Abstract
With increasing concern related to sustainable chemistry, we investigated the biosorption of Pb2+ ions from aqueous medium using an environmental friendly and economic biosorbent Bougainvillea spectabilis (BS). The BS was modified effectively using citric acid by hydrothermal method. The biosorbent(s) was characterized by scanning electron microscope (SEM), energy dispersion X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), and point of zero charge (pHpzc). Various process parameters including biosorbent dosage, time of contact, temperature, solution pH, and initial Pb2+ ions concentration were studied in batch mode. Kinetic modeling was performed to evaluate the kinetic data and results showed that the studied process followed the pseudo second order (PSO) kinetics. Equilibrium modeling was done using famous equilibrium models, i.e., Langmuir, Freundlich, Dubinin-Kaganer-Radushkevish, and Temkin in non-linear fashion to evaluate equilibrium data by varying initial Pb2+ ions concentration from 20 to 180 mg/L. Based on RMSE values, Langmuir model fits best. This paper also discusses thermodynamic parameters (i.e., enthalpy, entropy, and free energy) showing that the process was spontaneous and endothermic in nature. In comparison with BS (B. spectabilis), an appreciable increase in uptake capacity of CABS (citric acid modified B. spectabilis) was observed in sequestration of Pb2+ ions from aqueous medium showing advantage of citric acid modification making it industrially favorable and socially acceptable biosorbent for efficient removal of lead from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals are a group of metals that have density greater than 5 g/cm3. Many industries like mining, fertilizers, ore refineries, electroplating, tanning, batteries, and pesticides are the main cause of heavy metal pollution in water ways. USEPA has developed a chart for the permissible limits of these heavy metals above which these can cause health damage in different ways.
Concern about protection of our environment has gained much attention over the years from a global viewpoint and safe disposal of contaminated waste water is the major environmental issue for the industries to be resolved. Several ways have been adopted to remove this heavy metal pollution from water like precipitation, membrane filtration process, sand filters, ion exchange, reverse osmosis, solvent extraction, etc. (Unuabonah et al. 2007). But some of these methods produce even more toxic solid waste whose disposal becomes a problem. Some are not cost effective or ineffective to treat the metal ions of 100 mg/L concentration or below (Aydın et al. 2008). Hence, need of the day is to find a source, method, or technology that can address all the above problems. To date, in environmental chemistry, use of adsorption process to separate the contaminants has gained esthetic attention and deliberation abroad the nations due to low cost, simplicity of design, ease of operation, and complete removal of pollutants from solutions (Foo and Hameed 2010).
Recent literature showed the importance of low-cost agro-based materials for successful removal of heavy metals from waste water (Salman et al. 2015). Biomass like almond shell and hazelnut shell (Pehlivan et al. 2009), rice husk (Ajmal et al. 2003), wheat straw (Farooq et al. 2011), sawdust (Li et al. 2007), chitosan (Zulkali et al. 2006), mungbean husk (Saeed et al. 2009), sugarcane bagasse (Krishnan and Anirudhan 2003), cocoa shell (Meunier et al. 2003), Sorghum(Jawar) (Salman et al. 2013), and Gardenia jasminoides biomass (Rafiq et al. 2015) have been found as effective biosorbents. Literature reveals that sometime use of raw biosorbent causes secondary pollution and showed low metal uptake capacity. Many studies have been carried out and reported in literature to enhance the adsorption capacity of biosorbent using hydrothermal method (Rwiza et al. 2018) and chemical methods such as pre-treatment with organic acids, minerals, oxidizing agents, bases, and other chemicals like KOH, H2O2, NaCl, ZnCl2, Na2CO3, epichlorohydrin, and glycerol (Abdolali et al. 2014; Hokkanen et al. 2016).
Pb2+ belongs to heavy metal and found to be neurotoxin specially in children and cause mental debilities (Skerfving et al. 2015), dysfunction of kidneys and liver due to its cumulative property (Wan Ngah and Fatinathan 2010). Due to non-biodegradability, it belongs to high ranking persistent environmental toxic chemicals (Man et al. 2013). Sources of lead in the environment include industrial water, food, soil, paint, leaded gasoline, plastic materials, and cosmetic products (Markowitz 2000). Its industrial resources include lead mining, battery recycling industry, and electronic assembly plant effluents (Wan Ngah and Fatinathan 2010). Use of conventional techniques of treating water like flocculation, ultrafiltration, reverse osmosis, sedimentation, and coagulation is not good for lead removal due to high energy consumption (making it expensive) as well as large production of biological and chemical waste leading to metal hydroxides formation (Acheampong et al. 2010; Sud et al. 2008). So, there is need to choose an optimal process and a suitable biosorbent which should be environmental benign as well as cost effective for lead removal.
In the present study, Bougainvillea spectabilis has been used as a novel and environment friendly biosorbent. It is an ornamental plant having papery flowers. It is a drought tolerant, requiring a little water for growth and can grow in warm climate areas like in Pakistan with little or no care. Its branches are cut regularly for giving shape and beauty to plant; these branches are the waste material which can be used to clean metal contaminated water. There has been no work reported till now on Pb+2 removal from aqueous solution using biomass of B. spectabilis.
The main objectives of this study are (i) to explore a new biosorbent and check its efficiency for removal of lead from waste water; (ii) to produce and characterize carboxylic acid functionalized biosorbent that fulfills 4-E strategy, i.e., environmental benign, efficient, easy to prepare, and economical; (iii) elucidate its biosorption process by studying its kinetic, thermodynamics, equilibrium modeling; (iv) to investigate surface morphology of biosorbent by using characterization techniques; and (v) to compare the removal efficiency of raw and modified biosorbent.
