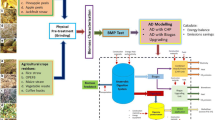
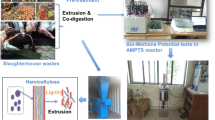
Abstract
Organic wastes from wholesale vegetable, fruit, and flower markets are one among the major sources of centralized waste generation in urban cities, which are predominantly disposed at dumpsites. Sustainable solution for the effective treatment and disposal of these wastes from Indian urban centers needs to be explored. Anaerobic digestion (AD) process coupled with extrusion as a pretreatment prior to AD was studied to increase methane production from vegetable, fruit, and flower market wastes. The detailed waste characterization and bio-methane potential (BMP) tests of vegetable, fruit, and flower market wastes were carried out. From the results of BMP tests, the highest specific methane yield (SMY) of 332.7 mLN/g oDM (31% increase) was obtained for extruded fruit market wastes compared with non-extruded wastes, which showed SMY of 253.9 mLN/g oDM. Similarly, the SMY of vegetable and flower market wastes was significantly increased (p < 0.05) to 319.6 mLN/g oDM (+ 35.5%) and 188.1 mLN/g oDM (+ 42.3%), respectively, as a result of extrusion pretreatment. Further, AD of extruded wastes showed 11.4–17.2% increase in the oDM reduction in concurrence with the increased SMY. The energy potential of extruded and non-extruded wastes varied between 6.4–10.9 MJ/kg DM and 4.7–9.4 MJ/kg DM, respectively. In addition, from the profile of bio-methane production, it is observed that the extruded wastes reached 95% of SMY in 14–17 days unlike non-extruded wastes, which took 17–23 days to reach the same value thereby attaining steady-state condition faster than non-extruded wastes. Among the three models used, the logistic model showed best fit with R2 value of 0.96–0.98 with minimum SMY deviation < 2.8% than first-order kinetic and modified Gompertz models. Results of the study revealed that extrusion pretreatment of wholesale vegetable, fruit, and flower markets wastes prior to AD was found to be effective in increasing the methane production during anaerobic treatment of these wastes from urban centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Growing industrialization and urbanization increase the challenges for managing the wastes being generated from urban cities (Joseph et al. 2012; Salehiyoun et al. 2019). This further increases the per capita waste generation rates and energy demands on the other hand (Shareefdeen et al. 2015). The organic fractions of municipal solid wastes (MSWs) not only increase the load to the landfills/dumpsites but also lead to the under-exploitation of the wastes’ potency for energy generation. These organic fractions include wastes from households, food waste, vegetable, fruit and flower market wastes, garden trimmings, litters etc. Food and Agriculture Organization (FAO) has reported that one third of the produced food is being wasted every year among which the highest wastage is being shared by horticultural commodities of about 60% (FAO 2019). This highest share of horticultural wastages results from various stages that include harvesting, transportation, classification/grading, storage, marketing, and processing (Sagar et al. 2018). Among these stages, by-products of vegetables and fruits discarded as waste during processing share a significant amount and the quantities of fruit and vegetable market waste generation vary among different fruit and vegetables. However, in general, about 18–30% is being discarded as waste materials from many fruits and vegetables (Narnaware et al. 2017; Sachdeva et al. 2013; Mozhiarasi et al. 2019a). In addition to fruits and vegetables, floral wastes are also being generated in huge amounts especially in countries where the flowers are used every day for worship in temples. It was also reported that the wastes from vegetable, fruit, and flower markets are one among the major source of centralized waste generation among organic fractions of MSW in urban cities (Jha et al. 2008). Hence, there is a need for utilization of vegetable, fruit, and flower market wastes from centralized horticultural markets in urban cities. Biological treatment of these wastes is a suitable option in terms of economic viability and environmental pollution reduction (Paraskeva and Diamadopoulos 2006; Bouallagui et al. 2005).
Anaerobic digestion (AD) of organic wastes is one among the biological treatment methods that has gained importance in the recent years since AD process gives output in the form of energy/fuel and nutrient rich bio-fertilizer (Porselvam et al. 2017; Zieliński et al. 2019). Thus environmental-friendly transformation of wastes can be done through AD process that meets the sustainable development goals (SDGs) of the United Nations to attain sustainability, affordable energy, and climate action (goals 7, 11, and 13 of SDGs) (Li et al. 2016; Song et al. 2019). Biological degradation of organic matter through AD involves a series of processes such as hydrolysis, acidogenesis, acetogenesis, and methanogenesis. The sequences of reactions involved in the conversion of biomass to biogas are detailed as follows.
Hydrolysis involves the breakdown of chemical bonds where the anions and cations react with water molecule to facilitate cleavage of H–O bonds by altering the pH (Den et al. 2018; Anukam et al. 2019). This is usually a slow step especially if a solid substrate is utilized, and the reaction involved is presented below (Van et al. 2020).
From Eq. (1), it could be seen that the hydrolysis of cellulose (C6H5O10)n forms glucose (C6H12O6) and releases hydrogen (H2) with the presence of water molecule. This Eq. (1) mainly involves the breakdown of the β-1,4-glycosidic linkages, which in turn creates the accessibility for catalytic transformation (Bertucci et al. 2019; Anukam et al. 2019). Homogeneous or heterogeneous acids catalyze this reaction to form a monosaccharide (C6H12O6) (Lee et al. 2014). Usually, protons (H+) and hydroxide anions (OH−) present in the acid catalysis result from the dissociation of water molecule that catabolizes the cellulose compound to simpler compounds with the release of energy (Karuppiah and Azariah 2019; Anukam et al. 2019). Similarly, the chemical bonds in biomass containing insoluble organics are broken down to form soluble compounds, which can be utilized by the bacteria (Shah et al. 2014). For instance, carbohydrates are broken down to saccharides whereas proteinaceous substances catabolized into amino acids and subsequently lipids into fatty acids (Sikora et al. 2018).
The second step is the acidogenesis, which is a fermentation process where the soluble compounds resulting from the hydrolysis process are converted into volatile fatty acids, alcohols, CO2, and H2 upon the act of acidogenic bacteria (Eqs. (2)–(4)) (Karuppiah and Azariah 2019). The foremost organic acids produced in this stage are acetic acid (CH3COOH), propionic acid (CH3CH2COOH), and butyric acid (CH3CH2CH2COOH), which are the predominate substrate utilized by the methanogens (Goswami et al. 2016).
The next process is the acetogenesis where the volatile fatty acids and alcohols formed from the acidogenesis stage are being converted into acetate and hydrogen by proton reducing bacteria, which will be utilized by methanogenic bacteria in the subsequent stage (Eqs. (5)–(7)) (Venkiteshwaran et al. 2015). The hydrogen released in this phase exhibits toxicity to the acetogenic microorganisms, which in turn utilized by the methanogenic bacteria, therein symbiosis exists between methanogenic and acetogenic bacteria (Shah et al. 2014). It is also stated that the VFA, CO2, and H2 are still being produced in this stage since there will be the presence of small amount of water molecule that acts an electron source for further conversion of the VFA (Moestedt et al. 2019; Anukam et al. 2019).
Methanogenesis is the last stage where the methanogenic bacteria convert the acetic acid and hydrogen into CH4 and CO2. This stage involves the following conversions (Fenchel et al. 2012).
The production of methane and carbon dioxide as a result of decarboxylation of acetic acid could be seen in Eq. (8) (Cazier et al. 2015; Hassan and Nelson 2012). There exists two groups of methane producing bacteria, i.e., acetophilic and hydrogenophilic (Goswami et al. 2016). The former species produces methane by acetate decarboxylation and the latter by reduction of CO2/H2.
Among these four series of processes, hydrolysis is the rate-limiting step of AD process since it has greater influence on the degradation efficiency of the substrate (Weichgrebe 2015; Khanal 2008; Hutñan et al. 1999). Lignin embeds the hemicelluloses and celluloses, and it is hard to degrade lignin because of the presence of three phenyl-propane precursor monomers (Fig. 1). Thus, it prevents the accessibility to the cellulose and hemicellulose compounds, which are biodegradable in nature. This causes the delay in the hydrolysis of these compounds, which often reduces the degradation efficiency of lignocellulosic biomass during AD process (Zheng et al. 2019). The pretreatment of lignocellulosic compounds usually involves the complete deconstruction of the cell wall into small fibers so as to create accessibility (Kucharska et al. 2018). Pretreatment not only helps in the breakdown of the lignin, which protects the hemicellulose and cellulose, but also reduces the susceptibility to enzymatic hydrolysis by reducing the crystallinity of cellulose and increasing the porosity of the compounds for higher accessibility. Thus, the pretreatment for disintegration of lignin would result in increased degradation efficiency (Kamdem et al. 2013; Zheng et al. 2019).
