Abstract
A combined process comprised of electro-Fenton and bioslurry (EF–bioslurry) was developed at lab scale for remediating a real coking plant soil with an initial polycyclic aromatic hydrocarbons (PAHs) content of 3605 mg/kg. Sodium citrate was used as a complexant to keep the iron in solution at near-neutral pH conditions for increasing the reaction rate. The appropriate order of application was to perform EF process followed by bioslurry, which was evaluated through analysis of degradation characteristics of individual processes. The optimum EF duration was assessed through an analysis of the induced changes in PAHs degradation and bacterial counts. The optimum application time of EF process was determined to be 24 h. The removal of PAHs was 95.2% for EF–bioslurry after 40 days, and the efficiency was increased by almost 150%, compared with the individual bioslurry treatment. The EF reaction caused significant cell death and high inhibition to polyphenol oxidase (PPO) activity of soil. The bacterial activity and counts in the slurry recovered rapidly after EF oxidation through the addition of raw soil (2%, w/w). Therefore, the combined process of EF–bioslurry process may be an efficient and promising approach for the remediation of highly organic-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, with the acceleration of urbanization in China, more and more coking plants in urban area causing high environmental pollution have been closed and relocated (Chen et al. 2016a). The contaminated sites were left over and may pose potential hazards to human health and the environment because of the presence of high levels of polycyclic aromatic hydrocarbons (PAHs) and heavy metals. Thus, the management of contaminated sites after the relocation has become a “hot spot” in the soil pollution control in China.
PAHs are main organic pollutants in the soil polluted by coking industry (Li et al. 2018). PAHs have strong hydrophobicity and high octanol water partition coefficient. Therefore, soil becomes the main carrier of PAHs. PAHs are of wide, international concern because of their strong carcinogenicity, teratogenicity and ecotoxicity. The difficulty in PAHs degradation increases with the increased number of benzene rings (Ghosal et al. 2016). In addition, PAHs molecules can adsorb strongly to soil organic matter, leading to low bioaccessibility and bioavailability of the pollutants in soil especially in aged soil.
Chemical oxidation has been widely used in site remediation engineering due to its advantages of high efficiency, short remediation duration, multiple types of repairable pollutants and small secondary pollution (Cheng et al. 2016). The most common oxidants employed in this technique are Fenton’s reagent, hydrogen peroxide, permanganate, ozone, and persulfate. Among these oxidants, Fenton’s reagent has shown promising potential to rapidly decompose PAHs in contaminated soil (Yap et al. 2011). As one of electrochemical advanced oxidation processes, electro-Fenton (EF) technique has demonstrated good performance towards treating biorefractory organic pollutants (Mousset et al. 2017). EF has advantages such as, generating in situ Fenton’s reagent leading to the formation of hydroxyl radicals (•OH) [Eq.(1) and (2)], good mass transfer rate, selective oxidation, no sludge production and high mineralization degree of organic pollutants (Mousset et al. 2017).
Nevertheless, it is not appropriate to completely degrade soil organic pollutants by EF because of high energy consumption and great damage to soil structure.
Bioremediation is a safe and environment-friendly soil remediation technology. However, the actual application of solid-phase bioremediation (such as landfarming, composting, bioventing) of organic-contaminated soil is restricted by the bioavailability, concentration, toxicity, molecular structure, hydrophobicity of the target pollutants, low mass transfer, soil heterogeneity and long duration. Bioslurry is an enhanced ex situ bioremediation technology, which can greatly improve the reaction rate and removal efficiency of pollutants compared with in situ bioremediation (Pant and Rai 2018). Typically, bioslurry process involves the mixture of contaminated soil with nutrient, water and microbial inoculum. Bioslurry reactor offers a good way to remediate PAHs contaminated soil by utilizing the existing soil microbes, thus decreasing the time for inoculums to acclimatize to the conditions. Nevertheless, for high-molecular-weight PAHs, especially for aged soil, the bioavailability of PAHs can be still a rate-limiting step to affect degradation efficiency. Thus, there is a need for integrating bioslurry with other methods to enhance remediation efficiency while reducing cost.
