Abstract
The effects of CuO NPs and bulk Cu at 0–1000 mg L−1 on the growth, photosynthesis and biochemical parameters were investigated in 30-day-old rice plants grown hydroponically. ICP-OES measurements showed that CuO NPs released ≤ 1 mg L−1 of Cu2+ ions compared with ≤ 81 mg L−1 by bulk Cu at their highest concentration. Both treatments showed that growth, photo-phosphorylation and carbon dioxide assimilation declined considerably. Bulk particles caused oxidative stress whereas NP showed no such effect. Electromicrographs showed that CuO NPs accumulated in chloroplasts resulting in destacking and distortions of thylakoid membranes while bulk Cu showed no such behaviour. Results suggest that NP affected the growth by accumulation in non-ionic form in chloroplasts causing damage to thylakoid membrane without oxidative damage, whereas the bulk Cu affected the growth by causing oxidative damage as a result of release of Cu2+ ions without affecting the ultrastructure of the chloroplasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Widespread application of nanoparticles in multidisciplinary fields has resulted in the large-scale production of engineered nanoparticles (NPs) in recent years and is expected to near 58,000 metric tonnes by 2020 (Maynard 2006). This could lead to the entry of large quantities of NPs into the environment; contaminating soil and aquatic ecosystems at a rapid rate (Klaine et al. 2008). Likewise, total copper (Cu2+) production was reported to be 45,266 metric tonnes in 2017 (www.statista.com) and is estimated to rise quickly in the near future. Hence, release of Cu due to mining, smelting and refineries will lead to its accumulation into the agricultural land. Since Cu does not undergo microbial or chemical degradation unlike organic pollutants, they become a potent toxicant to plants. In order to ensure a sustainable nanotechnology industry, and to protect the environment from harmful pollutants, it is essential to understand the environmental impacts of NPs and their bulk counterparts on growth and productivity in crop plants.
Metal oxide NPs (i.e. 1–100 nm) differ from their bulk counterparts (ionic radius (r) of Cu:128r; Cu+:77r; Cu2+:73r; Wells 2012) due to their size range. NPs have well-defined particle density and atomic mass, whereas, bulk particles show variation in density and mass owing to their freely settled or compact state (Buzea et al. 2007). Bulk Cu belongs to the group of nonbiodegradable, persistent inorganic heavy metals with a density higher than 8.96 g cm−3 (Wuana and Okieimen 2011). Unlike bulk particles, MNPs not only have a very large surface area thus high particle number per unit mass but also display quantum effects, modifying the electrical, visual, mechanical, optical, electric and magnetic properties as a result of its electron confinement ability, thus capable of changing the absorption wavelengths and conduction band gaps. CuO NPs are extensively used in heat transfer nanofluids, catalysts, gas sensors, semiconductors (Wang et al. 2012), as antimicrobial (Ren et al. 2009) and antibacterial agents (You et al. 2011), cosmetic and personal care products (Xiao et al. 2005) and wound healing (Rigo et al. 2013). Bulk Cu, on the other hand, is used as an electrical conductor (Pops 2008), in electrical motors (Peters et al. 2007), as an anti-biofoul (Edding and Tala 1996) and as fungicide in the form of Bordeaux mixture (CuSO4 + Ca (OH)2) in agriculture.
Copper is an essential micronutrient actively involved in maintaining normal metabolism in higher plants. It is required in many physiological processes such as photosynthetic and mitochondrial electron transport chain and can exist in multiple oxidative states (Cu2+, Cu+) in vivo (Yruela 2005). This redox property of Cu can also contribute to its toxicity by catalysing the production of damaging radicals as free ions (Manceau et al. 2008), altering membrane permeability, chromatin structure, protein synthesis, photosynthesis, and respiratory processes and bringing about senescence (Yruela 2005). Typical uncontaminated soils contain approximately 2–100 mg kg−1 Cu, whereas, agricultural amendments such as sewage sludge, compost refuse, farmyard manure and phosphate fertilisers contain Cu in the range of 50–8000 mg kg−1 (Nagajyoti et al. 2010). Uptake, distribution and mechanism of toxicity of bulk Cu in plants largely depend upon the availability of free Cu ions in bioavailable forms, which is well studied (Emamverdian et al. 2015; Yruela 2005; Yruela 2009); however, data on the amount and availability of CuO NPs in soil is currently unavailable. The entry of NPs into the living system may probably occur due to its surface physicochemical characteristics owing to its active sites (Buzea et al. 2007). Small size of NPs confers greater mobility, surface charge, material solubility and purity, crucial to its fate and toxicity in plants.
Plant responses to Cu vary depending on the level of exposure. At higher concentrations, bulk Cu can hamper normal plant functioning and block cellular metabolism affecting physiological and biochemical processes such as photosynthesis, respiration and enzymatic activities. This is achieved by increased generation of reactive oxygen species (ROS) and methylglyoxal (MG), leading to activation of programmed cell death (PCD) pathways (Emamverdian et al. 2015). However, the mode of NP response to plants is not well understood, whether it behaves like its bulk counterpart or shows a different mechanism of effect and is an area of active study. There are studies suggesting that the effect of metal NP could be due to the Cu ions (Franklin et al. 2007; Navarro et al. 2008) or the metal particle itself (Stampoulis et al. 2009; Lin and Xing 2008). Earlier, we have shown that MNPs, as a particulate form, affect the growth and productivity of rice (Da Costa and Sharma 2016). In this study, we compare the process of damage to plants due to MNPs and bulk Cu and, based on the data, suggest a different mechanism of damage to plants under CuO NP and bulk Cu stress in rice.
2 Materials and Methods
2.1 Plant Material and Treatments
Oryza sativa (var. Jyoti), a high yielding rice variety obtained from Indian Council of Agricultural Research, Goa, was stored in dark at 20 °C. CuO NPs (< 50 nm size, Sigma-Aldrich) and copper (II) sulphate pentahydrate (CuSO4·5H2O, M.W. 249.68 g mol−1, Sigma) were used as bulk Cu (ionic form). Both CuO NPs and bulk Cu solutions were prepared at concentrations of 0 (control), 2.5, 10, 50, 100 and 1000 mg L−1 (w/v) at pH 6.5 in Hoagland’s solution. The seeds were grown in the abovementioned preparation as described in Da Costa and Sharma (2016).
