Abstract
To determine the leaching behavior and mechanism of cadmium (Cd) from contaminated soil by nitrilotriacetic acid (NTA), batch leaching experiments and chemical extraction in combination with Fourier transform infrared spectroscopy (FTIR) analyses were conducted to investigate the changes of Cd species, the organic matter content, the functional groups of soil particles in the course of leaching. The batch experiment was conducted using one cadmium level (12.49 mg/kg), different levels of NTA (1, 5, 10, 100, 150, 200, 300, 400, and 500 mmol/L), different pH levels (6–11), and different contact times (10, 30, 60, 120, 240, 480, 1440 min). Moreover, based on the experiment results, factors like the input NTA concentration, the eluent pH values, as well as the leaching time for the amount of leached Cd were evaluated via a multiple linear regression simulation. The results showed that the leached Cd from contaminated soils increased with increasing input NTA concentration and absorbed NTA in soils. At the beginning of the leaching experiment, the functional groups of the soil particles changed from hydroxyl to ester, resulting in the release of Cd from soil particles. At the same time, the esterification reaction caused an increase of eluent pH. However, after Cd released into the liquid phase, the complexation reaction between NTA and Cd continually consumed NTA, and this resulted in a minor decrease in eluent pH. As the result, some amounts of Cd could be precipitated as Cd(OH)2 and immobilized again under high pH condition. Multiple linear regression results showed that the input NTA concentration is the main factor that influences the removal efficiency of Cd from contaminated soils. The study results provide vital support for the practice of Cd-contaminated soil remediation using NTA as chemical eluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) is a heavy metal which exhibits high chemical activity and permanent toxicity in the earth surface environments. It can influence human health through food chain accumulation, such as inducing kidney failure and cancers (Chen et al. 2018; Satarug et al. 2017; Xue et al. 2017). In recent years, soil contamination by Cd has gradually become a global environmental issue and attracted worldwide attention (Khan et al. 2017; Wu et al. 2010). Rehabilitating the Cd-contaminated soil to eliminate the negative impact on human health is extremely urgent (Pan et al. 2010; Chaney 2015; Wang et al. 2016).
The speciation of Cd in soils determines what remediation method could be effective in the removal of Cd (Arwidsson et al. 2010). The Cd in soils can be divided into soluble and insoluble speciation. Soluble Cd exists as free ions or complex ions, such as Cd2+, CdCl+, CdSO4, CdHCO3+, CdNO3+, CdOH+, CdHPO4 and organic complexes of Cd. Insoluble Cd mainly includes CdCO3 and colloid-adsorbed Cd (Van Poucke et al. 2018). To determine the speciation of solid-phase-associated metals, Tessier et al. (1979) proposed a sequential chemical extraction procedure operationally targeting exchangeable, carbonates-bounded, Fe-Mn oxides-bounded, organic matter-bounded and residual phases Cd. The mobilization and transformation of Cd in soils is closely related to the pH and organic matter content (Cheng et al. 2017; Cruz-Paredes et al. 2017; Tang et al. 2017; Van Poucke et al. 2018). The change of soil pH can alter the stability and surface site charges of soil particles, which further influences the speciation and environmental behaviors of Cd (Wang et al. 2013). In acidic environments where the bioavailability of Cd is high, the adsorption reaction controls the mobilization and transformation of Cd in soils (de Abreu et al. 2012), and the positive surface charges of the soil particles restrict the adsorption of positively charged Cd ions (Wang et al. 2013). The electronegativity of soil particles increases with the increasing of the soil pH, resulting in the increase of Cd adsorption on soil particles. However, Cd precipitation as carbonate and hydrolysis to form hydroxide under high pH condition can impact the mobility of Cd at the same time (Zeng et al. 2011). The occurrence of organic matter can cause the decrease of bioavailability and migration capability of Cd in the soils (Gadd 2000; Liu and Chen 2013; Van Poucke et al. 2018). Soil organic matter can transform exchangeable and Fe/Mn oxides-bounded Cd to Cd complexed with organic matters and thus decrease Cd migration capability (Li et al. 2015; Van Poucke et al. 2018). Soil organic matter contains large amounts of hydroxyl and carbonyl groups, which can complex with Cd ions to form stable Cd-containing organic chelate (Cui et al. 2017). Besides, soil organic matter can alter soil adsorption capacity through changing pH and Eh properties and cationic exchange capacity and thus influence the precipitation-dissolution equilibrium of Cd (Lee et al. 2011).
