Abstract
Feeding a growing population requires striking a balance between increasing production and decreasing environmental impacts in agricultural settings. We established 12 experimental mesocosms with silt loam atop a base of sand and examined the ability of three emergent aquatic plants common to the USA to remediate pesticides and nutrients in agricultural runoff. Mesocosms were planted in monocultures of Myriophyllum aquaticum, Polygonum amphibium, and Typha latifolia, or left unvegetated to serve as controls. All mesocosms were amended with target concentrations of 10 mg L−1 (each) nitrate, ammonium, and orthophosphate; 20 μg L−1 (each) of the pesticides propanil and clomazone; and 10 μg L−1 of the pesticide cyfluthrin. After a 6-h-simulated agricultural runoff with amended water, mesocosms sat idle for 48 h before flushing with unamended water for another 6 h. Outflow water samples were collected and analyzed for contaminant concentrations. Most significant differences between vegetated mesocosms and controls occurred when comparing mean contaminant transfer/transformation rates post-amendment. Differences among plant species occurred regarding retention of dissolved nutrients orthophosphate, ammonium, and nitrate. Similarly, all three plant species retained more propanil than controls during post-amendment (8–48 h), but individual plant differences occurred with regard to clomazone and cyfluthrin retention. While variation in mitigation of specific dissolved components of nutrients suggests different mechanisms involved in nutrient cycling within our mesocosms, consistent overall total nutrient and pesticide reduction during the post-amendment period indicate that holding runoff in vegetated ditches may reduce transport of agricultural contaminants to downstream aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global agricultural industry is responsible for providing food and fiber to nearly 7.7 billion people (United Nations 2019). According to the World Bank (2016), approximately 37% of the world’s land area are used for agricultural purposes. In many countries where arable land is decreasing due to urbanization, agriculture is forced to intensify their cropping practices, including the use of fertilizers and pesticides to increase crop yields and minimize losses.
Unfortunately, agricultural systems are incapable of complete utilization of applied fertilizers and pesticides. Residual nutrients or pesticides not utilized by plants or soil have the potential to be transported as nonpoint source pollution either in dissolved or particulate forms during storm or irrigation runoff. Such pollution has received increased attention, especially due to eutrophication of freshwater bodies and expansion of hypoxic zones along coastal waters of the USA. Van Meter et al. (2018) reported the largest measured hypoxic zone in the Gulf of Mexico, to date, occurred in August 2017 (22,720 km2). The 2019 hypoxic zone was the eighth largest on record at 18,005 km2 (USEPA 2019).
Climate change has been identified as a primary cause of environmental insecurity impacting freshwater systems globally (Heathwaite 2010). Current challenges of mitigating excess nutrients in agricultural runoff will be exacerbated by precipitation changes in the future. Sinha et al. (2017) suggested that by the end of the century, under business-as-usual operations in the continental USA, riverine total nitrogen (TN) loading will increase 19 ± 14% due to precipitation changes. In the Mississippi-Atchafalaya River Basin, an 18% increase in N loading is projected solely by climate-induced precipitation changes. Compounding these problems is the fact that positive changes in water quality are not always immediate, even after implementation of management practices. Inherent delays, also known as time lags, in water quality have been recorded in scientific literature and serve as a major constraint for water policy development (Vero et al. 2018). Meals et al. (2010) reported time lags between 4 and over 50 years for nitrate concentrations, while Tomer and Burkhart (2003) found time lags of greater than 30 years in two Iowa watersheds. For example, even if agricultural N use efficiency reached 100%, legacy N within the Mississippi River Basin would prevent meeting national target reduction goals for loadings to the Gulf of Mexico (Van Meter et al. 2018). Therefore, business-as-usual management practices or lack of support for conservation efforts will only result in continued water resource degradation. As single stressors are rarely responsible for nonpoint source pollution observed in agricultural ecosystems, research on management practice efficiency should include assessments of complex mixtures of contaminants or multiple stressors and their impact on ecosystem services (Chapman 2012, 2018).
