Abstract
Phytoremediation is the most promising and emerging technology for remediation of metal-contaminated sites. The aim of the current research was to investigate the metals accumulation potential of four weed species i.e., Parthenium, Cannabis, Euphorbia, and Rumex species. The endogenous free proline, total phenolic, carotenoids, and chlorophyll contents were assessed under metals stress and correlated with metals accumulation in plants. Plants of each species were allowed to grow in reference (uncontaminated) soil and other group on industrial effluent-contaminated soil under natural environment. Phytoextraction potentials of these plants were evaluated for removal of lead (Pb) and chromium (Cr). The heavy metal concentration in plant parts was analyzed by atomic absorption spectrophotometer. Varied accumulation of metals was found among different weed species. Moreover, metal accumulation was different within plant tissues i.e., roots, leaves, and stem. Cannabis and Parthenium showed bio-concentration BCF > 1 for Pb, reflecting their high metal accumulation potential and both plants were found superior than Rumex and Euphorbia for Cr accumulation. The endogenous free proline and phenolic contents showed significantly positive correlation with Pb accumulation in four plants i.e., Parthenium (R2 = 0.977, R2 = 0.9996), Cannabis (R2 = 0.9924, R2 = 0.9999), Euporbia (R2 = 0.9992, R2 = 0.9832), Rumex (R2 = 0.6033, R2 = 0.8272) respectively. Similarly, Cr accumulation in plants showed significantly positive correlation with proline and phenolic but the BCF ˂ 1.The chlorophyll and carotenoids contents in all plants negatively correlated with Pb and Cr concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial estates are set up to meet the demands of rising population, but they contribute in environmental pollution. In the last two decades, industrial revolution, population explosion, and uncontrolled anthropogenic activities have adversely affected natural resources existing on earth’s environment (Srivastava et al. 2012). It is alerting that most industries in Pakistan have no proper mechanism of wastewater/effluent treatment facilities. Such effluents containing heavy metals directly affect soil in industrial areas and also in agricultural fields, thus posing a severe threat to human and normal functioning of ecosystem (Singh et al. 2012). Toxic heavy metals such as Pb and Cr are most abundant metals in environment. Pb is biologically nonessential for normal growth and development of plants and animals and causes health hazards like neurotoxicity, anemia, lungs cancer, hypertension, impaired fertility, and even death (Sthanadar et al. 2013). Acute exposure to Cr causes severe health problems like nausea, diarrhea, kidney and liver damage, irritation and ulceration of gastrointestinal tract, lung cancer, and even death due to cardiovascular disorders (Zvinowando et al. 2009). Both Pb and Cr are toxic to plants and negatively affect growth, pigment contents, and enzymatic activities causing oxidative damage to plants (Peralta-Videa et al. 2009).
Conventional methods used for metal removal are usually expensive, destructive, labor intensive, and cause secondary problems (Wu et al. 2010). In comparison, phytoremediation is innovative, efficient, less expensive, eco-friendly remediation technique with good public acceptance, and most appropriate for developing countries (Revathi et al. 2011). Phytoremediation is one of the effective techniques for remediation of soils polluted with toxic heavy metals, which uses green plants to remove, detoxify, or sequester the contaminants from soil and ground water or to lower its mobility (Shakoor et al. 2013). Phytoextraction is also called phytoaccumulation, and it is a widely used strategy of phytoremediation. This clean up method uses the hyper accumulator plants that can accumulate metals in their shoots several times higher than in normal plants without showing toxic symptoms (Gosh and Singh 2005). Chaney (1983) suggested first time the role of hyper accumulator plants for phytoremediation of metal polluted areas. Hyper accumulators for Pb and Cr can be defined as “plant species which can accumulate > 1000 mgKg-1 of these metals in their shoots when growing on metals rich soils.” Screening of native plants for hyperaccumulator potential is a key step in development of phytoremediation technology. Phytoextraction efficiency of plants can be determined by two key factors: ability to hyper accumulate heavy metals and production of high biomass (McGrath and Zhao 2003).
Production of reactive oxygen species (ROS) is one of the responses of plants against different environmental stresses. Under stress conditions, the balance between ROS generation and degradation is necessary for maintaining normal metabolic functions, otherwise oxidative burst may result in cellular injuries. The level of ROS in plant tissues is controlled by an antioxidative system which consists of antioxidative enzymes (superoxide dismutase (SOD), peroxidase (POD), glutathione transferase, catalase), and non-enzymatic low molecular mass antioxidants (phenolics, proline, glutathione, organic acids) (Davis and Swanson 2001). Both of free proline and phenolics can function as osmoprotectants, metal chelators, hydroxyl radical scavengers, and inhibitor of lipid peroxidation, thus protecting plants from oxidative stress and playing key role in stress tolerance (Handique and Handique 2009; Ahmad et al. 2015; Hadi et al. 2016).