Chemical leaching is a widely used technology in heavy metal-contaminated soil remediation (Lee et al. 2011; Mahar et al. 2015; Zhou et al. 2011). By this method, the remediation or treatment of high-concentration heavy metal-contaminated soil can be achieved in a relatively short period of time. Because of the low cost and the reusability of remedied soils, this method has become more and more popular in the practical implication of soil retreatment (Liu et al. 2018). The leaching agent selection is the key factor for the performance of chemical leaching technology. Some kind of acids, chelating agents, and surfactants are commonly used for the remediation of heavy metal-contaminated soils (Dermont et al. 2008). The disadvantage of acids is that they can destroy soil organic matter and minerals and exhibit poor elution effects for soils with high buffering capacity (Dermont et al. 2008). The chelating agent, like EDTA, can form stable compounds with heavy metals over a very wide range of pH (Zupanc et al. 2014). However, because of the environmental and health risks arising from its chemical stability, non-biodegradability, and lack of ion selection, the use of EDTA has been questioned (Zupanc et al. 2014). Synthetic surfactants have exhibited excellent elution effects for heavy metal-contaminated soils, but their high price and low biodegradability restricts their application (Dermont et al. 2008). Although biosurfactants have the advantages of biodegradability and strong specificity, their high cost and low yield restricts their use in contaminated soil remediation (Bonal et al. 2018).
In recent years, naturally degradable chelating agents such as ethylenediamine-N,N′-disuccinic acid (EDDS) (Wang et al. 2012a), nitrilotriacetic acid (NTA) (Naghipour et al. 2016), and N,N-bis (carboxymethyl)-l-glutamic acid (GLDA) (Wang et al. 2016) have been used in chemical leaching for the remediation of heavy-metal-contaminated soil. These chelating agents exhibit excellent removal efficiency and environmental friendliness, and therefore have excellent application potential. The NTA is a biodegradable, natural multi-carboxyl amino acid chelating agent (Bucheli-Witschel and Egli 2001). It has strong reactivity with heavy metals in soils and is therefore able to decrease the amount of Cd adsorbed by soil particles. However, the detailed mechanism of Cd leaching from contaminated soils by NTA is far from full understanding. Therefore, in this study, the mechanism of NTA interaction with Cd in contaminated soils was studied through batch experiments and chemical extraction combined with Fourier-transform infrared spectroscopy analyses. In addition, a multiple linear regression model was built up to identify the key factor(s) impacting Cd leaching efficiency from contaminated soils. The results of this study provide technical support for the further application of NTA to remediate Cd-contaminated soils.
2 Materials and Methods
2.1 Experiments Methods
The soil samples were collected from a Cd-contaminated field and air-dried in the laboratory after residual plants and other debris were removed. The soil samples were then passed through 10-mesh sieve and stored in the dark for future use. At the same time, a subsection of soil samples was further grounded in an agate mortar to 50-mesh for storage and future use.
NTA solutions with concentrations of 1, 5, 10, 100, 150, 200, 300, 400, and 500 mmol/L were prepared, and their pH values were adjusted to 11 using 0.1 mol/L of NaOH and 0.1 mol/L of HCl. A total of 2 g 10-mesh soil was added into a 50 ml centrifuge tube. The NTA solution was added with a solid-to-solution ratio of 1:10 into the centrifuge tube, which was then shaken on a horizontal shaker for 4 h at a speed of 150 r/min and temperature of 25 °C. And then, the mixed solution was centrifuged at 4000 r/min for 10 min. The supernatant was filtered through 0.45-μm microfiltration membrane. The filtrate was acidized using nitic acid and stored in a 50 ml pre-cleaned PET bottle for Cd concentration determination.
By controlling the NTA concentration at 100 mmol/L, the solution pH was adjusted to 6, 7, 8, 9, 10, and 11 using 0.1 mol/L of NaOH and 0.1 mol/L of HCl to test the effect of solution pH on Cd leaching. A total of 2 g 10-mesh soil was added to a 50 ml centrifuge tube. The NTA solution with the corresponding pH and a solid-to-solution ratio of 1:10 was added to the centrifuge tube, which was then shaken for 4 h at a speed at 150 r/min and a temperature of 25 °C. The centrifuge tube was centrifuged at 4000 r/min for 10 min. The supernatant was filtered through a 0.45-μm microfiltration membrane. The filtrate was acidized using nitic acid and stored in a 50 ml pre-cleaned PET bottle to determine the concentration of Cd in filtrate.
By controlling the NTA concentration at 100 mmol/L, the solution pH was adjusted to 7 and 11 using 0.1 mol/L of NaOH and 0.1 mol/L of HCl. A total of 2 g of 10-mesh soil was added to a 50-mL centrifuge tube. The NTA solution with the corresponding pH and a solid-to-solution ratio of 1:10 was added to the centrifuge tube, and then shaken for 10 min, 30 min, 60 min, 120 min, 240 min, 480 min, and 1440 min at a speed of 150 r/min and a temperature of 25 °C. The centrifuge tube was taken out at the set time and centrifuged at 4000 r/min for 10 min. The supernatant was filtered through a 0.45-μm microfiltration membrane. The filtrate was acidized using nitic acid and stored in a 50 ml pre-cleaned PET bottle for Cd concentration analysis. Before and after the experiment, the five-step chemical extraction procedure proposed by Tessier et al. (1979) was used to extract the different Cd fractions from the soils.