2.2 Nanoparticle Size Determination
Scanning electron microscope (SEM, JSM 5800 LV, JEOL, Japan) for CuO NP characterisation and X-ray diffractometer (XRD ultraX 18HB–300, Rigaku, Japan) for particle size confirmation were used according to Da Costa and Sharma (2016). Dynamic light scattering (DLS) measurements were performed as previously described by Walczak et al. (2012), to determine the hydrodynamic size of CuO NPs. Measurements were performed at a scattering angle of 90° at 25 °C, and each correlation function accumulated for 30s was repeated 10 times using a ZetaPALS Analyzer (Brookhaven Instruments Corporation, New York, USA) at Centre for Nano Science and Engineering (CeNSE), Indian Institute of Science, Bangalore, India. The velocity distribution of particle movement was analysed using DLS by measuring dynamic fluctuations of light scattering intensity produced by the Brownian motion of the particle. The data obtained was then analysed in BIC Particle Sizing Software (Ver.5.23) which was used to calculate the hydrodynamic radius of the particles by applying the Stokes–Einstein equation. Suspensions of 100 μg mL−1(w/v) were analysed in triplicate and the results were presented as the mean ± SD.
2.3 Dissolution of Nano and Bulk Cu Suspensions
Metal ion concentration released from NP and bulk Cu solutions was measured according to Wu et al. (2012). Aliquots of CuO NPs and bulk Cu suspensions 0, 2.5, 10, 50, 100 and 1000 mg L−1 (w/v) were drawn after 48 h. The extracts were centrifuged at 12,000 rpm for 20 min, and supernatants were collected and filtered with 0.2 μm Ultipor®N66®Nylon membrane filter. Inductively coupled plasma mass spectroscopy (ICP–OES, Perkin–Elmer Optima 7300 DV, USA) was used to conduct concentration assays of the metal ion at the National Institute of Oceanography, Goa.
2.4 Growth, Morphology and Biomass Studies
Percentage of germination, shoot and root length were measured after 6 days, and the biomass was measured in 10 randomly selected plants grown for 30 days as described in our previous study (Da Costa and Sharma 2016) The external leaf and root morphology was studied by visualising lyophilised segments using SEM (JSM 5800 LV, JEOL, Japan). The internal leaf and root morphology were studied using transmission electron microscopy (TEM) at All India Institute of Medical Sciences (AIIMS), New Delhi, according to the method described by Da Costa and Sharma (2016).
2.5 Nanoparticle Uptake
Uptake of Cu was determined using atomic absorption spectrophotometer (Flame Furnace system–AAnalyst 200, AAS, Perkin Elmer, USA) at the National Institute of Oceanography, Goa. The Cu content in fresh tissue (1 g) of leaf and root (control and treated plant) was analysed as described by Da Costa and Sharma (2016).
2.6 Photosynthetic Measurements
Carbon dioxide fixation studies were carried out using a portable infrared gas analyser (IRGA, ADC Bioscientific, LCi–SD, Hansatech, UK) according to Sharma and Hall (1996). The photosynthetic rate (PN), transpiration rate (E) and stomatal conductance (gs) were measured at ambient temperature, CO2 concentration and light intensity of 1200 μmol m−2 s−1, by means of a detachable light source delivered by a dichroic lamp (Hansatech, UK). Chlorophyll fluorescence measurements were carried out using a fluorescence monitoring system (FMS–1, Hansatech, UK), and extraction and analysis of photosynthetic pigments were done as described by Da Costa and Sharma (2016) using an HPLC system (Waters, USA), with a two-solvent gradient.
2.7 Determination of Hydroxyl Radical (OH·)
OH· radical content was measured according to Liu et al. (2009). Fresh leaf tissue of 0.2 g was homogenised in 1.2 mL of 50 mM sodium phosphate buffer (pH 7.0) and centrifuged at 10,000×g for 10 min at 4 °C. Reaction mixture containing 0.5 mL supernatant, 0.5 mL of 50 mM of sodium phosphate buffer (pH 7.0) and 1 mL of 25 mM sodium phosphate buffer containing 2.5 mM 2-deoxyribose was incubated at 35 °C for 1 h in dark. After incubation, 1 mL of 1% thiobarbituric Acid (TBA, Sigma, USA) and 1 mL of glacial acetic acid were added and boiled for 10 min. Reaction was cooled immediately on ice for 10 min and absorbance was recorded at 532 nm. Concentration of OH· was expressed as Absc. units (Absorbance × 1000).
2.8 Lipid Peroxidation Assay
Lipid peroxidation assay was determined by estimation of the malondialdehyde (MDA) content in leaves following Da Costa and Sharma (2016). The amount of MDA (extinction coefficient of 155 mM−1 cm−1) was calculated by subtracting non-specific absorbance at 600 nm from absorbance at 532 nm using UV–Visible spectrophotometer (UV–2450, Shimadzu, Singapore).
2.9 Protein Carbonyl Colorimetric Assay Method
Protein carbonyl content was estimated according to Reznick and Packer (1994). Briefly, plant tissue (0.1 g) was taken and ground in 2.5 mL of sodium phosphate buffer (pH 7.4, Merck) and centrifuged at 7000 rpm for 15 min at 4 °C. One millilitre each of supernatant was used for sample (S) and control (C) reactions separately. To the control reactions, 4 mL of 2.5 N HCl (Merck), and to the sample reactions, 4 mL of 0.2% 2, 4-dinitrophenyl hydrazine (DNPH, Himedia AR grade) prepared in 2.5 N HCl were added. Both mixtures were incubated in dark for 1 h at room temperature, after which, 4 mL of 20% TCA (Himedia, AR grade) was added, mixed and centrifuged at 7000 rpm for 10 min at 4 °C. The supernatant was then discarded, and the pellet was re-suspended in 1 mL of 10% TCA, incubated in ice for 5 min and centrifuged at 7000 rpm for 10 min at 4 °C. One millilitre of ethyl acetate:ethanol (1:1) was carefully added to the pellet, vortexed and centrifuged at 7000 rpm for 10 min at 4 °C. The pellet was then finally dissolved in 1.5 mL of 6 N guanidine hydrochloride (Himedia ≥ 99% pure) prepared in sodium phosphate buffer (Qualigens) and centrifuged at 7000 rpm for 10 min at 4 °C. The O.D. was measured at 370 nm using UV–Visible spectrophotometer (UV–2450, Shimadzu, Singapore). Protein oxidation was measured as protein carbonyl using the calculated average (CA) = (S) − (C) and extinction coefficient of 0.022 μM−1 cm−1. The following formula was used. Protein carbonyl (nmol/mL) = Calculated average (CA)/0.022 μM−1 cm−1 (a/b), where extinction coefficient = 0.022 μM−1 cm−1, a = volume of sample used (μL) and b = volume of 6 N guanidine HCl (μL).
2.10 Proline Accumulation
Fresh leaves (0.1 g) were used for the determination of proline, according to the method of Bates et al. (1973), measured at an absorbance of 520 nm using UV–Visible spectrophotometer (UV–2450, Shimadzu, Singapore). l-Proline (Sigma) was used as standard.