The weed plants thriving naturally in the industrial area were selected to screen for heavy metals accumulation. Such plants are mostly unpalatable and prevent or minimize the entrance of metals into food chain. It is pivotal to manage such type of weeds and use them for beneficial purpose i.e., soil reclamation. Parthenium hysterophorus belongs to the family Asteraceae. It is native to the subtropics of North and South America (Reddy et al. 2007). It is rapidly spreading in Pakistan for about last 20 years and has become a serious weed, destroying other useful plants (Riaz and Javaid 2007). Cannabis sativa (L) is an erect, fast growing, annual dioecious herb and can reach the height of 4 m (Citterio et al. 2003). Euphorbia helioscopia of family Euphorbiaceae is commonly known as sun spurge. It is monoecious annual herb (Khan et al. 2014). The species Rumex dentatus (L) is commonly known as toothed-dock. It is an annual and biennial weed of family Polygonaceae. It occurs in waste places, shores, and cultivated fields (Siddiqui and Bajwa 2001). The emphasis of current research was to (i) assess total concentration of Pb and Cr in selected plants biomass growing in contaminated sites, (ii) evaluate the biochemical response of plants to heavy metals stress based on chlorophyll, carotenoids, proline, and phenolic contents, and (iii) identify efficient weeds among experimental plants having good potential for Pb and Cr phytoaccumulation and remediation of contaminated soil.
2 Materials and Methods
2.1 Site Description and Sample Collection
A site in the vicinity of marble industries and locomotive factory risalpur in Pakistan was selected for the growth of four different weed species for research purpose. The uniform size plants were collected carefully and transferred to laboratory in a labelled polythene bag along with soil. All the plants were collected at vegetative stage. The weed plants i.e., Parthenium hysterophorus, Cannabis sativa, Euphorbia helioscopia, and Rumex dentatus were selected for this study. The same species were collected from reference soil which was uncontaminated. The analysis of both soils was carried out for metal contents, pH, EC, and water holding capacity. The plants from reference soil were used as controls. Twenty replicate plants for each species were harvested from each soil and were selected randomly among plants. All the plants were transferred to the laboratory and rinsed thoroughly with solution of EDTA and then with dH2O to wash all the adhering soil particles and surface metals. Then, the plants were separated into leaves, stems, and roots and stored for further analysis.
2.2 Carotenoid Content Estimation of Plants
The carotenoid contents in leaves were estimated from the 90% acetonic extract of leaf and recorded the absorbance at 480 nm using spectrophotometer. Acetone (90%) was used as blank (Borker et al. 2013). The carotenoid content was calculated using the formula Carotenoids contents = A480 × volume of extract × 10 × 100/2500 × weight of plant material (g).
2.3 Estimation of Proline in Leaves and Roots
Proline extraction and its biochemical quantification were performed using the method of Bates et al. 1973. Fresh tissues (100 mg) of root and leaf were taken in 2 ml Eppendorf tubes, and 1.5 ml of 3% sulfosalicylic was added into it and homogenized. The homogenate was centrifuged for 5 min at 13000 rpm. Then, 300 all supernatant and 2 ml each of glacial acetic acid and acid ninhydrin were added in tube and allowed for 1 h at boiling temperature in water bath. The reaction was stopped by directly transferring the reacted mixture from water bath to ice bath. After this, 1 ml toluene was mixed with reaction mixture vigorously (for 10–30 s). The chromophore layer having toluene was taken from the upper aqueous phase with the help of micropipette, warmed to room temperature, and the absorbance of samples was recorded at wavelength of 520 nm using UV-visible spectrophotometer. Toluene was used as a blank. Each sample was run in triplicate for this reaction.
