 Wastewater Chemical Composition after Algae Growth
The ultimate goal of the algal treatment of PW was to clean it up to a level that can be used for irrigation and/or in industrial applications while producing biomass that can be converted to bioproducts including biofuels. Hence, in this study, irrigation water quality parameters (Table 1) were used to evaluate the effect of the algae growth on WW quality.
Almost no nitrate or ammonia was left in the medium after growing PO in PW-2 and AW/PW-2 mixtures (Table 6). Over 99% N removal is due to the algal cell uptake of this compound. A large portion of P was also taken up from AW and PW-2 by algae. It is interesting to note that as the PW ratio increased in the medium, P removal increased (Table 6), probably due to the lower amount of P availability in PW (Table 1).
For most of the cultures, pH of the medium dropped below 8 after algae growth (data not shown). This can be explained by the CO2-enriched air feed to the culture. CO2 dissolved in water formed carbonic acid which further dissociated and released H+ into the medium. As a rule of thumb, an increase in algae population in the culture medium would correspond to an increase in CO2/CO3−2 uptake by cells, and consequently the pH of the medium is expected to increase. However, when the biomass concentration is very low in the culture medium, as was the case in PW-2 (Table 2), it is expected that excess CO32− not taken up by the cells would lead to a decrease in pH of the medium.
The chemical composition of water determines its suitability for a given application such as irrigation. High concentrations of chloride, sodium, and boron in irrigation water or soil may cause toxicity problems in plants. Some plants are sensitive to high levels of boron in soil and water. Excess boron uptake by plant roots may result in complete plant growth inhibition in many agricultural regions (Brdar-Jakanović 2020). Cultivation of PO in PW-2 resulted in a 16% boron reduction while boron uptake from AW and AW/PW-2 mixtures was significantly lower, <5%, probably due to the lower boron concentrations in the latter water samples (Table 1).
Boron requirement for algae growth is strain specific (Bonilla et al. 1990; Lewin 1966; Smyth and Dugger 1981). There is evidence that boron is required by marine and freshwater diatoms (Smyth and Dugger 1981), while its requirement for green algae and cyanobacteria is not well understood (Fernandez et al. 1984; Mateo et al. 1986).
The results presented in Tables 1 and 6 clearly indicate that contaminant removal efficiency of PO depends not only on the growth medium composition but also on the amount of biomass present in the culture. Even the adsorption mechanism occurring on the surface of the microalgal cells and biomass flocs may contribute to the observed removal efficiency.
3.8 Proof of Concept for PW Treatment Scheme through Microalgae
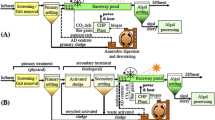
In the light of the information so far gathered, including those ones regarding the pre-treatment steps, a proof of concept for the treatment of PW/AW mixtures through microalgae is proposed in Fig. 4. The process is in its main section quite simple and is based on first section where residual oil is removed from the PW medium by an oil skimmer and then mixed with the AW medium. The resulting mixture is sent to an evaporator which removes residual VOCs (volatile organic compounds) and then filtered to remove solid particles. The obtained solution is then inoculated with PO and sent to an open pond where the microalgae are grown. Finally, the remediated water is separated from microalgae by a centrifuge. The separated microalgal biomass can be then sent to specific downstream processes which depend on the final use. It should be noted that the boiling section, which could involve relatively high energy consumptions, is mandatory in whatever treatment scheme of this water since VOCs should be removed before discharge. Open pond cultivation might be performed without significant bacteria contamination by imposing high salinity levels. In fact, given its high halo-tolerance, PO can be grown under high salinity conditions which instead are lethal for competitive and predator organisms. The possibility to apply such a selective cultivation strategy would permit to perform cultivation in open ponds which in turn would result in a great improvement of the economic sustainability of the process. Moreover, the oil recovered in the skimming and boiling sections might be eventually mixed with the lipids obtained from microalgae lipids to obtain a viable fuel. Our ongoing research is evaluating viability of the proposed model.