2 Materials and Methods
2.1 Chemicals and Reagents
Analytical grade chemicals and reagents were used in the present study and solutions were made using distilled water. Washing of glassware was done with nitric acid followed by distilled water. Chemicals used include sodium hydroxide, hydrochloric acid, sodium chloride, citric acid, and lead nitrate were bought from Merk/Scharlaw. Stock solution of lead ions (1000 mg/L) was prepared using lead nitrate salt. Standard solutions having different concentrations of lead ions were prepared from stock solution, diluting the calculated aliquots.
2.2 Biomass Preparation and Modification
Stalks of Bougainvillea spectabilis were collected from local area of Lahore Pakistan, which was then washed and dried in oven at 70 °C followed by grinding in to powder. It was then sieved through 80 mesh size and rinsed with distilled water to remove the dust particles. The biomass was then dried in oven again at 70 °C and stored in a dry box for future use.
The biosorbent was modified with citric acid using hydrothermal approach with slight modification in already reported method (Leyva-Ramos et al. 2012). Briefly, 20 g of BS was added in citric acid (1 M, 200 mL). The mixture was stirred for 2 h at 60 °C after which it was cooled at room temperature for 3 h. Acid was decanted and sorbent was washed with distilled water to remove any excess acid. Sorbent was dried at 80 °C for a day and stored in dry container for further use and labeled as CABS.
2.3 Instrumentation
Analysis of Pb2+ ions was done using atomic absorption spectrophotometer (AAS) (Perkin Elmer Model-A Analyst 100) at 217 nm. Orbital shaker (VORTEX Model no: OSM-747) was used for adsorption experiments. pH factor and pHpzc were done using digital pH meter (Model: Ino Lab WTW Series). Fourier transform infrared spectrophotometer (Aligent Technologies CARY 630) was used to characterize the biomass in range of 4000 to 600 cm−1 determining the surface functionality. A Nova Nano SEM 450 field-emission scanning electron microscope (FE-SEM) was used to determine the surface morphology comparing BS vs CABS and lead loaded BS (Pb-BS) vs lead loaded CABS (Pb-CABS). Energy dispersive X-ray spectroscopy EDX (FE-SEM) was also done for elemental analysis of CABS and Pb-CABS.
2.4 Adsorption Experiments
All the experiments were done using 50 mg/L of lead solution using CABS and BS in batch process and effect of different parameters on biosorption such as biosorbent dose, pH, time of contact, temperature, and initial concentration of Pb2+ ions were investigated. Effect of change of one parameter was studied keeping all other constant.
2.4.1 Experiment of Adsorption Dose
Fifty milliliters of 50 ppm Pb2+ solution was taken in 9 Erlenmeyer flasks of 250 mL and labeled them from no.1 to no.9. Then, 0.1 to 0.9 g of adsorbent concentrations (BS and CABS separately) were placed in flasks from 1 to 9, respectively. Flasks were agitated on orbital shaker at 125 rpm for 30 min at 25 °C and solution was then filtered. The filtrate was then subjected to atomic absorption for study of change in concentration of metal ions. Same procedure was repeated 3 times and concordant reading were taken for further study.
2.4.2 Experiment of pH
Fifty milliliters of 50 ppm solution of Pb2+ was taken in 9 Erlenmeyer flasks of 250 mL. pH of metal solutions was adjusted from 1.0 to 9.0 (on the basis of earlier reports) with the help of pH meter using HCl (0.1 M) and NaOH (0.1 M). 0.1 g of adsorbent (BS and CABS separately) was added in each flask and solution was shaken at 125 rpm for 30 min at 25 °C then filtered and filtrate then subjected to study through AAS. Same procedure was repeated 3 times and concordant reading were taken for further study.
2.4.3 Experiment of Temperature
Fifty milliliters of 50 ppm solution of Pb2+ was taken in 6 Erlenmeyer flasks of 250 mL. Effect of temperature on biosorption of metal ion was studied at temperature from 10, 20, 30, 40, 50, and 60 °C at 125 rpm for 30 min with 0.1 g of biosorbent (both with BS and CABS separately). Then filtered and subjected to AAS for study of change of metal ion concentration. Concordant value of three repeated experiments was reported.
2.4.4 Experiment of Contact Time and Initial Metal Ion Concentration
During the biosorption experiment (for both BS and CABS), samples of 50 ppm of Pb2+ solution were withdrawn at different time intervals from 5 to 45 min and filtered. Then after filtration, concentration of metal ions in solution was determined by flame atomic absorption spectrophotometer. Effect of initial metal ion concentration was studied by varying the metal ion concentration from 20 to 180 ppm using standard conditions described earlier. Concordant value of three repeated experiments was reported in the study.
Sorption capacity of biosorbent was investigated using the following formula (Eq. 1):
Here, qe (mg/g) is the uptake capacity of biosorbent, V (mL) is the volume of solution, Co (mg/L) is the initial metal ion concentration while Ce (mg/L) is the equilibrium concentration of metal ion, and m (mg/g) is the biosorbent dose.