2.2 Laboratory Analysis
A total of 10.0 ± 0.l g 10-mesh soil sample was added to a 50 ml PET bottle, and then 25 ml of CO2-free water was added. The mixture was stirred for 1–2 min. After standing for 30 min, the pH of the soil was measured using a calibrated HACH portable pH meter. A 0.1–0.5 g (with the precision of 0.0001 g) 50-mesh soil sample was added to a 50-mL Teflon tube. Then, 10 mL of 0.136 mol/L K2Cr2O7-H2SO4 solution was added to the tube, and the tube was heated in an oil bath for 5 min at 170–180 °C. Then, 0.2 mol/L of standard FeSO4 solution was used to measure the soil organic matter content (Wang et al. 2012b). Mixture of HCl + HNO3 + HF + HClO4 was used to digest the soil samples, and a flame atomic absorption spectrophotometer (FAAS) (iCE3000, Thermo Fisher) was used to measure the Cd concentration of the digested soil solution (Frentiu et al. 2013). Inductively coupled plasma mass spectrometry (ICP-MS) (7900, Agilent) was used to determine the concentration of Cd in extraction (Huang et al. 2014). The soil samples before and after NTA leaching experiments were dried in an oven at 105 °C for 2 h to remove the interference of water molecules. And then a small amount of sample was weighed and ground with KBr to obtain KBr pellets. The IR spectrum was obtained on a Fourier transform infrared (FTIR) spectrometer (Vertex 70, Bruker) by setting the step at 4 cm−1 (Knadel et al. 2017). A 30 g soil sample and 200 mL of pure water were added to a 500-mL beaker; after incubation for 24 h, 10 mL of 4% sodium hexametaphosphate was added to disperse the soil solution. A TM-85 soil densitometer was used to measure the density of the soil solution.
Three parallel samples were tested in leaching and chemical extraction experiments, and the relative deviation between the parallel samples for Cd concentration in leachate and extraction solution was within ± 10%. Standards or quality control samples were tested for all chemical analysis, and parallel double samples were tested with each batch samples with the error less than 5%.
2.3 Development of the Multiple Linear Regression Model
To determine the impacts of the input NTA concentration, the eluent pH, and the elution leaching time on the leaching efficiency of Cd, a multiple linear regression model was developed based on the leaching experiment results (Table 2). The quantitative relationships between the measured random variable y (leached Cd) and the three nonrandom variables (x1: NTA concentration, x2: pH of eluent, x3: elution time) were established through independent and dependent variable matrix (Table 2). The SPSS software package (SPSS19.0) was used to perform the linear regression analysis and develop the regression model using stepwise linear regression (Stokes et al. 2017).
3 Results and Discussion
3.1 Physicochemical Properties of the Contaminated Soil
The soil has a silty sand texture. The measured physicochemical properties of the contaminated soil are listed in Table 1. The pH value of the soil is 7.03, indicating that it is a neutral soil. The measured bulk Cd content in the soil is 12.49 mg/kg. The exchangeable Cd (4.72 mg/kg) is the highest fraction in the contaminated soil sample, which accounts for 37.8% of the total Cd, while the Cd bounded to carbonates (4.44 mg/kg) accounts for 35.5% of total Cd in the soil. The Cd bounded to Fe-Mn oxides (2.23 mg/kg) accounts for 17.9%, and this fraction is stable compared to the other fractions and, therefore, is difficult to migrate (Fan et al. 2012). The Cd bounded to organic matter (0.94 mg/kg) accounts for 7.6% of bulk Cd in soil sample. This fraction of Cd was formed through ion exchange, adsorption, and chelation between Cd and soil organic matter (Tang et al. 2017; Zeng et al. 2011). The residual Cd (0.16 mg/kg) is the most stable fraction in soil and has low content accounting for 1.3% of bulk Cd. This fraction Cd exists in the crystal lattices of silicate minerals of the soil and is difficult to migrate and transform (Butt et al. 2008). The IR spectrum of the soil samples (Fig. 1) shows that there are stretching vibration peak of O-H bonds at 3625.81 cm−1 and C-O bonds at 1031.18 cm−1, indicating that the soil organic matter contains -OH and/or -COOH structure. Soil organic matters are effective on complexing metal ions due to the occurrence of carboxylic (-COOH) and hydroxyl (-OH) groups (Van Poucke et al. 2018). According to the study conducted by Inyang et al. (2011), the Cd bounded to organic matter is believed to exist as -O-Cd.