Vegetated drainage ditches surrounding agricultural production acreage are more than simple conduits for water conveyance from the field to nearby rivers, lakes, and streams. Research over the last two decades has demonstrated these systems provide valuable ecosystem services in edge-of-field agricultural areas including, but not limited to, pollutant mitigation and processing, sedimentation, and flood control (Moore et al. 2001, 2008; Taylor et al. 2015; Christopher et al. 2017; Soana et al. 2017; Kumwimba et al. 2018; Vymazal and Březubivá 2018). Trade-offs exist, however, between necessary field drainage requirements, soil interaction, hydraulic retention time, and plant community succession and senescence. The objective of the current experiment was to determine capabilities of three different aquatic plants to mitigate a mixture of nutrients and pesticides potentially found in agricultural runoff.
2 Materials and Methods
Twelve mesocosms (1.3 × 0.7 × 0.6 m) were established using Rubbermaid® polyethylene stock tanks filled with a 22-cm base of sand overlain with 16 cm of Lexington silt loam. Three aquatic macrophytes common in Southern USA drainage ditches were chosen for evaluation of the current experiment: Myriophyllum aquaticum (parrot feather), Polygonum amphibium (water knotweed), and Typha latifolia (common cattail). All soils and plants were collected from previously unamended wetland cells at the University of Mississippi Field Station, Abbeville, MS in early spring. Triplicate mesocosms (similar density with mature plants) were planted in monocultures of each of the three species to be investigated, while three mesocosms were also left unvegetated (soil only) to serve as controls. Water (municipal source, city of Oxford, MS) (18–32 cm deep, depending on the individual mesocosm) was added to each system. Systems acclimated for 6 weeks prior to experimental initiation.
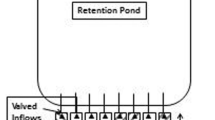
An experiment was designed to determine the mitigation capability of different plant species to nutrient and pesticide contaminants. Simulated runoff was made from municipal source water (city of Oxford, MS) amended with sodium nitrate, ammonium sulfate, and potassium phosphate dibasic as nutrients. Pesticides were also added to the simulated runoff including the herbicides propanil (3′,4′-dichloropropionanilide) (as RiceShot®) and clomazone [2-(2-chlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone] (as Command 3ME®), as well as the insecticide cyfluthrin [cyano(4-fluoro-3-phenoxyphenyl)methyl-3-(2,2-dichloroethenyl)-2,2-dimethyl-cyclopropanecarboxylate] (as Tombstone™). Target concentrations for the nutrient contaminants were 10 mg L−1 each for nitrate (NO3−), ammonium (NH4+), and orthophosphate (PO4−3), while pesticide concentrations were 20 μg L−1 each for propanil and clomazone and 10 μg L−1 for cyfluthrin. Simulated runoff (138–246 L) was prepared in polyethylene mixing chambers (189 L) and delivered to individual mesocosms via FMI® piston pumps (Fluid Metering Incorporated, Syosset, NY) calibrated to deliver the mixture (383–683 mL min−1) to ensure a 6 h hydraulic retention time (HRT) in each mesocosm. The mixture was delivered from the mixing chambers, through the pumps, to individual mesocosms via 0.64 cm × 0.95 cm (ID × OD) vinyl tubing. The same size tubing was placed at the outflow of each mesocosm and used to collect runoff samples in prewashed 500-mL amber glass jars for pesticides and new 237-mL polyethylene cups for nutrients (Fig. 1).
To help simulate effects of water retention structures (e.g., weirs) to improve system mitigation, the original individual mesocosm water level was reduced by 1/3 (46–82 L) prior to addition of simulated runoff. The simulated runoff was applied for 6 h, after which mesocosms were left undisturbed for 48 h before being flushed with unamended municipal water for 6 h (the same volume as used in the simulated storm runoff.) This combined scenario simulated drainages that, while having standing water, still had storage capacity before overflow would begin. The unamended flush after 48 h simulated a second stormflow that would move the water out of the system. In addition to background samples, aqueous outflow samples were collected at 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 24, and 48-h post-application. Samples were again collected at 49, 51, 54, 72, and 168 h to determine mitigation following the unamended water flush. Water quality measurements of dissolved oxygen (DO) (mg L−1), temperature (°C), and pH (s.u.) were taken at background, 2, 4, 8, 12, 24, 48, 54, 72, and 168 h using a Yellow Springs Instruments (YSI)-85 handheld portable meter (DO and temperature) and an Oakton pH meter.