In addition to the breakdown of lignin for a faster hydrolysis process, the particle size of the substrate also plays a vital role in the mass transfer, which in turn influences the methane production (Izumi et al. 2010; Kratky and Jirout 2011). Hence, for particle size reduction of substrate, several pretreatment units like shredder, ball mill, two-roll mill, colloid mill, extruder, etc. are being utilized (Rodriguez et al. 2017). Among these pretreatment units, extruder has been identified as a novel pretreatment unit for biomass conversion to methane since it combines multiple operations in a single unit (Chen et al. 2014; Hjorth et al. 2011; Novarino and Zanetti 2012). As the substrate moves along the extruder barrel, the biomass is subjected to friction heat, mixing, and shearing, which in turn disrupts the structure of the lignocellulosic biomass (Camire 1998). The pressure along with the heat is developed while feeding the biomass through the extruder barrel by a screw die with increasing restrictions, eventually releasing the extruded biomass (Kelley and Walker 2000). This causes the physical and chemical changes in the structure of the biomass as a result of extrusion process due to the sudden drop in the pressure (from approximately 2700 kPa) while the biomass leaves out of the extruder into the atmosphere (Pilarski et al. 2016; Kelley and Walker 2000). This further results in defibrillation, fibrillation, and cutting of the fibers, thus causes the accessibility of the microorganisms to cellulose and hemicelluloses, which increases the methane production (Karunanithy and Muthukumarappan 2011; Maurya et al. 2015). Hjorth et al. (2011) studied the effect of extrusion of agro-wastes and found 18–70% increase in the methane production for different agro-wastes. Chen et al. (2014) studied the impact of extrusion of rice straw on methane production during AD process and found 72% increase in the methane yield than untreated rice straw, which further showed shorter retention period and higher cellulose and hemicellulose degradation efficiencies. Apart from the few existing studies on the impact of extrusion pretreatment on biogas production, there are several studies on extrusion in which the effect of extrusion pretreatment on reducing the bacterial pathogen was mostly studied (Bianchini et al. 2012; Okelo et al. 2008; Kelley and Walker 2000). Further, the impact of extrusion pretreatment for increasing the bioethanol production from lignocellulosic biomass was also studied by Liu et al. (2013) and Lin et al. (2012). However, the existing literature information on the impact of extrusion on the methane production from vegetable, fruit, and flower market wastes from urban cities is not available to the best of our knowledge. While visualizing the existing literatures on vegetable, fruit, and flower market wastes, the pretreatments commonly studied were hydrothermal treatment, alkaline treatment, grinding, acid hydrolysis, mechanical, and ultrasonic pretreatments (Table 1). This information reveals the non-existence of prior information on the effect of extrusion for the treatment of wastes from wholesale vegetable, fruit, and flower market wastes. Hence, the major objective of the present study is to investigate the influence of extrusion on methane production from vegetable, fruit, and flower market wastes. Further, the experimental methane yields were modeled by using first-order kinetic model, modified Gompertz, and logistic models. The energy potential of the wastes was also calculated.
2 Materials and Methods
2.1 Sample Collection
The vegetable, fruit, and flower waste samples were collected from one of Asia’s largest horticultural market Koyambedu wholesale market complex (KWMC) at Koyambedu, Chennai, India. The sampling was carried out by following standard sampling protocol by LAGA protocol ( 2016) and Weichgrebe et al. (2017). About 50 individual waste samples of each 10 L were collected from three to four major heaps from vegetable, fruit, and flower markets separately. The collected waste samples were chilled during transportation at 4 °C. Once the waste samples were brought to the laboratory, they were individually and immediately weighed. Then, the samples were minced using a mincer (La Mineva, A/E12-05) and stored at 4 °C for further experimentation.
2.2 Extrusion of Wastes
The extrusion of vegetable, fruit, and flower market wastes was carried out by a pilot scale twin-screw Bio-Extruder by Lehmann-UMT GmbH (model: MSZ B 22e), Germany (Lehmann 2009). The extruder has a capacity of 200 kg/h. About 100 kg of the collected wastes was fed into the inlet hopper of the extruder, and the output from the extruder was collected from the screw conveyor connected at the outlet of the extruder. The extruder wastes were stored at 4 °C for further use, and the wastes were characterized.
The non-extruded and extruded wastes are represented as follows: vegetable market wastes (VWs), fruit market wastes (FRWs), flower market wastes (FLWs), extruded vegetable market wastes (EVWs), extruded fruit market wastes (EFRWs), and extruded flower market wastes (EFLWs).
2.3 Characterization of Waste Samples
The extruded and non-extruded wastes, VW, FRW, FLW, EVW, EFRW, and EFLW, were characterized in terms of dry matter (DM) and organic dry matter content (oDM), pH, and moisture content as per APHA standard methods (APHA 1998). The pH was measured using a pH meter (EUTECH cyberscan pH 310 meter). Protein and lipid estimations were done by following Bradford (1976) and Frings and Dunn (1970), respectively, based on the UV spectrophotometric method (UV spectrophotometer Shimadzu 1900). Carbohydrate estimation was performed according to Dubois et al. (1956) and fiber analysis by following the methods by Van Soest et al. (1991). The hemicellulose was measured by the differences between NDF and ADF and the calculation of nitrogen-free extract (NfE) by subtracting the oDM by the amounts of protein, lipid, and NDF (Pokój et al. 2018). The elemental analysis was carried out using an elemental analyzer (EA 3000, EURO VECTOR) by following ASTM D5373 standards (ASTM 2014).
2.4 Batch AD Assays
The specific methane yield (SMY) of extruded and non-extruded waste samples was conducted in triplicates in an Automatic Bio-Methane Potential Test System (bioprocess control, AMPTS II) by following standard method VDI 4630 with an analysis period of 30 days (VDI 4630 2006) (Fig. 2). The AMPTS system is provided with thermostatic water bath with 15 reactors (where the sample is fed), CO2 scrubbing unit (with 15 glass bottles), and a gas volume measuring device (flow cell arrangements connected to the data logging system). The substrate/inoculum ratio of 0.5 (g oDM of substrate/g oDM of inoculum sludge) and a temperature of 37 °C were maintained for conducting the batch tests by following VDI 4630 protocol. Once the reactors were fed with the substrate and inoculum, it was immediately sealed and flushed with nitrogen gas to maintain anaerobic environment at the headspace.
From AD tests, the results of SMY are presented as mLN of methane generated per gram of oDM added (mLN/g oDM). The inoculum was collected from an anaerobic digester from the sewage treatment plant, Chennai, which has pH of 7.2 and DM and oDM content of 4.1 wt% and 2.7 wt%, respectively. The pH and ammoniacal nitrogen concentration for digestate samples from AD process were measured according to APHA standard methods (APHA 1998). The ammoniacal nitrogen was measured using a Kjeldahl apparatus (KEL PLUS KES 06L(VA)).The volatile fatty acid (VFA)/alkalinity ratio for digestate samples was measured by direct titration method following Dilallo and Albertson (1961).
2.5 Estimation of Energy Potential
The resultant SMY values of the extruded and non-extruded wastes from the AD tests were converted into energy potential by following Demirbas (1997) by using the gross calorific value of methane as 39.8 MJ/m3, and the energy potentials are expressed on dry matter basis as MJ/kg DM.
2.6 Kinetic Modeling of SMY
Mathematical modeling of the methane production kinetics helps to evaluate the behavior of the substrate within the AD reactor (Ware and Power 2017). Further, modeling of the methane production helps to predict the kinetic parameters therein predicting and monitoring the performance of the AD reactor under different conditions (Pramanik et al. 2019; Chala et al. 2019). While designing full-scale systems, the results of kinetic modeling of methane production help to design and optimize the reactor parameters in comparison with the obtained experimental yields (Teng et al. 2014).
The current study applied the widely used models such as first-order kinetic model, modified Gompertz, and logistic models for modeling the methane production kinetics (Abudi et al. 2016; Duan et al. 2018; Li et al. 2018). Among which, first-order kinetic model is the simplest model and it is based on the assumption that hydrolysis is the rate-limiting step during AD process and this model does not take into account the conditions to reach maximum biological activity and digester failures (Kafle and Chen 2016). However, several researchers used this model to obtain hydrolysis kinetics using the batch BMP data (Li et al. 2018).
The modified Gompertz model is one among the widely used models for modeling the kinetics of biogas production (Salehiyoun et al. 2019; Abudi et al. 2016). Gompertz model was initially developed for human mortality data and organ growth prediction (Kafle and Chen 2016; Gil et al. 2011). However, Gibson et al. (1987) modified this model to describe cell density in terms of exponential growth rate and lag phase period during bacterial growth stage. This information about the lag phase from this model helps to find out the minimum time required for the bacteria to produce biogas once it acclimatizes to the environment (Ware and Power 2017). Further, it describes the specific growth rate (Rm) of methanogenic bacteria, and the higher the value of Rm, the higher is the rate of biogas production (Szlachta et al. 2018).
The logistic model has also been widely used for modeling the biogas production kinetics (Pramanik et al. 2019; Ghatak and Mahanta 2017). This model is based on the assumption that the methane production rate is proportional to the amount of methane produced (Chala et al. 2019). This model gives an estimate of lag phase delay and maximum methane production in addition to the methane production potential of substrate (Deepanraj et al. 2017; Ali et al. 2018).
Microsoft Excel 2010 was used to perform the first-order kinetic model, and the kinetic parameters such as first-order disintegration rate constant (k), statistical indicator (R2), and predicted SMY (Y) were calculated. The statistical software, IBM SPSS Statistics 20, was used for predicting the kinetic parameters of modified Gompertz and logistic models. The equations of first-order kinetic model (Eq. (11)), modified Gompertz (Eq. (12)), and logistic models (Eq. (13)) used for prediction are shown below.
where Y(t) = cumulative methane production (mL/g oDM), Y ultimate methane production potential (mL/g oDM), Rm maximum methane production rate (mL/g oDM/day), t time in day, k first-order model constant (1/day), e exp. (1) = 2.7183, and λ lag phase time (day).
2.7 Statistical Analysis
The evaluation of the datasets was carried out by statistical analyses by means of paired sample t test using IBM SPSS Statistics 20 software, and the statistical significance (in terms of p value) was measured with a confidence interval of 95%.
