Abstract
Microplastics have been identified as a widespread, persistent environmental pollutant. The investigation of microplastics in marine ecosystems has been prevalent in the literature; however, much less consideration has been given to this form of pollution in freshwater ecosystems. Relatively few studies have considered the uptake of microplastics in freshwater mussels. This study investigated the presence of microplastics in fluted-shell mussels (Lasmigona costata) collected from various sites in the Grand River watershed, Southern Ontario’s largest watershed and home to one million people. The soft tissue of adult mussels underwent enzyme digestion, followed by filtration to isolate undigested particles. Particles were removed and analyzed using Raman spectroscopy to determine their composition. Ten different polymers were identified in the sampled mussels, with polypropylene-co-polyethylene being the most prevalent. Microplastic particles were detected in 71% of mussels with the greatest number of particles observed in a single mussel being seven. No significant difference in microplastic particles per mussel was observed among the different sites sampled. A significant positive relationship between particles per mussel and size of upstream catchment was observed, but a relationship between particles per mussel and percentage of urban land use was not observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plastic debris is considered one of the most abundant and enduring forms of pollution to date. While plastics provide an inexpensive versatile material for consumers, the high demand and production of plastics has led to widespread contamination of ecosystems in every area of the globe (Andrady and Neal 2009). An estimated 4.8–15.1 million tons of plastic debris enter the ocean every year, with between 1.15 and 2.41 million tons sourced from rivers (Jambeck et al. 2015; Lebreton et al. 2017). Plastics can be manufactured in a wide range of sizes and have been shown to break down into smaller fibers and fragments when released in the environment; these smaller particles are then classified as microplastics. The definition of microplastics varies, but they are generally defined as plastic particles or fibers < 5 mm in diameter (Thompson et al. 2004; Browne et al. 2007; Arthur et al. 2009). Plastic particles < 100 μm have been classified as nanoplastics (Koelmans et al. 2015).
Microplastics have been shown to be widely dispersed in aquatic environments and can be found in the sediment, as well as the water column and have been shown to accumulate along coastlines (Derraik 2002; Browne et al. 2011). Browne et al. (2011) assessed microplastic contamination in 18 marine sites across six continents and found that microplastics were more abundant at sites near populated areas. Further, they found that a large proportion of microplastics come in the form of fibers originating from clothing that enter aquatic ecosystems through the release of municipal wastewater effluent (Browne et al. 2011). Wastewater treatment plants have been estimated to contribute 3% of the total microplastics reaching the environment, with fibers being the most prevalent microplastic in wastewater (Vollertsen and Hansen 2017; Burns and Boxall 2018). The potential impacts of microplastics on the environment are still not well understood.
Microplastics can be as small as plankton and can be present in both the sediment and water column (Wagner et al. 2014). This creates the risk of microplastic ingestion in a variety of organisms ranging from zooplankton to fish to large mammals, therefore, posing a potential risk to a variety of aquatic organisms (Browne et al. 2008; Thompson et al. 2004). Laboratory testing has revealed the ecotoxicological consequences of microplastic ingestion in biota such as oxidative stress, endocrine and reproductive disruption, physical and behavioral changes, and mortality (Browne et al. 2008; Rochman et al. 2014; Della Torre et al. 2014; Au et al. 2015; Sussarellu et al. 2016). For example, Rochman et al. (2013) noted fish exposed to polyethylene sorbed with environmental contaminants to display signs of hepatic stress. Cellular alterations have also been noted in marine mussel Mytilus galloprovincialis exposed to microplastic contaminated with pyrene, including alteration of immune response, neurotoxic effects, and changes in gene expression (Avio et al. 2015). A review by de Sá et al. (2018) notes the importance of studying relevant groups of organisms while also emphasizing the need for laboratory studies to accurately evaluate the potential effect of environmentally relevant concentration of microplastics. Therefore, a focus on field data regarding underrepresented environments and organisms in current research is critical to understanding the potential impacts of microplastics.
While many studies have characterized and quantified the presence of microplastic in marine environments, much fewer have investigated the presence of microplastic in freshwater environments (Wagner et al. 2014; de Sá et al. 2018). Only recently has microplastic been documented in lakes, rivers, and estuaries (Eerkes-Medrano et al. 2015). Literature suggests that microplastic concentrations in lakes and rivers are comparable or in some cases higher than ocean gyres (Eriksen et al. 2013; Yonkos et al. 2014; Baldwin et al. 2016). In the Great Lakes, microplastics have been reported in benthic and shoreline sediment, tributaries, and surface waters (Zbyszewski and Corcoran 2011; Eriksen et al. 2013; Baldwin et al. 2016; Lenaker et al. 2019). In general, fibers and fragments are reported as the dominant types of microplastic in the Great Lakes and their tributaries (Baldwin et al. 2016; Dean et al. 2018; Lenaker et al. 2019; Corcoran et al. 2020). An estimated 9987 tons of plastic enter the Great Lakes every year (Hoffman and Hittinger 2017), with sources of microplastic including wastewater effluent, storm water, and agricultural runoff, namely, urban areas are noted to be high contributors (Lenaker et al. 2019; Grbić et al. 2020). Lusher et al. (2017a) noted that of 120 microplastic studies reviewed, only 23 studies considered freshwater ecosystems. Further still, of the microplastic studies reviewed, only 25 studies considered the phyla Mollusca. Of the 25 studies investigating microplastics in mollusk species, only a single study considered a freshwater mollusk species. This highlights the general lack of microplastic research that has been conducted in freshwater ecosystems, as well the lack of attention to freshwater mollusk species.