4 Conclusions
This study investigated the growth profile of the microalgae strain PO in PW, AW, and their mixtures. The experimental results indicate that the cell growth in PW is limited with the nutrient availability. A significant increase in biomass concentration was achieved by nutrient (N and P) supplementation of PW by blending it with AW at a 1:1 volume ratio. The chemical composition of the algal biomass generated is strain specific and affected by growth medium composition. An inverse relationship was found between the PO growth rate and final lipid content: as the PW content in the AW mixture increased the algal growth rate decreased while the final lipid content of the biomass increased. A simple mathematical model was successfully used to evaluate microalgae growth kinetics. A significant amount of contaminants could be removed from WW by growing algae and harvesting the biomass. For example, 100% nitrate and ammonia; over 95% phosphate; over 30% magnesium, calcium, and zinc; and 16% boron reduction in PW could be achieved. Hence, PW and AW can be potential WW sources for algal biomass production, consequently minimizing the adverse environmental impact of WW generated during oil, gas, and animal production.
References
Acharya, H. R., Henderson, C., Matis, H. Kommepalli, H., Moore, B., Wang, H. (2011). Cost effective recovery of low-TDS frac flowback water for re-use. Final Report. Resource document. Department of Energy. https://netl.doe.gov/sites/default/files/2018-03/FE0000784_FinalReport.pdf. Accessed 9 April 2020.
American Public Health Association-APHA. (2005). Standard methods for the examination of water and wastewater 21th edition. In: American public health association (APHA), American Water Works Association, Water Pollution Control Federation, pp. 2–48.
Ammar, S. H., Khadim, H. J., & Mohamed, A. I. (2018). Cultivation of Nannochloropsis oculata and Isochrysis galbana microalgae in produced water for bioremediation and biomass production. Environmental Technology and Innovation, 10, 132–142.
Annan, J. N. (2008). Growth response of the green alga Picochlorum oklahomensis to nutrient limitation and slainity stress. (PhD dissertation), Oklahoma State University, Stillwater, OK.
Arriada, A. A., & Abreu, P. C. (2014). Nannochloropsis oculata growth in produced water: an alternative for massive microalgae biomass production. Brazilian Journal of Petroleum and Gas, 8(3), 119–125.
Arthur, J. D., Langhus, B. G., Patel, C. (2017). Technical summary of oil and gas produced water treatment technologies. http://www.all-llc.com/publicdownloads/ALLConsulting-WaterTreatmentOptionsReport.pdf. Accessed on 18 October 2017.
Banks, H. T., Collins, E., Flores, K., Pershad, P., Stemkovski, M., & Stephenson, L. (2017). Statistical error model comparison for logistic growth of green algae (Raphidocelis subcapitata). Applied Mathematics Letters, 64, 213–222.
Barrera Bernal, C., Várquez, G., Barceló Quintal, I., & Bussy, A. L. (2008). Microalgal dynamics in batch reactors for municipal wastewater tretament containing deiry sewage water. Water, Air, Soil and Pollution, 190, 259–270.
Benko, K. L., & Drewes, J. E. (2008). Produced water in the Western United States: geographical distribution, occurrence, and composition. Environmental Engineering Science, 25(2), 239–246.
Bhola, V. K., Swalaha, F. M., Nasr, M., Kumari, S., & Bux, F. (2016). Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. European Journal of Phycology, 51(2016), 401–412.
Bonilla, I., Garcia-González, M., & Mateo, P. (1990). Boron requirement in cyanobacteria. Its possible role in the early evolution of photosynthetic organisms. Plant Physiology, 94(4), 1554–1560.
Brdar-Jakanović, M. (2020). Boron toxicity and deficiency in agricultural plants. International Journal of Molecular Sciences, 21, 1424.
Chaiwong, K., Kiatsiriroat, T., Vorayos, N., & Thararax, C. (2013). Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass and Bioenergy, 56, 600–606.
Chen, C., Ma, X., & He, Y. (2012). Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresource Technology, 117, 264–273.
Cluff, M. A., Hartsock, A. M., MacRae, J. D., Carter, K., & Mouser, P. J. (2014). Temporal changes in microbial ecology and geochemistry in produced water from hydraulically fractured Marcellus shale gas wells. Environmental Science and Technology, 48(11), 6508–6517.
Concas, A., Pisu, M., & Cao, G. (2013). Mathematical modelling of Chlorella vulgaris growth in semi-batch photobioreactors fed with pure CO2. Chemical Engineering Transactions, 32, 1021–1026.
Concas, A., Pisu, M., & Cao, G. (2016a). A novel mathematical model to simulate the size-structured growth of microalgae strains dividing by multiple fission. Chemical Engineering Journal, 287, 252–268.