2.11 Ascorbic Acid Assay
Fresh leaves (0.1 g) were used for the estimation of ascorbate (ASA) according to the method of Kampfenkel et al. (1995), measured at an absorbance of 520 nm using UV–Visible spectrophotometer (UV–2450, Shimadzu, Singapore). Ascorbic acid (Hi-media) was used as standard.
2.12 Superoxide Dismutase Assay
The activity of superoxide dismutase (SOD) was assayed according to Boveris (1984). Leaf tissue (0.2 g) was homogenised in 1.5 mL of 50 mM sodium phosphate buffer (pH 7.8) and centrifuged at 12,000 rpm 4 °C for 1 min. The supernatant was used to carry out the SOD assay at 480 nm. Blank or reaction (1) contained 2.5 mL of 10 mM Na2CO3; 0.1 mL of 10 mM sodium phosphate buffer; 0.1 mL of 6 mM disodium EDTA; and 0.3 mL of deionised water. Reaction (2) contained 2.5 mL of 10 mM Na2CO3; 0.1 mL of 10 mM sodium phosphate buffer; 0.1 mL of 6 mM disodium EDTA; and 0.3 mL of 4.5 mM epinephrine (Sigma, USA). Reaction (3) contained 2.5 mL of 10 mM Na2CO3; 0.1 mL of supernatant; 0.1 mL of 6 mM disodium EDTA; and 0.3 mL of 4.5 mM epinephrine. Protein concentration of enzyme extract was measured using Bradford method at 595 nm, and the SOD activity was expressed as SOD activity (∆A) min−1mg−1protein.
2.13 Statistical Analysis
The data presented corresponded to the mean values ± standard deviation (SD). One-way analysis of variance (ANOVA) and Duncan’s multiple range test by using Microsoft Excel XLSTAT (version 2015.2.01.17315) were performed to confirm the variability of the results and the determination of significant (p ≤ 0.05) difference between treatment groups, respectively.
3 Results
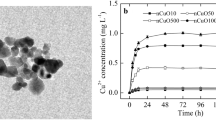
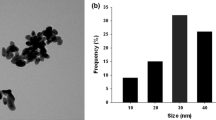
Copper oxide NPs were previously characterised for shape and size using XRD diffraction pattern and SEM imaging. It was found that CuO NPs were spherical in shape and had a size of < 50 nm (Da Costa and Sharma 2016). Dynamic light scattering analysis (DLS) of CuO NPs was later done to investigate the behaviour of CuO NPs in aqueous medium, and it was found that the size of NPs was 37.5 ± 15.0 nm in water.
The amount of ions released from nano and bulk Cu was estimated using inductively coupled plasma mass spectroscopy analysis. Copper oxide NPs released approximately 0.24% Cu ions at 2.5, 10 and 50 mg L−1 and 0.63% Cu ions at 1000 mg L−1 when dissolved in water (Table 1). Bulk copper, on the other hand, showed uniform percent distribution of ions (~ 8%; Table 1). On the whole, bulk Cu released 70-fold more Cu ions compared with CuO NPs at 1000 mg L−1.
The decrease in germination rate caused due to CuO NPs and bulk Cu, was found to be insignificant up to 100 mg L−1 concentration, but a significant decline of 7–8% was seen in germination at 1000 mg L−1 of both bulk Cu and CuO NP, as compared with their respective controls (Table 2). A decline in shoot and root length was observed due to both CuO NPs and bulk Cu stress (Fig. 1, Table 2). A decrease of 31% in shoot length and 23% in root length was observed at highest concentration of NPs while bulk Cu showed an inhibition of 74% in shoot length at the same concentration. Root growth increased slightly at 2.5 mg L−1 concentration of bulk Cu, but was completely inhibited at 1000 mg L−1 concentration. Root biomass of plants treated at the highest CuO NP concentration increased by 9%, shoot biomass decreased by 31% while root biomass was completely inhibited and shoot biomass decreased by 63% at the same concentration (Table 2).
TEM images of rice leaves treated with CuO NPs of 100 mg L−1 and above concentration revealed the accumulation of CuO NPs in the form of clusters inside the leaf especially in chloroplasts and cytosol, whereas, no such accumulation was observed with bulk Cu (Fig. 2C). CuO NP treatment also resulted in the significant destacking of thylakoids per granum at concentrations higher than 50 mg L−1. It was observed that thylakoid stacking was largely reduced to two vesicles. NP treatment also resulted in slight distortion (wavy arrangement) of the thylakoid membranes and swelling of intra-thylakoidal space (Fig. 2D–E), not seen in case of bulk Cu. Bulk Cu treatment resulted in greater stacking of grana region of chloroplasts. A slight increase in the number of mitochondria was also observed with some degradation of the inner matrix from the centre of the mitochondria extending to the peripheral region (Fig. 2I).
TEM images of leaf showing control leaf (A); accumulation of CuO NPs (A); no accumulation of bulk Cu (C); reduction in chloroplast thylakoid stacking (A–C) and changes in mitochondria structure (D-F) of rice plants treated with bulk Cu and CuO NPs after 30-day growth period. Data for CuO NP used in this study was taken from Da Costa and Sharma (2016)
Scanning electron microscopic images showed that at 1000 mg L−1 concentration, both CuO NPs and bulk Cu resulted in increased number and size of trichomes (Fig. 3A, B, C) and decrease in the size of stomata (Fig. 3D, E, F). Greater external damage to the root was observed in case of CuO NPs as compared with bulk Cu (Fig. 3I). A decrease in the epidermal wax layer, cuticular papillae, was observed in leaves treated with CuO NPs and bulk Cu (Fig. 3D, E, F).
Scanning electron microscope images of rice leaf (A–C), stomata (D–F) and root (G–I) upon treatment with CuO NP and bulk Cu at 1000 mg L−1 upon 30-day growth period. Data for CuO NP used in this study was taken from Da Costa and Sharma (2016)
Accumulation of Cu in roots was many-fold greater than that in leaves as a result of the treatment with both CuO NPs and bulk Cu (Table 3). Plants exposed to 100 mg L−1 CuO NPs and bulk Cu showed a 76-fold and 137-fold increase in the Cu accumulation in their root respectively in comparison to their control; furthermore, 1000 mg L−1 CuO NPs resulted in only about 9.4-fold increase of Cu in roots, while bulk Cu completely inhibited root growth. CuO NP–treated plants showed a linear increase in Cu accumulation in leaves. Treatment with 50 mg L−1 of NP resulted in 1.9-fold increase in Cu accumulation in leaves which increased to 5.5-fold at 1000 mg L−1. In comparison, plants treated with bulk Cu did not show linear accumulation of Cu in the leaves. Treatment with 2.5 mg L−1 of bulk Cu resulted in 3.2-fold accumulation of Cu which was further increased to 4.6-fold at 50 mg L−1 treatment as compared with its control. Further increase in the concentrations of bulk Cu, at 100 and 1000 mg L−1, did not show any increase in the Cu content in the leaves (Table 3).