3 Results and Discussion
3.1 Waste Characterization
The extruded and non-extruded wastes were characterized in terms of DM, oDM, pH, moisture, protein, lipid, carbohydrate, neutral detergent fiber (NDF), acid detergent fiber (ADF), nitrogen-free extract (NfE), and elemental analyses, and the results are presented in Table 2.
From Table 2, for non-extruded wastes (VW, FRW, FLW), the oDM share of DM ranged between 87.5 and 92.6 wt% for all the three wastes and the moisture content varied between 78.1 and 90.1 wt%. Thus, the wastes have high moisture and volatile contents and hence could be a potential source of feedstock for AD (Morales-Polo et al. 2018). The pH of the wastes lies in the acidic range of 4.6–5.5 although the optimal range for AD is 6.8–7.2 (Cioabla et al. 2012). The carbon content of the wastes ranged between 39.6 and 41.7 wt% (on dry basis), and the share of carbon decides the quantity of biogas production although it has influences on other parameters such as pH, C/N ratio, and temperature (Mulka et al. 2015). The results are comparable with the literature values stating the share of carbon in the VW, FRW, and FLW in the range of 39.0 to 44.7 wt% (Jain et al. 2019; Sharma et al. 2017; Choy et al. 2015).
The C/N ratio and temperature greatly affect the degradation efficiency during AD process (Wang et al. 2014). The higher C/N ratio of wastes results in the VFA accumulation due to the high carbon content of the wastes whereas the lower ratio results in the ammonia inhibition due to the high nitrogen content of the wastes. Hence, both higher and lower C/N ratios affect the AD process, which necessitates the waste suitability for AD process (Mozhiarasi et al. 2019b). However, in this study, the C/N ratio of all the three wastes lies in the range of 16–18 still lower than the optimal range of 20–30 reported for a stable AD process (Rabii et al. 2019; Alvarez et al. 2010).
The carbohydrate content of the wastes (VW, FRW, FLW) varied between 592.1 and 696.7 g/kg of DM, which is comparable with the reported literature values in the range of 433.7–830.0 g/kg of DM for similar wastes (Patil and Deshmukh 2015; Morales-Polo et al. 2018; Orozco et al. 2014). The protein content of wastes varied between 115.4 and 168.1 g/kg of DM, which is also comparable with the reported literatures in the range of 95.3–180.6 g/kg of DM (Angulo et al. 2012; Feumba et al. 2016; Sridevi et al. 2012). The lipid content of the wastes is lower compared with carbohydrates and protein content and found in the range of 52.4–84.0 g/kg of DM, which is comparable with the literatures values reported as 33.0–126.1 g/kg of DM (Alibardi and Cossu 2016; Feumba et al. 2016). Hence, carbohydrate is the major macromolecular organic matter that shares more than 59.2% of the DM for all the three wastes. Further, it can be observed that the NDF shares about 60.7% of carbohydrates for FRW and FLW; however, its share increased to 90.3% for FLW due to the presence of high amount of fiber (cellulose, hemicelluloses, and lignin). It could be also seen that the ADF (mixture of cellulose and lignin) shares about 26.7–27.6% of DM for VW and FLW whose presence slows down the rate of biological degradation thereby demanding the need for effective pretreatment prior to AD. Because ADF is one among the several parameters (such as lignin, cellulose, and crude fat) that greatly affects the methane production from a biomass mainly because of the presence of hardly biodegradable organic compounds of plant cell (Rath et al. 2013; Dandikas et al. 2014). For instance, Herrmann et al. (2016) observed 22–37% decrease in the methane yield while substituting maize silage with other fiber rich biomass. Similarly, He et al. (2008) observed 65.2% degradation of ADF after alkaline pretreatment of wastes as a result of hydrolysis of ester bond of lignin-carbohydrate complex. Further, several authors enhanced biological degradation of ADF by the use of white-rot fungi or specific bacterial culture (Pokój et al. 2018; Lianhua et al. 2010; Jamaluddin et al. 2014). Extrusion pretreatment also caused the devillication of fiber thereby augmenting microbial access to the cellulose and hemicellulose compounds in the biomass during AD process (Chen et al. 2014).
From Table 2, for extruded wastes, the moisture content of the wastes (EVW, EFRW, and EFLW) decreased by 0.7–1.0%, which results in an increase in the DM content of wastes after extrusion process by 2.4–8.0% compared with non-extruded wastes. However, the decrease in the moisture was not found to be statistically significant (p > 0.05). Similar observation of 0.5–2.0% loss in moisture was reported by Bianchini et al. (2012) during extrusion of carbohydrate-protein meal. The loss in moisture after extrusion could be due to water evaporation resulting from a sudden drop in the pressure while the substrate leaves out of the extruder barrel (Fallahi et al. 2013; Bianchini et al. 2012). Similar to moisture, the oDM share of DM of EVW, EFRW, and EFLW was decreased by 9.6%, 10.9%, and 4.7% compared with VW, FRW, and FLW, respectively. This decrease can be attributed to the losses in the specific macromolecules such as carbohydrate, protein, and lipid as a result of extrusion. For instance, the carbohydrate content of extruded waste lies within 55.7–61.8% (of DM), which is significantly lower than (p < 0.05) the non-extruded wastes whose share in DM ranged between 59.2 and 69.6%. Similar losses were observed in protein and lipid contents in the range of 3.4–8.6% (of DM) and 1.2–4.9% (of DM), respectively, for extruded wastes than non-extruded wastes. In accordance with the loss in the carbohydrate, the NDF of extruded wastes decreased significantly (p < 0.05) by 9.72%, 14.45%, and 3.87% for EVW, EFRW, and EFLW, respectively. Further, ADF of extruded wastes decreased significantly by 3.99–9.69% than non-extruded wastes (p < 0.05) as a result of structural breakdown of the lignocelluloses as a result of extrusion pretreatment (Kucharska et al. 2018; Chen et al. 2014). Similarly, Chen et al. (2014) and Pilarski et al. (2016) observed 3.1–12.0% losses in cellulose after extrusion of wastes, and these losses show evident that the extrusion process has positive effect on the breakdown of lignin therein creates access to the cellulose and hemicellulose molecules.
3.2 Specific Methane Yield from BMP Tests
The specific methane yield of extruded and non-extruded wastes, such as VW, FRW, FLW, EVW, EFRW, and EFLW, obtained from BMP tests are presented in Fig. 3.
The maximum SMY was attained for EFRW of 332.7 mLN/g oDM, which was significantly higher (p < 0.05) than that of FRW of 253.9 mLN/g oDM (+ 31.0%). The higher SMY of fruit wastes compared with other wastes indicates the presence of high amount of easily bioavailable organics that have facilitated enhanced methane yields (Ware and Power 2016). Further, the enhanced methane yield obtained for EFRW shows the positive effect of extrusion on the methane production from fruit wastes which could be the result of enhanced hydrolysis resulting from extrusion pretreatment (Wahid et al. 2015).
The SMY of VW and EVW is 235.8 mLN/g oDM and 319.6 mLN/g oDM, respectively, thus 35.5% increase in the methane yield as a result of extrusion. The impact of extrusion on SMY of EFLW is significantly higher (p < 0.05), compared with VW and FRW, that shows SMY of 188.1 mLN/g oDM, which is 42.3% higher than non-extruded FLW that yielded SMY of 132.1 mLN/g oDM. Thus, the increase in the SMY ranges from 31.0 to 42.3% as a result of extrusion. Several authors observed similar increase in the methane yield as a result of extrusion process. Chen et al. (2014) observed 72.2% increase in the methane yield of rice straw during extrusion pretreatment while Pilarski et al. (2016) observed 35.3% increase in methane yield of maize silage straw. Wahid et al. (2015) observed 29.0% increase in the methane yield of straw after 28 days of AD study. Among the extruded wastes, FLW showed higher increase in the SMY of about 42.3%, which could be due to the high DM content of the wastes compared with VW and FRW. Thus, the increase in the DM content of wastes is positively correlated with SMY during AD of extruded wastes (Pilarski et al. 2016).
The SMY of non-extruded wastes obtained in the current study is comparable with the previously reported literatures for VW and FRW in the range of 217.8–300.0 mLN/g oDM (Zeynali et al. 2017; Babaee and Shayegan 2011; Maile et al. 2016; Seswoya et al. 2019). Similarly, the SMY of FLW is higher than the reported SMY of 81.5 mLN/g oDM for rose stalk (Liang et al. 2016). However, while comparing with the previously reported literatures on various pretreatments, it could be found that the pretreatment enhanced the SMY of VW and FRW in the range of 232.4–326.0 mLN/g oDM (Zeynali et al. 2017; Qiao et al. 2011; Zhang et al. 2013). This is comparable with the results obtained in the current study as 332.7 mLN/g oDM and 319.6 mLN/g oDM for EFRW and EVW, respectively, as a result of extrusion pretreatment.
In addition to the increased methane yield during extrusion, it could also be observed that the extruded samples reached steady-state condition faster than non-extruded samples. For instance, VW reached 95% of its SMY in 23 days where EVW reached in 17 days. Similarly, FLW reached 95% of produced SMY in 18 days while EFLW yielded in 14 days. However, both FRW and EFRW yielded 95% SMY in 17 days; still, the differences in their SMY yields remain as 25.2% as an impact of extrusion. Thus, all the extruded samples finally reached a plateau in the maximum methane yield in almost less than 20 days. The presence of less elongated S-shaped curves for the non-extruded wastes could be attributed to the short delay in the intermediate hydrolysis unlike non-extruded samples where the uniform steady rise indicate that the extruded process favored enhanced hydrolysis of wastes. Hence, it is evident from the methane production profile that extrusion not only increases the methane production but also reduces the digestion period.