2.4 Estimation of Total Phenolics in Leaves and Roots
The plant tissues used for total phenolics estimation were air-dried in dark for one night and blended. Two hundred milligrams of sample was taken, and phenolic extraction was carried out with 10 ml of 80% methanol on stirrer for 30 min. Shaking was performed in closed flask to avoid loss of solvent. Two milliliters portion of extract was taken from the supernatant and centrifuged at 13000 rpm for 3 to 5 min. Quantification of total phenolics in extract was done by Folin Ciocalteau (FC) reagent method (Singleton and Rossi 1965). Two hundred fifty microliters of tenfold diluted FC reagent was added to 100 μL methanolic extract and kept for 3–5 min at room temperature in dark. Then, 500 μL of sodium carbonate (7% Na2CO3) solution was added to it and the final volume was raised upto 5 ml with distilled water and allowed for 2 h in dark at room temperature before measuring the absorbance. Absorbance was measured at 760 nm in spectrophotometer. Eighty percent methanol was used as a control (blank) solution. All sample reactions for analysis were performed in triplicates.
2.5 Chlorophyll Content Estimation
Fresh leaves (200 mg) were taken in a tube and roughly homogenized in 2 ml of 80% acetone. It was centrifuged at 1000 rpm for 5 min. The supernatant was taken and transferred to a clean test tube. Acetone was added up to 6 ml. The extracts were analyzed with UV-visible spectrophotometer for chlorophyll content estimation using 80% acetone as blank (Arnon 1949). All the samples were run in replicates. Chlorophyll “a” was measured at 663 nm and chlorophyll “b” at 645 nm. The chlorophyll contents were determined using the following formulae:
2.6 Plant Dry Biomass Analysis
The plants were kept at 80 °C in an oven (Beschickung-loading model 100-800) for 48 h and completely dried. Then, the dry biomass of all samples was measured with an electronic balance. All the plant parts were blended with a commercial blender, and the powder was kept in a labelled packing for further analysis.
2.7 Plant Analysis for Lead and Chromium Contents
The plants were kept at 80 °C in an oven (Beschickung-loading model 100-800) for 48 h and completely dried. All the plant parts were blended with a commercial blender, and the powder was kept in a labelled packing for further analysis. Powdered material (0.25 g) of each leaf, stem, and root was taken in a 50-ml flask. Five milliliters of nitric acid and 1 ml of sulfuric acid were added to it. It was kept on electric hot plate in fume hood, until white fumes come out and completed digestion of the tissues (Allen 1974; Hadi et al. 2010). The samples were placed at room temperature for cooling, and the final volume was raised up to 50 ml by adding dH2O. Then, extract was filtered with Whitman filter paper into a labelled bottle. Heavy metal contents of all the samples were ascertained with the help of an Atomic Absorption Spectrophotometer (AAS).
2.8 Physicochemical Characteristics and Acid Digestion of the Soil
The soil samples were collected from industrially contaminated sites and normal sites (used as control) respectively. The physicochemical parameters such as pH, EC, and WHC of all these samples were assessed using standard methods of APHA (2005). The soil was completely air-dried and finely grounded in pestle and mortar. Then passed through a 2-mm net (mesh) and transferred to labelled small packs. The soil samples were digested using acids as used for plants tissues digestion.
2.9 Phytoextraction Potential of Plants
Phytoremediation potential of plants was based on Biological Accumulation Coefficient (BAC) which is the ratio of metal concentrations accumulated in shoots to that of the same metal in the soil (Adesodun et al. 2010). Biological transfer coefficient (BTC) also known as translocation factor (TF) was represented as the concentration of a particular metal accumulated in shoot to that metal in the root. Bio-concentration factor (BCF) was evaluated as defined as the accumulated concentration of heavy metals in plant divided by concentration to that in respective soil (Yoon et al. 2006).
2.10 Data Analysis
Mean of the replicates and standard deviation was measured. Analysis of variance (ANOVA) was made and Tukey test was used for comparison, and mean values were presented in the form of figures and tables compared the values with control plants. Graph Pad Prism, MS Exce,l and SPSS were used for statistical analysis.
3 Results and Discussion
3.1 Physicochemical Characteristics and Metal Contamination Level in Soils
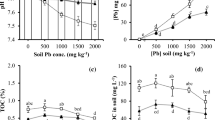
Soil samples from contaminated and reference sites were analyzed for various physicochemical parameters including heavy metals concentrations (Table 1). The soil from control site was with pH 7.01, and soil from polluted site was with pH 7.34. Results revealed that the contaminated soil was slightly alkaline in nature. The pH and EC of samples from contaminated site were higher than their respective controls. Yasmin et al. (2011) reported the pH of marble industrial effluents in the same ranges. Electrical conductivity of contaminated soil was higher as compared to control, which might be due to ions present in soil (Li et al. 2010). The lead (Pb) content at both the control and polluted site was higher as compared to the concentration of Cr. It is obvious that all the physicochemical parameters of contaminated soil were found higher than the respective control except for water-holding capacity. The heavy metal concentration was higher at the polluted site compared to control sites. Kumara et al. (2013) have reported higher heavy metal concentrations at site contaminated with industrial wastes when compared with control.