Glassware interference was checked using blank biosorption experiments of lead solution. No interference was observed due to glass ware. All experiments were performed in triplicate and their mean value is reported. Graphs were plotted using Microsoft Excel 2013 program. Root mean square error and regression analysis (R2) was done to check the suitability of kinetic and mathematical models. RMSE value is calculated by the following formula (Eq. 2):
2.5 pHpzc Experiment
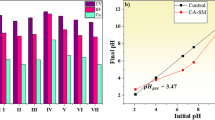
Point of zero charge of BS and CABS was determined by taking NaCl solution (0.1 M, 100 ml). Their pH of solutions was adjusted between 2 and 10 using 0.1 M HCl and 0.1 M NaOH. Then 500 mg of BS and CABS was added separately in solutions. The solutions were allowed to attain equilibrium for 48 h with occasional stirring. Final pH of the solutions was measured and a plot between ΔpH (initial pH–final pH) vs initial pH was obtained. The point of intersection of obtained curve with abscissa pointed out the pHpzc of the studied biosorbent.
3 Results and Discussion
3.1 Characterization of Biosorbent
3.1.1 FTIR Spectroscopy
FTIR is an important tool for detection of functional groups on biosorbent surface which may be possible binding sites for metal ions attachment. The BS and CABS were subjected to FTIR analysis before and after biosorption of Pb+2 ions and results are shown in Fig. 1. FTIR of BS identified the presence of hydroxyl group (3322 cm−1), carbonyl group (1617 cm−1), carboxyl group (1313 cm−1 and 1239 cm−1), and ether linkage (1027 cm−1) (Socrates 1994). Hence, BS is enriched in oxygen containing functional groups.
Notable changes were observed in the spectrum of CABS when compared with BS. CABS also contain similar functional groups as in BS but are greater in number due to incorporation of citric acid on the surface of BS. The shifting of carbonyl peak to 1719 cm−1 and appearance of carboxyl peak at 1157 cm−1 affirmed the modification of BS. In addition, the peak of hydroxyl group in CABS becomes more broadened that attributes to the increase in concentration of hydroxyl groups on the surface of CABS. This comparison indicated that CABS can offer more binding sites for Pb2+ attachment as compared to BS. FTIR of Pb-BS and Pb-CABS are also shown in Fig. 1. Both the spectra indicated the attachment of Pb2+ ions with oxygen containing functionalities. The broadness of hydroxyl peak becomes lessened, disappearance of peak at 1617 cm−1 (for BS) and 1719 cm−1 (for CABS) and shifting of carboxyl peaks affirmed the attachment of Pb2+ ions on BS and CABS.
3.1.2 SEM Analysis
Surface morphology of biomass was analyzed by scanning electron microscopy in SE mode. Fig. 2 a, b, c, and d is showing the SEM images of BS, CABS, Pb-BS, and Pb-CABS, respectively. Fantastic porous surface was observed in both BS and CABS that offers the appreciable attachment sites for the Pb2+ ions. SEM image of Pb-BS and Pb-CABS showed different morphology as compared to BS and CABS, respectively. This may be attributed to the attachment of Pb2+ ions on their surfaces. In addition, the SEM image of Pb-CABS is shinier than Pb-BS due to the greater concentration of Pb2+ ions on its surface. This indicated that CABS offered greater binding sites for Pb2+ ions attachment (greater metal uptake capacity) and hence can be considered as better biosorbent than BS (Yousaf et al. 2017).
3.1.3 EDX Analysis
As an evidence of Pb2+ ion attachment on to the surface of CABS, both CABS and Pb-CABS were subjected to EDX analysis. EDX mapping for CABS and Pb-CABS is shown in Fig. 3 (a, b). A clear signal of presence of Pb2+ ions was observed in Pb-CABS. So, EDX analysis provides direct evidence for sorption of lead ions onto CABS.
3.1.4 Thermogravimetric Analysis
Thermogravimetric analysis (TGA) is a technique used to monitor the weight loss of a substance as a function of temperature in controlled environment (Pezoti et al. 2016). To investigate the thermal stability of BS and CABS, TGA was performed. Biosorbent is mainly composed of lignin, cellulose, and hemicellulose. As depicted in Fig. 4, three weight loss steps were observed in both BS and CABS. In the first step from 60 to 270 °C, BS showed approximately 13% weight loss and CABS showed approximately 07% weight loss indicating more thermal stability in this region. This weight loss may be due to loss of free guest water adsorbed on the surface (Yuan et al. 2019).
In the second step from 270 to 370 °C, both biosorbents showed weight loss of almost 55% which can be attributed to degradation of lignin, cellulose, and hemicellulose (Pezoti et al. 2016). In range from 370 to 600 °C, weight of biosorbents decreased slowly. Relatively higher weight loss of CABS than BS is due to degradation of carboxylic acid groups attached on the CABS surface (Wan et al. 2014).
3.1.5 Point of Zero Charge (pHpzc)
pHpzc determines the pH value at which substance is found to be neutrally charged in virtue of pH of the medium (de O. Jorgetto et al. 2015). It is the isoelectric point when pH initial of the substance becomes equal to pH final and net surface charge on adsorbent is zero. This parameter is of great importance in determining the optimum conditions for adsorption in term of pH. If pH is greater than pHpzc, it shows that adsorbent surface bears negative charge due to deprotonation of functional groups indicating that adsorption of metal cations is feasible and vice versa. Literature shows that decrease in solution pH favors the desorption of metal cations (Hossain et al. 2012). So, pH of the system becomes economical factor in terms of regeneration of biosorbent. As shown in Fig. 5, pHpzc is evaluated to be 5.8 for raw biosorbent (BS) which indicates that adsorbent gains positive charge below pH 5.8 due to protonation of carboxylic and hydroxyl group. Above pH 5.8, surface of adsorbent is negatively charged which will be favorable for adsorption. Also acidic pHpzc suggests dominancy of acidic groups at adsorbent surface (A. O. Jorgetto et al. 2014). The pHpzc value for CABS was found at 4.9 which indicates the incorporation of citric acid onto the biosorbent surface. The decrease in pHpzc value in CABS may attribute to the increment in carboxyl groups onto its surface. Due to this reason, CABS can act as more affective biosorbent as compared to BS for metal cations uptake.