Concas, A., Malavasi, V., Costelli, C., Fadda, P., Pisu, M., & Cao, G. (2016b). Autotrophic growth and lipid production of Chlorella sorokiniana in lab batch and BIOCOIL photobioreactors: experiments and modeling. Bioresource Technology, 211, 327–338.
de Bashan, L. E., & Bashan, Y. (2010). Immobilized microalgae for removing pollutans: review of practical aspects. Bioresource Technology, 101, 1611–1627.
De Francisci, D., Su, Y., Iital, A., & Angelidaki, I. (2018). Evaluation of microalgae production coupled with wastewater treatment. Environmental Technology., 39(5), 581–592.
Estrada, J. M., & Bhamidimarri, R. (2016). A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel, 182, 292–303.
Fernandez, E., Sanchez, E., Bonilla, I., Mateo, P., & Ortega, P. (1984). Effect of boron on the growth and cell composition of Chlorella pyrenoidosa. Phyton, 44, 125–131.
Ferrer, I., & Thurman, E. M. (2015). Chemical constituents and analytical approaches for hydraulic fracturing waters. Trends in Environmental Analytical Chemistry, 5, 18–25.
Frac Focus Chemical Disclosure Registry. (2018). https://fracfocusdata.org/DisclosureSearch/Search.aspx. Accessed on 17 May 2018.
Gallegos, T. J., Varela, B. A., Haines, S. S., & Engle, M. A. (2015). Hydraulic fracturing water use variability in the United States and potential environmental implications. Water Resources. Research., 51(7), 5839–5845.
García, R., Pizarro, C., Lavín, A. G., & Bueno, J. L. (2013). Biomass proximate analysis using thermogravimetry. Bioresource Technology, 139, 1–4.
Godfrey, V. (2012). Production of biodiesel from oleaginous organisms using underutilized wastewaters (MS). Logan, UT: Utah State University.
Haugstad, B. H. (2017). Fuel characterization and process analysis of hydrothermal liquefacion of algae. Grimstad: (MS), University of Agder.
Henley, W. J., Major, K. M., & Hironaka, J. L. (2002). Response to salinity and heat stress in two halotolerant chlorophyte algae. Journal of Phycology, 38(4), 757–766.
Henley, W. J., Hironaka, J. L., Guillou, L., Buchheim, M. A., Buchheim, J. A., Fawley, M. W., et al. (2004). Phylogenetic analysis of the 'Nanochloris-like' algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Phycologia., 43(6), 641–652.
Hu, M., Chen, Z., Guo, D., Liu, C., Xiao, B., Hu, Z., et al. (2015). Thermogravimetric study on pyrolysis kinetics of Chlorella pyrenoidosa and bloom-forming cyanobacteria. Bioresource Technology, 177, 41–50.
Jackson, R. B., Vengosh, A., Carey, J. W., Davies, R. J., Darrah, T. H., O’Sullivan, F., et al. (2014). The environmental costs and benefits of fracking. Annual Review of Environment and Resources, 39, 327–362.
Kirkwood, A. E., & Henley, W. J. (2006). Algal community dynamics and halotolerance in a terrestrial, hypersaline environment. Journal of Phycology, 42(3), 537–547.
Kirkwood, A. E., Buchheim, J. A., Buchheim, M. A., & Henley, W. J. (2008). Cyanobacterial diversity and halotolerance in a variable hypersaline environment. Microbial Ecology, 55(3), 453–465.
Kirtania, K., & Bhattacharya, S. (2013). Pyrolysis kinetics and reactivity of algae–coal blends. Biomass and Bioenergy, 55, 291–298.
Kondash, A., & Vengosh, A. (2015). Water footprint of hydraulic fracturing. Environmental Sciences and Technology Letters., 2(10), 276–280.
Lammers, P. J., Huesemann, M., Boeing, W., Anderson, D. B., Arnold, R. G., Bai, X., et al. (2017). Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Research, 22, 166–186.
Lester, Y., Yacob, T., Morrissey, I., & Linden, K. G. (2014). Can we treat hydraulic fracturing flowback with a conventional biological process? The case of guar gum. Environmental Sciences and Technology Letters., 1(1), 133–136.
Lewin, J. C. (1966). Boron as a growth requirement for diatoms. Journal of Phycology, 2(4), 160–163.
Li, D., Chen, L., Zhao, J., Zhang, X., Wang, Q., Wang, H., et al. (2010). Evaluation of the pyrolytic and kinetic characteristics of Enteromorpha prolifera as a source of renewable bio-fuel from the Yellow Sea of China. Chemical Engineering Research and Design, 88(5), 647–652.
