Abstract
Sewage effluent has been identified as a potential source of metal(loid) contamination in the aquatic environment. The Sydney rock oyster, Saccostrea glomerata, can accumulate most metals and is well established as a biomonitor of metals in the marine environment. To determine if Burwood Beach wastewater treatment works (WWTW) is a source of metal(loid) contamination, S. glomerata was deployed for 6 weeks in effluent receiving waters (Burwood Beach near and Burwood Beach far) and at reference locations (Redhead, Fingal Island 1 and Fingal Island 2) at depths 4, 8 and 12 m. In dried oyster tissue, inductively coupled plasma mass spectrometry (ICPMS) was employed to measure concentrations of a suite of metal(loid)s including aluminium, arsenic, cadmium, copper, lead, iron, manganese, mercury, nickel, selenium, silver and zinc. It was found that for all metal(loid)s, S. glomerata tissue concentrations were not significantly higher at Burwood Beach locations in comparison to all reference locations. Concentrations of metal(loid)s were similar to those which have been detected in previous studies of background locations in New South Wales (NSW). Further, all metals fell below National Food Authority maximum residue levels (MRLs), except for arsenic and this does not appear uncommon for concentrations in biota within NSW. Comparisons to historical data suggested that concentrations of metal(loid)s in sewage effluent from Burwood Beach WWTW, assessed via concentrations in oyster tissue, are similar or lower, suggesting that changes in treatment processes initiated in the intervening time have lowered metallic inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metals and metalloids (synonymous with trace metal(loid)s: including both essential and non-essential metal(loid)s within the current context) enter the sewage treatment process via domestic and industrial sources. Incomplete removal during sewage treatment can result in entry into the aquatic environment via sewage effluent release. Following release into the aquatic environment, metal(loid)s in bioavailable forms may be accumulated by aquatic organisms (Naimo 1995). Uptake of metals by aquatic wildlife is of concern, due to potential toxicity effects. Although toxicity varies depending on the exposed species, metal type and concentration, some demonstrated effects include reproductive and growth impairments, and in some cases, mortality (Keller and Zam 1991; Naimo 1995; Norris and Carr 2006). Also of high concern is their potential environmental persistence, capacity to bio-accumulate in organisms and possible subsequent biomagnification through trophic levels for select metal(loids)s such as Cs, Se and Zn (Mathews and Fisher 2008). Thus, routine monitoring of metals in the aquatic environment is required.
Following release into the aquatic environment, there are various pathways for exposure (dermal and ingestion) and subsequent uptake by aquatic suspension feeders, such as oysters (Naimo 1995). Diet, however, has been suggested to be the major source of exposure (Boisson et al. 1998; Wang 2002; Rainbow and Wang 2001). Following uptake, the metal(loid) may be used for essential metabolic processes, excreted, bound to a biomolecule and/or stored in tissues (Rainbow 2002).
Bivalves are considered to be effective biomonitors of many non-essential metals (Phillips and Rainbow 1989; Scanes and Roach 1999), demonstrating the capacity to bioaccumulate with dose dependence at concentrations orders of magnitude higher than ambient environmental concentrations. Oysters are also considered to be effective accumulators of particular essential metals with dose dependence, such as copper and zinc, unlike other bivalves, such as mussels, where some regulation is observed (Phillips and Yim 1981; Phillips and Rainbow 1989; Brown and McPherson 1992; Rainbow 1995; Robinson et al. 2005). Accumulation of many metal(loid)s with dose allows mussels and oysters to effectively discriminate relative location differences in bioavailable environmental metal load and has led to the successful Mussel watch programs internationally (O’Connor 1998), and in Australia, the Oyster watch program (Scanes 1996). Thus, the capacity of oysters to bioaccumulate metal(loid)s, which reflect environmental concentrations, has resulted in their widespread use as biomonitors of bioavailable metal(loid)s in aquatic environments. In Australia, S. glomerata is commonly used for biomonitoring of metals/metalloids in the marine environment (Avery et al. 1996; Scanes 1996; Spooner et al. 2003; Lincoln-Smith and Cooper 2004; Robinson et al. 2005; Andrew-Priestley 2011).