Mussels have been found to be ideal ecological indicators as they are relatively widespread sessile feeders that have been shown to be sensitive to physical and chemical alterations in aquatic ecosystems (Grabarkiewicz and Davis 2008). Incidences of microplastic accumulation has been widely reported in marine bivalves, with numbers of microplastic ingested by mussels thought to be correlated with the abundance of microplastic in their surrounding environment (Qu et al. 2018). Marine mussels have been shown to take in microplastics from the surrounding environment and accumulate microplastics in their gills and/or gut (Browne et al. 2008; Von Moos et al. 2012). Further, translocation of microplastics from the digestive tract to the circulatory system has also been noted to occur in marine mussels (Browne et al. 2008).
Wastewater treatment facilities have been shown to be a source of microplastics to the environment. Municipal wastewater in Canada is the largest source of effluent by volume, with the majority of this effluent being discharged into freshwater systems (ECCC 2014). Freshwater mussels living downstream of urban areas and wastewater inputs have been noted to have reduced physical condition and life span (Gillis 2012). Little research has been done to determine whether microplastics may contribute to this decline in freshwater mussel populations downstream of urban areas. As freshwater mussel species are one of the North Americas’ most imperiled group of organisms (Williams et al. 1993), there is a need to study whether freshwater mussels, especially in watersheds that contain urban centers, may be ingesting microplastics. In order to characterize the risk of microplastics to freshwater mussels, the potential exposure needs to be quantified. For this reason, this study investigated the presence of microplastics in freshwater mussels across a large watershed that contains multiple urban centers.
2 Methods
2.1 Study Sites and Organism
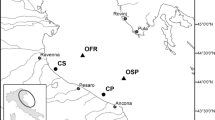
The watershed of the Grand River is the largest watersheds and one of the most populated watersheds in Southern Ontario (Fig. 1). Fluted-shell mussel (Lasmigona costata) is a freshwater mussel species found throughout the Mississippi watershed and Great Lakes basin, existing in moderate abundances throughout the Grand River watershed, therefore making it the ideal mussel species for this study (Grabarkiewicz and Davis 2008). The watershed contains 30 wastewater treatment facilities that service > 1,000,000 inhabitants (GRCA 2018). For this study, adult L. costata were sampled upstream and downstream of a major urban center (Kitchener/Waterloo) along the Grand River (Fig. 2; Table 1). Mussels were collected under a license to collect fish for scientific purposes granted by the Ontario Ministry of Natural Resources and Forestry (License No. 1089928). Characteristics of the upstream catchment for each site and the wastewater treatment facilities in the watershed are presented in Tables S1 and S2, respectively.
Map displaying Grand River watershed in Southern Ontario (shaded in red). Map created using the Ontario Ministry of Natural Resources and Forestry’s Ontario Flow Assessment Tool (OMNRF 2019)
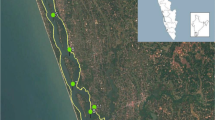
Map displaying the mussel collection sites (red) in relation to wastewater treatment plants (WWTP) (blue) along the Grand River. Inset map in top left corner shows the reference site (site Z) in relation to the other collection sites in the Grand River watershed. Inset map in bottom left shows the location of site E, site D, and the Preston wastewater treatment plant relative to each other
This study was seen as an initial investigative assessment of microplastics in mussels in a large watershed in southern Ontario. A relatively low number of mussels were collected for this study in order to limit the impact on freshwater mussel populations. Three adult L. costata were collected from the streambeds of six sites in the Grand River watershed (sites A to F) and a reference site (site Z) outside of the Grand River watershed in the Maitland River (total of 21 mussels) (Fig. 2). The Maitland River reference site is north-west of the Grand River watershed and empties into Lake Huron, while the Grand River empties into Lake Erie. It was chosen as a reference site due to its low urban and infrastructure land use in the upstream catchment relative to the Grand River watershed sites (Table 1); however, no prior microplastic research has been conducted in the Maitland River. Selection of the specific location within the rivers from which the mussels were collected was based on previous surveys of the rivers and knowledge of the location of suitable habitat for this species of freshwater mussels (Gillis 2012). To preserve the specimen and ensure that potential plastics ingested by the mussels were to be retained, mussels were immediately frozen and stored at − 20 °C before being analyzed for microplastics using digestion methods similar to those outlined by Catarino et al. (2017).
2.2 Soft Tissue Digestion
Frozen mussels were thawed for 15 min at room temperature and rinsed with deionized water prior to separating the soft tissue from the shell. Dissections were performed in glass dishes. The adductor muscles of the organism were cut using a stainless-steel scalpel in order to separate the valves of the mussel and remove the soft tissue. The fresh weight of the soft tissue and length of shell were recorded (Table 2). Subsequently, the soft tissue of the organism was placed in a 125-mL glass Erlenmeyer flask with a magnetic stir bar and 50 mL of 20% v/v protease enzyme from Bacillus licheniformis (Sigma-Aldrich, Oakville, Ontario, Canada). This enzyme digestion was selected based on the efficacy and rate of digestion along limiting any degradation of microplastics (Catarino et al. 2017). The flask was covered with aluminum foil to prevent airborne contamination, and the soft tissue was left to digest on a stir plate for 16 h at 37 °C with a stir rate of ~ 100 rpm. Following digestion, the digestate was transferred to 150-mL glass storage bottles and combined with 20 mL of deionized water that was used to rinse the Erlenmeyer flask. The storage bottles were then capped with Teflon lids and stored at 4 °C until filtration could occur.