In this work, in order to develop a hybrid technology that can cost-effectively, environmentally friendly and efficiently degrades PAHs in aged soil, the application of EF process and bioslurry in a slurry reactor was investigated. First, the order of combination was evaluated by analyzing the degradation characteristics of EF and bioslurry processes individually. Then, the processes were combined and optimized by controlling the application time of EF treatment. The soil parameters and microbial properties were determined to assess the influence of EF application time on the efficiency of bioslurry.
2 Materials and Methods
2.1 Tested Soil
The tested soil was collected from the top layer (0–10 cm) of a local coking plant in China. The soil was air-dried, thoroughly mixed, and then passed through a 60-mesh sieve to remove gravels, stones and leaves. The soil had the following properties: pH 6.2, total organic carbon (TOC) 9.3 g/kg, total iron (Fe2+ and Fe3+) 26.3 g/kg, Mn 0.43 g/kg, total nitrogen 530 mg/kg, total phosphorus 34 mg/kg. Table 1 lists the concentration of individual PAHs in the tested soil. It can be found that the soil was highly contaminated with PAHs. Refractory species (4-, 5- and 6-ring) accounted for 69.9% of total mass, indicating a difficulty in remediation of this soil.
To assess the mineralization of PAHs during soil treatment, the soil was spiked with [1,2,3,4,4a,9a-14C]-anthracene, [4,5,9,10-14C]-pyrene, [7,10-14C]benzo[a]pyrene and [2,3,6-14C]benzo[ghi]perylene. The four 14C-PAHs were dissolved in acetone, mixed, and then added dropwise to soil to obtain a content of 1.0 μCi/kg soil for each 14C-PAHs congener.
2.2 Experimental Design
A total of eight tests were designed in this work (Table 2). Nutrients [KNO3 and (NH4)2HPO4] were added at the beginning of bioslurry treatment to achieve a C:N:P molar ratio of 100:10:1 based on the PAHs loading as a measure of C (Smith et al. 2015). Test A was set up to evaluate the abiotic loss of PAHs during treatment. Tests B1 and C were used to assess the individual efficiencies of EF and bioslurry processes, respectively, in degrading PAHs. Test B2 was used to detect •OH concentration during the EF process. Tests D1 to D4 were EF reactors at first, and then were converted to bioslurry reactors at different time points; these tests were conducted to study the remediation efficiency of the combined process, with different application times of EF treatment.
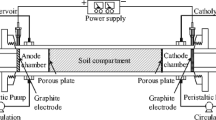
Both EF and bioslurry processes were performed in a series of identical reactors, which consisted of a glass cylindrical vessel with two electrode bars and an adjustable fitted cover. The reactor had a working volume of 0.50 L. A graphite bar (as cathode) and an iron bar (as anode) were connected to a direct power supply at a distance of 5 cm. A constant potential difference (10 V) was applied, resulting in a voltage gradient 2 V/cm. In all experiments, each reactor contained 100 g of soil (dry weight) and 300 mL of distilled water. An inert electrolyte Na2SO4 was added at its final concentration of 1.0 g/L. The reactors were constantly stirred at 300 rpm to avoid concentration gradients. To reach a stationary O2 concentration, aeration was started 15 min before electrolysis.
2.3 Determination of Soil Basic Physic-Chemical Properties
Soil moisture content was determined by gravimetry, in an aliquot of moist soil dried at 105 °C to a constant weight. Soil pH was determined by using a pH meter (model pHS-3B, Shanghai Precision Scientific Instrument Co., Ltd., China) with a soil:water ratio of 1:2.5. Total nitrogen was measured by a total nitrogen analyzer (model 5B-3BN; Lianhua Technology Co., Ltd., China). Total phosphorus was determined colorimetrically after perchloric/sulfuric acid digestion, by using inductively coupled plasma atomic emission spectrometry (ICP-AES) (Seiko Instruments, Chiba, Japan). Malvern Mastersizer 3000 laser particle size analyzer was used to determine the specific surface area and particle size distribution of soil samples.