3.2 Pb Concentration (ppm) in Root, Stem, and Leaf of Selected Plants
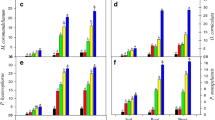
Concentration of Pb in different parts of the plants (Parthenium hysterophorus, Cannabis sativa, Rumex dentatus, and Euphorbia helioscopia) is presented in Fig. 1a–d. Increased Pb uptake was observed in plants from contaminated sites. Pb concentration was much higher in roots of all plants as compared to other parts of plants. Among these plants, the highest Pb concentrations in various parts were shown by Cannabis and Parthenium as compared to Euphorbia and Rumex. In all plants except Rumex, the Pb concentration was varied in order of root > leaves > stem except Rumex. Irshad et al. (2015) have mentioned high Pb concentrations (40–45 mg/kg) in roots and shoots of Cannabis and Parthenium. Peng and Yang (2007) observed that Cannabis growing on industrially contaminated site accumulated Pb mainly in root and less in stem and leaf. Hadi et al. (2014) reported that Parthenium hysterophorus highly accumulated Pb in the roots followed by leaves and stem under different treatments. Varun et al. (2012) have found that heavy metal Pb accumulated in different parts of Cannabis, Parthenium, and Rumex. Khan et al. (2014) during their studies on Euphorbia noticed the Pb concentrations in range of 0.11 to 0.63 mg/kg.
3.3 Lead Accumulation (μg/DBM) in Plants
Lead accumulation (μg/DBM), translocation, and bio concentration in plant parts are presented in Table 2. The highest and lowest Pb accumulations in roots were found in Parthenium (54.95 ± 12.00 μg/DBM) and Rumex (16.20 ± 1.30 μg/DBM) respectively at contaminated site. Pb accumulation in stem varied from 2.88 ± 1.35 μg/DBM in Rumex to15.38 ± 5.05 μg/DBM in Cannabis from polluted site. An increased Pb accumulation in leaves and in entire plant from contaminated site was observed in Parthenium plant (49.62 ± 13.24 μg/DBM and 115.82 ± 30.61 μg/DBM) respectively, whereas Rumex plant showed decreased accumulation (1.35 ± 0.61 μg/ DBM and 20.43 ± 3.23 μg/ DBM) respectively. Pb accumulation in root, stem, and in entire plant from control site was in the sequence i.e., Cannabis > Parthenium > Rumex > Euphorbia.
Highest Pb translocation from root to stem (0.47 ± 0.06) and from root to leaves (0.95 ± 0.03) was shown by Parthenium plant from contaminated site, while highest Pb translocation from root to stem (0.55 ± 0.04) and from root to leaves (1.05 ± 0.18) was found in Cannabis and Parthenium, respectively at control site (Table 2). In Table 2, it is obvious that uptake of Pb from contaminated site was much higher in Cannabis (1.03 ± 0.02) and Parthenium (1.01 ± 0.01) compared to Euphorbia and Rumex. Both of Parthenium (from contaminated site) and Cannabis plants (from control and contaminated site) showed bio-concentration factor > 1 for Pb which shows higher potential for its accumulation.
3.4 Cr Concentration (ppm) in Root, Stem, and Leaf of Selected Plants
Cr concentration in different parts of Parthenium hysterophorus, Cannabis sativa, Rumex dentatus, and Euphorbia helioscopia is shown in Fig. 2a–d. Plants from contaminated sites showed increased Cr concentrations. Roots of all plants showed higher Cr concentration compared to leaves and stem. Among the plants, Parthenium and Cannabis in all parts showed higher concentration of Cr as compared to Rumex and Euphorbia. The results revealed that Cr concentration in Parthenium and Cannabis was found in order i.e., in root > leaf > stem. Citterio et al. (2003) had found higher concentration of Cr in roots of Cannabis followed by leaves. Irshad et al. (2015) have also reported higher Cr concentrations (50 mg/kg) in roots of Cannabis and Parthenium. Zehra et al. (2009) demonstrated higher Pb accumulation in roots and leaves of Rumex dentatus. Cannabis sativa, and Rumex dentatus accumulated both Pb and Cr in various plant parts. Mahmood et al. (2013) have worked on various medicinal plants including Cannabis, Parthenium, and Euphorbia and have reported Pb and Cr accumulation in various plant parts.