The focus of this study was Burwood Beach wastewater treatment works (WWTW), Newcastle, NSW, Australia. Several studies have been conducted at Burwood Beach WWTW to assess metal loads in oysters (following short-term deployment periods in the receiving waters), resident fish, final treated sewage effluent and sediments. The Hunter Environmental Monitoring Program (Hunter EMP) was conducted between 1992 and 1996 (Ajani et al. 1999; NSW EPA 1996a) to assess metals in deployed oysters and sediments. Oysters, S. glomerata, were deployed in the receiving waters of Boulder Bay WWTW, Burwood Beach WWTW and at four reference locations (Point Stephens, Boat Harbour, Redhead and Terrigal) for 3 months at a time, with subsequent measurements of metals/metalloids (arsenic, cadmium, chromium, cobalt, copper, lead, manganese, mercury, nickel, selenium, silver and zinc). Deployments were repeated eight times during 1992–1994, with three deployments prior to and five following the commissioning of the extended Burwood Beach and Boulder Bay WWTWs. Within sediments, all metals/metalloids at the Burwood Beach location were comparable to background levels. For oysters, selenium at Burwood Beach was the only metalloid which was higher than the Australian and New Zealand Food Authority Maximum Residue Limits (ANZFA MRLs 2011). A possible explanation provided was that natural levels of selenium are higher within this region. For metals/metalloids it was found that there was high variability and there were no clear patterns with metal/metalloids concentrations and locations.
Several changes to Burwood Beach WWTW have occurred during and since the study of NSW EPA (1996b), which may have contributed to an improvement in the removal of metals. In 1992, the plant was upgraded from primary to secondary treatment which consists of physical screening to remove large particulates, biological filtration and activated sludge processing including aeration and settling stages (CH2MHill and Hunter Water Corporation 2007). Secondary treated effluent and waste activated sludge (biosolids) are the by-products of the treatment process and both are discharged into the ocean via separate outfall diffusers. It is well established that secondary treatment processes, in particular activated sludge, are considered more efficient at removing metals from effluent in comparison to primary treatment, through a combination of bacteria facilitated flocculation and settling during the activated sludge treatment stage (Brown et al. 1973; Gearnaey and Sin 2013; Santos and Judd 2010), and in the long term, this may have contributed to a decrease in environmental metallic concentrations. Further in 1994, the point of release of sewage sludge was transferred from the effluent pipe (solids and effluent were previously mixed and discharged together) to a separate diffuser pipe (CH2MHill and Hunter Water Corporation 2007). Indeed, changes in wastewater treatment technologies have historically been evidenced to lower metal loads in sediments and dependent biota in effluent receiving waters. Hornberger et al. (2000), for example, found concentrations of metals in sediments and bivalves adjacent to a WWTW in San Francisco Bay declined following the introduction of advanced treatment technologies such as trickle filters, nitrification processes, increases in retention time and clarifier additions.
Since this time at Burwood, BioAnalysis (2007) performed an assessment of metal(loid)s in sediments from the Burwood Beach outfall. Metal/metalloid concentrations in sediments were also low, and apart from manganese (which ranged from ~ 45 to 75 mg/kg at Burwood Beach), all reference locations had higher concentrations in comparison to the Burwood Beach WWTW sampling location. All metals/metalloids were below the ANZECC trigger guidelines (ISQ-Low) (BioAnalysis 2007).
The main aim of this study was to determine whether metal(loid)s, potentially present in effluent, were bioavailable to aquatic taxa through employing S. glomerata as a biomonitoring organism. This study forms part of a larger biomonitoring program assessing impacts of estrogenic endocrine-disrupting chemicals from effluent release on deployed oysters (Andrew-Priestley et al. 2012). We deployed S. glomerata in the receiving waters of Burwood Beach WWTW (Burwood Beach near and Burwood Beach far) and at three reference locations (Redhead, Fingal Island 1 and 2) for 6 weeks with subsequent measurement of metal(loid)s in tissue. The second aim was to compare present metal concentrations in S. glomerata to results obtained from NSW EPA (1996a), considering WWTW upgrades since the initial study. It was anticipated that metal concentrations in S. glomerata tissue would be similar among Burwood Beach and reference locations and decline with upgrades to treatment processes.