Soil dissolved organic carbon (DOC) was extracted by ultra pure water (Han et al. 2013). Briefly, 5.0 g soil was added to a 50 mL centrifuge tube containing 25 mL ultra pure water. The mixture was stirred at 180 rpm for 30 min, and then centrifuged at 5000 rpm for 10 min. The supernatant was collected and passed through a 0.22 μm microporous membrane. The filtrate was analyzed using a total organic carbon analyzer (TOC-5000, Shimadzu, Kyoto, Japan).
2.4 PAHs Extraction and Analysis
PAHs were extracted from soil samples using an accelerated solvent extraction system (ASE 200, Dionex, Sunnyvale, CA, USA) with a 1:1 (v/v) acetone/dichloromethane solvent mixture. Soil samples were extracted three times, and the extracts were combined, concentrated with a rotary evaporator, and solvent exchanged with 5 mL of n-hexane. The extracts were purified in a glass column filled with silica gel and aluminum oxide as described by Chen et al. (2016).
Quantification of PAHs was conducted by high performance liquid chromatography with fluorescence detection (HPLC-FL, model 1200, Agilent Technologies, Santa Clara, CA, USA), equipped with a Waters PAHs C18 column (250 mm × 4.6 mm × 5 μm). The run conditions consisted of a mobile phase of acetonitrile-water and a flow rate of 1.0 mL/min. Mobile phase gradient (acetonitrile/water, v/v): 0–10 min 60:40, 10–60 min 100:0.
HPLC was also used to evaluate the concentration of •OH, through the quantitative determination of the primary hydroxylated derivatives of salicylic acid, 2,3-dihydroxybenzoic acid and/or 2,5-dihydroxybenzoic acid (Jen et al. 1998). A Waters C18 reversed-phase column (250 mm × 4.6 mm × 5 μm) was used in this case.
2.5 Microbial Analysis
Total bacteria (TB) counts were measured on nutrient agar plates through plate spread method (five replicates per sample). The total number of colonies was counted after incubation at 28 °C in the dark for 2 days, and the results were expressed as colony forming units (CFU)/g soil or mL slurry.
Soil aerobic PAH-degrading bacteria (PDB) were enumerated by using the plate spread method following the method of Chen et al. (2016). The number was counted after 14 days of incubation at 28 °C.
Soil polyphenol oxidase (PPO) activity was determined following the colorimetric method of Yu et al. (2011). One gram of soil and 10 mL pyrogallic acid (1%) was mixed and incubated at 30 °C for 2 h, then 4 mL citric–phosphoric acid buffer (pH 4.5) was added to the mixture. The purpurigallin (PPG) formed was extracted with chloroform, and then measured by a spectrophotometer at 430 nm. The results are expressed as μg PPG/g soil or mL slurry.
2.6 Mineralization Assay
PAHs mineralization assay was conducted through wet combustion method (Lu and Zhang 2014). The 14CO2 produced was trapped with aquasol and 4 M NaOH solution. The radioactivity in trap solution was determined by liquid scintillation counting (LSC) using ACS II scintillation fluid (ASCII; Amersham, UK).
2.7 Statistical Analysis
All experiments were conducted in triplicate to obtain reliable results, and the results presented represent an average of the three parallel experiments. Statistical significance was evaluated using SPSS package (version 11.0) with two-way ANOVA and least significant difference (LSD) was applied to test for significance at P < 0.05 between the means.
3 Results and Discussion
3.1 EF Versus Bioslurry Process in a Single Unit
The degradation characteristics of one target pollutant differ between technologies. These differences provide the reasons and benefits behind combining the technologies, so need to be analyzed and evaluated. Three reactors (tests A, B1 and C) were employed to detect the treatment effects and to analyze different PAHs degradation characteristics of the EF and bioslurry processes individually. The EF and bioslurry treatment lasted for 4 and 60 days, respectively.