3.5 Chromium Accumulation (μg/DBM) in Plants
Cr accumulation, translocation, and bio-concentration in Parthenium hysterophorus, Cannabis sativa, Rumex dentatus, and Euphorbia helioscopia are given in Table 3. The table shows that higher Cr accumulation in root of Parthenium (11.08 ± 2.73 μg/ DBM) was observed, followed by Cannabis (9.35 ± 3.54 μg/ DBM), Rumex (5.83 ± 0.68 μg/ DBM), and Euphorbia (1.75 ± 0.45 μg/ DBM) respectively from contaminated site. Cr accumulation in stem was found in the range of 2.08 ± 0.77 μg/ DBM in Euphorbia to 3.92 ± μg/ DBM in Rumex. Cr accumulation in roots and stems of control plants varied in the same manner as that in plants from polluted site. Parthenium from contaminated site showed an increased Cr accumulation in leaf (7.23 ± 2.44 μg/DBM) and entire plant (21.15 ± 6.50 μg/DBM), whereas Euphorbia showed decreased accumulation in leaf (2.01 ± 1.38 μg/DBM) and in entire plant (5.84 ± 2.57 μg/DBM) respectively. Cr accumulation in studied plants was found much lesser than Pb (Table 2 and Table 3). The overall bio-concentration factor of Pb was much higher than Cr for all plants studied as shown in Fig. 5. Translocation factors (root to stem and root to leaf) for Cr in all plants from both of contaminated and control sites were less than 1 (Table 3). At contaminated site, Euphorbia (0.82 ± 0.03) showed the highest Cr translocation factor (from root to stem) and Cannabis (0.76 ± 0.03) showed the highest Cr translocation factor (from root to leaf), while in control plants, the highest Cr translocation factor (root to stem) was found in Cannabis (0.71 ± 0.03) and highest Cr translocation factor (root to leaf) was noted in Parthenium (0.89 ± 0.00). It is apparent from Table 3 that uptake of Cr from both contaminated and control sites was higher in Parthenium and lower in Euphorbia. But all the plants showed bio-concentration factor almost < 1 for Cr, which shows their low potential for the accumulation of Cr.
3.6 Effect of Pb and Cr on Proline Accumulation in Roots and Leaves
Proline accumulation in plants is an indicator of heavy metals stress which prevents oxidative injury in plants (Hare and Cress 1997). The effect of Pb and Cr on proline contents in root was determined by the comparison of control (reference site) with contaminated site (industrial contaminated soil). The results revealed that proline contents increased in roots of plants from contaminated site compared to control site as shown in Fig. 3a–d. The roots of Cannabis from contaminated site showed significantly higher proline accumulation as compared to its control (from reference site) and as compared to the roots of other three plants. Parthenium root has also significantly increased proline accumulation as compared to control. Similar increase was observed in Rumex and Euphorbia when compared with control, but their root proline contents were lower than Cannabis and Parthenium. The leaf of Parthenium showed significant increase in proline accumulation compared to control, followed by Rumex, Euphorbia, and Cannabis. Also, the results showed that proline content of leaf in plants from contaminated site was higher than plants from control site. According to our results, a positive correlation between proline accumulation and Pb and Cr concentration has been observed in roots and leaves of all the studied plants. A significantly higher proline accumulation was observed in plants from contaminated site than plants from reference site. Similarly, Rai et al. (2004) have reported higher accumulation of proline in Cr-treated Ocimum tenuiflorum. It has also been previously reported that Pb induced the proline accumulation in shoots of Brassica (John et al. 2009) and artichoke (Karimi et al. 2012).