3.2 Changes of Cd Fractions in Soils Before and After Leaching
The change of the Cd fractions before and after leaching is shown in Fig. 2. After NTA elution, the contents of exchangeable Cd, Cd bounded to carbonates, and Cd bounded to organic matter changed significantly from 4.71, 4.43, and 0.94 mg/kg to 0.50, 0.96, and 0.38 mg/kg, respectively, whereas the content of Cd bounded to Fe-Mn oxides and residual phase Cd did not change obviously. These results suggest that NTA can mobilize the exchangeable Cd and Cd associated with carbonates and organic matter. The exchangeable Cd has high activity and is extremely easy to migrate to the liquid phase (Fan et al. 2012). The fraction of Cd bounded to carbonates that exists as precipitates, such as CdCO3 in soils, is very sensitive to the pH and easily migrated. The input of NTA results in the change of the system pH; a low pH results in the dissolution of carbonates (Evangelou et al. 2004), whereas a high pH causes the transformation of Cd to multi-hydroxyl chelate (Davari et al. 2015). The fraction of Cd bounded to organic matter is formed through a series of reactions including ion exchange, adsorption, and chelation (Van Poucke et al. 2018). The NTA as an exogenous organic matter can enhance the activity of Cd in soil through chelation with Cd, resulting in the decrease of Cd adsorbed by soil particles and improving the bioavailability of Cd in the soil (Filipovic et al. 2018; Liu and Chen 2013). Cd normally adsorbs onto the surfaces of Fe-Mn oxide particles through a strong binding force or reacts with Fe-Mn oxides (Fan et al. 2012). Therefore, Cd bounded to Fe-Mn oxides is more stable than the other fractions. The NTA molecules cannot destroy the strong binding force between Cd and Fe-Mn oxides. As the result, the Cd bounded to Fe-Mn oxides did not significantly change after NTA elution (Fig. 2). The content of residual Cd in the soil samples is extremely low and does not change considerably after leaching because this fraction of Cd exists in the crystal lattices of the silicate minerals in soil and is difficult to migrate and transform (Butt et al. 2008).
The contents of different Cd fractions in contaminated soil before and after chemical leaching using 500 mmol/L NTA solution with pH of 11. During treatment, the mixture was shaken at 150 r/min and 25 °C for 4 h with a solid-to-solution ratio of 1:10. Exc exchangeable Cd, Carb Cd associated with carbonates, Fe-Mn Cd bounded to the Fe and Mn oxides, OM Cd associated with organic matters, Res the residual phases Cd
3.3 Effect of Input Concentration of NTA on Cd Leaching from Soil
The covariation of leached Cd and the adsorbed NTA with the input concentration of NTA (Fig. 3) clearly shows that the amount of adsorbed NTA has certain impacts on the leached Cd. The amount of leached Cd initially increased with the increasing of the input NTA concentration and then became stable at 0.83 mg/L when the input concentration of NTA was higher than 300 mmol/L. The similar trend was observed between the input NTA concentration and the amount of NTA adsorbed by the soil. Figure 4 shows that elution using NTA will result in an increase in soil organic matter content, and this increase was enhanced with the elevated NTA concentration from 100 to 400 mmol/L. However, it will no longer increase after the NTA concentration reached 400 mm/L. The IR spectrum of the soil after elution is significantly different from that prior to elution (Fig. 1). There are stretching vibrations of C=O bonds at 1639.72 cm−1, C=C unsaturated bonds at 1596.0 cm−1, and C-O bonds at 1027.9 cm−1, which indicates the existence of ester group in the soil after elution. The results of IR spectrum suggest that competitive adsorption onto the soil particles between the exogenous organic matter and Cd occurred (Bradl 2004). Because NTA is an organic substance with a low molecular weight and contains the carboxyl active functional group, it can easily react with organic matter in the soil (Song et al. 2016). During the elution process, the carboxyl groups in NTA react with the hydroxyl groups on the surface of soil particles to form esters and then promote the mobilization of Cd that is bounded through specific coordination bonds, thereby resulting in an increase of the Cd concentration in the liquid phase. And then the carboxyl active functional group of NTA can complex Cd ion with the increase of NTA concentration in leachate. With high NTA concentrations, the adsorption sites of the soil particles are saturated and reach adsorption equilibrium, and then the amount of leached Cd and the organic matter concentration of soil do not change.
3.4 Variation of Eluent pH during Cd Leaching
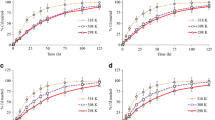
The maximum and minimum leached Cd concentration is 0.56 and 0.37 mg/L, which is obtained at pH 6 and 11, respectively (Table S1). This indicates that low pH condition favors the leaching of Cd from contaminated soil. However, in consideration it is a neutral contaminated soil (pH = 7.03), the experiments of eluent pH = 7 are addressed to obtain the variation of eluent pH during Cd leaching.