2.3 Filtering and Sorting
A novel approach was taken for the filtering and sorting of digested mussel tissue. The digestate was sequentially filtered through three filtering screens made of Nitex® (250 μm, 100 μm, and 50 μm) to capture any undigested particles. Filters were visually inspected under a stereomicroscope with a magnification range of × 7 to × 45 in combination with NightSea® (Lexington, Massachusetts, USA) stereomicroscope florescence adaptors in royal blue (emission of 500-nm longpass) and ultraviolet (emission of 415-nm longpass) florescent lighting paired with yellow and ultraviolet protective objective filters. Suspected particles or those observed to fluoresce were removed from the Nitex® filters using a wet 12-gauge needle and placed on a piece of silicon wafer protected within a 12-mm glass petri dish. To better combat sample losses from static and air flow, as well for greater transfer precision, a bead of deionized water was set on the silicon wafer. Moving forward, “particles” refers to a generalized term for suspected microplastic items, with shapes characterized as “fragments” lacking regular shape including semi-rounded, angular, and broken edges and “fibers” defined with a thread-like elongated structure.
2.4 Particle Identification
In order to determine whether isolated particles were microplastics, material identification was done using a Renishaw inVia Ramen Microscope coupled with WiRE 5.1™ software (Renishaw plc, Wotton-under-Edge, Gloucestershire, UK) (Imhof et al. 2012; Song et al. 2015). Of the particles collected from each mussel, 60% of the total particles present on the silicon wafer were subsampled for identification using Raman spectroscopy. Particles were identified at objective magnifications of × 50, × 200, and × 500 using a red laser (785 nm) in static mode with 3-s exposures × 10 accumulations at power ranging from 0.01 to 1%. Spectral measurement acquisitions were baseline subtracted, and spectra were compared to Renishaw’s organic, polymer, and inorganic spectral databases to determine the material compositions of each particle. Triplicate spectral measurements were conducted at different locations on each particle to provide greater certainty in identification. Objects were reported as a positive match if triplicates reproduced polymer match and with triplicate quality matches to library averaged greater than 10%. Images of identified particles were also taken on the attached microscope camera for reference to size, shape, and color of each particle (Fig. 2). Images were processed using ImageJ2 (Rueden et al. 2017) to obtain accurate measurements of particle size in conjunction with the microscope scale bar for reference.
2.5 Accounting for Contamination
Measures were taken to prevent contamination of samples with microplastics throughout the processing of samples. All equipment was rinsed with deionized water prior to use. A 100% cotton lab coat and nitrile gloves were worn while handling of all samples. All procedures took place in either laminar flow or fume hoods with surfaces cleaned with 70% ethanol and Kimwipes™. Additionally, a blank sample composed of deionized water and protease enzyme was set up prior to mussel dissection and run once for every three mussel samples to document procedural and airborne contamination (total of six blank samples). Overall, 7 of the total 24 particles analyzed from the blank samples (29%) were identified as polymers.
2.6 Statistical Analyses
All statistical testing was carried out in the statistical software R (R Core Team 2019) using a significance level of α = 0.05. The abundances of microplastics among sites were analyzed using a Shapiro-Wilk test for normality and a Bartlett test for homogeneity of variances. Due to lack of variance among the microplastic samples between sites, a non-parametric Kruskal-Wallis was used to identify differences among the sites was conducted. Following this, post hoc Tukey HSD test was used to conduct a pair-wise comparison. A linear regression was conducted to determine whether a significant relationship was present between the number or size of microplastics in the mussels and the land use in the upstream catchment of each site, the total area of the catchment upstream of each site, the mass of soft tissue in the mussels, the length of the mussels, distance from the nearest wastewater treatment plant outfall, and the level of treatment of the wastewater facility.
3 Results and Discussion
In general, there were a low number of microplastics detected in mussels collected from the sites in the Grand River watershed (Tables 2 and S3). The highest reported microplastic abundance was seven particles in an individual mussel, and no microplastics were detected in 6 of the 21 (28.5%) mussels analyzed. Relative to the total particles analyzed in each mussel, the percentage of particles that were microplastic in each mussel ranged from 0 to 21%, with the mean being 5% (Table 2). Although low in abundance, microplastics were detected in at least one mussel at every site. A comparison to other biota within the watershed cannot be made, as to date, no other studies have investigated the presence of microplastics in biota from the Grand River watershed. However, the presence of microplastics in the Grand River watershed has been confirmed by Dean et al. (2018), who reported that sediment collected from the Grand River inlet to Lake Erie contained 42 microplastic particles/kg, all of which were identified as fragments. Dean et al. (2018) also identified plastic producing companies in the region, eight of which were located in the Grand River watershed (Fig. 1). Sediment samples are useful in terms of documenting the presence of microplastics, but as sediment accumulates contamination over time, they cannot be directly related to the portion of microplastics that is bioavailable. Therefore, integrating studies of ingestion in biota, such as the current study, provides important insight into the availability of microplastics to aquatic organisms. There is a high risk of microplastic contamination from a variety of sources when processing samples; even though measures were taken to prevent contamination, five of six procedural blanks contained contamination totaling seven particles. However, the only polymer type identified in the blanks that was also identified in mussel sample was polypropylene (Table 2).