Once aqueous samples were collected, they were immediately taken inside to the United States Department of Agriculture (USDA), Agricultural Research Service (ARS) National Sedimentation Laboratory (NSL) for preservation. A 200-mL sample was filtered through a 0.45-μm membrane filter, and 14 mL were transferred to a polypropylene tube for dissolved nutrient (NO3−-N; NH4+-N; PO43−-P) analysis. Samples in the polypropylene tubes were then frozen until analysis on a Lachat QuikChem 8500 Series 2 Flow Injection Analysis (Hach, Loveland, Colorado). Nutrient analyses were based on APHA (2005) and QuikChem Methods 10-107-04-1-C (NO3−/NO2−), 10-107-06-1-J (NH4+), and 10-115-01-1A (PO43−). By multiplying the individual mesocosm outflow volume (over a prescribed time series) by that time series nutrient concentration, a contaminant load (mg) was calculated and used to determine system retention.
Pesticide samples were preserved with 100-mL ethyl acetate and 8-g KCl. Samples were concentrated to near dryness using an Organomation OA-SYS heating system with N-EVAP-112 nitrogen evaporator. An Agilent Model 7890 gas chromatograph (GC) (Agilent Technologies, Santa Clara, CA) equipped with an Agilent 7693 autosampler and dual G4513A autoinjectors, dual split-splitless inlets, dual capillary columns, Agilent ChemStation, and autoinjector set at 1.0 μL injection volume fast mode were used for all targeted pesticide analyses according to Smith and Cooper (2004) and Smith et al. (2007). The Agilent 7890 GC was equipped with two micro electron capture detectors (μECDs). Column oven temperatures were initially at 65 °C for 1 min; ramp at 10 °C min−1 to 175 °C and hold for 15 min; ramp at 10 °C min−1 to 225 °C and hold for 34 min. Carrier gas used was ultrahigh purity (UHP) helium at 54.5 mL min−1, and inlet temperature at 250 °C. The μECD temperature was 325 °C with a constant make-up gas flow of 60 mL min−1 UHP nitrogen. The analytical column was an Agilent HP 5MS capillary column, 30 m × 0.25 mm i.d. × 0.25 μm film thickness. Detection limit for propanil and cyfluthrin was 0.05 μg L−1, while clomazone’s detection limit was 0.10 μg L−1. Pesticide loads were calculated in an identical manner to nutrient loads as described earlier.
Simple linear regressions and least squares fit on retention of nutrients and pesticides, plant species, and timing were both conducted using (R Core Team 2017). Experimental phases were divided into initial flow (0–6 h), initial stagnation (8–48 h), unamended flush (49–54 h), and final stagnation (72–168 h). Significant differences in effluent nutrient and pesticide concentrations and loads between different plant species were determined with JMP 8.0 software (SAS, Cary, NC, USA) using analysis of variance (ANOVA) at an alpha of 0.05. A Tukey’s HSD followed to determine levels of differences. Individual treatments were compared to controls using Student’s t test at an alpha of 0.05.
3 Results and Discussion
3.1 Vegetation
Mesocosms with T. latifolia contained 38 (±1) plants m−2, while P. amphibium mesocosms had 141 (±10) plants m−2. Both these planting densities were similar to those observed in the field by authors (unpublished data). Individual stem counts for M. aquaticum were unable to be determined due to extensive branching and sensitivity of plant material structure; therefore, percent cover was used to quantify vegetative material in these mesocosms. Myriophyllum aquaticum mesocosms had a mean (±SD) vegetative coverage of 82 (± 28) % of the 0.75 m2 system, which is typical of vegetated drainage ditch coverage in the field.