2 Methods
2.1 Oyster Deployment at Burwood Beach WWTW
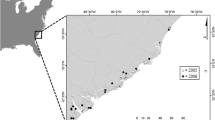
Burwood Beach WWTW releases ~ 44 ML sewage effluent/day as a secondary treatment plant for approximately 180,000 people in the Newcastle region. A deployment unit was deployed at 5 separate locations: 2 at Burwood Beach WWTW effluent receiving waters, in line with the predominant north-eastern current (based on hydrodynamic plume modelling, unpublished data) and 3 at reference locations within the region (Redhead, Fingal Island 1 and Fingal Island 2) (Fig. 1). Sydney rock oysters were deployed within Burwood Beach “near” (< 50 m from outfall) and “far” (100–150 m from outfall) to establish a gradient of effluent exposure with the assumption that oysters deployed at Burwood Beach near would receive higher effluent exposure compared to Burwood Beach far. Deployment units consisted of 2 bags of 40 oysters (1–2 m apart) attached to an anchored rope at 4, 8 and 12 m depth. Deployment units had a 120 cm diameter buoy with a flashing solar light (Sealite SLB1250 and SL60, respectively). Oysters were deployed for a period of 6 weeks, during the Australian summer in Dec 2007–Jan 2008. Oysters were all of similar size across all locations prior to deployment with an average wet tissue weight of 12.49 g ± 2.40 g (SD). Oysters were in the early to mid-stage of gonadal development at deployment and were in mature gonadal stages at harvest.
Map of Newcastle and Port Stephens, NSW, Australia. Deployment units of Sydney rock oysters, Saccostrea glomerata (indicated) were deployed adjacent to Burwood Beach Wastewater treatment works in effluent receiving waters. Location of the deployment units at reference locations, Redhead, Fingal Island 1 and Fingal Island 2 are also indicated. In inset, dotted line represents effluent plume and deployments are indicated in relation to ocean outfall diffuser
2.2 Preparation of Oyster Tissue for Metal Analysis
Eight individuals were selected at random from each bag (two bags/depth). In order to gain sufficient tissue for analyses, two female individuals within the same bag were combined. In total there were 4 replicates (of two pooled females) from each bag (× 2) at 4, 8 and 12 m depths at all locations: Burwood Beach near, Burwood Beach far, Redhead, Fingal Island 1 and Fingal Island 2. Tissue was excised, weighed, placed into the freeze dryer for 24–48 h, ground to a fine powder and dry weight was recorded. Oysters were not depurated post-deployment, thus some fraction of metal load may be sediment associated, but is likely to be low due to deployment in oceanic waters.
2.3 Metal(loid) Analysis in Dried Oyster Tissue
The selection of metal(loid)s was based on the past study performed by NSW EPA (1996a), where S. glomerata were also deployed at Burwood Beach and reference locations to assess metal(loid) bioaccumulation. Metal(loid)s for analysis included arsenic, aluminium, cadmium, chromium, copper, lead, iron, manganese, mercury, nickel, selenium, silver and zinc. Extraction of metal(loid)s was performed using a Hotblock Digestor at 120 °C for 1 h. Each sample consisted of 40 mg of dried oyster tissue digested with 10 mL concentrated nitric acid, which was made to a final volume of 25 mL prior to analysis. Quantification was performed using a Perkin Elmer Elan DRC-e inductively coupled plasma mass spectrometer. The method was verified using Institute for National Measurement (NIST) Certified Lobster Hepatopancreas Reference Material for trace metals (TORT-2). All metals had reasonable recoveries ranging from 108 to 183%, with the exception of chromium which was 316% and subsequently was excluded from the results (Table 1). All concentrations are expressed on a dry weight basis.
2.4 Statistical Analysis
To test for significant differences in metal(loid) concentrations measured in S. glomerata tissue, a two-way analysis of variance (ANOVA) assessing effects of location and depth was not possible due to two missing bag units at 8 m depth at Burwood Beach far and one missing bag unit at Redhead. Differences in metal(loid) concentrations among locations were therefore assessed for each depth separately using a nested ANOVA design, with bags treated as a random factor nested within location as a fixed factor (n = 2 bags per location/depth) (Underwood 1997). Where significant differences were detected, Student-Newman-Keuls (SNK) tests were performed as a post hoc comparison of means. All ANOVAs and SNK tests were performed using GMAV5 (Underwood and Chapman 1984).