From the SMY profile (Fig. 3), it can be seen that VW and FRW show greater fluctuations with subsequent rise and fall in the methane production profile. In contrast, EVW and EFRW show much steady and gradual rise in the methane production, thereby indicating the steady methane production during AD of extruded wastes.
3.3 Process Parameters After AD Tests
The digestate samples after AD tests were characterized for process parameters such as pH, VFA/alkalinity ratio, ammoniacal nitrogen, and oDM reduction, and the results are presented in Table 3.
Among the reactor parameters, pH is one of the important parameters that decide the reactor performance. Because anaerobic microorganisms, in specific, methanogens that gives the ultimate value added end product, i.e., methane from AD process, functions effectively at a pH range of 6.5–8.0, although the optimal lies between 7.0 and 7.2 for maximal methane yield (Carotenuto et al. 2016). In AD reactors, the drop in the pH is mainly due to acidic nature of the substrates or the higher concentrations of VFA produced during the process. At a pH value below 6.6, the metabolism of methanogens is greatly affected whereas the higher alkaline pH causes the disintegration of microbial granules, both of which influence the reactor stability (Reungsang et al. 2012). However, from Table 3, in this study, it can be observed that the pH of both extruded and non-extruded wastes lies within 7.0–7.2, which is optimal for a stable AD process (Cioabla et al. 2012).
VFA/alkalinity ratio is one of the reactor parameters that decide the AD reactor performance and the VFA/alkalinity ratio of all the digestate samples were less than 0.4, which is found to be in the acceptable limit for AD (Callaghan et al. 2002; Sridevi et al. 2012). The ammoniacal nitrogen concentration in the AD reactor also has inhibitory effect on the methane production, and several authors observed inhibitions while the concentrations in AD reactor exceed 1000–1500 mg/L (Hegde and Trabold 2019; Chen et al. 2008). The ammoniacal nitrogen is below the inhibitory concentration in all the reactor samples containing extruded and non-extruded wastes. These results are comparable with the reported ammoniacal nitrogen concentration during AD of organic fractions of MSW in the range of 97–337 mg/L (Shi et al. 2017; El-Hadj et al. 2009; Cuetos et al. 2008).
Upon extrusion of wastes, the oDM reduction of FRW increased significantly (p < 0.05) from 60.5 to 76.3% and the oDM reduction is positively correlated with the increase in the methane yields observed from AD tests. The higher oDM reduction is a result of high degree of biodegradability (Lee et al. 2019). Yu et al. (2008) observed 12% increase in the methane production when the biodegradability of substrate was increased as a result of pretreatment prior to AD process. Similar to EFRW, a significant increase in the oDM reduction (p < 0.05) from 55.5 to 72.7% and from 32.4 to 43.8% was observed for EVW and EFLW, respectively. Similarly, Novarino and Zanetti (2012) observed a maximum of 70% oDM reduction from AD of extruded organic fractions of MSW. The results of increased oDM reduction, in addition to higher SMY of extruded wastes, further confirm the soundness of the extrusion process as a pretreatment prior to AD process.
The various factors that influence the SMY values such as pH, VFA/alkalinity ratio, and ammoniacal nitrogen were found to be in the optimal limit for both the extruded and non-extruded wastes and hence do not impact the SMY value in this study (Fig. 3 and Table 3). However, in addition to the above process parameters, the waste composition also plays an inherent role in influencing the SMY value. In this study, while comparing the SMY value with the waste composition, it could be seen that the lower ADF contained substrate showed greater SMY value (FRW, EFRW) than the rest, which could be due to the higher rate of biodegradability that is evident from the higher oDM reduction. Also, it was observed that the higher the NfE content of waste, the higher is the SMY value obtained and found low for FLW due to its low NfE content since the NfE comprises predominantly of easily degradable compounds (sugars, starch, and pectin) thereby resulting in faster degradation (Costa et al. 2016). Also, the higher DM and oDM content of the wastes increased the efficiency of extrusion pretreatment with an increased SMY value of about 42.3% than non-extruded FLW.
3.4 Results of Estimation of Energy Potential of Wastes
The results of energy potential of wastes such as VW, FRW, FLW, EVW, EFRW, and EFLW are presented (Fig. 4).
The highest energy potential of 10.9 MJ/kg DM was obtained for EFRW, which is due to the higher SMY of the waste as a result of extrusion. The second and third highest energy potentials of 10.1 MJ/kg DM and 9.4 MJ/kg DM were obtained for EVW and FRW, respectively (Fig. 4). The energy contents of the remaining samples were 8.2, 6.4, and 4.7 MJ/kg DM for VW, EFLW, and FLW, respectively. Thus as a result of extrusion, the energy content of the wastes increased by 16.7%, 22.4%, and 35.7% for EFRW, EVW, and EFLW compared with FRW, VW, and FLW, respectively. The results are comparable with the previous studies on the energy potential of vegetable, cabbage, lettuce, fruits, MSW, and green wastes reported in the range of 7.8–14.1 MJ/kg DM (Hla and Roberts 2015; Mozhiarasi et al. 2019a; Alsabbagh 2019; Mahgoub et al. 2018; Srivastava et al. 2014).
3.5 Results of Kinetic Modeling of Methane Production
The results of kinetic modeling of SMY, obtained from AD tests, using first-order kinetic model, modified Gompertz model, and logistic model for VW, FRW, FLW, EVW, EFRW, and EFLW are presented in Table 4 and Fig. 5.
From the results of first-order kinetic model (Table 4), it was observed that the value of first-order disintegration constant (k) increased for all the extruded samples compared with the non-extruded samples. There is a faster rate of increase in the methane production with the increase in the k value (Budiyono and Sumardiono 2014). Thus, the value of k for non-extruded wastes ranged between 0.15 and 0.19 day−1 whereas for extruded samples, the k value lies between 0.21 and 0.23 day−1, and hence, the extruded wastes reveal faster methane production than the non-extruded wastes.
From the results of modified Gompertz and logistic models, it was found that the lag phase for all the wastes lies below 1.4 days, which could be due to the presence of soluble degradable matters and active inoculum (Kafle and Chen 2016). The maximum methane production rate (Rm) for non-extruded wastes varied between 10.7 and 16.6 mLN/g oDM day whereas for extruded wastes, the Rm increased to 25.0–29.0 mLN/g oDM day, which reveals an enhancement in the rate of methane production as a result of extrusion process.
From the methane production curves (Fig. 5), it could be seen that the first-order kinetic model deviates during the start of the incubation period whereas tends to coincide with the experimental methane yield during the stationary period. This could be because of the assumption of the first-order kinetic model that hydrolysis is the rate-limiting step; however, it fails often for a substrate containing low fat content where there is a comparatively faster rate of hydrolysis (Ware and Power 2017). In contrast, the curves obtained from both modified Gompertz and logistic models coincide well with the experimental methane yield both during the incubation and stationary periods. Further from the visualization of the SMY profile obtained from all the three models, the more balanced SMY pattern could be seen for extruded wastes than non-extruded wastes that could be due to the increased rate of methane production (Fig. 5). This shows that the extrusion as a pretreatment increased the bioavailability of organics that accelerated the rate of degradation (Ware and Power 2017). This can be further visualized by the decrease in the λ values for all the extruded wastes compared with non-extruded wastes.
It was also observed that the difference between the measured and calculated SMY values of first-order kinetic model, modified Gompertz, and logistic model were 0.1–0.9%, 0–4.5%, and 0–2.8%, respectively (Table 3). Thus, the low deviation between the calculated and measured SMY was observed for first-order kinetic model. But R2 value that represents the accuracy in calculation of a model varied between 0.68 and 0.95 for first-order kinetic model. Hence, the first-order model does not show its accuracy in prediction. In contrast, both modified Gompertz and logistic model exhibited high R2 value in the range of 0.96–0.99 and 0.96–0.98, respectively. Further, the maximum methane production rate (Rm) obtained from both modified Gompertz and logistic model was highest for EFMW in concurrence with the highest SMY. This further reveals the accuracy of the prediction models used. Hence, considering both the measured and predicted SMY and R2 value, logistic model shows its best fitness for predicting SMY from experimental SMY than other two models used.
4 Conclusion
The impact of extrusion as a pretreatment on methane production from wholesale vegetable, fruit, and flower market wastes was studied. From the results of characterization of extruded and non-extruded wastes, minor losses in the moisture and macromolecules were observed after extrusion pretreatment which evidence the occurrence of prehydrolysis as a result of extrusion pretreatment. This could be observed from the increase in the SMY of extruded wastes than non-extruded wastes. The highest SMY of 332.7 mLN/g oDM with oDM reduction of 76.3 wt% was obtained for EFRW. The extrusion process significantly increased (p < 0.05) the SMY of VW, FRW, and FLW by 35.5%, 31.0%, and 42.3%, respectively. Thus, extrusion has more effect on the FLW compared with VM and FRW due to the high DM content of FLW in comparison. The increase in the SMY of extruded wastes could be attributed to the increase in the oDM reduction of VM, FRW, and FLW by 17.2%, 15.8%, and 11.4% using extrusion pretreatment. In concurrence with SMY, the energy potential of the wastes increased by 22.4%, 16.7%, and 35.7% for VW, FRW, and FLW, respectively, with the highest value obtained for EFRW of 10.9 MJ/kg DM. In addition to the increased SMY and oDM reduction, extruded wastes reached a steady-state condition faster than non-extruded wastes. Thus, extrusion as a pretreatment prior to AD could be effectively used for increasing the methane yields from the vegetable, fruit, and flower market wastes. Further, results of kinetic modeling of SMY showed best fit from the kinetic yields obtained from logistic model than first-order kinetic model and modified Gompertz model.