The abiotic control (test A) showed 1.6% and 3.3% loss of PAHs in EF and bioslurry process, respectively (Fig. 1). This abiotic loss of PAHs was attributed to evaporation, sequestration and adsorption onto soil organic matter (SOM) particles, which was of minor significance in this work. The higher PAHs loss in bioslurry relative to the EF can be ascribed to the longer aeration duration of EF process. The EF process was an efficient remediation method for PAHs, whereas biodegradation behavior was slow and low-effective in bioslurry treatment (Fig. 1). At the end of experiments, there were significant differences (P < 0.05) in the total removal ratios of PAHs between EF (92.6%) and bioslurry (34.3%). Most of these reductions of PAHs can be attributed to chemical oxidation or biodegradation in view of the low abiotic loss of PAHs.
Different degradability characteristics were observed in the two tests. The process of PAHs degradation can be divided into two stages according to the degradability characteristics (Fig. 1). The degradation rate of EF reached the maximum value in a short time and then decreased gradually, while the degradation rate of bioslurry was much slower than that of EF. Especially, PAHs biodegradation gradually reached a plateau at the later stage.
The formation of •OH radicals was the key factor for PAHs removal during the EF process (test B2). The •OH radicals were generated right after the application of power and remained at a high level for most of the time (Fig. 2). This demonstrates that using sodium citrate can effectively keep the iron in solution by forming iron-citrate chelates and increase the reaction rate outside the ideal operating range pH for Fenton’s reagent (pH 3.0–4.0). Complexed iron oxidation systems have increased the operating range of the classical Fenton system to pH of 6.0–8.5, which precludes the requirement of pH adjustment for these systems (Lu et al. 2010).
During the bioslurry treatment (test C), the pattern of microbial growth was characterized by an initial increase in the number of PDB (Fig. 2). A decrease in PDB count was observed after 30 days, which can be attributed to the bioaccessibility of substrates. Thus, it is difficult to accomplish the soil remediation through bioslurry alone due to the low bioaccessibility and bioavailability of PAHs.
Solution pH is another important factor influencing treatment efficiency. The pH was initially 6.2, which gradually increased to 8.4 by the end of the experiment (Fig. 3). The increase in pH during the EF process can be mainly ascribed to the consumption of H+ ions [Eq.(1)] and the generation of OH−[Eq.(2)]. Although H+ ions are produced during the process [Eq.(3)], the rate constant of Eq.(3) is much lower than that of Eq.(2) [70 mol/L•s for Eq.(2), 0.001–0.01 mol/L•s for Eq. (3)] (de Luna et al. 2012).
3.2 Evaluation of the Combined EF–Bioslurry Process
Above all, EF and bioslurry processes have different degradation characteristics of PAHs. It is this difference that makes it feasible to combine the two remediation methods combined for treating the PAHs-contaminated soil. Therefore, combining the two processes with the EF prior to the bioslurry process was considered a reasonable approach. A good question is what time is good to stop the EF process and start the subsequent bioslurry process, for rigorous economic comparisons using an accepted standard measure of treatment efficiency are necessary. Thus, four EF treatment times were tested for its performance towards the downstream bioslurry treatment.
The different EF durations (12, 24, 36 and 48 h) resulted in 15.3%, 36.2%, 52.5% and 61.4% removal of PAHs (Fig. 4, the starting value on day 0). This result is easy to understand, since hydroxyl radicals can non-specifically oxidize organic compounds, and the longer the reaction time, the more PAHs can be oxidized. Nevertheless, during the subsequent bioslurry treatment, the biodegradation efficiency of PAHs diverged among different EF-pretreatment durations. In general, test D2 exhibited the highest treatment performance among the four tests (Fig. 4). After 40 days, the concentration of PAHs decreased to 1269, 173, 418 and 520 mg/kg for tests D1, D2, D3 and D4, respectively, corresponding to a total removal efficiency of 64.8%, 95.2%, 88.4% and 85.6%, respectively. As the time increasing, the degradation rate of PAHs increased first and then gradually decreased with time, which was a typical biodegradation model. Although oxidation efficiency was higher in D3 and D4 than in D2, biodegradation performance of bioslurry treatment was not improved by the higher EF-pretreatment efficiency of tests D3 and D4 (Fig. 4).