The quantum efficiency of photosystem II, measured as Fv/Fm ratio upon treatment with CuO NPs, remained unaffected up to 100 mg L−1 but decreased by 46% at the highest concentration (1000 mg L−1) as compared with its control (Fig. 4A). Bulk Cu, however, showed a gradual decrease of 59% in the Fv/Fm ratio at 1000 mg L−1 (Fig. 4A). Photochemical quenching (qP) showed no effect up to 10 mg L−1 concentration of CuO NPs treatment, but decreased by 20% at 100 mg L−1 and was completely inhibited at 1000 mg L−1 (Fig. 4B). Bulk Cu treatment, however, led to a gradual decrease of 86% in qP at 1000 mg L−1 (Fig. 4B). Non-photochemical quenching (qN), indicative of photochemical energy that is dissipated in the form of heat, increased and was observed to be more or less same (10–15%) for both NPs and bulk Cu at the highest concentration (Fig. 4C).
The maximum quantum efficiency of PSII (Fv/Fm) (A), photochemical quenching (qP) (B), non-photochemical quenching (qNP) (C), photosynthesis rate (PN) (D), transpiration rate (E) (E) and stomatal conductance (gs) (F) of rice plants treated with bulk Cu and CuO NPs after 30-day growth period. The error bars represent mean values ± S.D. (n = 5). Data in the column followed by the same letter do not differ significantly at p ≤ 0.05. Data for CuO NP used in this study was taken from Da Costa and Sharma (2016)
Net CO2 assimilation rate (PN), stomatal conductance (gs) and transpiration rate (E) declined linearly in plants treated with both CuO NP or bulk Cu; however, extent of decrease was slightly less in plants treated with NPs as compared with bulk particles (Fig. 4D–F). Plants treated with 1000 mg L−1 bulk Cu showed stunted growth, making it difficult for gas exchange measurements. CuO NP treatment resulted in a linear decrease in PN to 68% and 86% at 100 and 1000 mg L−1 in comparison to 86% decrease in plants treated with Cu bulk particle at 100 mg L−1 concentration. Likewise, gs declined to 72% and 90% as a result of 100 and 1000 mg L−1 CuO NP treatment respectively, as compared with 80% decrease in gs at 100 mg L−1due to bulk copper. Transpiration rate also decreased to 60% and 83% due to 100 and 1000 mg L−1 of CuO NP treatment and 79% due to 100 mg L−1of bulk copper.
Pigments, chlorophyll and carotenoids, showed an overall decrease due to both the treatments (Table 4). Total chlorophyll (chlorophyll a and b) declined to 86% at 1000 mg L−1of CuO NP and 73% at 100 mg L−1 bulk Cu, in comparison to controls. Carotenoids (lutein, β-carotene, violaxanthin) declined to 30%, 10% and 42% at 1000 mg L−1 CuO NP concentration and 44%, 14% and 29% due to bulk Cu at 100 mg L−1concentration.
The OH· content of rice leaves treated with bulk Cu increased up to 43% at 1000 mg L−1 compared with control; however, no significant change was observed at all concentrations upon treatment with CuO NPs (Fig. 5A). Lipid peroxidation of membrane lipids (MDA–TBA adduct formation) indicative of oxidative damage was greatly observed on treatment with bulk Cu, whereas, CuO NP showed no significant lipid peroxidation (Fig. 5B). Bulk Cu at highest concentration resulted in an increase of 27% in MDA content. In comparison, CuO NP–treated plants showed no significant change in MDA content at all concentration (Fig. 5B). Protein carbonyl formation, an indicator of oxidative damage to proteins, also showed an increase of 42% in the protein oxidation level at the highest concentration of the bulk Cu treatment, but again, no such oxidation of proteins was confirmed with CuO NP–treated plants at all concentrations as compared with its control (Fig. 5C). Results also showed a much greater increase in the antioxidant enzymes SOD activity in plants treated with bulk Cu than with CuO NP (Fig. 5D). Gene expression studies of SOD and APX also showed similar results (data not shown). Also, ascorbic acid (non-enzymatic antioxidant) content showed much greater accumulation in plants treated with bulk Cu than seen in plants treated with CuO NPs (Fig. 5E). Proline, an osmolyte and osmo-protectant, increased due to CuO NPs as well as bulk treatment under hydroponic conditions (Fig. 5F). A 2.5-fold increase in the proline content was seen as a result of the 1000 mg L−1 concentration of CuO NPs as compared with 3.4-fold increase in the proline due to the bulk Cu at the same concentration (Fig. 5F).
Hydroxyl ion content (A), malondialdehyde (B), protein carbonyl content (C), superoxide dismutase activity (D), ascorbate (E) and proline (F) content and of rice plants treated with CuO NPs and bulk Cu after 30 days. The error bars represent mean values ± S.D. (n = 5). Data in the column followed by the same letter do not differ significantly at p ≤ 0.05. Data for CuO NP in B, C, E and F was taken from Da Costa and Sharma (2016)
4 Discussion
This study was carried out to investigate the comparative effect of nano and bulk Cu on growth, photosynthesis, its accumulation and antioxidant response. Results demonstrated that seed germination was only slightly affected as a result of both the treatments (CuO NPs and bulk Cu; Table 2). This may be due to the selective permeability by the seed coat largely restricting the entry of Cu, thereby protecting the embryo. Wierzbicka and Obidzińska (1998) have reported about selective permeability of seed coat in general, thereby, exhibiting protection to embryo. Seed germination is reported to be influenced by copper in a dose- and time-dependent manner in mung bean (Vigna radiata L.), wheat, rice, haricot bean (Phaseolus vulgaris L.), soybean (Glycine max L.) and chickpea (Cicer arietinum L.) reviewed by Adrees et al. (2015). Similarly, Peralta et al. (2001) and Lou et al. (2004) observed a reduction in germination upon CuO NP stress in a dose-dependent manner in Alfalfa (Medicago sativa L.) and in Elsholtzia haichowensis respectively. Similar results on germination were reported by Lin and Xing (2007) in radish, rape, lettuce and cucumber with nanoparticles such as multi-walled carbon nanotube (MWCNT), Al and Al2O3 at 2000 mg L−1respectively and by Boonyanitipong et al. (2011) in rice with TiO2 NPs and ZnO NPs. Munzuroglu and Geckil (2002) also reported that metals such as Cd, Co, Cu, Pb and Zn had no effect on germination of Triticum aestivum and Cucumis sativus. Excess Cu is also reported to inhibit seed germination by downregulating the activity of alpha amylase and enolase (ENO) enzymes, the key enzymes of seed energy metabolism (Zhang et al. 2009), not reported in the case of CuO NPs.
