Abstract
The degradation of two sulfonylurea herbicides, nicosulfuron and thifensulfuron methyl in water by chlorine dioxide, was studied for the first time in this paper. In order to examine the optimal parameters for degradation of both herbicides, degradation was investigated under light or dark conditions with different amount of chlorine dioxide, different degradation periods, and at different pH values. Degradation efficiency of herbicides was monitored using high-performance liquid chromatography with photodiode array detection (HPLC-DAD). The degradation products were analyzed by gas chromatography with triple quadrupole mass detector (GC–QQQ). Three products were identified after degradation of nicosulfuron and two products after degradation of thifensulfuron methyl. Total organic analysis (TOC) gave insight into some differences in degradation mechanisms and degrees of mineralization after degradation of the herbicides using chlorine dioxide. A simple mechanism of herbicide degradation was proposed. Acute toxicity tests were performed on the products produced after degradation with chlorine dioxide, and the results showed that the degradation products were less toxic than the parent compounds. The findings of the present study are very useful for the treatment of wastewaters contaminated with herbicides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides are very hazardous pollutants that can persist in the aquatic environment for many years (Shukla et al. 2006). Contamination of soil and ground water with pesticides applied to soil and swept up by transport processes such as leaching or runoff is posing an increasingly serious environmental problem (Đorđević et al. 2014). Due to their long-term persistence in soil, high water solubility, and photochemical stability, contamination of water resources with pesticides used in agriculture is a cause for environmental concern (Ahmed et al. 2011; Jović et al. 2013; Lanchote et al. 2000). Faced with the rapid growth of the world’s population, modern agriculture has to retain and improve production capacity, but also try to minimize its environmental impact (Vela et al. 2004).
Pesticides can pose a threat to the environment because they are designed to have specific physiological effects on living cells and can be xenobiotics, mutagens, carcinogens, and teratogens. Long-term human exposure to pesticides, even at low concentrations, could result in serious health problems (George et al. 2011). Almost 80% of the world’s human population is assumed to be exposed to pesticides in water (Pileggi et al. 2012). Conventional biological treatments cannot completely remove pesticides. Their removal from the environment, especially from surface waters, is now an imperative and is the subject of studies which have involved numerous researchers for several years now.
Sulfonylureas are a large family of herbicides widely used for control of broad leaf weeds in various crops and vegetables as well as industrial weeds. They have gained attention more so than other pesticides due to their good crop selectivity, low application rates, and favorable environmental properties (Dugandžić et al. 2017). However, due to their high solubility in water, moderate to high mobility, and slow degradation, they are now being detected in surface and ground waters (Benzi et al. 2011; Battaglin et al. 2000). Moreover, they express low to acute mammalian toxicity. Therefore, effective, low-cost, and robust methods to decontaminate waters are needed, as long as they do not further stress the environment or endanger human health.
Nicosulfuron, chemically defined as 2-[(4,6-dimethoxypyrimidin-2-yl)carbamoylsulfamoyl]-N,N-dimethylpyridine-3-carboxamide (NS; Fig. 1), and thifensulfuron methyl, chemically defined as methyl 3-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]thiophene-2-carboxylate (TFSM; Fig. 1), are sulfonylurea herbicides typically used to control weeds in post-emergence treatments. Microbial degradation and chemical hydrolysis in soil and water are two primary degradation mechanisms of sulfonylurea pesticides (Berger et al. 1996). Sabadie (Sabadie et al. 2002; Sarmah et al. 2002) has showed that hydrolysis of nicosulfuron is much more rapid under acidic conditions, while the reaction follows first-order kinetics. Biodegradation of nicosulfuron by bacteria, such as Serratia marcescens N80 and by fungi, such as Talaromyces flavus LZM1 has also been reported (Ma et al. 2011; Song et al. 2013). In the environment, sulfonylurea herbicides