From the particles identified as polymers, ten types of polymer material were detected with polypropylene-co-ethylene and polypropylene as the dominant types of polymer (Table S3). This coincides with the most produced types of resin polymer in North America being polyethylene followed by polypropylene, used in both industrial and domestic applications (Andrady and Neal 2009; American Chemistry Council 2019). A study by Zbyszewski and Corcoran (2011) observed similar types of polymers from a sub-sample of forty-five particles collected from Lake Huron beaches, where 32, 12, and 1 consisted of polyethylene, polypropylene, and polyethylene terephthalate, respectively. Further, these polymer types have been reported as the main types of microplastic pollution in marine ecosystems, as their low-density allows them to be easily transported in flowing waters and their resistance to degradation allows them to persist in the environment (Erni-Cassola et al. 2019). Nylon (polyamide) is another polymer that has been typically found in both wild and farmed marine mussels (Birnstiel et al. 2019). The presence of nylon in marine ecosystems is thought to be due to the large volumes of nylon netting used in commercial fishing equipment (Lusher et al. 2017b). The absence of nylon in mussels collected from the Grand River likely due to significantly lower fishing pressures. Other particles collected from the mussels that were not identified as polymers included inorganic elements consistent with rock such as silicate and calcite groups. When identifying particles found in the mussels, some spectra reflected relatively low-quality matches to the library database. Other studies have reported that environmental conditions may degrade and/or coat plastics in biofilm (Käppler et al. 2015). In turn, this lowers the spectra quality match of the object in question to the library which reports the spectra of a pure standard. Because triplicate measures were taken in addition to subtraction of background noise, there is some certainty that items identified as polymers were indeed microplastics.
All the particles identified as polymers from mussels were categorized as fragments with only a single object identified as a polymer-based fiber. Other definitive shapes such as spherical beads, pellets, and films were not readily observed. With half of the selected sites being located downstream of wastewater treatment facilities that serve a population > 100,000 (Table S2), it was hypothesized that microfibers from textiles may compose the greatest proportion of microplastics detected in mussels. Although fibers were collected from mussels, none were identified as polymers that typically come from washing machines, such as polyesters, polyacrylics, and polyamides (Almroth et al. 2018) (Table 2; Table S3). Studies have reported fibers as the most frequently detected type of microplastic in freshwater watersheds, especially in urban watersheds (Baldwin et al. 2016; Luo et al. 2019; Grbić et al. 2020). McNeish et al. (2018) sampled fish from three watersheds of Lake Michigan, and microfibers composed the majority of microplastics found. While fibers were noted in samples from the present study, anthropogenic fibers such as dyed cellulose fibers were not considered as microplastic due to lack of polymer composition matches with Raman spectroscopy. A study by Woods et al. (2018) considered the uptake, ingestion, and egestion of microplastic fibers by blue mussels exposed at 30 microplastic fibers per mL, noting 71% of microfibers to promptly be rejected as pseudofeces, 9% ingested, and < 1% excreted in feces. Although Woods et al. (2018) did not consider other types of fragments, the degree to which fibers were rejected by mussels in their study may offer some explanation as to why the mussels in this study were found to contain few microfibers. Color did not appear to vary much between particles being described as white, off-white, or opaque as noted in Fig. 3 and Table S4; the majority were described as opaque. With regard to sizes of microplastics found, the mussels contained particles ranging in size from 21 to 298 μm with the average particle size being 114 μm (SD = 63 μm). The size of the opening of the inhalant aperture of an adult-fluted shell mussel is larger than 5 mm and therefore is not going to limit the uptake of microplastics. These mussels typically consume fine particulate matter, bacteria, detritus, and algal cells approximately 2.8–45 μm in size, with a preference for the smaller size range (Beck and Neves 2003). A study conducted by Kolandhasamy et al. (2018) found that blue mussels can ingest microplastics ranging in size from 50 μm to 5 mm, with the smaller size range of 50–205 μm being observed in the greatest proportion in these marine mussels. Qu et al. (2018) found that the majority of microplastics observed in the tissues of blue mussels from the coast of China ranged in size from 0.25 μm to 1 mm. The size of particles observed in L. costata from this study (21–298 μm) corresponds with the size of those observed in studies with marine bivalves. Upon investigation, a small number of particles less than the 50-μm filter size were observed, likely due to particles aggregating in the digestion and filtration processes.
In terms of variation in the number of microplastics per mussel among the sites, a significant difference was not detected among the sites (p = 0.206). It is important to note that the lack of a significant difference among the sites may be attributed to limitations of the study design in terms of the trade-off between the relatively low numbers of replicate mussels collected at each site and minimizing the removal of reproductively mature adult mussels from the populations in the river. While this study did not identify a relationship between the location of sites and the presence of microplastic in the mussels, other studies suggest that the location of study sites can influence the abundance of microplastic within biota. A study conducted by Luo et al. (2019) examined the pathways of origin and transport of microplastics into water bodies (city creeks, rivers, estuary, and coastal waters) outside the highly urbanized and industrialized area of Shanghai. From water samples, they observed that microplastic abundance varied across different spatial scales; for example, smaller water bodies appeared more impacted by microplastic pollution than estuaries, with freshwater sites holding a greater amount of microplastics than coastal water bodies. Additionally, it was observed that microplastic abundance in water along rivers increased from upstream to downstream, with locations downstream of urban centers containing the greatest amount. This pattern is also reflected in a study conducted by Berglund et al. (2019), who collected 64 duck mussels (Anodonta anatina) from a rural upstream area and a downstream area with influence from a large municipality and two wastewater treatment facilities in the Höje River in Sweden. It was observed that 62.5% of mussels from the site downstream contained microplastic particles compared with only 37.5% of mussels sampled upstream. The greatest number of microplastic particles were detected in mussels from site C (mean = 4) and site F (mean = 4) in this study (Table 2). This corresponds with the previously observed trend of microplastic density increasing downstream along rivers, with the exception of the site E (mean = 1.7) (Fig. 2; Table 2). Site E was downstream of the site C; however, mussels from this site contained lower numbers of microplastic particles (Fig. 2; Table 2).