The observed decrease in shoot length, root length and fresh biomass of root and shoot at higher concentration of both CuO NPs and bulk Cu in our study (Table 2), could be primarily due to greater accumulation of Cu in both root and shoot tissue in plants treated with bulk Cu than seen in plants treated with NPs (Table 3). The greater accumulation of Cu, in case of bulk Cu–treated plants, resulted in the production of higher Cu ions (Table 1) which not only caused oxidative damage to cell (Fig. 5A, B, C) but also may have also interfered with the ionic balance of cell membrane across the tissue. Increased level of electrolyte leakage as a result of CuO NPs in Cucumis sativus (Mosa et al. 2018) and bulk Cu in Phaseolus vulgaris (Younis et al. 2018) has been reported. Others have suggested that direct contact of Cu ions with young radicles could inhibit their growth (Lin and Xing 2008). Also, binding and accumulation of Cu has been suggested to cause cell wall stiffening and rupture of tissue (Kopittke et al. 2008) resulting in compromising root growth. Inhibitions of root length and biomass due to CuO NPs in the range of 10–100 mg L−1 have been reported (Wang et al. 2012; Musante and White 2012). These reports suggest that the decrease in biomass was mainly due to the limited absorption of nutrients and water by the roots as a result of particle exposure. In another study, Yang and Watts (2005) reported that alumina NP at 2000 mg L−1 reduced root length in corn, cucumber, soybean, cabbage and carrot. Zhou et al. (2011) and Schwabe et al. (2013) reported adsorption of NPs (CuO and CeO2 respectively) on the root surface in wheat and pumpkin. Slight increase observed in root biomass (fresh) in plants treated with CuO NP in our study (Table 2) could be the result of such adsorption of the NP rather than increase in biomass.
Our results showed that NP accumulation formed metal clusters inside the chloroplasts which resulted in destacking, forming single vesicles and distortion (wavy) of thylakoid membranes (Fig. 2). On the other hand, no such destacking and distortion in thylakoid arrangement was observed with the bulk Cu treatment (Fig. 2D–F). Also, metal accumulation reported was higher in bulk Cu–treated plants than in NP-treated plants (Table 3) and bulk Cu treatment showed structural damage to mitochondria (Fig. 2G–I). Our DLS data suggest that most of the CuO NPs remained in non-ionic form while bulk Cu resulted in much greater Cu ions. This suggests that CuO NPs behave as an entity while bulk Cu behave as Cu ions. Buzea et al. (2007) also reported such behaviour of Cu in bulk and as a NP. The destacking of thylakoids observed in our study with CuO NP was not followed with MDA or protein carbonyl ion formation (oxidative damage to lipid and proteins) (Fig. 5C) nor ROS generation (Fig. 5A), whereas oxidative damage due to ROS was observed with bulk Cu (Fig. 5A), which, however, did not show any destacking in thylakoid; rather, a greater stacking of thylakoids was observed (Fig. 2D–F), but some structural damage to mitochondria was seen (Fig. 2I). Also, activity of antioxidants like APX and ascorbate (Fig. 5E) was much greater in plants treated with bulk Cu than CuO NP again indicating oxidative stress response with bulk Cu. The oxidative damage observed with bulk Cu was not seen with CuO NPs as NPs remained largely in the particulate form and did not change to its ionic form. If metal ions are redox active like Cu and Fe, then ROS generated is directly involved in oxidation of biomolecules through metal protein attenuating compounds, whereas, metallic nanoparticle may lead to different biological functions depending on particle size, shape, surface area and chemistry (Uversky and Fink 2007). Our results indicate that the negative effect on growth and development observed seems to be due to structural changes taking place in thylakoids due to the accumulation of the CuO NPs, whereas the main cause of damage to the physiological processes leading to decrease growth and productivity seems to be due to oxidative damage as a result of generation of Cu2+ ions. Our study clearly shows that CuO NPs did not result in significant production of its ionic form (Table 1), and therefore, no oxidative damage to biological molecules was observed (Fig. 5A, B, C). Further studies need to be carried out to look into this aspect.
Reduced number (Fig. 3A–C) and size of stomata (Fig. 3D–F), and increase in the leaf trichomes (Fig. 3A–C) as well as external morphological changes to the roots and shoots were observed largely in plants treated with CuO NPs, followed by bulk Cu, indicating an adaptation process of the plant to the treatments. Similar symptoms have also been observed during osmotic stress. This may be due to the physical adsorption of NPs to the root surface decreasing the trans-root potential which is essential for water and ion uptake (Kennedy and Gonsalves 1987). Very high accumulation of Cu in the roots of plants may also lead to physiological drought, thus the symptoms. The accumulation of proline, an osmolyte and a compatible solute, observed in present study (Fig. 5F) has been reported to accumulate in response to osmotic stress, preventing membrane distortion (Alia and Saradhi 1991), decreasing damage to enzymes and biopolymers at cellular level and mitigating metal-induced lipid peroxidation (Mehta and Gaur 1999).
Reduction in the root morphology (total root length, mean root diameter, root surface area, and root volume) due to Cu stress was reported to be a cost-effective plant adaptation process limiting the uptake of metals (Elisa et al. 2007). The absence of root hairs due to reduction in root length may also cause osmotic stress (Yruela 2009). Ouzounidou et al. (1995) reported that Cu caused extensive damage to root epidermal cells affecting root elongation. Thounaojam et al. (2012) showed that rice plant when treated with different Cu concentrations showed maximum inhibition to root growth. These adaptations in root are mainly to overcome excess uptake of Cu (nano and bulk) leading to osmotic stress.