3 Results
3.1 Metal(loid) Concentrations Among Locations and Depths
Metal(loid) concentrations were compared via nested ANOVAs, for each depth of deployment, to determine if metal(loid)s were significantly elevated at Burwood Beach locations compared to reference locations (Table 2, Figs. 2 and 3). There were no trends in metal(loid) concentrations to indicate that S. glomerata at Burwood Beach near or far had accumulated higher concentrations compared to all reference locations. For some metal(loid)s, there were significantly higher concentrations detected at a Burwood Beach location compared to one or two reference locations only. These metal(loid)s included arsenic (4 m), lead (4 m) and manganese (8 m). However, many metal(loid) concentrations in S. glomerata tissue were significantly higher at one or more reference location compared to Burwood Beach near and/or Burwood Beach far. This occurred for aluminium (4 m), cadmium (at all depths), iron (8 m), mercury (8 m, 12 m), selenium (4 m) and silver (4 m). There were no significant differences in metal concentrations detected among locations at any depths for copper, nickel or zinc.
Comparison of concentrations of aluminium, arsenic, cadmium, copper, lead and iron (mg/kg dry weight) measured in dried tissue samples of Sydney rock oyster, Saccostrea glomerata, deployed at three depths (4, 8 and 12 m) in sewage impacted locations (Burwood Beach near and Burwood Beach far) and reference locations (Redhead, Fingal Island 1 and Fingal Island 2) for 6 weeks. BN = Burwood Beach near, BF = Burwood Beach far, RH = Redhead, F1 = Fingal Island 1 and F2 = Fingal Island 2. NSW BG = NSW median background concentrations (Scanes and Roach 1999) (n = 2 bags per location/depth). Different letters represent statistically significant differences among locations
Comparison of concentrations of manganese, mercury, nickel, selenium, silver and zinc (mg/kg dry weight) measured in dried tissue samples of Sydney rock oyster, Saccostrea glomerata, deployed at three depths (4, 8 and 12 m) in sewage impacted locations (Burwood Beach near and Burwood Beach far) and reference locations (Redhead, Fingal Island 1 and Fingal Island 2) for 6 weeks. BN = Burwood Beach near, BF = Burwood Beach far, RH = Redhead, F1 = Fingal Island 1 and F2 = Fingal Island 2. NSW BG = NSW median background concentrations (Scanes and Roach 1999) (n = 2 bags per location/depth). Different letters represent statistically significant differences among locations
For all metal(loid)s, concentrations in S. glomerata deployed at Burwood Beach near and Burwood Beach far were not significantly different from all reference locations, i.e. fell within the range of natural variability among references. These findings provide evidence that S. glomerata exposed to sewage effluent from Burwood Beach WWTW did not accumulate significantly higher quantities of metal(loid)s compared to individuals at reference locations. Overall, concentrations of metal(loid)s were low and most (apart from some locations for nickel, selenium and lead) were within the range of NSW median background metal(loid) concentrations (Scanes and Roach 1999).
In terms of depth, there were no consistent differences in metal(loid)s accumulated to oyster tissue among the depths for all metals examined, i.e. most metals were similar among depths, with the exception of mercury which exhibited trends for greater accumulation at lower depths.
3.2 Comparisons of Metal(loid) Concentrations in Oyster Tissue to Historical Data in Sediment and S. glomerata Individuals at Burwood Beach
In the previous study of NSW EPA (1996b), metal(loid) concentrations were assessed in sediments and in S. glomerata deployed in the receiving waters of Burwood Beach WWTW at Burwood Beach near (within 50 m of outfall; which corresponds with the Burwood Beach near location employed for the current study). NSW EPA (1996a) employed a similar methodology and experimental design, with the exception of a 12-week deployment period. It was considered that 6 weeks would be a sufficient period for the equilibration of most metal(loid)s to oyster tissue; thus, comparisons between studies are possible (Boisson et al. 1998; Boisson et al. 2003; Scanes 1998). Concentrations of metal(loid)s in S. glomerata were compared to historical data, and minimum and maximum values were consistently similar and/or lower (Table 3). Mean concentrations of metal(loid)s were also compared to available MRLs (ANZFA 2011). With the exception of arsenic, all mean concentrations were below ANZFA MRLs.