3.2 Water Quality
Control mesocosms consistently measured higher levels of both DO (range 6.15–10.5 mg L−1) and pH (range 5.57–7.43) than vegetated mesocosms. Mean (±SD) unvegetated control DO measurements were always significantly higher (α = 0.05) than M. aquaticum and P. amphibium (Table 1). Control mesocosms ranged in pH from 5.57–7.43 and most often had significantly greater pH measurements than in vegetated systems. Except one data point (M. aquaticum at 54 h), all measured plant mesocosm pH values were less than 7, indicating that accelerated breakdown of pesticide contaminants through alkaline hydrolysis would likely not occur (Brogan and Relyea 2014).
3.3 Pesticides
Only T. latifolia and P. amphibium mesocosms exhibited initial (6 h) clomazone load retention above 40% (Tables 2 and 3), yet there was still no significant difference among any of the plant species and the control nor among the plant species themselves. The only significant difference (p = 0.0222) was noted at the initial stagnation period (8–48 h) where neither Typha nor Polygonum retained clomazone loads, while Myriophyllum and the unvegetated control retained 100% and 67%, respectively. Some retention with the mesocosms may have occurred with binding of clomazone to limited quantities of organic matter, although that parameter was not measured in the current study. Van Scoy and Tjeerdema (2014) noted microbial degradation of clomazone is possible in favorable conditions. With a log KOW of 2.55, Henry’s Law Constant of 4.14 × 10−8 atm m3 m−1, and hydrolytic stability at a pH range of 4.5–9.5 for 41 d, the physicochemical characteristics of clomazone indicate the tendency of the chemical to reside in the aqueous phase (Van Scoy and Tjeerdema 2014). Because of its high water solubility, hydrolytic stability, low Kd, and slow degradation in natural sunlight, a relatively large amount of clomazone was lost during the unamended water flush (49–54 h) (Table 3).
Propanil load retention in the first 6 h of experimentation indicated no significance between treatments (three plant species) and control, nor between individual plant species themselves. However, significant differences did occur between the control and all three plant species in the initial stagnation period (8–48 h). During this time, P. amphibium, T. latifolia, and M. aquaticum retained 100%, 98%, and 86% of the applied propanil loads, respectively, with p values < 0.05. During the unamended flush (49–54 h), M. aquaticum also managed to retain 100% of the applied propanil load, which was significantly different from the control (p < 0.0001), and by the final stagnation period (72–168 h), all mesocosms had retained 100% of measured propanil loads, including the control (Tables 2 and 3). Propanil is hydrophilic with a log KOW of 2.29 and has a Henry’s Law Constant of 1.74 × 10−4 atm m3 m−1, yet with a KOC ranging between 152 and 800, there is the potential for the pesticide to bind with soil particles (USDA ARS 1995). Danchour et al. (1986) noted the stability of propanil in aqueous solutions ranging from pH 5–8. The more acidic the solution, the slower the observed biodegradation (Danchour et al. 1986). Mesocosm water in the current experiment ranged in pH from 4.90 to 7.60 (Table 1), suggesting propanil would be stable in the aqueous phase. However, based on other physicochemical parameters of propanil (KOW and Henry’s Law Constant), it is possible that the pesticide could passively diffuse through plants, move through the lipid bilayer, and travel into cell fluids (Pilon-Smits 2005). Brogan and Relyea (2017) discovered that submerged aquatic plants were capable of increasing both the surrounding environmental pH and DO, which could drive alkaline hydrolysis of certain pesticides. All plant species utilized in the current study were emergent; thus, gas exchange is facilitated directly through shoots and leaves above the water column rather than through gas films on submerged leaf surfaces (Colmer and Pedersen 2008). Although plant samples were not taken during the experiment, this may provide some evidence for the observed rapidly declining propanil concentrations in outflow water.