absorb solar light and undergo direct photodegradation. During the last decade, several studies have demonstrated that some sulfonylurea pesticides can be photoremoved from waters by the use of semiconductor materials, mainly titanium dioxide. Dugandžić et al. (2017) studied the photocatalytic degradation of nicosulfuron using TiO2 as a catalyst under UV light, and Maurino et al. (1999) studied TiO2 photocatalytic degradation of chlorsulfuron and thifensulfuron methyl. Some studies have reported photolysis of nicosulfuron: photo-induced aqueous degradation (Benzi et al. 2011) and photolysis on a simulated cuticular wax film (Halle et al. 2010). The photocatalytic degradation of five sulfonylurea herbicides, including nicosulfuron, using ZnO (with or without Na2S2O8), WO3, SnO2, and ZnS as photocatalysts under natural sunlight, has been also investigated (Fenoll et al. 2012), as well as the photocatalytic degradation of 30 sulfonylurea herbicides, including nicosulfuron and thifensulfuron methyl, with TiO2 and ZnO in tandem with Na2S2O8 (Fenoll et al. 2013). Adsorption and desorption of nicosulfuron in soils (Gonzalez et al. 1996; Fan et al. 2008) and clay minerals (Ukrainczyk et al. 1995) have also been studied. Furthermore, a study of the adsorption of nicosulfuron on a calcined Mg–Al hydrotalcite in the presence and absence of various anions found that calcined hydrotalcite is an effective adsorbent for the removal of nicosulfuron from contaminated water (Otero et al. 2013). To minimize the risk of pesticide pollution, it is advisable to develop new technologies that promote easy degradation of pesticides.
To the best of our knowledge, no detailed optimization study of nicosulfuron and thifensulfuron methyl degradation using chlorine dioxide has been published so far. ClO2 is a strong oxidizing agent, bactericide, fungicide, algaecide, and antiseptic. It is a powerful oxidant (E0 = 0.936 V) which can remove many organic contaminants, including pesticides. The literature contains data for removal of some pesticides (such as isoproturon, chlortoluron, diuron, ametryn, methiocarb, phorate, diazinon, etc.) as well as for removal of diclofenac, antipyrine, and sulfonamide antibiotics (Lopez et al. 1997; Tian et al. 2010, 2014; Chen et al. 2014; Jia et al. 2017; Wang et al. 2015; Ben et al. 2017; Chamberlain et al. 2012; Hwang et al. 2002). ClO2 is also suitable for the treatment of apples, lettuce, and minced meat in order to reduce microbial activity, for the degradation of certain drugs, as well as for the removal of pesticides on fresh fruits and vegetables (Chen et al. 2014; Sharma et al. 2008). Moreover, ClO2 is used as a disinfecting/oxidizing agent in the treatment of drinking water. In comparison with chlorine, it has a stronger antimicrobial activity, and compared with ozone and chlorine, does not produce toxic products such as trihalomethane, halogen acids, or ketones.
The objectives of this study were to: (a) investigate and optimize degradation of nicosulfuron and thifensulfuron methyl using ClO2 in water, (b) investigate the degradation efficiency of herbicides using high-pressure liquid chromatography with photodiode array detection (HPLC-DAD), (c) identify the products of herbicide degradation using gas chromatography with triple quadrupole mass detector (GC-QQQ), and (d) examine the toxicity of herbicide degradation products after ClO2 treatment. Analysis of total organic carbon (TOC) was also used to monitor nicosulfuron and thifensulfuron methyl mineralization.
2 Materials and Methods
2.1 Chemicals
Nicosulfuron and thifensulfuron methyl (both technical grade, 98%) were both supplied by the Institute for Plant Protection, Belgrade and were applied without further purification. The pure stock solution of ClO2 (3 g/L) was prepared by mixing sodium chlorite (Superior Water Disinfection Power, TwinOxide®) and sodium bisulfate (Superior Water Disinfection Power, TwinOxide®), in 1 L of distilled water. The exact concentration of ClO2 in the stock solution was quantified using 4500-ClO2 DPD method according to the Standard Method (American Public Health Association 1998). Sodium-thiosulfate (Na2S2O3, p.a., Merck) was used as received.
Acetonitrile (> 99.9%, Sigma-Aldrich HPLC grade), formic acid (Fluka analytical HPLC grade), and water (HPLC grade water, Sigma-Aldrich) were used for HPLC analysis. Syringe filters (25 mm, PTFE membrane 0.45 μm) were obtained from Agilent Technologies. Methylene chloride (> 99.5%) was supplied from LGC and was used for GC-QQQ analysis.