4 Discussion
Comparisons of metal(loid) concentrations in oyster tissue among locations suggested that S. glomerata did not accumulate significant quantities of metal(loid)s at the Burwood Beach locations within the deployment period. This suggested that the receiving waters of Burwood Beach WWTW were not a significant source of aquatic metal exposure. In general, concentrations of metal(loid)s at all locations were low and similar to median background levels detected in oysters in Australian waters. Although in some instances, elevations were detected between Burwood Beach locations and select reference locations (e.g. arsenic (4 m), lead (4 m) and manganese (8 m)), metal(loid)s in oysters at Burwood Beach locations were not consistently elevated compared to all reference locations. This suggests that metal(loid) exposure and accumulation at Burwood Beach were within the boundaries of natural variation observed for the region. Comparison of the Burwood Beach locations provides further evidence that sewage effluent from Burwood Beach WWTW was not a significant source of metallic contamination during the study. Oysters were deployed at Burwood Beach near (< 50 m from outfall) and Burwood Beach far (100–150 m from the outfall) to establish a gradient of effluent exposure. It was assumed that if sewage effluent contained significant quantities of metal(loid)s, then S. glomerata at Burwood Beach near would accumulate significantly higher concentrations in comparison to Burwood Beach far. For all metal(loid)s; however, there were no significant differences detected in concentrations between Burwood Beach near and Burwood Beach far. Interestingly, counter to predictions, some metal(loid)s were significantly higher in oyster tissue at select reference locations compared to putative impact locations. This occurred for cadmium (at all depths), iron (8 m), mercury (8 m, 12 m), selenium (4 m) and silver (4 m) and aluminium (4 m). It is unclear why metal(loid)s may be elevated at references locations, but may in part be attributable to variability in the physicochemical nature of sediments among locations. Aluminium, for example, is associated with the silt/clay fractions of sediments (Harbison 1986) and sediment grain size may vary significantly among locations, though as no sediment physicochemical data were collected, this remains speculative.
Further, although it was predicted that metal accumulation may vary with depth, due to effluent being most concentrated in the upper depths of the water column, no consistent differences were observed in metal(loid) accumulation to tissue with depth.
Spatial variation in metal(loid) concentrations poses difficulties in the measurement of metal(loid)s and the capacity to differentiate between background concentrations and elevated concentrations due to anthropogenic input (Cantillo 1997; Phillips and Rainbow 1993; Scanes and Roach 1999). Therefore, to determine if metal(loid) concentrations are significantly elevated at a location, it is useful to compare data to concentrations from reference locations within the same region. Scanes and Roach (1999) calculated background metal(loid) concentrations for S. glomerata for 12 locations in NSW which were identified as having a low risk of metallic contamination. It should be acknowledged that possible differences may exist in background concentrations between Scanes and Roach (1999) and the current study, as the locations they assessed were estuarine rather than marine. All mean concentrations of metal(loid)s in the current study were considerably lower than NSW median background concentrations determined by Scanes and Roach (1999) both at impact and reference locations, with the exception of nickel, selenium and lead. For some locations (Burwood Beach and/or references), concentrations of nickel, selenium and lead exceeded NSW median background concentrations. It may be possible that background concentrations of selenium and lead are higher for Newcastle and Port Stephens in comparison to other NSW locations. For example, Cole (1990) detected similar concentrations of a similar suite of metal(loid)s in an assessment of metals in fish species, the red morwong, Cheilodactylus fuscus and the blue groper, Achoerodus viridis in several locations across Newcastle, including Burwood Beach and reference locations. Lead and selenium were detected at similar concentrations in fish among locations, whereas only nickel was elevated at Burwood Beach (Cole 1990). For the current study, nickel was found to be elevated above the NSW median background concentration at 12 m for Burwood Beach far; however, no significant differences were detected among locations suggesting that concentrations were still comparable to concentrations at reference locations. Nickel has been measured in biosolids from Burwood Beach WWTW, from 2006–2013, at concentrations 30–180 μg/L, with an average of 47.21 μg/L (N = 152; Hunter Water 2013).