Though the Cu content was much higher in the roots than in shoots on treatment with CuO NPs or bulk Cu, only a small fraction of Cu was transported from the roots to the shoots, leading to major toxicity in the roots (Table 3). The observed increase of Cu in roots may be due to as a defense mechanism, which includes sub-cellular compartmentalisation and sequestration by organic compounds to withstand toxicity, wherein plants store metals in the root to avoid its transport to the physiologically more active part, shoot (Hall 2002; Wang et al. 2004). High amounts of these low molecular weight organic acids (LMWOAs) such as citrate and malate are known to complex Cu and sequestrate it in the vacuoles (Dresler et al. 2014; Chaffai et al. 2006). LMWOAs in a concentration-dependent manner in response to Cu treatment is also reported in the shoot vacuoles of Oryza sativa (Lidon and Henriques (1998), P. radiates and T. aestivum (Lee et al. 2008) and in yellow horned poppy, Glaucium (Cambrollé et al. 2011), facilitating sequestration of Cu to protect shoot tissue. Presence of non-protein thiols (NPTs) and sulphur ligands in the root cells may also complex with Cu inside the root cells (Drążkiewicz et al. 2004; Shahbaz et al. 2010). This was also observed in our study showing that high Cu concentration resulted in a 3-fold increase of NPT levels in shoots (data not shown). Mucilage containing negatively charged pectin components secreted by the root tips and hairs may also have caused the electrostatic binding of NPs and bulk Cu by physical adsorption, adding to the metal uptake (Zhang et al. 2011; Lombardi et al. 2002). However, the same may work as a mechanism to prevent uptake of Cu from medium to root. Andrés-Colás et al. (2006, 2010) showed that increased accumulation of bulk Cu in roots may also be due to increase in Cu transporters such as COPT 1, COPT3 and Ptype ATPase. The counter flow of Cu from the shoot to the root tissue may also have contributed for the higher amount of Cu in the root tissue (Liao et al. 2000; Ducic and Polle 2005). Ando et al. (2013) showed the presence of nicotianamine and histidine in the phloem which is a Cu complexing compound indicating the counter flow of Cu.
The maximum quantum efficiency of PSII denoted as Fv/Fm ratio and photochemical quenching (qP) decreased with both CuO NP and bulk Cu, while the non-photochemical quenching (qN) increased to almost the same extent with both the treatments at 1000 mg L−1 (Fig. 4A, B, C). We also observed a decline in net photosynthetic rate (PN), transpiration rate (E) and stomatal conductance (gs) with both CuO NPs and bulk Cu (Fig. 4D, E, F); however, the extent of decrease in these parameters was greater in plants treated with bulk Cu. The decrease in both light and dark reaction of photosynthesis observed may partly be due to the decline in the photosynthetic pigments specially Chl a (Table 4) and the increase in the Chl a/b ratio, resulting in lowered photosynthetic efficiency affecting plant growth. Both bulk and NP treatment resulted in overall pigment decline (Table 4). The observed decline in the photosynthetic pigments in plants treated with CuO NP may be due to reduction in thylakoid stacking and the distortion of the thylakoid membranes (Fig. 2D–F) as the PSII–LHCII pigment complexes important for light harvesting are present on thylakoid membranes. Vinit-Dunand et al. (2002) reported that Cu affected isolated chloroplast in vitro causing damage to photosynthetic membrane, altering the lipid–protein interaction in the chloroplast membrane disturbing the light reactions associated with PSII, whereas the decline in photosynthetic pigments in bulk Cu–treated plants may be due to the oxidative damage to pigments, thus both the treatments leading to decreased Fv/Fm ratio and photochemical quenching (qP).
The decline observed in the photosynthetic rate (PN) measured as the carbon dioxide assimilation rate was associated with a parallel decrease in the transpiration rate (E) and stomatal conductance (gs) at the highest concentration of CuO NPs and bulk Cu. This may be due to the inhibition of enzymatic reactions in the photosynthetic carbon reduction cycle (Vinit-Dunand et al. 2002) mainly rubisco activity on high copper dosage (Lidon and Henriques 1991). Decrease in transpiration and stomatal conductance may be attributed to copper-induced stomatal closure via reduction in the guard cell turgor, ascorbate redox state control, lower water potential in roots and transport of abscisic acid (ABA) from root to shoot occurring during metal stress (Moradi and Ismail 2007).
Increased SOD activity with bulk Cu treatment with insignificant activity in case of CuO NPs in this study also substantiates our data with direct measurement of ROS (Fig. 5A) and suggests that bulk copper caused ROS generation and to mitigate their oxidative effect, plants produced antioxidants such as ASA and SOD, whereas, CuO NP did not show higher antioxidant activities as there was insignificant production of ROS. There are several reports of bulk particle resulting in increased antioxidant activities (Peng et al. 2006; Panda and Khan 2004; Gao et al. 2008) but only a few reporting oxidative damage to plants under metallo-nanoparticle stress. Rico et al., 2013 showed that 250 mg L−1 of CeO2 NPs resulted in increased SOD activity in rice, but higher concentrations showed insignificant change in enzyme activity. Mosa et al. (2018) also showed that CuNPs (10–30 nm size) resulted in increased SOD gene expression at concentrations higher than 100 mg L−1 in cucumber (Cucumis sativus) plants; however, NP treatment was given only for 4-day to 6-week-old plants.
5 Conclusion
This study highlights two different mechanisms of adverse effects of CuO NPs and bulk Cu on plant growth in terms of photosynthetic efficiency, carbon dioxide fixation and pigment loss as well as oxidative and osmotic stress in Oryza sativa var. Jyoti resulting in loss of productivity. It was observed that bulk Cu affected growth and photosynthesis by inducing oxidative stress as a result of generation of Cu2+ ions, whereas, CuO NPs resulted in damage to productivity largely due to structural damage to thylakoid membranes due to NP itself, without causing oxidative damage as insignificant amount of Cu2+ ion formation was observed due to the treatment of CuO NPs.
References
Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F., Farid, M., et al. (2015). The effect of excess copper on growth and physiology of important food crops: a review. Environmental Science and Pollution Research, 22(11), 8148–8162.
Alia, & Saradhi, P. P. (1991). Proline accumulation under heavy metal stress. Journal of Plant Physiology, 138(5), 554–558.
Ando, Y., Nagata, S., Yanagisawa, S., & Yoneyama, T. (2013). Copper in xylem and phloem saps from rice (Oryza sativa): the effect of moderate copper concentrations in the growth medium on the accumulation of five essential metals and a speciation analysis of copper-containing compounds. Functional Plant Biology, 40(1), 89–100.
Andrés-Colás, N., Sancenón, V., Rodríguez-Navarro, S., Mayo, S., Thiele, D. J., Ecker, J. R., et al. (2006). The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. The Plant Journal, 45(2), 225–236.
Andrés-Colás, N., Perea-García, A., Puig, S., & Penarrubia, L. (2010). Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiology, 153(1), 170–184.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Boonyanitipong, P., Kositsup, B., Kumar, P., Baruah, S., & Dutta, J. (2011). Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. International Journal of Bioscience, Biochemistry and Bioinformatics, 1(4), 282.
Boveris, A. (1984). Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods in Enzymology, 105, 429–435.
Buzea, C., Pacheco, I. I., & Robbie, K. (2007). Nanomaterials and nanoparticles: sources and toxicity. Biointerphases, 2, MR17–MR71.
Cambrollé, J., Mateos-Naranjo, E., Redondo-Gómez, S., Luque, T., & Figueroa, M. E. (2011). Growth, reproductive and photosynthetic responses to copper in the yellow-horned poppy, Glaucium flavum Crantz. Environmental and Experimental Botany, 71(1), 57–64.