2.2 Experimental Setup
In all experiments, the concentration of herbicides was 10 mg/L dissolved in deionized water. Degradation of aqueous herbicide solution by ClO2 was performed in 200-mL closed flasks on a rotary shaker. Different dosages of ClO2 stock solution (5 and 10 mg/L) were added to herbicide solution (10 mg/L) to initiate the reaction on room temperature. At different time intervals (30 min, 1 h, 2 h, 3 h, 6 h, and 24 h of degradation), 10 mL of reaction mixture was taken and ClO2 residues were quenched with a standard 0.1 mol/dm3 solution of Na2S2O3 (in 10 mL of sample was added approximately 0.3 mL of a solution of Na2S2O3) prior to HPLC analysis, which was used to evaluate degradation efficiency. In order to optimize the conditions for degradation of herbicides, degradation was investigated in the dark and in the light, as well as at different pH values (see below). In order to check the reproducibility of results, all degradation experiments were repeated three times, and the experimental error was found to be within 5%.
2.3 Analytical Procedure
Degradation efficiency of herbicides was monitored using high-performance liquid chromatography (HPLC) (Thermo Ultimate 3000 RS) with photodiode array detection (DAD) on a Hypersil Gold aQ C18 analytical column (150 mm × 3 mm, 3 μm) at 40 °C. The mobile phase consisted of 0.1% formic acid water solution as component A and acetonitrile as component B. The chromatographic elution was conducted at a flow rate of 0.6 mL/min in gradient mode: 0.0–0.5 min 5% B, 0.5–6.0 min from 5 to 45% B, 6.0–8.0 min from 45 to 95% B, 8.0–8.1 min from 95 to 5% B, then 5% B for 6 min. The detector was set at 197, 224, 233, and 240 nm for detection of thifensulfuron methyl and at 197 nm for detection of nicosulfuron. Injection volume was 25 μL. Data analysis was performed with software Chromeleon, v6.8 (ThermoFisher Scientific, Bremen, Germany). At appropriate time intervals, samples were taken from the reaction mixture and quenched using Na2S2O3. Degradation efficiency was calculated for each sample by the equation:
where η was the degradation efficiency (%); P0 was the peak area of initial herbicide concentration; Pt (mAU/min) was the peak area of residual herbicide concentration.
pH values were measured using a pH meter (Orion Star A221, Thermo Scientific). The pH of herbicide solutions was adjusted to three different values (pH 3.00, 7.00, and 9.00) by adding sulfuric acid (conc. 98%, Sigma-Aldrich) or sodium hydroxide (analytical grade Sigma-Aldrich).
Major degradation products of both herbicides were determined by Agilent Technologies gas chromatograph with triple quadrupole mass detector (GC-QQQ) 7890B/7010, capillary column HP5-MS (30 m length, 0.25 mm inner diameter, and 0.25 μm film layer), and He gas (5.0 specification) as the carrier gas at a flow rate of 1.5 mL/min. The GC temperature program consisted of an initial temperature of 40 °C, which was held for 1 min, followed by decrease at a rate of 25 °C/min to 160 °C, which was held for 1 min, then a rate of 5 °C/min to 300 °C, which was held for 0.5 min. Transfer lines were held at 280 °C, and the injection port was controlled at 150 °C. The temperature of the ion source was 230 °C and the temperature of quadrupole was 150 °C. The spectrum was obtained at a scan range from m/z 40 to 400. Samples for GC-QQQ analysis were prepared by triplicate methylene chloride extraction and concentrating the organic extract to 1 mL.