3.2 Effect of Adsorbent Dose
Experiments were conducted to check the effect of biosorbent dose on Pb2+ ions removal from aqueous solution using an initial concentration of 50 mg/L of Pb2+ while the dose of BS and CABS varied in a range of 0.1–0.9 g/50 mL. The results obtained are shown in a comparative plot (Fig. 6). The plot showed a quick uptake of Pb2+ initially followed by the equilibrium and decrease in uptake capacity (qe) with increase in biosorbent dose. BS shows 86.82% removal efficiency of Pb2+ at a dose of 0.1 g/50 mL, increasing with the increase in dose rises to maximum of 99.02% at a dose of 0.8 g/50 mL. While using CABS, the removal efficiency has gone up from 90.73% at a dose of 0.1 g/50 mL to a maximum of 99.51% at 0.5 g/50 mL, remaining almost constant at higher dosages. The increase in adsorption with the increase in adsorbent dose is attributed to the availability of adsorption sites for metal adsorption (Fawzy et al. 2016; Manzoor et al. 2013).
Figure 6 also illustrates that under similar condition (say 0.1 g/50 mL), CABS showed high removal efficiency (90.73%) and adsorption capacity (22.63 mg/g) relative to BS, i.e., 86.82% and 21.71 mg/g, respectively. From this discussion, the conclusion can be made that modification of B. spectabilis with citric acid increases its functionality which makes it responsible for good efficiency of CABS for Pb2+ removal even at low dosages.
3.3 Effect of pH on Pb2+ Adsorption
pH of the medium has very close relationship while studying the metal ions attachment to biosorbent (Yuan et al. 2019). It not only directs the speciation of metal ions in solution but also controls the charge on biosorbent surface. Different functional groups are present on lignocellulosic surface of BS as confirmed by FTIR. These functional groups show different behavior at different pH value. The deciding factor which explains the behavior of functional group on biosorbent surface is its pHpzc value. Lead ions are present in the solution as divalent cation (Pb2+) below pH 6 and start forming precipitates of Pb(OH)2 at higher pH values (Soyol-Erdene et al. 2018
The adsorption of Pb2+ ions was investigated over the pH range of 1–9 for both CABS and BS keeping the rest of the factors constant. The comparative pH profile of both BS and CABS is shown in Fig. 7. From graph, it is clear that the adsorption increases with increase in pH up to pH 5–6 then starts decreasing with further increase. In more acidic condition, the surface of adsorbent remains covered with H3O+ ions; this protonation repels the positive metal ions and there is also chance for competitive biosorption of H+ ions with respect to metal cations because of its higher concentration at low pH values. With the increase in pH due to deprotonation, the adsorption sites become available for metal ion attachment (Yousaf et al. 2017) as well as decrease in probability of H+ ions biosorption due to decrease in its concentration. But at high pH value, precipitation of lead hydroxide occurs so biosorption decreases.
pH profile is also helping in finding the beneficial effect of modification of raw BS. Uptake capacity of Pb2+ ions was found to be increased significantly using CABS as compared to BS. This increment is due to induction of more functional groups in biomass as a result of citric acid modification.
3.4 Kinetic Study (Effect of Contact Time)
To investigate optimum contact time for maximum removal of Pb2+ ions, experiments were carried out at different time from 5 to 30 min keeping other parameters constant for both BS and CABS. The kinetic curve for Pb2+ uptake by BS and CABS is shown in Fig. 8. Rate of uptake of Pb2+ ions decrease with the time eventually attain equilibrium stage. Optimum time to attain equilibrium for Pb2+ ions biosorption is found to be 20 min for BS and 10 min for CABS. This shows that modified biosorbent take less time to attain equilibrium making the process more rapid. Equilibrium time is the time when maximum binding sites on biosorbent are available due to concentration gradient initially after which vacant surface sites become less accessible due to potential electrostatic repulsion (Zhang et al. 2018). In addition to active sites, ease in accessibility of metal ions to biosorbent surface also plays important role (Yousaf et al. 2017).
Kinetic data of Pb2+ ions biosorption by BS and CABS was also investigated using pseudo first order (PFO) and pseudo second order (PSO) kinetic models. Linear mathematical form of PFO (Eq. 3) and PSO (Eq. 4) are as follows:
Here, qe (mg/g) and qt (mg/g) are the uptake capacities at equilibrium and t is the contact time, respectively. K1 (min−1) and K2 (mg/g/min) are the rate constant for PFO and PSO reaction, respectively.
The results for kinetic parameters for biosorption of Pb2+ in the case of BS and CABS are tabulated in Table 1 and their trend is shown in Fig. 8.
Regression analysis of PFO model shows that R2 value for both BS and CABS is less than 0.98 which shows that the adsorption mechanism does not follow PFO. Moreover, there is large difference between its calculated qe value and experimental qe value which reinforces the above statement. The experimental data for kinetics of Pb2+ adsorption better fits for PSO model which provides near ideal R2 value for both BS and CABS biosorbent, i.e., 0.999. Moreover, in PSO, there is very small difference (less than 1%) between calculated and experimental qe value. Therefore, kinetic data was described better by pseudo second order kinetics than pseudo first order kinetic model.