Taking in account the huge amount of wastes generated from centralized wholesale markets from urban centers and the attained SMY results as a result of extrusion pretreatment, it can be stated that the anaerobic treatment coupled with extrusion pretreatment of wastes could be a viable solution for reducing the wastes entering into the dumpsites thereby yielding a “green fuel,” which can be used as an alternative source of fuel. Further research on the effect of extrusion by increasing the dry matter content of wastes by anaerobic co-digestion of organic wastes needs to be explored for selecting the most efficient organic fractions suitable for extrusion in terms of energy and economic efficiency.
Abbreviations
- AD:
-
anaerobic digestion
- BMP:
-
bio-methane potential
- SMY:
-
specific methane yield
- MSW:
-
municipal solid wastes
- FAO:
-
Food and Agriculture Organization
- SDGs:
-
sustainable development goals
- VW:
-
vegetable market wastes
- FRW:
-
fruit market wastes
- FLW:
-
flower market wastes
- EVW:
-
extruded vegetable market wastes
- EFRW:
-
extruded fruit market wastes
- EFLW:
-
extruded flower market wastes
- DM:
-
dry matter
- oDM:
-
organic dry matter
- VFA:
-
volatile fatty acids
- NDF:
-
neutral detergent fiber
- ADF:
-
acid detergent fiber
- NfE:
-
nitrogen free extract
- mLN :
-
normalized milliliter, volume of methane gas corrected to standard temperature and pressure
- BMBF:
-
German Federal Ministry of Education and Research
- DST:
-
Department of Science and Technology
- IGSTC:
-
Indo-German Science and Technology Centre
- CLRI:
-
Central Leather Research Institute
- RESERVES:
-
Resource and energy reliability by co-digestion of veg-market and slaughterhouse waste
References
Abudi, Z. N., Hu, Z., Sun, N., Xiao, B., Rajaa, N., Liu, C., & Guo, D. (2016). Batch anaerobic co-digestion of OFMSW (organic fraction of municipal solid waste), TWAS (thickened waste activated sludge) and RS (rice straw): influence of TWAS and RS pretreatment and mixing ratio. Energy, 107, 131–140. https://doi.org/10.1016/j.energy.2016.03.141.
Achinas, S., Li, Y., Achinas, V., & Euverink, G. J. W. (2019). Biogas potential from the anaerobic digestion of potato peels: process performance and kinetics evaluation. Energies, 12(12), 1–16. https://doi.org/10.3390/en12122311.
Ali, M. M., Bilai, B., Dia, N., Youm, I., & Ndongo, M. (2018). Modeling the kinetics of methane production from slaughterhouse waste and Salvinia Molesta: Batch digester operating at ambient temperature. Energy and Power, 8(3), 61–70. https://doi.org/10.5923/j.ep.20180803.01.
Alibardi, L., & Cossu, R. (2016). Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Management, 47, 69–77. https://doi.org/10.1016/j.wasman.2015.07.049.
Alsabbagh, M. (2019). Mitigation of CO2 emissions from the municipal solid waste sector in the Kingdom of Bahrain. Climate, 7(8), 1–24. https://doi.org/10.3390/cli7080100.
Alvarez, J. A., Otero, L., & Lema, J. M. (2010). A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresource Technology, 101(4), 1153–1158. https://doi.org/10.1016/j.biortech.2009.09.061.
American Public Health Association (APHA). (1998). Standard Methods for the Examination of Water & Wastewater. Washington DC: American Public Health Association.
Anukam, A., Mohammadi, A., Naqvi, M., & Granström, K. (2019). A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes, 7(8), 1–19. https://doi.org/10.3390/pr7080504.
ASTM (2014). American Society for Testing and Materials. Standard test methods for determination of carbon, hydrogen and nitrogen in analysis samples of coal and carbon in analysis samples of coal and coke. ASTM D5373. ASTM International, West Conshohocken.
Angulo, J., Mahecha, L., Yepes, S. A., Yepes, A. M., Bustamante, G., Jaramillo, H., Valencia, E., Villamil, T., & Gallo, J. (2012). Quantitative and nutritional characterization of fruit and vegetable waste from marketplace: a potential use as bovine feedstuff. Journal of Environmental Management, 95, 203–209. https://doi.org/10.1016/j.jenvman.2010.09.022.
Babaee A., Shayegan, J. (2011). Effect of organic loading rates (OLR) on production of methane from anaerobic digestion of vegetables waste. World Renewable Energy Congress – Sweden, Linköping; Sweden. https://doi.org/10.3384/ecp11057411.
Bertucci, M., Calusinska, M., Goux, X., Rouland-Lefèvre, C., Untereiner, B., Ferrer, P., Gerin, P. A., & Delfosse, P. (2019). Carbohydrate hydrolytic potential and redundancy of anaerobic digestion microbiome exposed to acidosis uncovered by metagenomics. Applied and Environmental Microbiology, 85(15), 1–16. https://doi.org/10.1128/aem.00895-19.
Bianchini, A., Stratton, J., Weier, S., Hartter, T., Plattner, B., Rokey, G., Hertzel, G., Gompa, L., Martinez, B., & Eskridge, K. M. (2012). Validation of extrusion as a killing step for Enterococcus faecium in a balanced carbohydrate-protein meal by using a response surface design. Journal of Food Protection, 75(9), 1646–1653. https://doi.org/10.4315/0362-028x.jfp-12-085.
Bouallagui, H., Touhami, Y., Cheikh, R., & Hamdi, M. (2005). Bioreactor performance in anaerobic digestion of fruit and vegetable waste. Biochemistry Process, 40(3), 989–995. https://doi.org/10.1016/j.procbio.2004.03.007.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1006/abio.1976.9999.
Budiyono, I. S., & Sumardiono, S. (2014). Kinetic model of biogas yield production from vinasse at various initial pH: comparison between modified Gompertz model and first order kinetic model. Research Journal of Applied Sciences, Engineering and Technology, 7(13), 2798–2805. https://doi.org/10.19026/rjaset.7.602.
Calabrò, P. S., Greco, R., Evangelou, A., & Komilis, D. (2015). Anaerobic digestion of tomato processing waste: effect of alkaline pretreatment. Journal of Environmental Management, 163, 49–52. https://doi.org/10.1016/j.jenvman.2015.07.061.
Callaghan, F., Wase, D., Thayanithy, K., & Forster, C. (2002). Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass and Bioenergy, 27, 71–77. https://doi.org/10.1016/S0961-9534(01)00057-5.
Camire, M. E. (1998). Chemical changes during extrusion cooking: recent advances. In F. Shahidi, C. T. Ho, & N. Van Chuyen (Eds.), Process-induced chemical changes in food (pp. 109–121). New York: Plenum Press.
Carotenuto, C., Guarino, G., Morrone, B., & Minale, M. (2016). Temperature and pH effect on methane production from buffalo manure anaerobic digestion. International Journal of Heat and Technology, 34(2), S425–S429. https://doi.org/10.18280/ijht.34S233.
Cazier, E. A., Trably, E., Steyer, J. P., & Escudie, R. (2015). Biomass hydrolysis inhibition at high hydrogen partial pressure in solid-state anaerobic digestion. Bioresource Technology, 190, 106–113. https://doi.org/10.1016/j.biortech.2015.04.055.
Chala, B., Oechsner, H., & Müller, J. (2019). Introducing temperature as variable parameter into kinetic models for anaerobic fermentation of coffee husk, pulp and mucilage. Applied Sciences, 9(3), 1–15. https://doi.org/10.3390/app9030412.
Chen, Y., Cheng, J. J., & Creamer, K. S. (2008). Inhibition of anaerobic digestion process: a review. Bioresource Technology, 99, 4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057.
Chen, X., Zhang, Y., Gu, Y., Liu, Z., Shen, Z., Chu, H., & Zhou, X. (2014). Enhancing methane production from rice straw by extrusion pretreatment. Applied Energy, 122, 34–41. https://doi.org/10.1016/j.apenergy.2014.01.076.
Choy, S. Y., Wang, K., Qi, W., Wang, B., Chen, C. L., & Wang, J. Y. (2015). Co-composting of horticultural waste with fruit peels, food waste, and soybean residues. Environmental Technology, 36(11), 1448–1456. https://doi.org/10.1080/09593330.2014.993728.
Cioabla, A., Ionel, I., Dumitrel, G. A., & Popescu, F. (2012). Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnology for Biofuels, 5(1), 1–9. https://doi.org/10.1186/1754-6834-5-39.
Costa, M. S. S. M., Lucas, J., Costa, L. A. M., & Orrico, A. C. A. (2016). A highly concentrated diet increases biogas production and the agronomic value of young bull’s manure. Waste Management, 48, 521–527. https://doi.org/10.1016/j.wasman.2015.09.038.
Cuetos, M. J., Gomez, X., Otero, M., & Moran, A. (2008). Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: Influence of co-digestion with the organic fraction of municipal solid waste (OFMSW). Biochemical Engineering Journal, 40, 99–106. https://doi.org/10.1016/j.bej.2007.11.019.