The changes of the eluent pH and the amount of leached Cd are shown in Fig. 5. The eluent pH increased significantly during the first 15 min from 7.0 to 9.49 and then kept at approximately 9.0 after 60 min. However, the amount of leached Cd increased significantly during the first 15 min from 0.039 to 0.58 mg/L, and then decreased gradually to 0.54 mg/L at about 120 min. At last, the concentration of leached Cd keeping at 0.54 mg/L. The results show that the amount of leached Cd is closely related to the eluent pH. The observed variation of eluent pH and leached Cd with the time is the joint result of Cd complexation and exchange reaction with NTA. In the first 15 min, the amount of leached Cd increased rapidly with increasing pH and reached a peak value of 0.58 mg/L. This can be attributed to the following:
- 1.
The competitive adsorption between NTA and Cd and accompanied esterification between carboxyl group in NTA reacts and the hydroxyl group of soil particles. This process can be described by the following chemical reaction:
where “—” represents coordination binding; “Surf” represents the surfaces of the soil particles; and “…” represents binding by electrostatic interaction.
- 2.
The complexation between Cd and NTA, which can result in the decrease of pH of eluent. This process can be described by the following reaction:
The observed variation of eluent pH (Fig. 5) indicates that the competitive adsorption between NTA and Cd (reaction 1) is the primary reaction during the leaching experiment. The decrease of leached Cd during about 10 to 120 min may be related to the formation of Cd-bearing precipitation as Cd(OH)2 under high pH conditions. From Fig. 1, it can be seen that at high pH values between 8 and 10, Cd(OH)2 precipitates are formed, which results in a decrease of the amount of leached Cd. In addition, the hydroxyl complex of Cd, CdOH+, is generated at the pH of 9. The CdOH+ has a higher affinity to the adsorption sites on soil particles than Cd ion (Hooda and Alloway 1998), and therefore is easily adsorbed by the negatively charged soil particles. Ultimately, with the stabilization of the eluent pH, the dissolution and precipitation reactions reach equilibrium; as a result, the amount of leached Cd in the liquid phase also stabilizes.
3.5 Effect of the Elution Time on the Leaching of Cd
Figure 6 clearly indicates that the effect of the elution time on the leaching of Cd is mainly reflected in the first 120 min of elution. During this period, the leaching of Cd from soil is controlled by reactions (1) and (2). As discussed above, at the first 15 min, the competitive adsorption between NTA and Cd accompanied with the esterification reaction controls the leaching of Cd resulting in the increase of eluent pH. And then, complexation between Cd and NTA caused the decrease of eluent pH. High pH condition controls the formation of Cd-bearing precipitation as Cd(OH)2 resulting in the decrease of leached Cd from soil. During the leaching period, the competitive adsorption between NTA and Cd determines the degree of complexation reaction between NTA and Cd and then the formation amount of Cd-bearing precipitation. Once the reactions (1) and (2) reach equilibrium, time no longer restricts the desorption of Cd, and the amount of leached Cd also stabilizes.
3.6 Leaching Mechanism of Cd from Soil by NTA
The exchangeable Cd, Cd bounded to carbonates, and Cd bounded to organic matter are leached under the action of NTA. The leaching process is influenced by the NTA concentration and pH of eluent. The addition of NTA destroys the coordination bond between Cd and soil particles, promotes the desorption of Cd from the soil particles, and causes the change of Cd speciation. Based on above discussion, the conceptual model for Cd leaching from soil by NTA can be proposed and described in Fig. 7.
The conceptual model of NTA leaching Cd from contaminated soil. ① The exchange and esterification caused the release of Cd into liquid phase and the significantly increase of eluent pH. ② The complexation reaction between NTA and Cd ion which resulted in the decrease of eluent pH. ③ The combinated effect of ① and ② resulted in the increase of eluent pH. High pH condition promoted the precipitation of Cd as multi-hydroxyl Cd (e.g., Cd(OH)2)h)
The addition of NTA promotes the occurrence of competitive adsorption between NTA and the Cd. In the reaction, the initially adsorbed Cd by the soil is replaced by NTA. This process is accompanied by the breaking of the original coordination bonds and the generation of new covalent bonds. The carboxyl group in NTA reacts with the hydroxyl groups on the surface of soil particles to form esters and mobilizes the bound Cd following the reaction (1). When the high concentration of NTA reaches saturation on the adsorption sites of the soil particles, the competitive adsorption reaches equilibrium. At the same time, the amount of leached Cd also stabilizes (Fig. 3).