3.5 Effect of Initial Pb2+ Ions Concentration (Equilibrium Modeling)
Adsorption isotherm is a mathematical model equation which describes the relation between biosorbent and sorbate. These are also used to find out the required amount of biosorbent for a particular sorbate (Hj Ismail et al. 2013).
The equilibrium data for varying initial Pb2+ ions concentration (20–180 mg/L) was evaluated by using famous mathematical models including Langmuir, Freundlich, Temkin, and Dubinin-Kaganer-Radushkevish (DKR) models in non-linear fashion. Comparison was done by their RMSE values calculated using Eq. 2 and reported in Table 2. The results in the present study are tabulated in comparison with already reported studies in Table 3.
3.5.1 Langmuir Isotherm
Langmuir isotherm (Eq. 5) assumes that adsorption occurs on monolayer surface (Athar et al. 2013; Chen et al. 2017).
where KL (L/mg) is the Langmuir constant, Ce (mg/g) is the equilibrium concentration, qe is the equilibrium adsorption capacity, and qmax is the maximum theoretical adsorption capacity (J. Zhou et al. 2018). It tells that some specific homogenous sites are more active for biosorption (Nadeem et al. 2016) and each active site behaves independently of the neighbor molecule (Yousaf et al. 2017). Monolayer adsorption capacity (qmax) calculated by Langmuir model for Pb2+ in case of BS and CABS is 50.25 mg/g and 67.11 mg/g, respectively. Higher value of R2, i.e., 0.99 for both BS and CABS showed that Langmuir model fits best on adsorption data. RMSE value is 1.20 in case of Langmuir that is minimum as compared to all other models.
3.5.2 Freundlich Isotherm
Freundlich isotherm (Eq. 6) is most commonly used isotherm for equilibrium study. Freundlich isotherm is an empirical equation describing adsorption on heterogeneous surface escorted by the presence of multifunctional groups and various interactions (L. Zhou et al. 2016).
Here, KF and n are the Freundlich adsorption factor (showing adsorption capacity) and heterogeneity factor (expressing intensity of adsorption), respectively. Relevant parameter results of Freundlich isotherm (listed in Table 2) show that value of n is 2.73 for BS and 2.24 for CABS, indicating that lead ions are feasibly attached with biosorption sites (J. Zhou et al. 2018). Degree of non-linearity between concentration of solution and adsorption is dependent on “n.” Unity value of n shows linear adsorption while value of n below unity points towards chemical adsorption and if value of n is greater than unity it indicates physical adsorption. Value of adsorption intensity (1/n) is greater for CABS than BS indicating preferential sorption by CABS (Jnr et al. 2004).
3.5.3 D.K.R (Dubinin-Kaganer-Radushkevish) Isotherm
D.K.R model gives information about apparent energy of adsorption and characteristic porosity of adsorbent towards adsorbate. It describes adsorption on uniform pores and distinguishes between physical and chemical adsorption. It does not consider surface of biosorbent as homogenous (Wan Ngah and Fatinathan 2010).
Non-linear D.K.R isotherm expression is as under:
Here, qe is the amount of solute adsorbed per unit adsorbent (mg/g), qm is the maximum adsorption capacity (mg/g), K is the activity coefficient related to energy of sorption (mol2/KJ2), and ε is the Polanyi potential (j/mol) which can be calculated from the following equation:
Value of E that is mean free energy of adsorption per adsorbate molecule when transferred to surface of solid from solution is calculated from equation (Wan Ngah and Fatinathan 2010):
E value tells about the type of adsorption process. Value of E between 8 and 16 KJ/mol shows adsorption process involve chemical sorption (Laus et al. 2010) and below 8 KJ/mol indicates that adsorption process is physical in nature (Wan Ngah and Fatinathan 2010). DKR parameters are calculated from graph and listed in Table 2. Results show that value of E for lead ions on BS and CABS is 1.20 and 1.45 kJ/mol, respectively, which is lower than the range of 8–16 KJ/mol. Results indicate that adsorption mechanism involved here is physical sorption (J. Zhou et al. 2018).
3.5.4 Temkin Isotherm
Temkin model helps in calculating equilibrium binding constant corresponding to maximum binding energy. If binding energy decreases with increase in temperature, it points towards the exothermic process which is favored at high temperature (Aljeboree et al. 2017). This model gives information about interaction between metal ions and material.
Equation of Temkin is as follows:
Here, BT is the Temkin constant which is related to adsorption heat (KJ/mol) and KT is the empirical Temkin constant related to maximum binding energy (L/mg). BT for BS is less than 8 showing weak interaction suggesting process to be physisorption (Theivarasu and Mylsamy 2010) while in case of CABS greater BT value shows strong attractive force.
A comparitive plot of non-linear modeling of biosorption of Pb2+ on BS and CABS is shown in Fig. 9 (a) and (b), respectively. It can be concluded from figure and RMSE value that description of equilibrium data by Langmuir model is superior to other studied models.
3.6 Effect of Temperature: Thermodynamic Study
Temperature plays an important role on biosorption process. So, study of thermodynamics is important to understand the adsorption process (Nazir et al. 2019). Adsorbent metal uptake capacity as a function of temperature is illustrated in Fig. 10 which shows notably increase in Pb2+ uptake capacity of biosorbent with increase in temperature up to a certain level suggesting that lead uptake may be an endothermic process (Yuan et al. 2019). This increase in biosorption with temperature may be due to increase in active sites for metal binding or increase in diffusion rate of metal with temperature. Thermodynamic data was obtained by thermodynamic parameters including ΔHo (standard enthalpy), ΔSo (standard entropy), and ΔGo Gibbs free energy using the following formulas and listed in Table 4.