A linear regression did not identify a significant relationship (p > 0.5) between community/infrastructure (urban) land use in the upstream catchment of a site and the microplastic particles collected in mussels (Table 1). Proximity to the nearest wastewater treatment plant outfall, level of treatment of the wastewater facility, or population served by the nearest wastewater treatment plant did not have a significant relationship (p > 0.5) with the number or size of microplastic particles observed in mussels. In contrast, the linear regression conducted to investigate the size of the upstream catchment of the collection sites to the total number of microplastics collected in mussel tissue identified a statistically significant relationship (p < 0.05). This observed relationship parallels the positive relationship between microplastic particle concentration in water and downstream movement observed in other studies (Luo et al. 2019). The drainage area increases with downstream movement, consequently, the area where plastics can be washed into surface increases with downstream movement. It is important to consider impoundments in the upstream catchments, as impoundments have been found to serve as a sink for microplastics. Sediment within impoundments has been found to contain a significantly greater quantity of microplastics than sediments upstream and downstream of the catchments (Watkins et al. 2019). In this study, all of the impoundments were upstream of the major urban centers. If urban centers are the major contributor to microplastic contamination, as discussed above, this would mean that the modification that impoundments would make to microplastic exposure in this study would be limited. The catchment size may explain the relatively low number of microplastic particles in mussels from the site D despite the catchment having a relatively high proportion of community/infrastructure (urban) land use (13.1%; 102.3 square km) among the sampling sites (Tables 1 and S1). A significant relationship between microplastic particles in mussels and the percent of community/infrastructure (urban) land use was not observed in this study. However, mussels from the site furthest downstream (site F) had the greatest mean microplastic number (mean = 4) and the greatest quantity of community/infrastructure (urban) land use in the upstream catchment (383.0 square km) of the sites (Tables 1 and 2). Baldwin et al. (2016) surveyed microplastics in the water of 29 tributaries of the Great Lakes. They observed that the concentration of fragments, films, foams, and beads was positively correlated with the percentage of watershed that was urban. Yonkos et al. (2014) also observed this positive relationship between microplastic concentration in water and population density and proportion of urban/suburban development in the upstream catchment of sampling sites. A number of studies have observed a positive relationship between coastal urban development and microplastic pollution in marine systems (Tanaka and Takada 2016; Bollmann et al. 2019). Su et al. (2018) also noted urbanization as a potential influence of microplastics found in the Asian clam (Corbicula fluminea). In addition to urban land cover, factors such as river morphology, water flow, substrate, and organic matter have been proposed to influence microplastic distribution and retention in sediment (Nel et al. 2018; Corcoran et al. 2020), and these factors could influence uptake of microplastics in freshwater mussels. Studies have suggested that mussels could be used to monitor microplastic contamination of aquatic ecosystems (Qu et al. 2018; Su et al. 2018). However, it is not advisable to use mussels alone as a proxy for measuring the level of microplastic pollution, as environmental concentration may not always be reflected in biota (EPA US 2000; Lusher et al. 2017c). Findings from this study suggest that the number of microplastics in freshwater mussels could be related to the proportion of urban land use in the catchment upstream of mussel populations, as has been observed in other bivalves. However, more detailed sampling to document the environmental exposure of microplastics to L. costata such as levels sediment and water should be conducted.
The linear regression between the shell length and microplastics abundance in the mussels did not reveal a statistically significant relationship (p = 0.09) nor did the linear regression between soft tissue mass and microplastics per site (p > 0.5). Overall, the abundances of microplastics in mussels ranged from 0 to 0.16 microplastic particles per gram wet weight and 0–7 microplastic particles per individual; this is comparably lower than what has been reported in other studies. In general, there was not a discernible relationship between the size or mass of mussels in regard to number of microplastics found. This could be a product of the relatively low number of microplastics observed in mussels in this study. Few studies report on microplastic abundance in relation to the length or mass of mussels. Qu et al. (2018) reported that field-collected marine mussels Mytilus edulis and Perna viridis contained 1.52 to 5.36 microplastic particles per gram wet weight and 0.77 to 8.22 microplastic particles per individual. Su et al. (2018) observed that Asian clams (Corbicula fluminea) contained 0.3–4.9 microplastics per gram and 0.4–5.0 microplastics per individual. A recent study by Catarino et al. (2018) reported a potential size-dependent relationship between microplastic abundance and the soft tissue wet weight in the marine mussel species Mytilus spp. and Modiolus modiolus from an urban estuary in Edinburgh, UK. Catarino et al. (2018) note that normalization of data to mass of tissue may be necessary to more accurately compare between studies.