There was no significant difference in retention of cyfluthrin loads within the first 6 h of experimentation between the unvegetated control and vegetated treatments (Tables 2 and 3). Cyfluthrin load retention within the first 6 h ranged from 76 to 86% among all treatments and controls. This is likely due to cyfluthrin’s strong tendency to sorb to soil and low tendency to penetrate plant tissue (Casjens 2002). With a log KOW of 5.95, cyfluthrin is not anticipated to be significantly taken up through the plant cell membrane but instead through the root epidermis (Imfeld et al. 2009). Like clomazone and propanil, significant differences existed in the initial stagnation period (8–48 h), where P. amphibium and T. latifolia significantly reduced cyfluthrin loads as compared to the controls (p = 0.0035 and p = 0.0037, respectively) (Table 3). Polygonum amphibium retained significantly less cyfluthrin after the flush event as compared to the control (p = 0.0063), T. latifolia (p = 0.0104), and M. aquaticum (p = 0.0273). Aqueous photolysis and hydrolysis are two primary pathways for abiotic degradation of cyfluthrin, and this may help explain cyfluthrin breakdown in open, unvegetated control treatments.
3.4 Nutrients
No significant differences were noted in PO4−3-P retention within the first 6 h after simulated drainage water was added. Vegetated systems mitigated 39–53% of PO4−3-P loads, while control systems retained 42% (Tables 2 and 3). Significant differences existed during the initial stagnation period, where M. aquaticum (37 ± 11%) and T. latifolia (43 ± 1%) both significantly decreased (either through sorption or uptake) more PO4−3-P than control systems (15 ± 2%) (p = 0.0157 and 0.0407, respectively). The second stagnation period likewise showed significant differences, with T. latifolia (89 ± 4%) and P. amphibium (91 ± 1%) significantly decreased (sorption or uptake) more PO4−3-P than control systems (39 ± 4%) (p = 0.0233 and 0.0208, respectively). Current study results are similar to those of Kasak et al. (2018) who found T. latifolia PO4−3 removal up to 41.8% during the warm period of study. They also noted a strong negative correlation between PO4−3 removal efficiency and flow rate, indicating shorter retention times decrease overall P removal capability when PO4−3 inflow concentrations range from 0.04 to 0.1 mg L−1) (Kasak et al. 2018). Moore and Krӧger (2011) and Moore et al. (2013) reported T. latifolia PO4−3 mitigation of 40 ± 1% and 46.5 ± 11.9%, respectively, with both studies utilizing a 4-h HRT. These retentions are slightly lower than the current study’s T. latifolia 6-h HRT PO4−3-P mitigation of 53 ± 2%. Mitigation of PO4−3 in control (unvegetated) systems after 4 h was 41.7% and 40.8% in Moore and Krӧger (2011) and Moore et al. (2013), compared to the current study results at 6 h of 42 ± 14%. Orthophosphate mitigation by M. aquaticum in the current study (50 ± 6%) at 6 h was similar to that reported by Moore and Krӧger (2011) (59 ± 5%). Moore and Krӧger (2011) utilized an inflow PO4−3-P concentration of 5 mg L−1, while Moore et al. (2013) used a series of PO4−3-P concentrations (5, 2.5, 2.5, and 2.5 mg L−1) spreading over four different application times (first two in the summer, last two in the winter).
As with PO4−3-P retention, no significant differences were noted for NH4+-N retention within the first 6 h, after simulated drainage water was added between vegetated systems and the control (Tables 2 and 3). Both T. latifolia and P. amphibium significantly retained more NH4+-N than control mesocosms during the initial stagnation period (p = 0.0116 and p = 0.0092, respectively). Additionally, P. amphibium retained significantly more NH4+-N than the control during the unamended water flush (49–54 h) (p = 0.0324). However, after the final stagnation period, the control retained significantly more NH4+-N than M. aquaticum (p = 0.0339), T. latifolia (p = 0.0348), and P. amphibium (p = 0.0042). Mean NH4+-N retention values in the current study (Table 3) were similar to those reported by Moore and Krӧger (2011) who found 65 ± 3%, 67 ± 0.2%, and 70 ± 6% NH4+ retention after a 4-h HRT in control, T. latifolia, and Myriophyllum spicatum mesocosms when target influent NH4+-N concentrations were 5 mg L−1. Lu et al. (2018) reported 93% NH4+ removal efficiency of influent NH4+-N concentrations ranging from 7.48 to 13.68 mg L−1 in both control and M. spicatum microcosms (0.6 m3), but only after a 20-d HRT.