3.7 Effect of Pb and Cr on Phenolic Accumulation in Roots and Leaves
Plants produce certain important metabolites i.e., total phenolics to cope with heavy metal stress (Ferraris et al. 1987). These compounds provide defense against oxidative stress by acting as metal chelators and quenching of ROS. The accumulation of phenolics contents in roots and leaves of the studied plants under Pb and Cr stress both from the control and contaminated sites is presented in Fig. 4a–d. According to the results, both the heavy metals increased the phenolics contents of the root in all plants at contaminated site when compared to control site. Parthenium plant showed a significant increase in phenolic contents in roots compared to its control, followed by Euphorbia, Cannabis, and Rumex. Leaves of both Parthenium and Euphorbia showed high accumulation of phenolics at contaminated site, and their phenolic content was higher than Cannabis and Rumex plants. Significant increase in phenolic content of leaves of plants from contaminated site was observed when compared to their respective reference plants. The results revealed that total phenolic contents were increased in both roots and leaves of plants from contaminated site than from control sites. Metal toxicity induces increased phenolic accumulation in several plants, such as Arabidopsis thaliana (Lummerzheim et al. 1995) and Cannabis sativa (Ahmad et al. 2015). A similar effect of Pb and Cr was found on all the studied plants in present investigation (i.e., significant increase was found in phenolic contents of leaves and roots of plants from polluted site compared to non-polluted site).
3.8 Effect of Pb and Cr on Chlorophyll, Carotenoids, and Dry Biomass
The chloroplast, as the set of chlorophyll pigments in plants, occupies a unique position in the economy of the green cell. Carotenoids are non-enzymatic accessory pigments which help to protect the plants during photo-oxidation. The heavy metals Pb and Cr are known to negatively affect the most vital process in plants i.e., photosynthesis by disturbing the synthesis of chlorophyll or due to its increased degradation (Somashekaraiah et al. 1992). The effects of these metals on chlorophyll a, chlorophyll b, total chlorophyll as well as carotenoid contents of all the plants both at polluted and non-polluted sites were assessed. The results revealed that chlorophyll and cartenoid contents decreased significantly in all plants i.e., Parthenium, Cannabis, Euphorbia, and Rumex under Pb and Cr stress at contaminated sites when compared to their control plants as shown in Table 4. The polluted site plants significantly decreased the plants dry biomass when compared with plants from control (reference) sites as shown in Fig. 6. Several researchers have reported similar results on other crop plants. Rai et al. (2004) working on Ocimum tenuiflorum reported that chromium significantly (p < 0.05) reduced the level of photosynthetic pigments (total chlorophyll, chlorophyll a, chlorophyll b, and carotenoids). Chlorophyll contents are considered as indicator of the plant growth and biomass production. Ralph and Burchett (1998) demonstrated that chlorophyll a, b, and total chlorophyll were significantly lower (p < 0.05) in 5 and 10 mgL−1 Pb-treated Halophila ovalis plant compared to control.
3.9 Correlations among Different Parameters
The proline and phenolic contents of the roots and leaves showed a positive correlation, while chlorophyll and carotenoid contents showed negative correlation with Pb and Cr concentration in all the studied plants. All the correlations are represented in Table 5.
4 Conclusions
Our results established that the heavy metal (Pb and Cr) contents were higher at polluted site compared to reference site. The lead content in soils of study area was higher compared to chromium. Both the metals accumulated are much higher in roots. The Cannabis sativa and Parthenium hysterophorus showed BCF > 1 for Pb (Fig. 5 and 6) which reveals their better phytoaccumulation potential for Pb accumulation. Endogenous proline and phenolic contents of the root and leaves were highly increased in all studied plants with increasing metal accumulation in tissues. Present findings suggest that weeds, Cannabis, and Parthenium growing at industrial sites can naturally tolerate and accumulate higher levels of studied metals in their various parts and can be used for phytoremediation of lands contaminated with toxic metals. Further study is recommended to screen these plants for other heavy metals and more native plants species for hyperaccumulation from the same area.
References
Adesodun, J. K., Atayese, M. O., Agbaje, T. A., Osadiaye, B. A., Mafe, O. F., & Soretire, A. A. (2010). Phytoremediation potentials of sunflowers (Tithoniadiversifoliaand Helianthus annus) for metals in soils contaminated with zinc and lead nitrates. Water Air &Soil Pollution, 207, 195–201.
Ahmad, A., Hadi, F., & Ali, N. (2015). Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. International Journal of Phytoremediation, 17, 56–65.
Allen, S. E. (1974). Chemical analysis of ecological materials. Oxford: Blackwell scientific publication.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington DC: American public health association.
Arnon, D. I. (1949). Copper enzyme in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiology, 130, 267–272.
Bates, L. S., Waldren, S. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Borker, A. R., Mane, A. V., Saratale, G. D., & Pathade, G. R. (2013). Phytoremediation potential of Eichhornia crassipes for the treatment of cadmium in relation with biochemical and water parameters. Emirates Journal of Food and Agriculture, 25, 443–456.