Unionid mussels, such as L. costata, have been estimated to filter up to 1 L/h/individual (Vaughn et al. 2008). This makes it clear why freshwater mussels are susceptible to taking in microplastics from overlying water. However, while suspension feeding provides a pathway for uptake of microplastics from surrounding water, freshwater mussels possess the ability to select the particles they will ingest into their digestive tract (Rosa et al. 2018). Material enters the mussel through the inhalant aperture that is outlined with papillae that act as a course filter (Haag 2012). Particles that enter the inhalant aperture and are captured by the gill are moved to the labial palps by ciliary action. The final sorting of edible and nonedible particles is conducted by the labial palps (Haag 2012). Material not taken into the digestive tract is bound in mucus to create pseudofeces, which is released in the mantle cavity to be periodically expelled through the inhalant aperture (Thorp and Rogers 2011). This ability to select the particles that are ingested may explain the overall low number of microplastics found in the mussel tissue. Ward et al. (2019) demonstrated the ability of bivalves to selectively ingest and egest microplastic particles in a laboratory setting using two marine species, blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica). It was found that both species excreted microspheres as pseudofeces with increasing size (> 1000 μm), likely attributed to the organism’s ability to discriminate between size and shape for particle selection. It is also important to recognize that only microplastics approximately ≥ 50 μm were quantified in the tissues of mussels in this study. This may have also contributed to the relatively low number of microplastic particles observed in this study, as others have noted microplastics in bivalves < 10 μm in size (Browne et al. 2008; Van Cauwenberghe and Janssen 2014; Li et al. 2018). Another potential variable is the residence time of microplastic in the organism. Studies have found residence time can range from hours to more than 40 days in mussels (Woods et al. 2018; Browne et al. 2008). Residence time can also vary with location in a mussel as Woods et al. (2018) reported retention time of microplastic fibers to be longer in the gut of mussels than the gill.
4 Conclusions
A variety of studies have used mussels as ecological indicators of microplastic pollution, with most studies being conducted in marine ecosystems. While a small number of samples were collected, this study is the first to find evidence of the freshwater unionid mussel L. costata ingesting microplastic in a natural setting. With the ever-growing presence of plastics in the environment, it is increasingly important that watersheds and their biota are monitored for the presence of microplastics. Since exposure has been confirmed in this study, it is important that future studies be conducted to determine the threshold at which microplastics can have an adverse effect on the health of different life stages of freshwater mussels.
References
Almroth, B. M. C., Åström, L., Roslund, S., Petersson, H., Johansson, M., & Persson, N. K. (2018). Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-017-0528-7.
American Chemistry Council. (2019). ACC Resin Statistics Annual Summary 2017 vs. 2018. https://plastics.americanchemistry.com/Year-End-Resin-Stats.pdf. Accessed 15 Aug 2019.
Andrady, A. L., & Neal, M. A. (2009). Applications and societal benefits of plastics. Philosophical Transactions of the Royal Society B: Biological Sciences. https://doi.org/10.1098/rstb.2008.0304.
Arthur, C., Baker, J., & Bamford, H. (2009). Proceedings of the International Research Workshop on the Occurrence , Effects , and Fate of Microplastic Marine Debris. Group.
Au, S. Y., Bruce, T. F., Bridges, W. C., & Klaine, S. J. (2015). Responses of Hyalella azteca to acute and chronic microplastic exposures. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.3093.
Avio, C. G., Gorbi, S., Milan, M., Benedetti, M., Fattorini, D., D’Errico, G., et al. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution. https://doi.org/10.1016/j.envpol.2014.12.021.
Baldwin, A. K., Corsi, S. R., & Mason, S. A. (2016). Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environmental Science and Technology. https://doi.org/10.1021/acs.est.6b02917.
Beck, K., & Neves, R. J. (2003). An evaluation of selective feeding by three age-groups of the rainbow mussel Villosa iris. North American Journal of Aquaculture. https://doi.org/10.1577/c02-031.
Berglund, E., Fogelberg, V., Nilsson, P. A., & Hollander, J. (2019). Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.134192.
Birnstiel, S., Soares-Gomes, A., & da Gama, B. A. P. (2019). Depuration reduces microplastic content in wild and farmed mussels. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2019.01.044.
Bollmann, U. E., Simon, M., Vollertsen, J., & Bester, K. (2019). Assessment of input of organic micropollutants and microplastics into the Baltic Sea by urban waters. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2019.07.014.
Browne, M. A., Galloway, T., & Thompson, R. (2007). Microplastic-an emerging contaminant of potential concern? Integrated Environmental Assessment and Management. https://doi.org/10.1002/ieam.5630030412.
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., & Thompson, R. C. (2008). Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental Science and Technology. https://doi.org/10.1021/es800249a.
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., & Thompson, R. (2011). Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environmental Science and Technology. https://doi.org/10.1021/es201811s.
Burns, E. E., & Boxall, A. B. A. (2018). Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.4268.
Catarino, A. I., Thompson, R., Sanderson, W., & Henry, T. B. (2017). Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environmental Toxicology and Chemistry. https://doi.org/10.1002/etc.3608.
Catarino, A. I., Macchia, V., Sanderson, W. G., Thompson, R. C., & Henry, T. B. (2018). Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environmental Pollution. https://doi.org/10.1016/j.envpol.2018.02.069.
Corcoran, P. L., Belontz, S. L., Ryan, K., & Walzak, M. J. (2020). Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environmental Science and Technology. https://doi.org/10.1021/acs.est.9b04896.
de Sá, L. C., Oliveira, M., Ribeiro, F., Rocha, T. L., & Futter, M. N. (2018). Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2018.07.207.