Here, T is the temperature (K), R is the universal gas constant (8.314 J/mol/K), and KD is the equilibrium constant calculated from Equation no. 13. ΔHo and ΔSo were calculated from graph.
Positive value of ΔHo confirmed the endothermic nature of process of adsorption supporting the results clinched from Fig. 10. Positive value of ΔSo suggested increase in randomness at solid solution interface enhancing metal ions adsorption stability. Negative value of ΔGo revealed the spontaneous nature of process proving that it is thermodynamically favorable (Wang et al. 2017) (Table 4).
3.7 Adsorption Mechanism
The carboxyl functionality of B. spectabilis has been increased by incorporation of citric acid onto its surface. This increases the biosorption efficiency and metal uptake capacity of CABS. Plant biomass consists of carbohydrates in the form of lignin, cellulose, and hemicellulose. Large amount of oxygen containing groups (-OH, -CO, -OCH3) which have been determined by FTIR. Many -OH groups are present on surface of biosorbent which can be esterified by carboxylic group (Pezoti et al. 2016). Citric acid contained three carboxylic groups from which one carboxylic group attaches to -OH group of B. spectabilis through esterification process and the remaining two become available to bind the metal ions. This increase of oxygen containing functional group will lead to higher electronegativity that will ultimately increase the adsorption capacity of heavy metal cations, i.e., lead ions. It is notable here that these functional groups offer considerable active binding sites for strong interaction with lead ions on surface of biosorbent thus improving the biosorption ability of biosorbent. A schematic diagram showing possible modification and adsorption mechanism is shown in Fig. 11.
4 Conclusions
An environmental benign, low-cost citric acid modified B. spectabilis (CABS) was synthesized by simple steps using citric acid. The modification of CABS was confirmed by FTIR, EDX, TGA, and SEM. Comparative biosorption of most toxic heavy metal ions Pb2+ on BS and CABS from aqueous solution was the focus of the study.
The main focus of the study was to understand kinetics, nature of binding through different equilibrium models, and rate determining step as well as effect of pH for both BS and CABS. The biosorption of Pb2+ ions was shown to be dependent on solution pH with optimum pH value 5 and 6 for BS and CABS, respectively. Using modified biosorbent, the process became more rapid as it need less time to attain equilibrium. Kinetic study showed that adsorption followed pseudo second order model in both BS and CABS. Non-linear approach was used in equilibrium modeling. Langmuir model was found to be the best fit in both cases revealing maximum adsorption capacity 50.25 and 67.11 mg/g for BS and CABS, respectively. From DKR, isotherm value of mean free energy of adsorption shows that mechanism of biosorption is predominantly due to physical process providing feasibility to regenerate the biosorbent via desorption of metal ions. Thermodynamic parameters showed that process was feasible, spontaneous, and endothermic in nature. Increase in adsorption capacity and decrease in time to attain equilibrium shows the effectiveness of modification. It points towards effective use of modified novel biosorbent for treatment of waste water. In short, it is a cheap biowaste with impressive biosorption ability. Its easy availability on industrial scale and reusability makes it an economically favorable and socially acceptable for efficient removal of lead ions from aqueous solution.
References
Abdolali, A., Guo, W. S., Ngo, H. H., Chen, S. S., Nguyen, N., & Tung, K. L. (2014). Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresource Technology, 160, 57–66.
Acheampong, M. A., Meulepas, R. J. W., & Lens, P. N. L. (2010). Removal of heavymetals and cyanide from gold mine wastewater. Journal of Chemical Technology and Biotechnology, 85, 590–613.
Ajmal, M., Rao, R. A. K., Anwar, S., Ahmad, J., & Ahmad, R. (2003). Adsorption studies on rice husk: removal and recovery of Cd (II) from wastewater. Bioresource Technology, 86(2), 147–149.
Aljeboree, A. M., Alshirifi, A. N., & Alkaim, A. F. (2017). Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arabian Journal of Chemistry, 10, S3381–S3393. https://doi.org/10.1016/j.arabjc.2014.01.020.
Athar, M., Farooq, U., Aslam, M., & Salman, M. (2013). Adsorption of Pb (II) ions onto biomass from Trifolium resupinatum: equilibrium and kinetic studies. Applied Water Science, 3(3), 665–672.
Aydın, H., Bulut, Y., & Yerlikaya, Ç. (2008). Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. Journal of Environmental Management, 87(1), 37–45.
Bingöl, D., Hercan, M., Elevli, S., & Kılıç, E. (2012). Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresource Technology, 112, 111–115.
Chen, H., Dou, J., & Xu, H. (2017). Removal of Cr(VI) ions by sewage sludge compost biomass from aqueous solutions: reduction to Cr(III) and biosorption. Applied Surface Science, 425, 728–735. https://doi.org/10.1016/j.apsusc.2017.07.053.
de O. Jorgetto, A., da Silva, A. C. P., Wondracek, M. H. P., Silva, R. I. V., Velini, E. D., Saeki, M. J., et al. (2015). Multilayer adsorption of Cu(II) and Cd(II) over Brazilian orchid tree ( Pata-de-vaca ) and its adsorptive properties. Applied Surface Science, 345, 81–89. https://doi.org/10.1016/j.apsusc.2015.03.142.
Dos Santos, W. N., Cavalcante, D. D., da Silva, E. G. P., das Virgens, C. F., & de Souza Dias, F. (2011). Biosorption of Pb (II) and Cd (II) ions by Agave sisalana (sisal fiber). Microchemical Journal, 97(2), 269–273.