Table 3 shows the changes of TB and PDB counts in bioslurry during the biological treatment phase. It is clear that the numbers of microbial counts were strongly affected by EF oxidation. The counts of TB and PDB in raw slurry were 3.38 × 107 and 2.05 × 106 CFU/mL, respectively, which declined by 1 to 3 orders of magnitude after 12–48 h EF treatment (Table 3). Apparently, slurry microbial numbers suffer great loss during EF oxidation. Gong (2012) found a significant decrease of about two orders of magnitude of soil bacteria number after Fenton oxidation in soil slurries. Cell wall disintegration can be caused by H2O2 and radicals produced during Fenton oxidation (Gong 2012). Thus, with respect to this work, the disruption of cellular membrane is a reasonable reason for cell death in this work.
In view of the substantial loss of microbial amounts caused by EF oxidation, it is necessary to add exogenous microbes to the slurry for accelerating the recovery of microbial community structure and amounts and shortening the biotreatment duration. Bioaugmentation is one of the most common methods for bioremediation of contaminated sites worldwide. Nevertheless, many factors including strain selection, pollutant type, microbial ecology, and environmental constraints can cause a failure of bioaugmentation practices (Chen et al. 2016b). Moreover, indigenous microorganisms can outcompete exogenous inoculants during bioremediation, leading to low survival and growth of inoculants. Therefore, in this work, 2 g of raw contaminated (nonoxidized) soil was added to each bioslurry reactor to act as an inoculum source. This operation had negligible influence on PAHs concentration (around 2%), but could increase the microbial amount by up to several times (Table 3). It can be anticipated that the introduced soil microbes could survive and adapt to the slurry environment easily since they originated from the soil. This was evident from the significant increase in the population of TB and PDB in slurry during bioslurry treatment (Table 3). After 15 days, bacterial density basically recovered to raw slurry levels and then increased. Although no significant difference was observed in the final counts of bacteria among four tests, the recovery of bacterial counts of D1 and D2 was apparently faster than that of D3 and D4. This indicates that the long-term EF oxidation had profoundly changed the soil environment, resulting in a negative impact on the recovery of microbes.
3.3 Effect of EF Oxidation on Soil Physic-Chemical Properties
Figure 5 shows the specific surface area, size distribution and DOC content of the soil after different times of EF oxidation. It can be seen that at the treatment time of 12, 24, 36 and 48 h, the specific surface area of soil was 236.3, 285.6, 364.2 and 423.7 m2/kg (Fig. 5A), respectively, which was 45.6–161.0% higher than that of raw soil, and the specific surface area of soil was positively correlated with the treatment time (P < 0.05). As far as the particle size distribution be concerned, after 12–48 h of oxidation, the sand content decreased by 12.5%–24.3%, whereas the content of silt and clay increased by 4.6%–9.3% and 13.5%–19.6%, respectively (Fig. 5B). This indicates that the oxidation destroyed the cohesion and cementation of soil, leading to the destruction of soil aggregate structure and the thinning and dispersion of soil particles. The high alteration of specific surface area and size distribution may be detrimental to microbial recovery and microbial activity.
The DOC content was 0.68 g/kg in raw soil, which increased to 0.85, 1.42, 1.76 and 1.95 g/kg after 12, 24, 36 and 48 h of oxidation, respectively (Fig. 5C). Apparently, the DOC content was positively correlated with oxidation time (P < 0.05), indicating that EF oxidation could transform hydrophobic soil organic matter into soluble organic matter. Thus, the bioaccessibility and bioavailability of PAHs could be enhanced during EF oxidation by desorption of PAHs from soil organic matter.