Chaffai, R., Tekitek, A., & El Ferjani, E. (2006). A comparative study on the organic acid content and exudation in maize (Zea mays L.) seedlings under conditions of copper and cadmium stress. Asian Journal of Plant Sciences.
Da Costa, M. V. J., Sharma, P. K. (2016). Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica, 54(1), 110–119.
Drążkiewicz, M., Skórzyńska-Polit, E., & Krupa, Z. (2004). Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals, 17(4), 379–387.
Dresler, S., Hanaka, A., Bednarek, W., & Maksymiec, W. (2014). Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiologiae Plantarum, 36(6), 1565–1575.
Ducic, T., & Polle, A. (2005). Transport and detoxification of manganese and copper in plants. Brazilian Journal of Plant Physiology, 17(1), 103–112.
Edding, M., & Tala, F. (1996). Copper transfer and influence on a marine food chain. Bulletin of Environmental Contamination and Toxicology, 57(4), 617–624.
Elisa, B., Marsano, F., Cavaletto, M., & Berta, G. (2007). Copper stress in Cannabis sativa roots: morphological and proteomic analysis. Caryologia, 60(1–2), 96–101.
Emamverdian, A., Ding, Y., Mokhberdoran, F., & Xie, Y. (2015). Heavy metal stress and some mechanisms of plant defense response. The Scientific World Journal, 2015.
Franklin, N. M., Rogers, N. J., Apte, S. C., Batley, G. E., Gadd, G. E., & Casey, P. S. (2007). Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environmental Science & Technology, 41(24), 8484–8490.
Gao, S., Yan, R., Cao, M., Yang, W., Wang, S., & Chen, F. (2008). Effects of copper on growth, antioxidant enzymes and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedling. Plant Soil Environment, 54(3), 117–122.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53(366), 1–11.
Kampfenkel, K., Vanmontagu, M., & Inze, D. (1995). Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry, 225(1), 165–167.
Kennedy, C. D., & Gonsalves, F. A. N. (1987). The action of divalent zinc, cadmium, mercury, copper and lead on the trans-root potential and H+, efflux of excised roots. Journal of Experimental Botany, 38(5), 800–817.
Klaine, S. J., Alvarez, P. J., Batley, G. E., Fernandes, T. F., Handy, R. D., Lyon, D. Y., et al. (2008). Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry, 27(9), 1825–1851.
Kopittke, P. M., Blamey, F. P. C., & Menzies, N. W. (2008). Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant and Soil, 303(1–2), 217–227.
Lee, W. M., An, Y. J., Yoon, H., & Kweon, H. S. (2008). Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environmental Toxicology and Chemistry: An International Journal, 27(9), 1915–1921.
Liao, M. T., Hedley, M. J., Woolley, D. J., Brooks, R. R., & Nichols, M. A. (2000). Copper uptake and translocation in chicory (Cichorium intybus L. cv. Grasslands Puna) and tomato (Lycopersicon esculentum Mill. cv. Rondy) plants grown in NFT system. I. Copper uptake and distribution in plants. Plant and Soil, 221(2), 135–142.
Lidon, F. C., & Henriques, F. S. (1991). Limiting step on photosynthesis of rice plants treated with varying copper levels. Journal of Plant Physiology, 138(1), 115–118.
Lidon, F. C., & Henriques, F. S. (1998). Role of rice shoot vacuoles in copper toxicity regulation. Environmental and Experimental Botany, 39(3), 197–202.
Lin, D., Xing, B. (2007). Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environmental Pollution, 150(2), 243–250
Lin, D., & Xing, B. (2008). Root uptake and phytotoxicity of ZnO nanoparticles. Environmental Science & Technology, 42(15), 5580–5585.
Liu, D., Jiang, W., Meng, Q., Zou, J., Gu, J., & Zeng, M. (2009). Cytogenetical and ultrastructural effects of copper on root meristem cells of Allium sativum L. Biocell, 33(1), 25–32.
Lombardi, A. T., Vieira, A. A., & Sartori, L. A. (2002). Mucilaginous capsule adsorption and intracellular uptake of copper by Kirchneriella aperta (Chlorococcales). Journal of Phycology, 38(2), 332–337.
Lou, L. Q., Shen, Z. G., & Li, X. D. (2004). The copper tolerance mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environmental and Experimental Botany, 51(2), 111–120.
Manceau, A., Nagy, K. L., Marcus, M. A., Lanson, M., Geoffroy, N., Jacquet, T., & Kirpichtchikova, T. (2008). Formation of metallic copper nanoparticles at the soil− root interface. Environmental Science & Technology, 42(5), 1766–1772.
Maynard, A. D. (2006). A research strategy for addressing risk. Nanotechnology, Woodrow Wilson International Center for Scholars, 444, 267–269.
Mehta, S. K., & Gaur, J. P. (1999). Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. The New Phytologist, 143(2), 253–259.
Moradi, F., & Ismail, A. M. (2007). Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany, 99(6), 1161–1173.
Mosa, K. A., El-Naggar, M., Ramamoorthy, K., Alawadhi, H., Elnaggar, A., Wartanian, S., et al. (2018). Copper nanoparticles induced genotoxicty, oxidative stress, and changes in superoxide dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Frontiers in plant science, 9.
Munzuroglu, O., & Geckil, H. (2002). Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Archives of Environmental Contamination and Toxicology, 43(2), 203–213.
Musante, C., & White, J. C. (2012). Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environmental Toxicology, 27(9), 510–517.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters, 8(3), 199–216.
Navarro, E., Piccapietra, F., Wagner, B., Marconi, F., Kaegi, R., Odzak, N., et al. (2008). Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environmental Science & Technology, 42(23), 8959–8964.
Ouzounidou, G., Čiamporová, M., Moustakas, M., & Karataglis, S. (1995). Responses of maize (Zea mays L.) plants to copper stress—I. Growth, mineral content and ultrastructure of roots. Environmental and Experimental Botany, 35(2), 167–176.
Panda, S. K., & Khan, M. H. (2004). Changes in growth and superoxide dismutase activity in Hydrilla verticillata L. under abiotic stress. Brazilian Journal of Plant Physiology, 16(2), 115–118.
Peng, H. Y., Yang, X. E., Yang, M. J., & Tian, S. K. (2006). Responses of antioxidant enzyme system to copper toxicity and copper detoxification in the leaves of Elsholtzia splendens. Journal of Plant Nutrition, 29(9), 1619–1635.
Peralta, J. R., Gardea-Torresdey, J. L., Tiemann, K. J., Gomez, E., Arteaga, S., Rascon, E., & Parsons, J. G. (2001). Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bulletin of Environmental Contamination and Toxicology, 66(6), 727–734.