With the exception of arsenic, all metal(loid)s fell below available Australian and New Zealand Food Authority’s Maximum Residue Limits (MRLs) for concentrations of metals permitted for human consumption of molluscs (ANZFA 2011). Arsenic was consistently higher for all locations, but lower than median NSW background concentrations (Scanes and Roach 1999). However, arsenic has also been detected in similar or higher concentrations in fish from Newcastle locations, C. fuscus, A. viridis (Cole 1990) and in oysters, S. glomerata (NSW EPA 1996a). Furthermore, arsenic concentrations in S. glomerata from background locations in NSW were also found to exceed the ANZFA MRL and mean overseas concentrations (Scanes and Roach 1999). It was suggested by Scanes and Roach (1999) that Australia may be likely to contain higher natural sediment concentrations of arsenic.
Concentrations of metal(loid)s in S. glomerata from this study were compared to those observed by NSW EPA (1996a) who similarly deployed S. glomerata at the Burwood Beach near location (within 50 m of WWTW) and at reference locations for 3 months which was repeated over 8 sampling periods between 1992 and 1994. Comparisons of present metals in S. glomerata deployed at the Burwood Beach near location demonstrate that all metal(loid) concentrations were similar or lower than historical data. Although 6 weeks has been demonstrated to be sufficient for equillibration of metals (such as cadmium and lead) (Boisson et al. 1998; Boisson et al. 2003), it is possible that the longer deployment period, employed by NSW EPA (1996b), may have allowed for greater accumulation of metal(loid)s. In one of the only field-based studies examining equilibration, Scanes (1998) deployed oysters in a contaminated estuary in NSW for 24 weeks and sampled oysters at 10 random intervals over deployment. The metals Pb and Cd equilibrated in oyster tissue after 12 weeks, while concentrations of Se were still increasing after 24 weeks. For other metals such as Cu, Zn, Hg and Ag equilibration occurred in under 6 weeks. Thus, a 6-week deployment in the current study was likely sufficient equilibration time for some metal(loids), but perhaps not others, which may explain some reductions in metal(loid) load observed in the current study.
S. glomerata in the current study, due to the changes in sewage treatment process and upgrades to secondary treatment, were less likely to be exposed to sewage sludge compared to NSW EPA (1996a). Since 1994, there have been no other major changes to treatment processing at Burwood Beach WWTW. Changes in metal usage since 1992, such as the phasing out of lead from petrol since 1985–86 (Spooner et al. 2003), may have also decreased input of metals into the sewage treatment process. Many other factors influence the rate of metal uptake by oysters and contribute to variability among studies, even those which employ the same species as a biomonitors. This could include factors that are biological such as age, sex, size, feeding, gonadal development and/or pre-exposure history to metal(loid)s (Ayling 1974; Boening 1999) or environmental factors such as organic carbon, temperature, pH, dissolved oxygen and/or hydrologic features such as sewage plume dynamics (Elder and Collins 1991).
In conclusion, this study has confirmed that concentrations of most metal(loid)s were not significantly different in S. glomerata deployed at Burwood Beach locations compared to reference locations. Concentrations of metal(loid)s were similar to those which have been detected in previous studies in NSW which employed S. glomerata as a biomonitor of metal(loid)s. All metal(loid)s in S. glomerata tissue fell below National Food Authority MRLs, except for arsenic and this does not appear uncommon for concentrations in biota within NSW. Furthermore, comparisons to historical data suggest that concentrations of metal(loid)s in sewage effluent, assessed via S. glomerata metal tissue concentrations, have remained similar or decreased in the last two decades.
References
NSW EPA. 1996a. Hunter environmental monitoring program, “OysterWatch”1992–1996. NSW Environment Protection Authority Report, August 1996, 124 pp.
Ajani, P., Roberts, D., Smith, A., & Krogh, M. (1999). The effect of sewage on two bio-indicators at Port Stephens, New South Wales, Australia. Ecotoxicology, 8, 253–267.
Andrew-Priestley, M. 2011. Molluscan biomonitor for quantification and impact assessment of estrogenic and metallic contaminants in Australian marine ecosystems. PhD Thesis, Discipline of Biological Sciences, University of Newcastle.