3.4 Degradation of Individual PAHs
Figure 6 demonstrates the concentration and mass abundance of 2- to 6-ring PAHs after different times of EF oxidation and bioslurry treatment. In raw soil, the abundance of 2-, 3-, 4-, 5- and 6-ring PAHs was 1.26%, 28.78%, 44.47%, 18.38%, 7.11%, respectively. It can be found that, with the time extension of oxidation, the concentration of various PAHs groups declined continuously, and 2-ring PAH (namely naphthalene) disappeared at last (Fig. 6A).
During EF oxidation, the abundance of various PAHs changed slightly, demonstrating the non-specificality of Fenton oxidation (Fig. 6B). During bioslurry treatment, higher ring PAHs were concentrated, and finally 5- and 6-ring PAHs were the dominant species and constituted more than 70% of the total PAHs. This indicates that higher ring PAHs are more biorefractory than lower ring ones. Moreover, it was observed that the abundance of 5- and 6-ring PAHs was higher in test D2 than in other tests after biotreatment (Fig. 6B), which was in accordance with the highest biodegradation of PAHs of test D2 among four tests.
3.5 PAHs Mineralization
Table 4 demonstrates the cumulative mineralization efficiency of mixture of spiked 14C-PAHs during the EF-bioslurry treatment period. As shown, mineralization efficiency increased with EF treatment time, but a 5-day lag period was observed for various biotic treatments. At the end of experiments, the mineralization efficiency was 41.5%, 68.6%, 55.4% and 50.8% for tests D1, D2, D3 and D4, respectively. By comparing PAHs removal efficiency (64.8–85.6%, Fig. 4) with mineralization efficiency (41.5–68.6%, Table 4), it can be inferred that there existed large amounts of PAHs intermediates such as oxygenated PAHs in the soil. To solve this problem, static biological treatment, such as composting, may be carried out to further mineralize these metabolites in the soil at low cost.
3.6 Polyphenol Oxidase (PPO) Activity
Soil PPO is an oxygen-transferring enzyme acting on specific recalcitrant pollutants through precipitation or transformation to other products, and thus PPO activity is closely related to PAHs degradation in soil (Lu and Lu 2014). Figure 7 shows variations in PPO activity during the EF-bioslurry treatment period. It can be found that the changing trend of PPO activity was similar to that of PDB numbers and correlated to PAHs degradation. The PPO activity was 26.3 μg PPG/mL slurry before treatment, and remarkably decreased in the EF process (Fig. 7). PPO activity was low in the initial period of bioslurry treatment, which increased remarkably after 15 days of incubation. PPO activity was highest in test D2 at day 20, with the value of around 74.2 PPG/mL slurry. In the later phase, PPO activity declined with time in all tests. Test D3 showed higher PPO activity than D1 at most times (Fig. 7), although the latter had higher density of degraders than the former (Table 3). This indicates that PPO activity is not always positively correlated with the count of degraders during bioremediation. In general, microbial enzyme activity depends on several factors including microbial number, substrate induction, inhibitory substances, etc. (Yu et al. 2011).
4 Conclusions
The above results suggest the feasibility of applying the EF-bioslurry process to enhance the remediation of PAHs polluted soil. The EF treatment may diminish the pollutant content and improve its bioaccessibility due to the partial oxidation of SOM. In the process of combined remediation, EF time was a main factor affecting the remediation efficiency. When EF treatment time was 24 h, PAHs removal was maximized. The Bioslurry process was highly efficient in the second half of the experiment. Elimination of pollutants was accompanied by an increment in bacterial counts and activity. Further investigations are now under way to design a new process for higher PAHs mineralization.
References
Chen, F., Tan, M., Ma, J., Li, G., & Qu, J. (2016a). Restoration of manufactured gas plant site soil through combined ultrasound-assisted soil washing and bioaugmentation. Chemosphere, 146, 289–299.