Li, R., Yang, J., Pan, J., Zhang, L., & Qin, W. (2018). Effect of immobilization on growth and organics removal of Chlorella in fracturing flowback fluids treatment. Journal of Environmental Management, 226, 163–168.
Li, R., Pan, J., Yan, M., Yang, J., Qin, W., & Liu, Y. (2020). Treatment of fracturing wastewater using microalgae-bacteria consortium. The Canadian Journal of Chemical Engineering, 98(2), 484–490.
López-González, D., Fernandez-Lopez, M., Valverde, J. L., & Sanchez-Silva, L. (2014). Kinetic analysis and thermal characterization of the microalgae combustion process by thermal analysis coupled to mass spectrometry. Applied Energy, 114, 227–237.
Lutzu, G. A., & Dunford, N. T. (2019a). Algal treatment of wastewater generated during oil and gas production using hydraulic fracturing technology. Environmental Technology., 40(8), 1027–1034.
Lutzu, G. A., & Dunford, N. T. (2019b). Growing algae in produced water generated during oil and gas production using hydraulic fracturing technique. Chemical Engineering Transactions, 74, 1261–1266.
Lutzu, G. A., Locci, A. M., & Cao, G. (2012). Effect of medium composition on the growth of Nannochloris eucaryotum in batch photobioreactors. Journal of Biobased Material and Bioenergy, 6(1), 94–100.
Ma, X., Zhou, W., Fu, Z., Cheng, Y., Min, M., Liu, Y., Zhang, Y., Chen, P., & Ruan, R. (2014). Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresource Technology, 167, 8–13.
Maddi, B., Viamajala, S., & Varanasi, S. (2011). Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresource Technology, 102(23), 11018–11026.
Maguire-Boyle, S. J., & Barron, A. R. (2014). Organic compounds in produced waters from shale gas wells. Environmental Science: Processes and Impacts, 16(10), 2237–2248.
Mateo, P., Bonilla, I., Fernandez-Valiente, E., & Sanchez-Maeso, E. (1986). Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC 711. Plant Physiology, 81(2), 430–433.
Mauter, M. S., Alvarez, P. J. J., Burton, A., Cafaro, D. C., Chen, W., Gregory, K. B., et al. (2014). Regional variation in water-related impacts of shale gas development and implications for emerging international play. Environmental Science and Technology., 48(15), 8298–8306.
Mayers, J. J., Vaiciulyte, S., Malmhäll-Bah, E., Alcaide-Sancho, J., Ewald, S., Godhe, A., et al. (2018). Identifying a marine microalgae with high carbohydrate productivities under stress and potential for efficient flocculation. Algal Research, 31, 430–442.
Mendes, L.B., Cunha, P. C. R., Montes D’Oca, M., Abreu, P. C., Primel, E. (2010). US Patent 7955505 B2.
NAABB, The National Alliance for Advanced Biofuels and Bioproducts final report. (2014). https://www.energy.gov/sites/prod/files/2014/06/f16/naabb_synopsis_report.pdf. Accessed on 23 October 2014.
NCMA, The National Center for Marine Algae and Microbiota at Boothbay, Maine (NCMA). (2016). https://ncma.bigelow.org/products/algae. Accessed on 23 July 2016.
Notte, C., Allen, D. M., Gehman, J., Alessi, D. S., & Goss, G. G. (2017). Comparative analysis of hydraulic fracturing wastewater practices in unconventional shale developments: regulatory regimes. Canadian. Water Resources. Journal., 42(2), 122–137.
Orosz, M. S., & Forney, D. (2008). A comparison of algae to biofuel conversion pathways for energy storage off-grid. Boston, MA: Massachusetss Institute of Technology.
Racharaks, R., Ge, X., & Li, Y. (2015). Cultivation of marine microalgae using shale gas flowback water and anaerobic digestion effluent as the cultivation medium. Bioresource Technology, 191, 146–156.
Ross, A., Jones, J., Kubacki, M., & Bridgeman, T. (2008). Classification of macroalgae as fuel and its thermochemical behaviour. Bioresource Technology, 99(14), 6494–6504.
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P. (1998). A look back at the US Department of Energy's aquatic species program: biodiesel from algae (Vol. 328): National Renewable Energy Laboratory Golden.