Total organic analysis (TOC) was performed with a model Zellweger LabTOC 2100 TOC Analyzer. Percent of degradation (i.e., degree of mineralization) was expressed as “TOC (%)” and calculated using data obtained by TOC analysis using the following formula:
2.4 Toxicity Tests Using Daphnia magna
Acute toxicity tests with Daphnia magna (MicroBioTests Inc.) were performed according to standardized guidelines OECD 20234 at 21 ± 1 °C and photoperiod of 16 h light/8 h dark. The initial herbicides and degradation products of herbicides were analyzed after 24 h of ClO2 treatment. Herbicides were first dissolved in the specified medium (OECD Guideline 202, 2004) then exposed to sonification, and in order to achieve better dissolution, the sonified compounds were left overnight. Then, the sonified compounds were diluted to desired concentrations using the same medium. Five D. magna neonates not older than 24 h were placed in each vessel with 25 mL of the test medium (medium was diluted according to Klüttgen et al. 1994). Acute toxicity tests were performed in borosilicate glasses with control and five test dilutions (6.25, 12.5, 25, 50, and 100%) with four replicates per dilution and four replicates per control solution. Neonates were not fed during the exposure. Endpoint was the mortality of neonata. Toxic effect for each sample was expressed as the percentage of mortality. Tests were considered valid if the mortality in the controls did not exceed 10%. Immobilization of the neonates was observed after 24 and 48 h, and the results were compared to the control. The LC50 (lethal concentration which caused 50% mortality of the test organism) values with 95% confidence intervals were estimated by regression model: Spearman-Karber using TesTox software, version 1.0. Toxicity assessment was carried out according to a wastewater toxicology classification scale proposed by Persoone et al. (2003), where the results of toxicity tests were calculated as toxicity units acute (TUa). The TUa of an effluent is the inverse of its LC50 value: TU = 100/LC50.
3 Results and Discussion
3.1 Influence of Chlorine Dioxide Dosage, pH and Light on Degradation of Herbicides
In view of the increasing usage of ClO2 in water treatment, it is important to investigate its reactivity with common organic contaminants, such as pesticides. This paper studies the reactivity of sulfonylurea pesticides, nicosulfuron and thifensulfuron methyl, with ClO2 under conditions that have relevance in water treatment. Optimization of ClO2 dosage (5 and 10 mg/L), degradation duration (30 min, 1, 2, 3, 6, and 24 h), and pH (3.00, 7.00, and 9.00) was performed in systems with deionized water. ClO2 was added to deionized water containing 10 mg/L pesticides.
3.2 HPLC Analysis
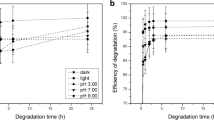
The percentage of degradation was monitored by HPLC analysis on the basis of the herbicide peak area reduction after degradation compared to the peak area of the herbicides prior to degradation. The degradation efficiency of sulfonylurea herbicides (10 mg/L) with different dosages of ClO2, pHs, and under dark and light conditions in deionized water is presented in Figs. 2 and 3. Measurements were based on the changes in nicosulfuron and thifensulfuron methyl parent signal at retention times (Rt) of 7.650 and 8.153, respectively (Figs. S1 and S2 (Supplementary Material)).
Under light and dark conditions, there was decrease in the parent signal for nicosulfuron and thifensulfuron methyl with time, and therefore, increase in degradation efficiency (Figs. 2 and 3). The results also showed somewhat higher degradation efficiency in light compared to dark conditions.
Analysis of nicosulfuron showed that at concentrations of 5 and 10 mg/L ClO2, only low efficiency of degradation was achieved in the dark (after 24 h, 52.55% was degraded for 5 mg/L and 64.17% for 10 mg/L). However, high degradation efficiency was achieved in the light at a concentration of 10 mg/L ClO2 (after 24 h, 91.43%), which was greater degradation than in the light at a concentration of 5 mg/L ClO2 (after 24 h, 65.31%). The results showed that the best degradation efficiency of 93.22% was achieved after 6 h at pH 3.00, at a concentration of 10 mg/L ClO2 (after 24 h, degradation efficiency was unchanged, remaining at 92.77%), while at the same pH and at a concentration of 5 mg/L ClO2, slightly lower efficiency of degradation was achieved (after 6 h, 86.25% and after 24 h, 88.40%). At the other pH values (7.00 and 9.00) at a concentration of 5 mg/L ClO2, unsatisfactory degradation efficiency was measured (at pH 7.00, after 24 h, 67.15%; at pH 9.00, after 24 h, 59.27%), but at a concentration of 10 mg/L ClO2, at pH 7.00 and 9.00 somewhat higher degradation efficiency was achieved (at pH 7.00, after 24 h, 83.13%; at pH 9.00, after 24 h, 84.72%). The results obtained are consistent with another study reported by Tian et al. (2014) concerning some phenylurea pesticides. They found that the degradation rate of diuron increased progressively as pH decreased.