Chen, F., Tan, M., Ma, J., Zhang, S., Li, G., & Qu, J. (2016b). Efficient remediation of PAH-metal co-contaminated soil using microbial-plant combination: A greenhouse study. Journal of Hazardous Materials, 302, 250–261.
Cheng, M., Zeng, G., Huang, D., Lai, C., Xu, P., Zhang, C., & Liu, Y. (2016). Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chemical Engineering Journal, 284, 582–598.
de Luna, M. D., Veciana, M. L., Su, C. C., & Lu, M. C. (2012). Acetaminophen degradation by electro-Fenton and photoelectro-Fenton using a double cathode electrochemical cell. Journal of Hazardous Materials, 217–218, 200–207.
Han, W., Luo, L., & Zhang, S. (2013). Adsorption of tetrabromobisphenol a on soils: Contribution of soil components and influence of soil properties. Colloids and Surfaces A, 428, 60–64.
Jen, J. F., Leu, M. F., & Yang, T. C. (1998). Determination of hydroxyl radicals in an advanced oxidation process with salicylic acid trapping and liquid chromatography. Journal of Chromatography. A, 796, 283–288.
Li, X., Li, J., Sui, H., He, L., Cao, X., & Li, Y. (2018). Evaluation and determination of soil remediation schemes using a modified AHP model and its application in a contaminated coking plant. Journal of Hazardous Materials, 353, 300–311.
Lu, M., & Zhang, Z. Z. (2014). Phytoremediation of soil co-contaminated with heavy metals and deca-BDE by co-planting of Sedum alfredii with tall fescue associated with Bacillus cereus JP12. Plant and Soil, 382, 89–102.
Lu, M., Zhang, Z., Qiao, W., Wei, X., Guan, Y., Ma, Q., & Guan, Y. (2010). Remediation of petroleum-contaminated soil after composting by sequential treatment with Fenton-like oxidation and biodegradation. Bioresource Technology, 101, 2106–2113.
Lu, Y. F., & Lu, M. (2014). Remediation of pah-contaminated soil by the combination of tall fescue, arbuscular mycorrhizal fungus and epigeic earthworms. Journal of Hazardous Materials, 285, 535–541.
Mousset, E., Trellu, C., Oturan, N., Rodrigo, M. A., & Oturan, M. A. (2017). Soil remediation by electro-Fenton process, In electro-Fenton process (pp. 399–423). Singapore: Springer.
Ghosal, D., Ghosh, S., Dutta, T. K., & Ahn, Y. (2016). Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Frontiers in Microbiology, 7, 1369.
Gong, X. B. (2012). Remediation of weathered petroleum oil-contaminated soil using a combination of biostimulation and modified Fenton oxidation. International Biodeterioration & Biodegradation, 70, 89–95.
Pant, A., & Rai, J. P. N. (2018). Bioremediation of chlorpyrifos contaminated soil by two phase bioslurry reactor: Processes evaluation and optimization by Taguchi's design of experimental (DOE) methodology. Ecotoxicology and Environmental Safety, 150, 305–311.
Smith, E., Thavamani, P., Ramadass, K., Naidu, R., Srivastava, P., & Megharaj, M. (2015). Remediation trials for hydrocarbon-contaminated soils in arid environments: Evaluation of bioslurry and biopiling techniques. International Biodeterioration & Biodegradation, 101, 56–65.
Yap, C. L., Gan, S., & Ng, H. K. (2011). Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere, 83, 1414–1430.
Yu, X. Z., Wu, S. C., Wu, F. Y., & Wong, M. H. (2011). Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. Journal of Hazardous Materials, 186, 1206–1217.
Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (no. 51768018) for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Gao, Z. & Lu, X. An Integrated Approach to Remove PAHs from Highly Contaminated Soil: Electro-Fenton Process and Bioslurry Treatment. Water Air Soil Pollut 231, 314 (2020). https://doi.org/10.1007/s11270-020-04696-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04696-7