Farooq, U., Khan, M. A., Athar, M., & Kozinski, J. A. (2011). Effect of modification of environmentally friendly biosorbent wheat (Triticum aestivum) on the biosorptive removal of cadmium (II) ions from aqueous solution. Chemical Engineering Journal, 171(2), 400–410.
Fawzy, M., Nasr, M., Abdel-Gaber, A., & Fadly, S. (2016). Biosorption of Cr (VI) from aqueous solution using agricultural wastes, with artificial intelligence approach. Separation Science and Technology, 51(3), 416–426.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156(1), 2–10.
Hj Ismail, M. G. B., Weng, C. N., & Zakaria, N. A. (2013). Freundlich isotherm equilibrium equastions in determining effectiveness a low cost absorbent to heavy metal removal in wastewater (Leachate) at Teluk Kitang landfill, Pengkalan Chepa, Kelantan, Malaysia. Journal of Geography and Earth Science, 1(1), 01–08.
Hokkanen, S., Bhatnagar, A., & Sillanpaa, M. (2016). A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Research, 91, 156–173. https://doi.org/10.1016/j.watres.2016.01.008.
Hossain, M., Ngo, H., Guo, W., & Setiadi, T. (2012). Adsorption and desorption of copper (II) ions onto garden grass. Bioresource Technology, 121, 386–395.
Jnr, M. H., Spiff, A. I., & Abia, A. A. (2004). Studies on the influence of mercaptoacetic acid (MAA) modification of cassava (Manihot sculenta Cranz) waste biomass on the adsorption of Cu2+ and Cd2+ from aqueous solution. Bulletin of the Korean Chemical Society, 25(7), 969–976.
Jorgetto, A. O., Silva, R. I. V., Saeki, M. J., Barbosa, R. C., Martines, M. A. U., Jorge, S. M. A., et al. (2014). Cassava root husks powder as green adsorbent for the removal of Cu(II) from natural river water. Applied Surface Science, 288, 356–362. https://doi.org/10.1016/j.apsusc.2013.10.032.
Krishnan, K. A., & Anirudhan, T. (2003). Removal of cadmium (II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: kinetics and equilibrium studies. Water SA, 29(2), 147–156.
Laus, R., Costa, T. G., Szpoganicz, B., & Favere, V. T. (2010). Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. Journal of Hazardous Materials, 183(1–3), 233–241. https://doi.org/10.1016/j.jhazmat.2010.07.016.
Leyva-Ramos, R., Landin-Rodriguez, L. E., Leyva-Ramos, S., & Medellin-Castillo, N. A. (2012). Modification of corncob with citric acid to enhance its capacity for adsorbing cadmium(II) from water solution. Chemical Engineering Journal, 180, 113–120. https://doi.org/10.1016/j.cej.2011.11.021.
Li, Q., Zhai, J., Zhang, W., Wang, M., & Zhou, J. (2007). Kinetic studies of adsorption of Pb (II), Cr (III) and Cu (II) from aqueous solution by sawdust and modified peanut husk. Journal of Hazardous Materials, 141(1), 163–167.
Man, M., Naidu, R., & Wong, M. H. (2013). Persistent toxic substances released from uncontrolled e-waste recycling and actions for the future. Science of The Total Environment, 463-464, 1133–1137.
Manzoor, Q., Nadeem, R., Iqbal, M., Saeed, R., & Ansari, T. M. (2013). Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb (II) and Cu (II) from aqueous media. Bioresource Technology, 132, 446–452.
Markowitz, M. (2000). Lead poisoning: a disease for the next millennium. Current Problems in Pediatrics, 30, 62–70.
Meunier, N., Laroulandie, J., Blais, J., & Tyagi, R. (2003). Cocoa shells for heavy metal removal from acidic solutions. Bioresource Technology, 90(3), 255–263.
Nadeem, R., Manzoor, Q., Iqbal, M., & Nisar, J. (2016). Biosorption of Pb (II) onto immobilized and native Mangifera indica waste biomass. Journal of Industrial and Engineering Chemistry, 35, 185–194.
Nazir, H., Salman, M., Athar, M., Farooq, U., Akram, M., & Saleem, N. (2019). A novel biosorbent B. spectabilisis stalks leaves for removal of Cd(II) and Cu(II) from wastewater. Desalination and Water Treatment, 148, 222–228. https://doi.org/10.5004/dwt.2019.23706.
Pehlivan, E., Altun, T., Cetin, S., & Bhanger, M. I. (2009). Lead sorption by waste biomass of hazelnut and almond shell. Journal of Hazardous Materials, 167(1–3), 1203–1208.
Pezoti, O., Cazetta, A. L., Bedin, K. C., Souza, L. S., Martins, A. C., Silva, T. L., et al. (2016). NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chemical Engineering Journal, 288, 778–788. https://doi.org/10.1016/j.cej.2015.12.042.
Pitsari, S., Tsoufakis, E., & Loizidou, M. (2013). Enhanced lead adsorption by unbleached newspaper pulp modified with citric acid. Chemical Engineering Journal, 223, 18–30. https://doi.org/10.1016/j.cej.2013.02.105.
Rafiq, M., Farooq, U., Athar, M., Salman, M., Aslam, M., & Raza, H. M. H. (2015). Gardenia jasminoides: an ornamental plant for the biosorption of lead and cadmium ions. Desalination and Water Treatment, 57(22), 10432–10442. https://doi.org/10.1080/19443994.2015.1035341.