The eluent pH is closely related to the amount of leached Cd; the amount of leached Cd first increases and then decreases with the increase of pH and finally reaches a stable value (Fig. 5). This phenomenon is the joint result of competitive adsorption and complexation of Cd with NTA, in which competitive adsorption reaction plays a major role. Due to the addition of NTA, exchange reaction occurs between NTA and the adsorbed Cd; ultimately, the Cd bounded to the surfaces of the soil particles becomes free Cd2+. At the same time, the production of OH− in this reaction results in the increase of eluent pH. And then, the complexation between NTA and Cd continuously consumes NTA and release H+, which results in a minor decrease of pH. The environmental pH stabilizes when reactions (1) and (2) reach the equilibrium. In the end, the increase of the eluent pH results in the formation of multi-hydroxyl Cd as CdOH+ or Cd(OH)2. The Cd(OH)2 is insoluble, and CdOH+ has a higher affinity to the adsorption sites on the soil particles and therefore is easily adsorbed by the negatively charged soil particles, which results in re-fixation of Cd by the soil (Hooda and Alloway 1998). With the stabilization of the eluent pH, the Cd concentration in the liquid phase also stabilizes (Fig. 6).
3.7 Regression Model Analysis for Cd Leaching by NTA
During actual chemical elution and remediation practices, the operating parameters, including the amount of eluent, pH of the eluent, elution time, shaking frequency, and liquid-to-solid ratio determine the leaching efficiency and the cost of remediation project (Khalid et al. 2017). The effects of these multiple factors can be described by a multiple linear regression model based on the experiment data using stepwise linear regression. The standardized coefficient of the model can determine the direction and extent of the impacts of different factors and thus provides guidance for the remediation practice. In a remediation project, the factor that has the greatest influence on the leaching of Cd is chosen to optimize the chemical elution process in the hope of improving the elution efficiency and reducing operation cost. The regression equation and model obtained by SPSS are shown in Tables 2 and 3.
Table 2 shows that the F value of the regression equation is 43.89. This regression equation passes the significance test (p < 0.01), suggesting that there is significant linear relationship between the independent variable and the dependent variable. R2 is 0.830 indicating that 83% of the variation of the dependent variable can be explained by the independent variable through the regression equation. The regression model result implies that the input NTA concentration and the pH of the eluent explain 83% of the variation of the amount of leached Cd. Table 3 shows that the t value of the variables x1 and x2 that enter the equation after the step-wise regression is 9.229 and − 2.977, respectively. The corresponding p values are 0 and 0.008, respectively, and p < 0.01 for both x1 and x2. Therefore, both x1 and x2 pass the t test, which indicates that x1 and x2 have significant impacts on y. This result suggests that the input NTA concentration and the pH of the eluent have significant impacts on the amount of leached Cd, which is consistent with the above discussion. The independent variable x3 does not enter the equation, and its p is greater than 0.01. Therefore, it does not pass the t test, which indicates that x3 (i.e., the elution time) is not the major factor for Cd leaching. The standardized regression equation eliminates the impact of the dimension of the independent variable and thus can be used to indicate the impact of the independent variable on the dependent variable. The higher standard regression coefficient of the independent variable indicates the greater impact on the dependent variable (Hooda and Alloway 1998). The absolute value of the coefficient of the independent variable x1 is 0.908, which is greater than that of x2, which suggests that the input NTA concentration is the key factor that influences the amount of leached Cd, followed by the pH of the eluent.
4 Conclusions
The chemical extraction results indicate that NTA can leach exchangeable Cd, Cd bounded to carbonates, and Cd bounded to organic matter, which changes from the original 4.71, 4.43, and 0.94 mg/kg to 0.50, 0.96, and 0.38 mg/kg, respectively. The input NTA concentration and pH of eluent have significant impacts on the amount of leached Cd. The amount of leached Cd increases with increasing of input NTA concentration ranging from 10 to 300 mmol/L and then keep stable at high NTA concentrations (> 300 mmol/L). The pH of the eluent is closely related to the leaching of Cd; the amount of leached Cd first increases and then decreases with increasing pH and finally stabilizes. The complexation and esterification reaction between NTA and Cd and soil particles control the variation of elution pH. The mechanism of Cd leaching by NTA is that esterification activates the soil particles and the Cd that is bound through specific coordination bonds by hydroxyl groups and results in the leaching of Cd. The standard regression model shows that the dominant controlling factor in the elution process is the input NTA concentration. Therefore, more attention should be paid to optimize NTA concentration to enhance the leaching efficiency in Cd-contaminated soil remediation practice.
References
Arwidsson, Z., Elgh-Dalgren, K., von Kronhelm, T., Sjöberg, R., Allard, B., & van Hees, P. (2010). Remediation of heavy metal contaminated soil washing residues with amino polycarboxylic acids. Journal of Hazardous Materials, 173(1–3), 697–704.