Dean, B. Y., Corcoran, P. L., & Helm, P. A. (2018). Factors influencing microplastic abundances in nearshore, tributary and beach sediments along the Ontario shoreline of Lake Erie. Journal of Great Lakes Research. https://doi.org/10.1016/j.jglr.2018.07.014.
Della Torre, C., Bergami, E., Salvati, A., Faleri, C., Cirino, P., Dawson, K. A., & Corsi, I. (2014). Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environmental Science and Technology. https://doi.org/10.1021/es502569w.
Derraik, J. G. B. (2002). The pollution of the marine environment by plastic debris: A review. Marine Pollution Bulletin. https://doi.org/10.1016/S0025-326X(02)00220-5.
Eerkes-Medrano, D., Thompson, R. C., & Aldridge, D. C. (2015). Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Research. https://doi.org/10.1016/j.watres.2015.02.012.
Environment and Climate Change Canada. (2014). Wastewater pollution. Retrieved from: https://www.canada.ca/en/environment-climate-change/services/wastewater/pollution.html
EPA, US. (2000). Estuarine and coastal marine waters : Bioassessment and biocriteria technical guidance. Technology.
Eriksen, M., Mason, S., Wilson, S., Box, C., Zellers, A., Edwards, W., et al. (2013). Microplastic pollution in the surface waters of the Laurentian Great Lakes. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2013.10.007.
Erni-Cassola, G., Zadjelovic, V., Gibson, M. I., & Christie-Oleza, J. A. (2019). Distribution of plastic polymer types in the marine environment; A meta-analysis. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2019.02.067.
Gillis, P. L. (2012). Cumulative impacts of urban runoff and municipal wastewater effluents on wild freshwater mussels (Lasmigona costata). Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2012.05.061.
Grabarkiewicz, J. D., & Davis, W. S. (2008). An introduction to freshwater mussels as biological indicators. Environmental Protection.
Grand River Conservation Authority. (2018). Wastewater. Retrieved from Grand River Conservation Authority website: https://www.grandriver.ca/en/our-watershed/Wastewater.aspx. Accessed 20 Aug 2019.
Grbić, J., Helm, P., Athey, S., & Rochman, C. M. (2020). Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Research. https://doi.org/10.1016/j.watres.2020.115623.
Haag, W. R. (2012). North American freshwater mussels: Natural history, ecology, and conservation. North American Freshwater Mussels: Natural History, Ecology, and Conservation. https://doi.org/10.1017/CBO9781139048217.
Hoffman, M. J., & Hittinger, E. (2017). Inventory and transport of plastic debris in the Laurentian Great Lakes. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2016.11.061.
Imhof, H. K., Schmid, J., Niessner, R., Ivleva, N. P., & Laforsch, C. (2012). A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnology and Oceanography: Methods. https://doi.org/10.4319/lom.2012.10.524.
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Plastic waste inputs from land into the ocean. Science. https://doi.org/10.1126/science.1260352.
Käppler, A., Windrich, F., Löder, M. G. J., Malanin, M., Fischer, D., Labrenz, M., et al. (2015). Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Analytical and Bioanalytical Chemistry. https://doi.org/10.1007/s00216-015-8850-8.
Koelmans, A. A., Besseling, E., & Shim, W. J. (2015). Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter. https://doi.org/10.1007/978-3-319-16510-3_12.
Kolandhasamy, P., Su, L., Li, J., Qu, X., Jabeen, K., & Shi, H. (2018). Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2017.08.053.
Lebreton, L. C. M., Van Der Zwet, J., Damsteeg, J. W., Slat, B., Andrady, A., & Reisser, J. (2017). River plastic emissions to the world’s oceans. Nature Communications. https://doi.org/10.1038/ncomms15611.
Lenaker, P. L., Baldwin, A. K., Corsi, S. R., Mason, S. A., Reneau, P. C., & Scott, J. W. (2019). Vertical distribution of microplastics in the water column and surficial sediment from the Milwaukee River basin to Lake Michigan. Environmental Science and Technology. https://doi.org/10.1021/acs.est.9b03850.
Li, J., Green, C., Reynolds, A., Shi, H., & Rotchell, J. M. (2018). Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environmental Pollution. https://doi.org/10.1016/j.envpol.2018.05.038.
Luo, W., Su, L., Craig, N. J., Du, F., Wu, C., & Shi, H. (2019). Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environmental Pollution. https://doi.org/10.1016/j.envpol.2018.11.081.
Lusher, A. L., Welden, N. A., Sobral, P., & Cole, M. (2017a). Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Analytical Methods. https://doi.org/10.1039/c6ay02415g.
Lusher, A., Hollman, P., & Mandoza-Hill, J.J. (2017b). Microplastics in fisheries and aquaculture. FAO fisheries and aquaculture technical paper.
Lusher, A., Bråte, I. L. N., Hurley, R., Iversen, K., & Olsen, M. (2017c). Testing of methodology for measuring microplastics in blue mussels (Mytilus spp) and sediments, and recommendations for future monitoring of microplastics (R & D-project).
McNeish, R. E., Kim, L. H., Barrett, H. A., Mason, S. A., Kelly, J. J., & Hoellein, T. J. (2018). Microplastic in riverine fish is connected to species traits. Scientific Reports. https://doi.org/10.1038/s41598-018-29980-9.
Nel, H. A., Dalu, T., & Wasserman, R. J. (2018). Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an austral temperate urban river system. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2017.08.298.