Rwiza, M. J., Oh, S. Y., Kim, K. W., & Kim, S. D. (2018). Comparative sorption isotherms and removal studies for Pb(II) by physical and thermochemical modification of low-cost agro-wastes from Tanzania. Chemosphere, 195, 135–145. https://doi.org/10.1016/j.chemosphere.2017.12.043.
Saeed, A., Iqbal, M., & Höll, W. H. (2009). Kinetics, equilibrium and mechanism of Cd2+ removal from aqueous solution by mungbean husk. Journal of Hazardous Materials, 168(2–3), 1467–1475.
Salman, M., Athar, M., Farooq, U., Nazir, S., & Nazir, H. (2013). Insight to rapid removal of Pb(II), Cd(II), and Cu(II) from aqueous solution using an agro-based adsorbentSorghum bicolorL. Biomass Desalination and Water Treatment, 51(22–24), 4390–4401. https://doi.org/10.1080/19443994.2012.749186.
Salman, M., Athar, M., Farooq, U., Rauf, S., & Habiba, U. (2014). A new approach to modification of an agro-based raw material for Pb(II) adsorption. Korean Journal of Chemical Engineering, 31(3), 467–474. https://doi.org/10.1007/s11814-013-0264-8.
Salman, M., Athar, M., & Farooq, U. (2015). Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Reviews in Environmental Science and Bio/Technology, 14(2), 211–228. https://doi.org/10.1007/s11157-015-9362-x.
Skerfving, S., Löfmark, L., Lundh, T., Mikoczy, Z., & Strömberg, U. (2015). Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology, 49, 114–120.
Socrates, G. (1994). Infrared characteristic frequencies, 2nd. Wiley&Sons.
Soyol-Erdene, T. O., Oyungerel, S., Dorj, D., Lin, S., Tseveendorj, E., & Enkhdul, T. (2018). Biosorption of lead (II) from an aqueous solution using biosorbents prepared from water plants. Mongolian Journal of Chemistry, 18(44), 52–61. https://doi.org/10.5564/mjc.v18i44.937.
Sud, D., Mahajan, G., & Kaur, M. P. (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - a review. Bioresource Technology, 99, 6017–6027.
Theivarasu, C., & Mylsamy, S. (2010). Equilibrium and kinetic adsorption studies of Rhodamine-B from aqueous solutions using cocoa (Theobroma cacao) shell as a new adsorbent. International Journal of Engineering, Science and Technology, 2(11), 6284–6292.
Unuabonah, E., Olu-Owolabi, B., Adebowale, K., & Ofomaja, A. (2007). Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated kaolinite clay. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 292(2–3), 202–211.
Wan Ngah, W. S., & Fatinathan, S. (2010). Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. Journal of Environmental Management, 91, 958–969.
Wan, T., Huang, R., Xiong, L., Zhao, Q., Luo, L., Zhang, H., et al. (2014). Swelling behaviors and gel strength studies of wheat straw-composite superabsorbent. Journal of Composite Materials, 48(19), 2341–2348.
Wang, Y., Hu, L., Zhang, G., Yan, T., Yan, L., Wei, Q., et al. (2017). Removal of Pb (II) and methylene blue from aqueous solution by magnetic hydroxyapatite-immobilized oxidized multi-walled carbon nanotubes. Journal of Colloid and Interface Science, 494, 380–388.
Yousaf, A., Athar, M., Salman, M., Farooq, U., & Chawla, F. S. (2017). Biosorption characteristics of Pennisetum glaucum for the removal of Pb(II), Ni(II) and Cd(II) ions from aqueous medium. Green Chemistry Letters and Reviews, 10(4), 462–470. https://doi.org/10.1080/17518253.2017.1402093.
Yu, J.-X., Wang, L.-Y., Chi, R.-A., Zhang, Y.-F., Xu, Z.-G., & Guo, J. (2013). Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Applied Surface Science, 268, 163–170. https://doi.org/10.1016/j.apsusc.2012.12.047.
Yuan, G., Tu, H., Li, M., Liu, J., Zhao, C., Liao, J., et al. (2019). Glycine derivative-functionalized metal-organic framework (MOF) materials for Co(II) removal from aqueous solution. Applied Surface Science, 466, 903–910. https://doi.org/10.1016/j.apsusc.2018.10.129.
Zhang, S., Wang, Z., Chen, H., Kai, C., Jiang, M., Wang, Q., et al. (2018). Polyethylenimine functionalized Fe3O4/steam-exploded rice straw composite as an efficient adsorbent for Cr(VI) removal. Applied Surface Science, 440, 1277–1285. https://doi.org/10.1016/j.apsusc.2018.01.191.
Zhou, L., Liu, Y., Liu, S., Yin, Y., Zeng, G., Tan, X., et al. (2016). Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresource Technology, 218, 351–359.
Zhou, J., Liu, Y., Zhou, X., Ren, J., & Zhong, C. (2018). Magnetic multi-porous bio-adsorbent modified with amino siloxane for fast removal of Pb(II) from aqueous solution. Applied Surface Science, 427, 976–985. https://doi.org/10.1016/j.apsusc.2017.08.110.
Zulkali, M. M. D., Ahmad, A. L., Norulakmal, N. H., & Sharifah, N. S. (2006). Comparative studies of Oryza sativa L. husk and chitosan as lead adsorbent. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 81(7), 1324–1327.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazir, H., Salman, M., Athar, M. et al. Citric Acid Functionalized Bougainvillea spectabilis: a Novel, Sustainable, and Cost-effective Biosorbent for Removal of Heavy Metal (Pb2+) from Waste Water. Water Air Soil Pollut 230, 303 (2019). https://doi.org/10.1007/s11270-019-4360-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4360-1