Dandikas, V., Heuwinkel, H., Lichti, F., Drewes, J. E., & Koch, K. (2014). Correlation between biogas yield and chemical composition of energy crops. Bioresource Technology, 174, 316–320. https://doi.org/10.1016/j.biortech.2014.10.019.
Deepanraj, B., Sivasubramanian, V., & Jayaraj, S. (2017). Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. International Journal of Hydrogen Energy, 42(42), 26522–26528. https://doi.org/10.1016/j.ijhydene.2017.06.178.
Demirbas, A. (1997). Calculation of higher heating values of biomass fuels. Fuel, 76(5), 431–434. https://doi.org/10.1016/S0016-2361(97)85520-2.
Den, W., Sharma, V. K., Lee, M., Nadadur, G., & Varma, R. S. (2018). Lignocellulosic biomass transformations via greener oxidative pretreatment processes: access to energy and value-added chemicals. Frontiers in Chemistry, 6, 1–23. https://doi.org/10.3389/fchem.2018.00141.
Dilallo, R., & Albertson, O. E. (1961). Volatile acids by direct titration. Journal Water Pollution Control Federation, 33(4), 356–365.
Duan, N., Ran, X., Li, R., Kougias, P., Zhang, Y., Lin, C., & Liu, H. (2018). Performance evaluation of mesophilic anaerobic digestion of chicken manure with algal digestate. Energies, 11(7), 1–11. https://doi.org/10.3390/en11071829.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugar S and related substances. Analytical Chemistry, 28, 350–356. https://doi.org/10.1021/acs.energyfuels.9b01089.
El-Hadj, T. B., Astals, S., Gali, A., Mace, S., & Mata-Alvarez, J. (2009). Ammonia influence in anaerobic digestion of OFMSW. Water Science and Technology, 59, 1153–1158. https://doi.org/10.2166/wst.2009.100.
Fallahi, P., Rosentrater, K. A., Muthukumarappan, K., & Tulbek, M. (2013). Effects of steam, moisture, and screw speed on physical properties of DDGS-based extrudates. Cereal Chemistry Journal, 90(3), 186–197. https://doi.org/10.1094/cchem-08-12-0102-r.
Fenchel, T., King, G. M., & Blackburn, T. H. (2012). Bacterial metabolism. In Book: Bacterial Biogeochemistry, chapter 1, 1–34. https://doi.org/10.1016/b978-0-12-415836-8.00001-3.
Feumba, D. R., Ashwini, R. P., & Ragu, S. M. (2016). Chemical composition of some selected fruit peels. European Journal of Food Science and Technology, 4(4), 12–21.
Food and Agriculture Organization (FAO) of the United Nations (2019). http://www.fao.org/food-loss-and-food-waste/en/. Accessed on 19 October 2019.
Forgács, G., Pourbafrani, M., Niklasson, C., Taherzadeh, & M. J., Hováth, I. S. (2011). Methane production from citrus wastes: process development and cost estimation. Journal of Chemical Technology & Biotechnology, 87(2), 250–255. https://doi.org/10.1002/jctb.2707.
Frings, C. S., & Dunn, R. T. (1970). A colorimetric method for determination of total serum lipids by the sulpho-phospho-vanillin reaction. American Journal of Clinical Pathology, 53, 89–91. https://doi.org/10.1093/ajcp/53.1.89.
Ghatak, M. D., & Mahanta, P. (2017). Kinetic model development for biogas production from cattle dung. In International conference on functional materials, characterization, solid state physics, power, thermal and combustion energy. AIP conference proceedings 1859, 020010 (pp. 1–7). https://doi.org/10.1063/1.4990163.
Gibson, A. M., Bratchell, N., & Roberts, T. A. (1987). The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type a in pasteurized pork slurry. Journal of Applied Bacteriology, 62(6), 479–490. https://doi.org/10.1111/j.1365-2672.1987.tb02680.x.
Gil, M. M., Miller, F. A., Brandão, T. R. S., & Silva, & Cristina, L. M. S. (2011). On the use of the Gompertz model to predict microbial thermal inactivation under isothermal and non-isothermal conditions. Food Engineering Reviews, 3(1), 17–25. https://doi.org/10.1007/s12393-010-9032-2.
Goswami, R., Chattopadhyay, P., Shome, A., Banerjee, S. N., Chakraborty, A. K., Mathew, A. K., & Chaudhury, S. (2016). An overview of physico-chemical mechanisms of biogas production by microbial communities: a step towards sustainable waste management. 3 Biotech, 6(1), 1–33. https://doi.org/10.1007/s13205-016-0395-9.
Hassan, A. N., & Nelson, B. K. (2012). Invited review: anaerobic fermentation of dairy food wastewater. Journal of Dairy Science, 95(11), 6188–6203. https://doi.org/10.3168/jds.2012-5732.
He, Y., Pang, Y., Liu, Y., Li, X., & Wang, K. (2008). Physicochemical characterization of Rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy & Fuels, 22(4), 2775–2781. https://doi.org/10.1021/ef8000967.
Hegde, S., & Trabold, T. A. (2019). Anaerobic digestion of food waste with unconventional co-substrates for stable biogas production at high organic loading rates. Sustainability, 11, 1–15. https://doi.org/10.3390/su11143875.
Herrmann, C., Idler, C., & Heiermann, M. (2016). Biogas crops grown in energy crop rotations: linking chemical composition and methane production characteristics. Bioresource Technology, 206, 23–35. https://doi.org/10.1016/j.biortech.2016.01.058.
Hjorth, M., Granitz, K., Adamsen, A. P. S., & Moller, H. B. (2011). Extrusion as a pretreatment to increase biogas production. Bioresource Technology, 102, 4989–4994. https://doi.org/10.1016/j.biortech.2010.11.128.
Hla, S. S., & Roberts, D. (2015). Characterisation of chemical composition and energy content of green waste and municipal solid waste from Greater Brisbane, Australia. Waste Management, 41, 12–19. https://doi.org/10.1016/j.wasman.2015.03.039.
Hutñan, M., Drtil, M., Derco, J., & Mrafková, L. (1999). Methanogenic and nonmethanogenic activity of granulated sludge in anaerobic baffled reactor. Chemical Papers, 53, 374–378.
Izumi, K., Okishio, Y., Nagao, N., Niwa, C., Yamamoto, S., & Toda, T. (2010). Effects of particle size on anaerobic digestion of food waste. International Biodeterioration & Biodegradation, 64(7), 601–608. https://doi.org/10.1016/j.ibiod.2010.06.013.
Jain, M. S., Daga, M., & Kalamdhad, A. S. (2019). Variation in the key indicators during composting of municipal solid organic wastes. Sustainable Environment Research, 29(1), 1–8. https://doi.org/10.1186/s42834-019-0012-9.
Jamaluddin, M. F., Zainol, N., Abdul-Rahmanb, R., Abdul-Ghaffar, N. F., & Salihon, J. (2014). Comparison of anaerobic lignin degradation of banana stem waste using mixed culture from Malaysian soil and pure strains from soil culture. Asian Journal of Microbiology, Biotechnology and Environmental Sciences, 16(3), 551–560.
Jha, A. K., Sharma, C., Singh, N., Ramesh, R., Purvaja, R., & Gupta, P. K. (2008). Greenhouse gas emissions from municipal solid waste management in Indian mega-cities: a case study of Chennai landfill sites. Chemosphere, 71(4), 750–758. https://doi.org/10.1016/j.chemosphere.2007.10.024.
Joseph, K., Rajendiran, S., Senthilnathan, R., & Rakesh, M. (2012). Integrated approach to solid waste management in Chennai: an Indian metro city. Journal of Material Cycles and Waste Management, 14, 75–84. https://doi.org/10.1007/s10163-012-0046-0.
Kafle, G. K., & Chen, L. (2016). Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Management, 48, 492–502. https://doi.org/10.1016/j.wasman.2015.10.021.
Kamdem, I., Hiligsmann, S., Vanderghem, C., Bilik, I., Paquot, M., & Thonart, P. (2013). Comparative biochemical analysis during the anaerobic digestion of lignocellulosic biomass from six morphological parts of Williams Cavendish banana (triploid Musa AAA group) plants. World Journal of Microbiology and Biotechnology, 29(12), 2259–2270. https://doi.org/10.1007/s11274-013-1392-3.
Karunanithy, C., & Muthukumarappan, K. (2011). Optimization of alkali soaking and extrusion pretreatment of prairie cord grass for maximum sugar recovery by enzymatic hydrolysis. Biochemical Engineering Journal, 54(2), 71–82. https://doi.org/10.1016/j.bej.2011.02.001.
Karuppiah, T., & Azariah, E. V. (2019). Biomass Pretreatment for Enhancement of Biogas Production. In Anaerobic Digestion. IntechOpen. https://doi.org/10.5772/intechopen.82088.
Kelley, T. R., & Walker, P. M. (2000). Bacterial concentration reduction in swine waste amended livestock feed using a single-screw dry-extrusion process. Bioresource Technology, 75(3), 189–195. https://doi.org/10.1016/s0960-8524(00)00063-8.
Khanal, S. K. (2008). Anaerobic biotechnology for bioenergy production: principles and applications (p. 2008). West Sussex: Wiley-Blackwell, Wiley.
Kratky, L., & Jirout, T. (2011). Biomass size reduction machines for enhancing biogas production. Chemical Engineering & Technology, 34, 391–399. https://doi.org/10.1002/ceat.201000357.