Chaney, R. L. (1983). Plant uptake of inorganic waste constitutes. In J. F. Parr, P. B. Marsh, & J. M. Kla (Eds.), Land treat hazard wastes (pp. 50–76). Park Ridge: Noyes Data Corp.
Citterio, S., Santagostino, A., Fumagalli, P., Prato, N., Ranalli, P., & Sgorbati, S. (2003). Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant and Soil, 256, 243–252.
Davis, D. G., & Swanson, H. R. (2001). Activity of stress-related enzymes in the perennial weed leafy spurge (Euphorbia esulaL.). Environmental and Experimental Botany, 46, 95–108.
Ferraris, L., Abbatista-Gentile, I., & Matta, A. (1987). Variations of phenolics concentrations as a consequence of stress that induce resistance to Fusarium wilt of tomato. Journal of Plant Diseases and Protection, 94, 624–629.
Gosh, M., & Singh, S. P. (2005). A review on phytoremediation of heavy metals and utilization of its byproducts. Applied Ecology and Environmental Research, 3, 1–18.
Hadi, F., Bano, A., & Fuller, M. P. (2010). The improved phytoextraction of lead (Pb) and the growth of maize (ZeamaysL.): the role of plant growth regulators (GA 3 and IAA) and EDTA alone and in combinations. Chemosphere, 80, 457–462.
Hadi, F., Ali, N., & Ahmad, A. (2014). Enhanced phytoremediation of cadmium-contaminated soil by Partheniumhysterophorus plant: effect of gibberellic acid (GA3) and synthetic chelator, alone and in combinations. Bioremediation Journal, 18, 46–55.
Hadi, F., Ali, N., & Fuller, M. P. (2016). Molybdenum (Mo) increases endogenous phenolics, proline and photosynthetic pigments and the phytoremediation potential of the industrially important plant Ricinus communis L. for removal of cadmium from contaminated soil. Environmental Science and Pollution Research, 23, 20408–20430.
Handique, G. K., & Handique, A. K. (2009). Proline accumulation in lemongrass (CymbopogonflexuosusStapf.) due to heavy metal stress. Journal of Environmental Biology, 30, 299–302.
Hare, P. D., & Cress, W. A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regulation, 21, 79–102.
Irshad, M., Ahmad, S., Pervez, A., & Inoue, M. (2015). Phytoaccumulation of heavy metals in natural plants thriving on wastewater effluent at Hattar industrial estate, Pakistan. International Journal Phytoremdiation, 17, 154–158.
John, R., Ahmad, P., Gadgil, K., & Sharm, S. (2009). Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. International Journal of Plant Production, 3, 65–76.
Karimi, L. N., Ahmadi, M. K., & Moradi, B. (2012). Accumulation and phytotoxicity of lead in Cynarascolymus. Indian Journal of Technology, 5, 3634–3641.
Khan, F. A., Ahmad, R., Khan, H., & Ullah, N. (2014). Geographical impact of heavy metals on Euphorbia heliscopia. Life Science Journal, 11, 236–239.
Kumara, N., Bauddha, K., Kumara, S., Dwivedia, N., Singha, D. P., & Barmanb, S. C. (2013). Accumulation of metals in weed species grown on the soil contaminated with industrial waste and their phytoremediation potential. Ecological Engineering, 61, 491–495.
Li, C., Xie, F., Ma, Y., Cai, T., Li, H., Huang, Z., & Yuan, G. (2010). Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. Journal Hazardous Material, 178, 823–833.
Lummerzheim, M., Sandroni, M., Castresana, C., Oliveira, D. D. E., Vanmontagu, M., Roby, D., & Timmerman, B. (1995). Comparative microscopic and enzymatic characterization of the leaf necrosis induced in Arabidopsis thaliana by lead nitrate and by Xanthomonas campestris pv. Campestris after foliar spray. Plant Cell Environment, 18, 499–509.
Mahmood, A., Rashid, S., & Malik, R. N. (2013). Determination of toxic heavy metals in indigenous medicinal plants used in Rawalpindi and Islamabad cities, Pakistan. Journal Ethno Pharmacology, 148, 158–164.
McGrath, S. P., & Zhao, F. J. (2003). Phytoextraction of metals and metalloids from contaminated soils. Current Opinion Biotechnology, 14, 277–282.
Peng, H., & Yang, X. (2007). Characteristics of copper and lead uptake and accumulation by two species of Elsholtzia. Bulletin of Environmental Contamination and Toxicology, 78, 152–157.