Andrew-Priestley, M. N., O’Connor, W. A., Dunstan, R. H., Van Zwieten, L., Tyler, T., Kumar, A., & MacFarlane, G. R. (2012). Estrogen mediated effects in the Sydney rock oyster, Saccostrea glomerata, following field exposures to sewage effluent containing estrogenic compounds and activity. Aquatic Toxicology, 120, 99–108.
ANZFA. (2011). Australian food standards code. Australia New Zealand Food Authority. Australia: ACT.
Avery, E. L., Dunstan, R. H., & Nell, J. A. (1996). The detection of pollutant impact in marine environments of the Hunter Region, Australia: condition index, oxidative DNA damage, and their associations with metal bioaccumulation in the Sydney rock oyster Saccostrea commercialis. Archives of Environmental Contamination and Toxicology., 31, 192–198.
Ayling, G. M. (1974). Uptake of cadmium, lead, zinc, copper, and chromium in the Pacific Oyster. Water Research, 8, 729–739.
BioAnalysis, (2007). Contaminants in sediments associated with the Ocean Outfalls at Boulder Bay, Burwood Beach and Belmont Beach – Ocean Outfall Contaminant Study. BioAnalysis Pty Ltd. Editors: Roberts, D.E., Cummins, S. P. & Murray, S. R.
Boening, D. (1999). An evaluation of bivalves as biomonitors of metals pollution in marine waters. Environmental Monitoring and Assessment., 55(3), 459–470.
Boisson, G., Hensley, G., McKinney, G., & Robinson, J. (2003). Comparative radiotracer study of cadmium uptake, storage, detoxification and depuration in the oyster Crassostrea gigas: potential adaptive mechanisms. Marine Ecology Progress Series., 254, 177–186.
Boisson, F., Cotret, O., & Fowler, S. (1998). Bioaccumulation and retention of lead in the mussel Mytilus galloprovincialis following uptake from seawater. Science of the Total Environment., 222(1–2), 55–61.
Brown, K., & McPherson, R. (1992). Concentrations of copper, zinc and lead in the Sydney rock oyster, Saccostrea commercialis (Iredale and Roughley) from the Georges River. New South Wales. Science of the Total Environment., 126, 27–33.
Brown, H. G., Hensley, H. P., McKinney, G. L., & Robinson, J. L. (1973). Efficiency of metals removal in municipal sewage treatment plants. Environmental Letters., 5, 103–114.
Cantillo, A.Y., 1997. World mussel watch data. National Oceanic and Atmospheric Administration technical memorandum NOS ORCA 109. NOAA, Silver Spring, MD.
CH2MHill and Hunter Water Corporation. (2007). Review of environmental monitoring for Burwood Sludge Outfall.
Cole, B. 1990. Fish studies 1989–1990. Hunter Water board scientific services, Newcastle.
Elder, J., & Collins, J. (1991). Freshwater molluscs as indicators of bioavailability and toxicity of metals in surface-water systems. Reviews of Environmental Contamination and Toxicology., 122, 36–79.
Gearnaey, K. V and Sin, G. 2013. Wastewater treatment models, reference module in earth systems and environmental Sciences, Elsevier. ISBN 9780124095489.
Harbison, P. A. T. (1986). Mangrove muds—a sink and a source for trace metals. Marine Pollution Bulletin, 17(6), 246–250.
Hornberger, M. I., Luoma, S. N., Cain, D. J., Parchaso, F., Brown, C. L., Bouse, R. M., & Thompson, J. K. (2000). Linkage of bioaccumulation and biological effects to changes in pollutant loads in south San Francisco Bay. Environmental Science & Technology, 34(12), 2401–2409.
Hunter Water. (2013). Chemistry compliance data for physiochemical, metal/metalloids and organics parameters monitored in Burwood Beach effluent and biosolids from 2006–2013.
Keller, A., & Zam, S. (1991). The acute toxicity of selected metals to the freshwater mussel. Anodonta imbecilis. Environmental Toxicology and Chemistry., 10, 539–546.
Lincoln-Smith, M., & Cooper, T. (2004). Combining the use of gradients and reference areas to study bioaccumulation in wild oysters in the Hunter River estuary, New South Wales, Australia. Marine Pollution Bulletin., 48, 873–883.
Mathews, T., & Fisher, N. S. (2008). Trophic transfer of seven trace metals in a four-step marine food chain. Marine Ecology Progress Series, 367, 23–33.