Kucharska, K., Rybarczyk, P., Hołowacz, I., Łukajtis, R., Glinka, M., & Kamiński, M. (2018). Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules, 23(11), 2937. https://doi.org/10.3390/molecules23112937.
Kulkarni, M. B., & Ghanegaonar, P. M. (2020). Anaerobic digestion, enhancement in biogas production and quality improvement. In P. Pawar, B. Ronge, R. Balasubramaniam, A. Vibhute, & S. Apte (Eds.), Techno-Societal 2018. Cham: Springer. https://doi.org/10.1007/978-3-030-16848-3_33.
LAGA (2016). LAGA - Methodensammlung – Abfalluntersuchung. Version 3.0. Stand: 14. Oktober 2016.Bund/Länder-Arbeitsge- meinschaftAbfall.
Lee, H. V., Hamid, S. B. A., & Zain, S. K. (2014). Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. The Scientific World Journal, 2014, 1–20. https://doi.org/10.1155/2014/631013.
Lee, B., Park, J., Shin, W., Kim, B., Byun, B., & Jun, H. (2019). Maximizing biogas production by pretreatment and by optimizing the mixture ratio of co-digestion with organic wastes. Environmental Engineering Research, 24(4), 662–669. https://doi.org/10.4491/eer.2018.375.
Lehmann, T. (2009). Errichtungeiner Biogas- u. Biobrennstofferzeugungsanlage- Demonstrationsvorhaben, Abschlussbericht K II b1–001333, BundesministeriumfürUmwelt, Naturschutz und Reaktorsicherheit, Deutschland. https://www.umweltinnovationsprogramm.de/sites/default/files/benutzer/36/dokumente/lehmann_maschinenbau_abschlussbericht_k_ii_b1_-_001333_141.pdf. Accessed 20 Oct 2019.
Li, Y., Jin, Y., Li, J., Li, H., & Yu, Z. (2016). Effects of thermal pretreatment on the biomethane yield and hydrolysis rate of kitchen waste. Applied Energy, 172, 47–58. https://doi.org/10.1016/j.apenergy.2016.03.080.
Li, P., Li, W., Sun, M., Xu, X., Zhang, B., & Sun, Y. (2018). Evaluation of biochemical methane potential and kinetics on the anaerobic digestion of vegetable crop residues. Energies, 12(1), 26. https://doi.org/10.3390/en12010026.
Liang, Y. G., Cheng, B., Si, Y. B., Cao, D. J., Li, D. L., & Chen, J. F. (2016). Effect of solid-state NaOH pretreatment on methane production from thermophilic semi-dry anaerobic digestion of rose stalk. Water Science and Technology, 73(12), 2913–2920. https://doi.org/10.2166/wst.2016.145.
Lianhua, L., Dong, L., Yongming, S., Longlong, M., Zhenhong, Y., & Xiaoying, K. (2010). Effect of temperature and solid concentration on anaerobic digestion of rice straw in South China. International Journal of Hydrogen Energy, 35(13), 7261–7266. https://doi.org/10.1016/j.ijhydene.2010.03.074.
Lin, Z., Liu, L., Li, R., & Shi, J. (2012). Screw extrusion pretreatments to enhance the hydrolysis of lignocellulosic biomass. Journal of Microbial & Biochemical Technology, S12, 1–5. https://doi.org/10.4172/1948-5948.S12-002.
Liu, C., Heide, E., Wang, H., Li, B., Yu, G., & Mu, X. (2013). Alkaline twin-screw extrusion pretreatment for fermentable sugar production. Biotechnology for Biofuels, 6(97), 1–11. https://doi.org/10.1186/1754-6834-6-97.
Mahgoub, O., Kadim, I., Eltahir, Y., Al-Lawatia, S., & Al-Ismaili, A. (2018). Nutritional value of vegetable wastes as livestock feed. SQU Journal for Science, 23(2), 78–84. https://doi.org/10.24200/squjs.vol23iss2pp78-84.
Maile, I., Muzenda, E., & Mbohwa, C. (2016). Biogas production from anaerobic digestion of fruit and vegetable waste from Johannesburg market. In: 7th International Conference on Biology, Environment and Chemistry, 98, 100–104. https://doi.org/10.7763/IPCBEE.2016.V98.15.
Maurya, D. P., Singla, A., & Negi, S. (2015). An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech, 5(5), 597–609. https://doi.org/10.1007/s13205-015-0279-4.
Moestedt, J., Westerholm, M., Isaksson, S., & Schnürer, A. (2019). Inoculum source determines acetate and lactate production during anaerobic digestion of sewage sludge and food waste. Bioengineering, 7(1), 1–19. https://doi.org/10.3390/bioengineering7010003.
Morales-Polo, C., Cledera-Castro, D. M., & Moratilla Soria, B. Y. (2018). Reviewing the anaerobic digestion of food waste: from waste generation and anaerobic process to its perspectives. Applied Sciences, 8(10), 1804. https://doi.org/10.3390/app8101804.
Mozhiarasi, V., Speier, C. J., Benish Rose, P. M., Mondal, M. M., Pragadeesh, S., Weichgrebe, D., & Srinivasan, S. V. (2019a). Variations in generation of vegetable, fruit and flower market waste and effects on biogas production, exergy and energy contents. Journal of Material Cycles and Waste Management, 3, 1–16. https://doi.org/10.1007/s10163-019-00828-2.
Mozhiarasi, V., Speier, C. J., Michealammal, B. R. P., Shrivastava, R., Rajan, B., Weichgrebe, D., & Venkatachalam, S. S. (2019b). Bio-reserves inventory—improving substrate management for anaerobic waste treatment in a fast-growing Indian urban city, Chennai. Environmental Science and Pollution Research, 1–17. https://doi.org/10.1007/s11356-019-07321-1.
Mulka, R., Szulczewski, W., Szlachta, J., & Prask, H. (2015). The influence of carbon content in the mixture of substrates on methane production. Clean Technologies and Environmental Policy, 18(3), 807–815. https://doi.org/10.1007/s10098-015-1057-z.
Narnaware, S. L., Srivastava, N. S. L., & Vahora, S. (2017). Gasification: an alternative solution for energy recovery and utilization of vegetable market waste. Waste Management and Research, 35(3), 276–284. https://doi.org/10.1177/0734242X16679257.
Novarino, D., & Zanetti, M. C. (2012). Anaerobic digestion of extruded OFMSW. Bioresource Technology, 104, 44–50. https://doi.org/10.1016/j.biortech.2011.10.001.
Okelo, P. O., Joseph, S. W., Wagner, D. D., Wheaton, F. W., Douglass, L. W., & Carr, L. E. (2008). Improvements in reduction of feed contamination: an alternative monitor of bacterial killing during feed extrusion. The Journal of Applied Poultry Research, 17(2), 219–228. https://doi.org/10.3382/japr.2007-00060.
Orozco, S. R., Hernández, B. P., Morales, R. G., Núñez, U. F., Villafuerte, O. J., Lugo, L. V., Ramírez, N. F., Díaz, C. E. B., & Vázquez, C. P. (2014). Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. BioResources, 9(2), 1873–1885. https://doi.org/10.15376/biores.9.2.
Paraskeva, P., & Diamadopoulos, E. (2006). Technologies for olive mill wastewater (OMW) treatment: a review. Journal of Chemical Technology and Biotechnology, 81(9), 1475–1485. https://doi.org/10.1002/jctb.1553.
Patil, V. S., & Deshmukh, H. V. (2015). Biomethanation potential study of individual and combined vegetable market wastes. International Research Journal of Environment Sciences, 4(7), 75–80 ISSN 2319–1414.
Pilarski, K., Pilarska, A. A., Witaszek, K., Dworecki, Z., Żelaziński, T., Ekielski, A., Makowska, A., & Michniewicz, J. (2016). The impact of extrusion on the biogas and biomethane yield of plant substrates. Journal of Ecological Engineering, 17(4), 264–272. https://doi.org/10.12911/22998993/64563.
Pokój, T., Klimiuk, E., Bułkowska, K., Kowal, P., & Ciesielski, S. (2018). Effect of individual components of lignocellulosic biomass on methane production and methanogen community structure. Waste and Biomass Valorization, 1–13. https://doi.org/10.1007/s12649-018-0434-3.
Porselvam, S., Mahendra, B., Srinivasan, S. V., Ravindranath, E., & Suthanthararajan, R. (2017). Enhanced biogas production from co-digestion of intestine waste from slaughterhouse and food waste. Energy & Fuels, 31(11), 12133–12140. https://doi.org/10.1021/acs.energyfuels.7b01764.
Pramanik, S. K., Suja, F. B., Porhemmat, M., & Pramanik, B. K. (2019). Performance and kinetic model of a single-stage anaerobic digestion system operated at different successive operating stages for the treatment of food waste. Processes, 7(9), 600. https://doi.org/10.3390/pr7090600.
Qiao, W., Yan, X., Ye, J., Sun, Y., Wang, W., & Zhang, Z. (2011). Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renewable Energy, 36(12), 3313–3318. https://doi.org/10.1016/j.renene.2011.05.002.
Rabii, A., Aldin, S., Dahman, Y., & Elbeshbishy, E. A. (2019). Review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies, 12(6), 2–25. https://doi.org/10.3390/en12061106.
Rath, J., Heuwinkel, H., & Herrmann, A. (2013). Specific biogas yield of maize can be predicted by the interaction of four biochemical constituents. Bioenergy Research, 6(3), 939–952. https://doi.org/10.1007/s12155-013-9318-3.