Shores, A., Laituri, M., & Butters, G. (2017). Produced water surface spills and the risk for BTEX and nafthalene groundwater contamination. Water Air and Soil Pollution, 228(11), 435.
Smyth, D. A., & Dugger, W. M. (1981). Cellular changes during boron-deficient culture of the diatom Cylindrotheca fusiformis. Physiologia Plantarum, 51, 111–117.
Soria-Verdugo, A., Goos, E., Morato-Godino, A., García-Hernando, N., & Riedel, U. (2017). Pyrolysis of biofuels of the future: sewage sludge and microalgae – thermogravimetric analysis and modelling of the pyrolysis under different temperature conditions. Energy Conversion and Management, 138, 261–272.
Soru, S., Malavasi, V., Concas, A., Caboni, P., & Cao, G. (2019). A novel investigation of the growth and lipid production of the extremophile microalga Coccomyxa melkonianii SCCA 048 under the effect of different cultivation conditions: Experiments and modeling. Chemical Engineering Journal, 377, 120589.
Stemkovski, M., Baraldi, R., Flores, K. B., & Banks, H. T. (2016). Validation of a mathematical model for green algae (Raphidocelis subcapitata) growth and implications for a coupled dynamical system with Daphnia magna. Applied Sciences, 6(5), 155.
Sudhakar, K., & Premalatha, M. (2015). Characterization of micro algal biomass through FTIR/TGA/CHN analysis: application to Scenedesmus sp. Energy Source. Part A: Recovery, Utilization, and Environmental Effects, 37(21), 2330–2337.
Takáčová, A., Smolinská, M., Ryba, J., Mackuľak, T., Jokrllová, J., Hronec, P., et al. (2014). Biodegradation of Benzo[a]Pyrene through the use of algae. Central European Journal of Chemistry, 12, 1133–1143.
US Environmental Protection Agency (EPA). 1980. Standard method 5220 D. Washington D.C., USA.
UTEX, The culture collection of algae at the University of Texas at Austin (UTEX). (2012). http://web.biosci.utexas.edu/utex/media.aspx. Accessed on 04 December 2012.
Wang, D., Sun, Y., Tsang, D. C., Hou, D., Khan, E., Alessi, D. S., Zhao, Y., Gong, J., & Wang, L. (2020a). The roles of suspended solids in persulfate/Fe2+ treatment of hydraulic fracturing wastewater: Synergistic interplay of inherent wastewater components. Chemical Engineering Journal, 388, 124243.
Wang, D., Sun, Y., Tsang, D. C., Khan, E., Cho, D. W., Zhou, Y., Qi, F., Gong, J., & Wang, L. (2020b). Synergistic utilization of inherent halides and alcohols in hydraulic fracturing wastewater for radical-based treatment: A case study of di-(2-ethylhexyl) phthalate removal. Journal of Hazardous Materials, 384, 121321.
Wood, J. L., Miller, C. D., Sims, R. C., & Takemoto, J. Y. (2015). Biomass and phycocyanin production from cyanobacteria dominated biofilm reactors cultured using oilfield and natural gas extraction produced water. Algal Research, 11, 165–168.
Xu, Y., & Boeing, W. J. (2014). Modeling maximum lipid productivity of microalgae: review and next step. Renewable and Susteneable Energy Reviews, 32, 29–39.
Zhou, N., & Dunford, N. T. (2017a). Characterization of green microalgae and cyanobacteria isolated from Great Salt Plains. Transaction of the ASABE, 60(2), 283–290.
Zhou, N., & Dunford, N. T. (2017b). Thermal degradation profile and microwave aided pyrolysis of green algae and cyanobacteria isolated from Great Salt Plains. Transaction of the ASABE, 60, 561–569.
Zhu, Y., & Dunford, N. T. (2013). Growth and biomass characteristics of Picochlorum oklahomensis and Nannochloropsis oculata. Journal of the American Oil Chemists' Society, 90(6), 841–849.
Acknowledgements
This research was supported by the Oklahoma Center for the Advancement of Science and Technology, Basic Plant Science Program, Project # PS13-007, and Oklahoma Water Resources Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lutzu, G.A., Marin, M.A., Concas, A. et al. Growing Picochlorum oklahomensis in Hydraulic Fracturing Wastewater Supplemented with Animal Wastewater. Water Air Soil Pollut 231, 457 (2020). https://doi.org/10.1007/s11270-020-04826-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04826-1