Mitigation of NO3−-N loads after 6 h was similar among control and vegetated systems with no significant differences, ranging from 50 ± 10% (P. amphibium) to 59 ± 2% (T. latifolia) (Tables 2 and 3). The only significant difference existed during the initial stagnation period (8–48 h) retention between control mesocosms (10 ± 4%) and P. amphibium (49 ± 8%) (p = 0.0402). Results from the current study regarding T. latifolia NO3−-N load retention after 6 h (59 ± 2%) were similar to those reported by Moore and Krӧger (2011) (50 ± 4%) and Moore et al. (2013) (59 ± 6%). In both the aforementioned studies, HRTs were 4 h. Although the current study found no significant differences between the unvegetated controls and vegetated systems for retention after 6 h, Soana et al. (2017) and Castaldelli et al. (2018) found that vegetated sediments were able to convert and remove more NO3− than bare or unvegetated sediments via denitrification. In low NO3− loading situations, Messer et al. (2017) reported that plant uptake was responsible for 2–3 times more N removal than denitrification, while the processes were responsible for nearly the same N removal when NO3− loading was high. In pulse events, like those observed in drainage ditches surrounding agricultural fields, plant uptake and microbial assimilation are likely responsible for the majority of NO3− removal. However, they serve as temporary sinks, and NO3− may be remineralized over a longer period of time (Messer et al. 2017).
Novel approaches to mitigation of pesticides and nutrients in agricultural runoff will be necessary as storm runoff extremes attributed to climate and anthropogenic changes pose significant threats to aquatic ecosystems (Yin et al. 2018). In order to meet goals established to reduce environmental hotspots, such as hypoxia in the Gulf of Mexico, nations must agree to not only large-scale but also long-term agricultural management practices (Van Meter et al. 2018). One possible solution is the integration of vegetated drainage ditches, already in the production landscape, as a part of a watershed management approach. The current study examined the ability, on a mesocosm scale, of three emergent aquatic plants (T. latifolia, M. aquaticum, and P. amphibium) to mitigate pesticides and nutrients. Of the three species examined, P. amphibium demonstrated the greatest capacity to mitigate both pesticides and nutrients, followed closely by T. latifolia. Myriophyllum aquaticum was the least effective species, with minimal demonstrated mitigation capability for selected pesticides and nutrients. Success of P. amphibium in contaminant mitigation may be tied to its role as a primary producer, sequestering and releasing nutrients, as well as acting as a substrate for macro- and microfauna (Partridge 2001). It is commonly theorized that biofilms present on stems of aquatic plants serve as important microbial habitat and likely play a significant role in pesticide and other contaminant mitigation using aquatic plants. Even if M. aquaticum was found to be an effective treatment, caution should be exercised regarding its use in certain areas where it is considered a non-native invasive plant. Research should continue to identify aquatic vegetation capable of mitigating pollutant mixtures to address current and future water quality challenges. The importance of prioritizing physical (e.g., plant density) and structural characteristics (e.g., emergent vs. submerged or floating, leaf surface area, stem lipid content, etc.) of aquatic vegetation could then be appropriately used for pollutant mitigation measures in various edge-of-field conservation practices.
References
American Public Health Association (APHA). (2005). Standard Methods for the Examination of Water and Wastewater (21st ed.). DC: Washington.
Brogan III, W. R., & Relyea, R. A. (2014). A new mechanism of macrophyte mitigation: how submerged plants reduce malathion’s acute toxicity to aquatic animals. Chemosphere, 108, 405–410.
Brogan III, W. R., & Relyea, R. A. (2017). Multiple mitigation mechanisms: Effects of submerged plants on the toxicity of nine insecticides to aquatic animals. Environmental Pollution, 220, 688–695.
Casjens, H. (2002). Environmental fate of cyfluthrin. www.cdpr.ca.gov.
Castaldelli, G., Aschonitis, V., Vincenzi, F., Fano, E. A., & Soana, E. (2018). The effect of water velocity on nitrate removal in vegetated waterways. Journal of Environmental Management, 215, 230–238.