Bonal, N. S., Paramkusam, B. R., & Basudhar, P. K. (2018). Enhancement of surfactant efficacy during the cleanup of engine oil contaminated soil using salt and multi-walled carbon nanotubes. Journal of Hazardous Materials, 351, 54–62.
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. Journal of Colloid and Interface Science, 277(1), 1–18.
Bucheli-Witschel, M., & Egli, T. (2001). Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiology Reviews, 25(1), 69–106.
Butt, D., Dowling, K., & Vinden, P. (2008). Assessment of cadmium distribution in some australian krasnozems by sequential extraction. Water, Air, and Soil Pollution, 190(1–4), 157–169.
Chaney, R. L. (2015). How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Current Pollution Reports, 1, 13–22.
Chen, H., Yang, X., Wang, P., Wang, Z., Li, M., & Zhao, F. J. (2018). Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Science of the Total Environment, 639, 271–277.
Cheng, M., Wang, A., & Tang, C. (2017). Ammonium-based fertilizers enhance Cd accumulation in Carpobrotus rossii grown in two soils differing in pH. Chemosphere, 188, 689–696.
Cruz-Paredes, C., Wallander, H., Kjøller, R., & Rousk, J. (2017). Using community trait-distributions to assign microbial responses to pH changes and Cd in forest soils treated with wood ash. Soil Biology and Biochemistry, 112, 153–164.
Cui, J., Luo, C., Tang, C. W., Chan, T., & Li, X. (2017). Speciation and leaching of trace metal contaminants from e-waste contaminated soils. Journal of Hazardous Materials, 329, 150–158.
Davari, M., Rahnemaie, R., & Homaee, M. (2015). Competitive adsorption-desorption reactions of two hazardous heavy metals in contaminated soils. Environmental Science and Pollution Research, 22, 13024–13032.
de Abreu, C. A., Coscione, A. R., Pires, A. M., & Paz-Ferreiro, J. (2012). Phytoremediation of a soil contaminated by heavy metals and boron using castor oil plants and organic matter amendments. Journal of Geochemical Exploration, 123, 3–7.
Dermont, G., Bergeron, M., Mercier, G., & Richer-Laflèche, M. (2008). Soil washing for metal removal: A review of physical/chemical technologies and field applications. Journal of Hazardous Materials, 152, 1–31.
Evangelou, M. W. H., Daghan, H., & Schaeffer, A. (2004). The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere, 57, 207–213.
Fan, W., Jia, Y., Li, X., Jiang, W., & Lu, L. (2012). Phytoavailability and geospeciation of cadmium in contaminated soil remediated by Rhodobacter sphaeroides. Chemosphere, 88(6), 751–756.
Filipovic, L., Romic, M., Romic, D., Filipovic, V., & Ondrasek, G. (2018). Organic matter and salinity modify cadmium soil (phyto)availability. Ecotoxicology and Environmental Safety, 147, 824–831.
Frentiu, T., Ponta, M., & Hategan, R. (2013). Validation of an analytical method based on the high-resolution continuum source flame atomic absorption spectrometry for the fast-sequential determination of several hazardous/priority hazardous metals in soil. Chemistry Central Journal, 7(1), 43.
Gadd, G. M. (2000). Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Current Opinion in Biotechnology, 11(3), 271–279.
Hooda, P. S., & Alloway, B. J. (1998). Cadmium and lead sorption behaviour of selected English and Indian soils. Geoderma, 84(1–3), 121–134.
Huang, Q., Liu, X., & Zhang, Q. (2014). Application of ICP-MS and AFS to detecting heavy metals in phosphorus fertilizers. Spectroscopy and Spectral Analysis, 34(5), 1403–1406.
Inyang, M. D., Gao, B., Ding, W. C., Pullammanappallil, P., Zimmerman, A. R., & Cao, X. D. (2011). Enhanced lead sorption by biochar derived from anaerobically digested sugarcane bagasse. Separation Science and Technology, 46(12), 1950–1956.
Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 182, 247–268.
Khan, M. A., Khan, S., Khan, A., & Alam, M. (2017). Soil contamination with cadmium, consequences and remediation using organic amendments. Science of the Total Environment, 601-602, 1591–1605.
Knadel, M., Gislum, R., Hermansen, C., Peng, Y., Moldrup, P., de Jonge, L. W., et al. (2017). Comparing predictive ability of laser-induced breakdown spectroscopy to visible near-infrared spectroscopy for soil property determination. Biosystems Engineering, 156, 157–172.
Lee, Y. C., Kim, E. J., Ko, D. A., & Yang, J. W. (2011). Water-soluble organo-building blocks of aminoclay as a soil-flushing agent for heavy metal contaminated soil. Journal of Hazardous Materials, 196, 101–108.