Reungsang, A., Pattra, S., & Sittijunda, S. (2012). Optimization of key factors affecting methane production from acidic effluent coming from the sugarcane juice hydrogen fermentation process. Energies, 5(11), 4746–4757. https://doi.org/10.3390/en5114746.
Rodriguez, C., Alaswad, A., Benyounis, K. Y., & Olabi, A. G. (2017). Pretreatment techniques used in biogas production from grass. Renewable and Sustainable Energy Reviews, 68, 1193–1204. https://doi.org/10.1016/j.rser.2016.02.022.
Sachdeva, S., Sachdev, T., & Sachdeva, R. (2013). Increasing fruit and vegetable consumption: challenges and opportunities. Indian Journal of Community Medicine, 38(4), 192–197. https://doi.org/10.4103/0970-0218.120146.
Sagar, N. A., Pareek, S., Sharma, S., Yahia, E. M., & Lobo, M. G. (2018). Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Comprehensive Reviews in Food Science and Food Safety, 17(3), 512–531. https://doi.org/10.1111/1541-4337.12330.
Salehiyoun, A. R., Sharifi, M., Di Maria, F., Zilouei, H., & Aghbashlo, M. (2019). Effect of substituting organic fraction of municipal solid waste with fruit and vegetable wastes on anaerobic digestion. Journal of Material Cycles and Waste Management. https://doi.org/10.1007/s10163-019-00887-5.
Seswoya, R., Fen, A. S., Yang, L. K., & Sulaiman, S. M. (2019). Performance of anaerobic digestion of fruit and vegetable waste (FVW). International Symposium on Green and Sustainable Technology, AIP Conference Proceedings, 2157, 020026. https://doi.org/10.1063/1.5126561.
Shah, F., Mahmood, Q., Maroof Shah, M., Pervez, A., Ahmad Asad, S. (2014). Microbial ecology of anaerobic digesters: the key players of anaerobiosis. The Scientific World Journal, 1–21. https://doi.org/10.1155/2014/183752.
Shareefdeen, Z., Elkamel, A., & Tse, S. (2015). Review of current technologies used in municipal solid waste-to-energy facilities in Canada. Clean Technologies and Environmental Policy, 17, 1837–1846. https://doi.org/10.1007/s10098-015-0904-2.
Sharma, D., Varma, V. S., Yadav, K. D., & Kalamdhad, A. S. (2017). Evolution of chemical and biological characterization during agitated pile composting of flower waste. International Journal of Recycling of Organic Waste in Agriculture, 6(1), 89–98. https://doi.org/10.1007/s40093-017-0155-9.
Shi, X., Lin, J., Zuo, J., Li, P., Li, X., & Guo, X. (2017). Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. Journal of Environmental Sciences, 55, 49–57. https://doi.org/10.1016/j.jes.2016.07.006.
Sikora, A., Detman, A., Mielecki, D., Chojnacka, A., & Błaszczyk, M. (2018). Searching for metabolic pathways of anaerobic Digestion: A Useful List of the Key Enzymes, Anaerobic Digestion, J. Rajesh Banu, IntechOpen, https://doi.org/10.5772/intechopen.81256.
Song, L., Li, D., Fang, H., Cao, X., Liu, R., Niu, Q., & Li, Y. Y. (2019). Revealing the correlation of biomethane generation, DOM fluorescence, and microbial community in the mesophilic co-digestion of chicken manure and sheep manure at different mixture ratio. Environmental Science and Pollution Research, 26(19), 19411–19424. https://doi.org/10.1007/s11356-019-05175-1.
Sridevi, D., Srinivasan, S. V., Kayalvizhi, R., & Bhuvaneswari, R. (2012). Studies on conversion of carbohydrate content in the mixture of vegetable wastes into biogas in a single stage anaerobic reactor. Research Journal of Chemical Sciences, 2(6), 66–71 ISSN 2231-606X.
Srivastava, N. S. L., Narnaware, S. L., Makwana, J. P., Singh, S. N., & Vahora, S. (2014). Investigating the energy use of vegetable market waste by briquetting. Renewable Energy, 68, 270–275. https://doi.org/10.1016/j.renene.2014.01.047.
Szlachta, J., Prask, H., Fugol, M., & Luberański, A. (2018). Effect of mechanical pre-treatment of the agricultural substrates on yield of biogas and kinetics of anaerobic digestion. Sustainability, 10(10), 1–16. https://doi.org/10.3390/su10103669.
Teng, Z., Hua, J., Wang, C., & Lu, X. (2014). Design and optimization principles of biogas reactors in large scale applications. Reactor and Process Design in Sustainable Energy Technology, 99–134. https://doi.org/10.1016/b978-0-444-59566-9.00004-1.
Van Soest, P. J., Robertson, J. B., & Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74, 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2.
Van, D. P., Fujiwara, T., Tho, B. L., Toan, P. P. S., & Minh, G. H. (2020). A review of anaerobic digestion systems for biodegradable waste: configurations, operating parameters, and current trends. Environmental Engineering Research, 25(1), 1–17. https://doi.org/10.4491/eer.2018.334.
VDI - Guideline 4630 (2006). Fermentation of organic materials. Characterization of substrate, sampling, collection of material data, fermentation tests, BeuthVerlag GmbH.
Venkiteshwaran, K., Bocher, B., Maki, J., & Zitomer, D. (2015). Relating anaerobic digestion microbial community and process function : supplementary issue: water microbiology. Microbiology Insights, 8(s2), 37–44. https://doi.org/10.4137/mbi.s33593.
Wahid, R., Hjorth, M., Kristensen, S., & Møller, H. B. (2015). Extrusion as pretreatment for boosting methane production: effect of screw configurations. Energy & Fuels, 29(7), 4030–4037. https://doi.org/10.1021/acs.energyfuels.5b00191.
Wang, X., Lu, X., Li, F., & Yang, G. (2014). Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PLoS One, 9(5), 1–7. https://doi.org/10.1371/journal.pone.0097265.
Ware, A., & Power, N. (2016). What is the effect of mandatory pasteurisation on the biogas transformation of solid slaughterhouse wastes. Waste Management, 48, 503–512. https://doi.org/10.1016/j.wasman.2015.10.013.
Ware, A., & Power, N. (2017). Modeling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renewable Energy, 104, 50–59. https://doi.org/10.1016/j.renene.2016.11.045.
Weichgrebe, D. (2015). Kompendium Biogas. Habilitation. Publications of the Institute of Sanitary Engineering and Waste Management (ISAH), Leibniz University Hannover. Book 155, Hannover.
Weichgrebe, D., Speier, C., & Mondal, M. (2017). Scientific approach for municipal solid waste characterization. In S. Goel (Ed.), Advances in solid and hazardous waste management (1st ed., pp. 63–96). New Delhi: Capital Publishing Company.
Wikandari, R., Nguyen, H., Millati, R., Niklasson, C., & Taherzadeh, M. J. (2015). Improvement of biogas production from orange peel waste by leaching of limonene. BioMed Research International, 1–6. https://doi.org/10.1155/2015/494182.
Yu, G. H., He, P. J., Shao, L. M., & Zhu, Y. S. (2008). Extracellular proteins, polysaccharides and enzymes impact on sludge aerobic digestion after ultrasonic pretreatment. Water Research, 42, 1925–1934. https://doi.org/10.1016/j.watres.2007.11.022.
Zeynali, R., Khojastehpour, M., & Ebrahimi-Nik, M. (2017). Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustainable Environment Research, 27(6), 259–264. https://doi.org/10.1016/j.serj.2017.07.001.
Zhang, C., Li, J., Liu, C., Liu, X., Wang, J., Li, S., Fan, G., & Zhang, L. (2013). Alkaline pretreatment for enhancement of biogas production from banana stem and swine manure by anaerobic codigestion. Bioresource Technology, 149, 353–358. https://doi.org/10.1016/j.biortech.2013.09.070.
Zheng, Y., Wang, X., & Yang, F. (2019). Improving the anaerobic digestion of switch-grass via the co-fermentation of rumen microorganisms (rumen bacteria, protozoa, and fungi) and a biogas slurry. Energy & Fuels, 33, 1185–1195. https://doi.org/10.1021/acs.energyfuels.8b03496.
Zieliński, M., Kisielewska, M., Dębowski, M., & Elbruda, K. (2019). Effects of nutrients supplementation on enhanced biogas production from maize silage and cattle slurry mixture. Water, Air & Soil Pollution, 230(117), 1–7. https://doi.org/10.1007/s11270-019-4162-5.
Acknowledgments
The authors thank the Director, Central Leather Research Institute (CLRI) for permitting to carry out the research work. The authors also acknowledge both German and Indian Industrial partners (Lehmann-UMT GmbH, Germany & Ramky Enviro Engineers Private Ltd., India) of this project for their support.
Funding
This work was funded by the German Federal Ministry of Education and Research (BMBF) and the Indian Department of Science and Technology (DST) under the Indo-German Science and Technology Centre (IGSTC) (Grant number 01DQ15007A) for the 2+2 Project “RESERVES—Resource and energy reliability by co-digestion of veg-market and slaughterhouse waste.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mozhiarasi, V., Weichgrebe, D. & Srinivasan, S.V. Enhancement of Methane Production from Vegetable, Fruit and Flower Market Wastes Using Extrusion as Pretreatment and Kinetic Modeling. Water Air Soil Pollut 231, 126 (2020). https://doi.org/10.1007/s11270-020-04469-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04469-2