Chapman, P. M. (2012). Management of coastal lagoons under climate change. Estuarine,Coastal and Shelf Science, 110, 32–35.
Chapman, P. M. (2018). Negatives and positives: Contaminants and other stressors in aquatic ecosystems. Bulletin of Environmental Contamination and Toxicology, 100, 3–7.
Christopher, S. H., Tank, J. L., Mahl, U. H., Yen, H., Arnold, J. G., Trentman, M. T., Sowa, S. P., Herbert, M. E., Ross, J. A., White, M. J., & Royer, T. V. (2017). Modeling nutrient removal using watershed-scale implementation of the two-stage ditch. Ecological Engineering, 108, 358–369.
Colmer, T. D., & Pedersen, O. (2008). Underwater photosynthesis and respiration in leaves of submerged wetland plants: Gas films improve CO2 and O2 exchange. New Phytologist, 177, 918–926.
Danchour, A., Bitton, G., Coste, C. M., & Bastide, J. (1986). Degradation of the herbicide propanil in distilled water. Bulletin of Environmental Contamination and Toxicology, 36, 556–562.
Heathwaite, A. L. (2010). Multiple stressors on water availability at global to catchment scales: understanding human impact on nutrient cycles to protect water quality and water availability in the long term. Freshwater Biology, 55, 241–257.
Imfeld, G., Braeckevelt, M., Kuschk, P., & Richnow, H. H. (2009). Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere, 74, 349–362.
Kasak, K., Kill, K., Pärn, J., & Mander, Ü. (2018). Efficiency of a newly established in-stream constructed wetland treating diffuse agricultural pollution. Ecological Engineering, 119, 1–7.
Kumwimba, M. N., Meng, F., Iseyemi, O. O., Moore, M. T., Zhu, B., Tao, W., Liang, T. J., & Ilunga, L. (2018). Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): design, mechanism, management strategies, and future directions. Science of the Total Environment, 639, 742–759.
Lu, B., Xu, Z., Li, J., & Chai, X. (2018). Removal of water nutrients by different aquatic plant species: an alternative way to remediate polluted rural rivers. Ecological Engineering, 110, 18–26.
Meals, D. W., Dressing, S. A., & Davenport, T. A. (2010). Time lag in response to best management practices: a review. Journal of Environmental Quality, 39, 85–96.
Messer, T. L., Burchell, M. R., Bӧhlke, J. K., & Tobias, C. R. (2017). Tracking the fate of nitrate through pulse-flow wetlands: a mesocosm scale 15N enrichment tracer study. Ecological Engineering, 106, 597–608.
Moore, M. T., & Krӧger, R. (2011). Evaluating plant species-specific contributions to nutrient mitigation in drainage ditch mesocosms. Water Air Soil Pollution, 217, 445–454.
Moore, M. T., Bennett, E. R., Cooper, C. M., Smith Jr., S., Shields Jr., F. D., Milam, C. D., & Farris, J. L. (2001). Transport and fate of atrazine and lambda-cyhalothrin in an agricultural drainage ditch in the Mississippi Delta, USA. Agriculture, Ecosystems and Environment, 87, 309–314.
Moore, M. T., Denton, D. L., Cooper, C. M., Wrysinski, J., Miller, J. L., Reece, K., Crane, D., & Robins, P. (2008). Mitigation assessment of vegetated drainage ditches for collecting irrigation runoff in California. Journal of Environmental Quality, 37, 486–493.
Moore, M. T., Krӧger, R., Locke, M. A., Tyler, H. L., & Cooper, C. M. (2013). Seasonal and interspecific nutrient mitigation comparisons of three emergent aquatic macrophytes. Bioremediation Journal, 17(3), 148–158.
Partridge, J. W. (2001). Biological flora of the British Isles: Persicaria amphibia (L.) Gray (Polygonum amphibium L.). Journal of Ecology, 89, 487–501.
Pilon-Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology, 56, 15–39.
R Core Team. (2017). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org. Accessed 27 Feb 2020.