Li, Z., Wu, L., Zhang, H., Luo, Y., & Christie, P. (2015). Effects of soil drying and wetting-drying cycles on the availability of heavy metals and their relationship to dissolved organic matter. Journal of Soils and Sediments, 15(7), 1510–1519.
Liu, C. C., & Chen, G. B. (2013). Reclamation of cadmium-contaminated soil using dissolved organic matter solution originating from wine-processing waste sludge. Journal of Hazardous Materials, 244, 645–653.
Liu, L., Li, W., Song, W., & Guo, M. (2018). Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Science of the Total Environment, 633, 206–219.
Mahar, A., Wang, P., Li, R., & Zhang, Z. (2015). Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere, 25, 555–568.
Naghipour, D., Gharibi, H., Taghavi, K., & Jaafari, J. (2016). Influence of EDTA and NTA on heavy metal extraction from sandy-loam contaminated soils. Journal of Environmental Chemical Engineering, 4(3), 3512–3518.
Pan, J., Plant, J. A., Voulvoulis, N. et al. (2010). Cadmium levels in Europe: implications for human health. Environmental Geochemistry and Health 32, 1–12.
Satarug, S., Vesey, D. A., & Gobe, G. C. (2017). Current health risk assessment practice for dietary cadmium: Data from different countries. Food and Chemical Toxicology, 106, 430–445.
Song, Y., Ammami, M. T., Benamar, A., Mezazigh, S., & Wang, H. (2016). Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environmental Science and Pollution Research, 23(11), 10577–10586.
Stokes, C., Masselink, G., Revie, M., Scott, T., Purves, D., & Walters, T. (2017). Application of multiple linear regression and Bayesian belief network approaches to model life risk to beach users in the UK. Ocean and Coastal Management, 139, 12–23.
Tang, W., Zhong, H., Xiao, L., Tan, Q., Zeng, Q., & Wei, Z. (2017). Inhibitory effects of rice residues amendment on Cd phytoavailability: A matter of Cd-organic matter interactions? Chemosphere, 186, 227–234.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Van Poucke, R., Ainsworth, J., Maeseele, M., Ok, Y. S., Meers, E., & Tack, F. M. G. (2018). Chemical stabilization of Cd-contaminated soil using biochar. Applied Geochemistry, 88, 122–130.
Wang, A., Luo, C., Yang, R., Chen, Y., Shen, Z., & Li, X. (2012a). Metal leaching along soil profiles after the EDDS application – A field study. Environmental Pollution, 164, 204–210.
Wang, J. B., Zhu, L. P., Wang, Y., Gao, S. P., & Daut, G. (2012b). A comparison of different methods for determining the organic and inorganic carbon content of lake sediment from two lakes on the Tibetan Plateau. Quaternary International, 250, 49–54.
Wang, T., Liu, W., Xiong, L., Xu, N., & Ni, J. (2013). Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chemical Engineering Journal, 215-216, 366–374.
Wang, G., Zhang, S., Xu, X., Zhong, Q., Zhang, C., Jia, Y., et al. (2016). Heavy metal removal by GLDA washing: Optimization, redistribution, recycling, and changes in soil fertility. Science of the Total Environment, 569-570, 557–568.
Wu, F., Yang, W., Zhang, J., & Zhou, L. (2010). Cadmium accumulation and growth responses of a poplar (Populus deltoids×Populus nigra) in cadmium contaminated purple soil and alluvial soil. Journal of Hazardous Materials, 177(1–3), 268–273.
Xue, S., Shi, L., Wu, C., Wu, H., Qin, Y., Pan, W., et al. (2017). Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environmental Research, 156, 23–30.
Zeng, F., Ali, S., Zhang, H., Ouyang, Y., Qiu, B., Wu, F., et al. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental Pollution, 159(1), 84–91.
Zhou, J. L., Wu, Q. T., Wei, Z. B., Guo, X. F., Qiu, J. R., & Huang, Z. J. (2011). Effects of mixed chelators on the leaching of cadmium in contaminated soils under intercropping system. Environmental Sciences, 32(11), 3440–3447 (in Chinese).
Zupanc, V., Kastelec, D., Lestan, D., & Grcman, H. (2014). Soil physical characteristics after EDTA washing and amendment with inorganic and organic additives. Environmental Pollution, 186, 56–62.
Acknowledgments
This study was jointly supported by the Natural Science Foundation of China (Nos. 41772255, 41521001, and 41372254) and Hubei Science and Technology Innovation Project (2016ACA167).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 154 kb)
Rights and permissions
About this article
Cite this article
Xie, X., Yang, S., Liu, H. et al. The Behavior of Cadmium Leaching from Contaminated Soil by Nitrilotriacetic Acid: Implication for Cd-Contaminated Soil Remediation. Water Air Soil Pollut 231, 166 (2020). https://doi.org/10.1007/s11270-020-04545-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04545-7