Ontario Ministry of Natural Resources and Forestry (OMNRF)- Ontario Flow Assessment Tool (OFAT). (2019). Toronto: Queen’s Printer for Ontario. http://www.gisapplication.lrc.gov.on.ca/webapps/OFAT/Viewer/Viewer.html?lang=en-US. Accessed 20 Aug 2019.
Qu, X., Su, L., Li, H., Liang, M., & Shi, H. (2018). Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2017.11.284.
R Core Team. (2019). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/. Accessed 10 Oct 2019.
Rochman, C. M., Hoh, E., Kurobe, T., & Teh, S. J. (2013). Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Scientific Reports. https://doi.org/10.1038/srep03263.
Rochman, C. M., Kurobe, T., Flores, I., & Teh, S. J. (2014). Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2014.06.051.
Rosa, M., Ward, J. E., & Shumway, S. E. (2018). Selective capture and ingestion of particles by suspension-feeding bivalve Molluscs: A Review. Journal of Shellfish Research. https://doi.org/10.2983/035.037.0405.
Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T., & Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. https://doi.org/10.1186/s12859-017-1934-z.
Song, Y. K., Hong, S. H., Jang, M., Han, G. M., Rani, M., Lee, J., & Shim, W. J. (2015). A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2015.01.015.
Su, L., Cai, H., Kolandhasamy, P., Wu, C., Rochman, C. M., & Shi, H. (2018). Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environmental Pollution. https://doi.org/10.1016/j.envpol.2017.11.075.
Sussarellu, R., Suquet, M., Thomas, Y., Lambert, C., Fabioux, C., Pernet, M. E. J., et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1519019113.
Tanaka, K., & Takada, H. (2016). Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Scientific Reports. https://doi.org/10.1038/srep34351.
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., McGonigle, D., & Russell, A. E. (2004). Lost at sea: Where is all the plastic? Science, 304(5672), 838–838.
Thorp, J. H., & Rogers, D. C. (2011). Mussels and Clams: Phylum Mollusca, Class Bivalvia. Field Guide to Freshwater Invertebrates of North America. https://doi.org/10.1016/B978-0-12-381426-5.00010-7.
Van Cauwenberghe, L., & Janssen, C. R. (2014). Microplastics in bivalves cultured for human consumption. Environmental Pollution. https://doi.org/10.1016/j.envpol.2014.06.010.
Vaughn, C. C., Nichols, S. J., & Spooner, D. E. (2008). Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society. https://doi.org/10.1899/07-058.1.
Vollertsen, J., & Hansen, A. A. (2017). Microplastic in Danish wastewater: Sources, occurrences and fate. Danish Environmental Protection Agency.
Von Moos, N., Burkhardt-Holm, P., & Köhler, A. (2012). Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environmental Science and Technology. https://doi.org/10.1021/es302332w.
Wagner, M., Scherer, C., Alvarez-Muñoz, D., Brennholt, N., Bourrain, X., Buchinger, S., et al. (2014). Microplastics in freshwater ecosystems: What we know and what we need to know. Environmental Sciences Europe. https://doi.org/10.1186/s12302-014-0012-7.
Ward, J. E., Zhao, S., Holohan, B. A., Mladinich, K. M., Griffin, T. W., Wozniak, J., & Shumway, S. E. (2019). Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environmental Science and Technology. https://doi.org/10.1021/acs.est.9b02073.
Watkins, L., McGrattan, S., Sullivan, P. J., & Walter, M. T. (2019). The effect of dams on river transport of microplastic pollution. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.02.028.
Williams, J. D., Warren, M. L., Cummings, K. S., Harris, J. L., & Neves, R. J. (1993). Conservation status of freshwater mussels of the United States and Canada. Fisheries. https://doi.org/10.1577/1548-8446(1993)018<0006:csofmo>2.0.co;2.
Woods, M. N., Stack, M. E., Fields, D. M., Shaw, S. D., & Matrai, P. A. (2018). Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis). Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2018.10.061.
Yonkos, L. T., Friedel, E. A., Perez-Reyes, A. C., Ghosal, S., & Arthur, C. D. (2014). Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environmental Science and Technology. https://doi.org/10.1021/es5036317.
Zbyszewski, M., & Corcoran, P. L. (2011). Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water, Air, and Soil Pollution. https://doi.org/10.1007/s11270-011-0760-6.
Acknowledgments
The authors would like to thank all individuals who participated in this project. Dr. Grzegorz Szymanski from the Department of Chemistry, University of Guelph for his valued training and assistance with Raman spectroscopy, Dr. Patricia Gillis (Environment and Climate Change Canada, Canadian Centre for Inland Waters) and colleagues, Jim Bennett and Joseph Salerno, for assistance in collecting of mussels in the Grand River, Nicole Wardell for the assistance in reviewing samples, and Dylan Bowes for the assistance in engineering filtering equipment.
Funding
Funding for this project was provided by a Natural Science and Engineering Research Council. Discovery Grant to R.S. Prosser.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Capsule: Microplastics observed in the tissues of freshwater mussels (Lasmigona costata) in the Grand River watershed. A greater number of microplastics observed in mussels downstream of wastewater treatment facilities.
Electronic Supplementary Material
ESM 1
(DOCX 58.3 kb)
Rights and permissions
About this article
Cite this article
Wardlaw, C., Prosser, R.S. Investigation of Microplastics in Freshwater Mussels (Lasmigona costata) From the Grand River Watershed in Ontario, Canada. Water Air Soil Pollut 231, 405 (2020). https://doi.org/10.1007/s11270-020-04741-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04741-5