Sinha, E., Michalak, A. M., & Balaji, V. (2017). Eutrophication will increase during the 21st century as a result of precipitation changes. Science, 357, 405–408.
Smith Jr., S., & Cooper, C. M. (2004). Pesticides in shallow groundwater and lake water in the Mississippi Delta MSEA. In M. Nett, M. A. Locke, & D. Pennington (Eds.), Water Quality Assessments in the Mississippi Delta: Regional Solutions, National Scope. ACS Symposium Series 877 (pp. 91–103). Oxford University Press.
Smith Jr., S., Cooper, C. M., Lizotte Jr., R. E., Locke, M. A., & Knight, S. S. (2007). Pesticides in lake water in the Beasley Lake watershed, 1998–2005. International Journal of Ecology and Environmental Science, 33, 61–71.
Soana, E., Balestrini, R., Vincenzi, F., Bartoli, M., & Castaldelli, G. (2017). Mitigation of nitrogen pollution in vegetated ditches fed by nitrate-rich spring waters. Agriculture, Ecosystems and Environment, 243, 74–82.
Taylor, J. M., Moore, M. T., & Scott, J. T. (2015). Contrasting nutrient mitigation and denitrification potential of agricultural drainage environments with different emergent aquatic macrophytes. Journal of Environmental Quality, 44, 1304–1314.
Tomer, M. D., & Burkhart, M. R. (2003). Long-term effects of nitrogen fertilizer use on ground water nitrate in two small watersheds. Journal of Environmental Quality, 32, 2158–2171.
United Nations. (2019). World population prospects 2017. https://population.un.org/wpp/. Accessed 15 Dec 2019
United States Department of Agriculture, Agricultural Research Service (USDA ARS). (1995). Pesticide properties database: Propanil. https://www.ars.usda.gov/ARSUserFiles/00000000/DatabaseFiles/PesticidePropertiesDatabase/IndividualPesticideFiles/PROPANIL.TXT. Accessed 27 Feb 2020.
United States Environmental Protection Agency (USEPA). (2019). https://www.epa.gov/ms-htf/northern-gulf-mexico-hypoxic-zone. Accessed 15 Dec 2019.
Van Meter, K. J., Van Cappellen, P., & Basu, N. B. (2018). Legacy nitrogen may prevent achievement of water quality goals in the Gulf of Mexico. Science, 360, 427–430.
Van Scoy, A. R., & Tjeerdema, R. S. (2014). Environmental fate and toxicology of clomazone. Reviews of Environmental Contamination and Toxicology, 229, 35–49.
Vero, S. E., Basu, N. B., Van Meter, K., Richards, K. G., Mellander, P. E., Healy, M. G., & Fenton, O. (2018). Review: the environmental status and implications of the nitrate time lag in Europe and North America. Hydrogeology Journal, 26, 7–22.
Vymazal, J., & Březubivá, T. D. (2018). Removal of nutrients, organics and suspended solids in vegetated agricultural drainage ditch. Ecological Engineering, 118, 97–103.
World Bank. (2016). Agricultural land (% of land area). https://data.worldbank.org/indicator/AG.LND.AGRI.ZS. Accessed 27 Feb 2020.
Yin, J., Gentine, P., Zhou, S., Sullivan, S. C., Wang, R., Zhang, Y., & Guo, S. (2018). Large. increase in global storm runoff extremes driven by climate and anthropogenic changes. Nature Communications, 9, 4389. https://doi.org/10.1038/s41467-018-06765-2.
Acknowledgments
Authors thank Lisa Brooks and Renee Russell for sample analyses. The use of trade or corporation names in this publication is for the information and convenience of the reader. Use does not constitute an official endorsement or approval by the USDA or the ARS of any product to the exclusion of others that may be suitable. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moore, M.T., Locke, M.A. Experimental Evidence for Using Vegetated Ditches for Mitigation of Complex Contaminant Mixtures in Agricultural Runoff. Water Air Soil Pollut 231, 140 (2020). https://doi.org/10.1007/s11270-020-04489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04489-y