Peralta-Videa, J. R., Lopez, M. L., Narayan, M., Saupe, G., & Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. International Journal of Biochemistry and Cell Biology, 41, 1665–1677.
Rai, V., Vajpayee, P., Singh, S. N., & Mehrotra, S. (2004). Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorumL. Plant Science, 167, 1159–1169.
Ralph, P. J., & Burchett, M. D. (1998). Photosynthetic response of Halophila ovalis to heavy metal stress. Environmental Pollution, 103, 91–101.
Reddy, K. N., Bryson, C. T., & Burke, I. C. (2007). Ragweed parthenium (Parthenium hysterophorus) control with pre emergence and post emergence herbicides. Weed Technology, 21, 982–986.
Revathi, K., Harbabu, T. E., & Sudha, P. N. (2011). Phytremediation of chromium contaminated soil using sorghum plant. International Journal of Environmental Science, 2, 417–428.
Riaz, T., & Javaid, A. (2007). Invasion of exotic weed partheniumhysterophorus l. in district Sheikhupura, Pakistan. International Journal of Biology and Biotechnology, 4, 163–166.
Shakoor, M. B., Ali, S., Farid, M., Farooq, M. A., Tauqeer, H. M., Iftikhar, U., Hannan, F., & Bharwana, S. A. (2013). Heavy metal pollution, a global problem and its remediation by chemically enhanced phytoremediation: a review. Journal of Biodiversity and Environmental Sciences, 3, 12–20.
Siddiqui, I., & Bajwa, R. (2001). Variation in weed composition in wheat fields of Lahore and Gujranwala divisions. Pakistan Journal of Biological Science, 4, 492–504.
Singh, S., Srivastava, P. K., Gupta, M., & Mukherjee, S. (2012). Modeling mineral phase change chemistry of groundwater in a rural-urban fringe. Water Science and Technology, 66, 1502–1510.
Singleton, V. L., & Rossi, J. A. (1965). Calorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Somashekaraiah, B. V., Padmaja, K., & Prasad, A. R. (1992). Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiologia Plantarum, 85, 85–89.
Srivastava, P. K., Gupta, M., & Mukherjee, S. (2012). Mapping spatial distribution of pollutants in groundwater of a tropical area of India using remote sensing and GIS. Applied Geomatics, 4, 21–32.
Sthanadar, I. A., Sthanadar, A. A., Yousaf, M., Muhammad, A., & Zahid, M. (2013). Bioaccumulation profile of heavy metals in the gills tissue of Wallago attu (Mulley) from Kalpani River Mardan, Khyber Pakhtunkhwa Pakistan. International Journal of Bioscience, (9), 165–174.
Varun, M., Dsouza, R., Pratas, J., & Paul, M. S. (2012). Metal contamination of soils and plants associated with the glass industry in North Central India. Prospects of phytoremediation. Environmental Science and Pollution Research, 19, 269–281.
Wu, G., Kang, H., Zhang, X., Shao, H., Chu, L., & Ruan, C. (2010). A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. Journal Hazardous Material, 174, 1–8.
Yasmin, A., Nawaz, S., & Ali, S. M. (2011). Impact of industrial effluents on germination and seedling growth of Lens esculentumvarieties. Pakistan Journalof Botany, 43, 2759–2763.
Yoon, J., Cao, X., Zhou, Q., & Ma, Q. L. (2006). Accumulation of Pb, Cu and Zn in native plants growing on a contaminated Florida site. Science Total Environment, 368, 456–464.
Zehra, S. S., Arshad, M., Mahmood, T., & Waheed, A. (2009). Assessment of heavy metal accumulation and their translocation in plant species. African Journalof Biotechnology, 8, 2802–2810.
Zvinowando, C. M., Okwonko, P. N., Shabalala, P. N., & Aggai, N. M. (2009). Novel adsorbent for heavy metals remediation in aqueous environment. International Journalof Environmental Science and Technology, 6, 425–434.
Acknowledgements
The Central Resources Laboratory (CRL) of Peshawar and its staff is acknowledged for their support and facilities provided.
Funding
The study is financially supported by the HEC of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ullah, R., Hadi, F., Ahmad, S. et al. Phytoremediation of Lead and Chromium Contaminated Soil Improves with the Endogenous Phenolics and Proline Production in Parthenium, Cannabis, Euphorbia, and Rumex Species. Water Air Soil Pollut 230, 40 (2019). https://doi.org/10.1007/s11270-019-4089-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4089-x