Naimo, T. J. (1995). A review of the effects of metals on freshwater mussels. Ecotoxicology, 4(6), 341–362.
Norris, D., & Carr, J. (2006). Endocrine disruption: biological bases for health effects in wildlife and humans. New York: Oxford University Press.
NSW EPA 1996b. Hunter environmental monitoring program 1992-1996. NSW Environment Protection Authority Report, August 1996, 124 pp.
O'Connor, T. P. (1998). Mussel watch results from 1986 to 1996. Marine Pollution Bulletin, 37(1–2), 14–19.
Phillips, D. and Rainbow, P. (1989) Strategies of trace metal sequestration in aquatic organisms. Marine Environmental Research. (28): 207–210.
Phillips, D., & Rainbow, P. (1993). Biomonitoring of trace aquatic contaminants. Barking: Applied Science Publishers.
Phillips, D. J., & Yim, W. W. (1981). A comparative evaluation of oysters, mussels and sediments as indicators of trace metals in Hong Kong waters. Marine Pollution Bulletin., 6, 285–293.
Rainbow, P. S., & Wang, W.-X. (2001). Comparative assimilation of Cd, Cr, Se, and Zn by the barnacle Elminius modestus from phytoplankton and zooplankton diets. Marine Ecology Progress Series., 218, 239–248.
Rainbow, P. (1995). Biomonitoring of metal availability in the marine environment. Marine Pollution Bulletin., 31(4–12), 183–192.
Rainbow, P. S. (2002). Trace metal concentrations in aquatic invertebrates: why and so what? Environmental Pollution., 120, 497–507.
Robinson, W., Maher, W., Kirkowa, F., Nell, J., & Hand, R. (2005). The use of the oyster Saccostrea glomerata as a biomonitor of trace metal contamination: Intra sample, local scale and temporal variability and its implications for biomonitoring. Journal of Environmental Monitoring., 7, 1–16.
Santos, A., & Judd, S. (2010). The fate of metals in wastewater treated by the activated sludge process and membrane bioreactors: a brief review. Journal of Environmental Monitoring., 12, 110–118.
Scanes, P. R. (1996). “Oyster Watch”: monitoring trace metal and organochlorine concentrations in Sydney’s coastal waters. Marine Pollution Bulletin., 33, 226–238.
Scanes, P.R., (1998). The use of bivalves to investigate trace metal and organochlorine contamination in the marine and estuarine waters of New South Wales, Australia. PhD thesis, University of Sydney, Sydney.
Scanes, P., & Roach, A. (1999). Determining natural ‘background’ concentrations of trace metals in oysters from New South Wales, Australia. Environmental Pollution., 105, 437–446.
Spooner, D., Maher, W., & Otway, N. (2003). Trace metal concentrations in sediments and oysters of Botany Bay, NSW, Australia. Archives of Environmental Contamination and Toxicology., 45, 92–101.
Underwood, A. J., & Chapman, M. G. (1984). GMAV-5. Sydney: University of Sydney.
Underwood, A. (1997). Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge: Cambridge University Press.
Wang, W.-X. (2002). Interaction of trace metals and different marine food chains. Marine Ecology Progress Series., 243, 295–309.
Acknowledgments
Gratitude is extended to individuals that significantly contributed to this project including: Bruce Cole from Hunter Water, Dr. Michael Dove, Steve O’Connor and Kyle Johnston from Industry and Investment NSW, Bruce Petersen from Port Stephens Council, Dr. Peter Scanes, Richard Gardiner and Joe Neilson from the New South Wales Environmental Protection Authority.
Funding
We would like to acknowledge funding sources including the Australian Research Council, New South Wales Department of Primary Industries, Hunter Water Corporation and Port Stephens Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The study was in part funded by Hunter Water Corporation and M. Andrew-Priestley is now an employee of Hunter Water Corporation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrew-Priestley, M.N., O’Connor, W.A., Dunstan, R.H. et al. An Impact-Control Study to Assess the Potential Accumulation of Metals and Metalloids from Sewage Effluent and Biosolids to Sydney Rock Oysters, Saccostrea glomerata. Water Air Soil Pollut 231, 197 (2020). https://doi.org/10.1007/s11270-020-04570-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04570-6