Peters, D. T., Brush, E. F., Kirtley, J. L. (2007). Die-cast copper rotors as strategy for improving induction motor efficiency. In 2007 Electrical Insulation Conference and Electrical Manufacturing Expo, 322-327, IEEE.
Pops, H. (2008). Processing of wire from antiquity to the future. Wire Journal International, 41(6), 58–66.
Ren, G., Hu, D., Cheng, E. W., Vargas-Reus, M. A., Reip, P., & Allaker, R. P. (2009). Characterisation of copper oxide nanoparticles for antimicrobial applications. International Journal of Antimicrobial Agents, 33(6), 587–590.
Reznick, A. Z., & Packer, L. (1994). Oxidative damage to proteins: spectrophotometric method for carbonyl assay. In In Methods in enzymology, 233, 357–363. Academic: Press.
Rico, C. M., Hong, J., Morales, M. I., Zhao, L., Barrios, A. C., Zhang, J. Y., Peralta-Videa, J. R., Gardea-Torresdey, J. L. (2013). Effect of Cerium Oxide Nanoparticles on Rice: A Study Involving the Antioxidant Defense System and In Vivo Fluorescence Imaging. Environmental Science & Technology, 47(11), 5635–5642
Rigo, C., Ferroni, L., Tocco, I., Roman, M., Munivrana, I., Gardin, C., et al. (2013). Active silver nanoparticles for wound healing. International Journal of Molecular Sciences, 14(3), 4817–4840.
Schwabe, F., Schulin, R., Limbach, L. K., Stark, W., Bürge, D., & Nowack, B. (2013). Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere, 91(4), 512–520.
Shahbaz, M., Tseng, M. H., Stuiver, C. E. E., Koralewska, A., Posthumus, F. S., Venema, J. H., et al. (2010). Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. Journal of Plant Physiology, 167(6), 438–446.
Sharma, P. K., & Hall, D. O. (1996). Effect of photoinhibition and temperature on carotenoids in sorghum leaves. Indian Journal of Biochemistry & Biophysics, 33(6), 471–477.
Stampoulis, D., Sinha, S. K., & White, J. C. (2009). Assay-dependent phytotoxicity of nanoparticles to plants. Environmental Science & Technology, 43(24), 9473–9479.
Thounaojam, T. C., Panda, P., Mazumdar, P., Kumar, D., Sharma, G. D., Sahoo, L., & Sanjib, P. (2012). Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiology and Biochemistry, 53, 33–39.
Uversky, V. N., & Fink, A. (Eds.). (2007). Protein misfolding, aggregation and conformational diseases: Part B: Molecular mechanisms of conformational diseases (Vol. 6). Springer Science & Business Media.
Vinit-Dunand, F., Epron, D., Alaoui-Sossé, B., & Badot, P. M. (2002). Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Science, 163(1), 53–58.
Walczak, A. P., Fokkink, R., Peters, R., Tromp, P., Herrera, R., Z. E., Rietjens, I, M. C. M., Hendriksen P. J. M., Bouwmeester H. (2012). Behaviour of silver nanoparticles and silver ions in an human gastrointestinal digestion model. Nanotoxicology, 7(7), 1198–1210
Wang, S. H., Yang, Z. M., Yang, H., Lu, B., Li, S. Q., & Lu, Y. P. (2004). Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Botanical Bulletin of Academia Sinica, 45, 203–212.
Wang, Z., Xie, X., Zhao, J., Liu, X., Feng, W., White, J. C., & Xing, B. (2012). Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environmental Science & Technology, 46(8), 4434–4441.
Wells, A. F. (2012). Structural inorganic chemistry. 5th ed., Clarendon Press, Oxford, 1984, p. 1288 (metallic radii for 12-coordination) (ed.) Oxford university press, 2012.
Wierzbicka, M., & Obidzińska, J. (1998). The effect of lead on seed imbibition and germination in different plant species. Plant Science, 137, 155–171.
Wu, S. G., Huang, L., Head, J., Chen, D. R., Kong, I. C., & Tang, Y. J. (2012). Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. Journal of Petroleum and Environmental Biotechnology, 3(4), 126.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 402647.
Xiao, L., Takada, H., Maeda, K., Haramoto, M., & Miwa, N. (2005). Antioxidant effects of water-soluble fullerene derivatives against ultraviolet ray or peroxylipid through their action of scavenging the reactive oxygen species in human skin keratinocytes. Biomedicine & Pharmacotherapy, 59(7), 351–358.
Yang, L., & Watts, D. J. (2005). Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicology Letters, 158(2), 122–132.
You, J., Zhang, Y., & Hu, Z. (2011). Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Colloids and Surfaces B: Biointerfaces, 85(2), 161–167.
Younis, M. E., Tourky, S. M. N., & Elsharkawy, S. E. A. (2018). Symptomatic parameters of oxidative stress and antioxidant defense system in Phaseolus vulgaris L. in response to copper or cadmium stress. South African Journal of Botany, 117, 207–214.
Yruela, I. (2005). Copper in plants. Brazilian Journal of Plant Physiology, 17(1), 145–156.
Yruela, I. (2009). Copper in plants: acquisition, transport and interactions. Functional Plant Biology, 36(5), 409–430.
Zhang, H., Lian, C., & Shen, Z. (2009). Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Annals of Botany, 103(6), 923–930.
Zhang, Z., He, X., Zhang, H., Ma, Y., Zhang, P., Ding, Y., & Zhao, Y. (2011). Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics, 3(8), 816–822.
Zhou, D., Jin, S., Li, L., Wang, Y., & Weng, N. (2011). Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. Journal of Environmental Sciences, 23(11), 1852–1857.
Acknowledgements
Maulana Azad National Fellowship (2013–2016) to MVJDC is acknowledged. Sincere thanks to National Institute of Oceanography (NIO), Goa for ICP–OES, SEM and AAS measurements; Physics Department, Goa University for XRD size determination; All India Institute of Medical sciences (AIIMS), New Delhi for TEM imaging.
Contributions
Author MVJ Da Costa (PhD student) has contributed towards CuO NP–related data collection, analysis, interpretation and drafting the manuscript, N Kevat (PhD student) has contributed towards bulk Cu–related data collection and analysis and Prof. PK Sharma (supervisor) has contributed towards conception of the work, critical revision of the manuscript and final approval of the version to be published.
Funding
This work was funded by the Department of Science & Technology (DST), New Delhi (grant number SR/SO/PS–63/2009) and University grants commission–UGC–SAP (Grant number F. 3–50/2009 (SAP–II).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Da Costa, M.V.J., Kevat, N. & Sharma, P.K. Copper Oxide Nanoparticle and Copper (II) Ion Exposure in Oryza sativa Reveals Two Different Mechanisms of Toxicity. Water Air Soil Pollut 231, 258 (2020). https://doi.org/10.1007/s11270-020-04592-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04592-0