Analysis of TFSM showed that at a concentration of 5 mg/L ClO2 in the dark, only low efficiency of degradation was achieved, while in the light at the same ClO2 concentration, somewhat better efficiency of degradation was achieved, but it was still not satisfactory (in the dark, after 24 h, 34.38%; in the light, after 24 h, 50.83%). At a concentration of 10 mg/L ClO2 in the dark, the efficiency of degradation was unsatisfactory, while in the light, good degradation efficacy was achieved (in the dark, after 24 h, 46.75%; in the light, after 24 h, 73.18%). Satisfactory degradation efficiency was not achieved at any of the observed pH values with a concentration of 5 mg/L ClO2 (at pH 3.00, after 24 h, 55.86%; at pH 7.00, after 24 h, 36.96%; at pH 9.00, after 24 h, 46.29%). However, at a concentration of 10 mg/L ClO2 and at all pH values, relatively good degradation efficiency was achieved (at pH 3.00, after 24 h, 67.23%; at pH 7.00, after 24 h, 61.36%; at pH 9.00, after 24 h, 67.26%), but it was lower compared to degradation in the light.
Analysis of degradation products and evaluation of their toxicity were conducted using the most efficiently degraded materials, i.e., by treating NS with 10 mg/L ClO2 at pH 3.00 for 6 h and by treating TFSM with 10 mg/L ClO2 for 24 h without pH adjustment. However, given these optimal conditions, more NS than TFSM was degraded. Under these optimal conditions for degradation of both herbicides, the signal did not become totally flat, indicating that neither herbicide was completely degraded. At the same time, new peaks were detected, with increasing intensities indicating the formation of new intermediates during the course of the reactions. For NS, new peaks occurred at retention times of 3.217, 4.713, and 6.090, and for TFSM, new peaks occurred at retention times of 3.367 and 5.827 (Figs. S1 and S2 (Supplementary Material)).
3.3 GC-QQQ Analysis
Identification of degradation products is significant for the evaluation of the specific conditions that can occur in the environment. The degradation products can provide information about degradation mechanisms and aid in discovering the key steps in the degradation. Therefore, this type of research is very topical, given the problems with pesticides and herbicides that can cause deterioration of environmental quality.
The degradation products of NS and TFSM obtained under optimal conditions were analyzed by GC-QQQ. Gas chromatograms and mass spectra of degradation products for nicosulfuron and thifensulfuron methyl are shown in Figs. S3, S4, and S5 (Supplementary Material). Three degradation products for NS and two degradation products for TFSM were identified according to their respective spectral characteristics: mass spectra, accurate mass, and characteristic fragmentation. Table 1 shows the GC-QQQ data for degradation products of both herbicides with respect to retention time. Proposed structures for the degradation products formed by degrading NS and TFSM under optimal conditions are shown in Figs. 4 and 5.
Herbicide degradation with ClO2 started with an attack on the urea groups. This type of reaction occurred in both herbicides we investigated. However, there was a slight difference in behavior between these two herbicides. Analyzing degradation products using GC-QQQ, we deduced that in NS, the urea group reacts first, with one bond breaking and two degradation products forming 2-(N-formylsulfamoyl)-N,N-dimethylnicotinamide (N-M1) with characteristic ion m/z 259 and 4,6-dimethoxypyrimidin-2-amine (N-M3) with characteristic ion m/z 155. After that, further degradation of N-M1 occurred and led to formation of N,N-dimethylnicotinamide (N-M2) with characteristic ion m/z 151 and N-hydrosulfonylformamide with characteristic ion m/z 109, as shown in Fig. S4 (Supplementary Material). A completely different set of reactions applied to TFSM, in which the urea group also reacted first, but at the same time, this group also reacted last, because the degradation reaction occurred on both sides of the carbonyl group (Fig. S5 (Supplementary Material)). This type of TFSM degradation produced only two organic products: 4-methoxy-6-methyl-1,3,5-triazin-2-amine (TM1) with characteristic ion m/z 140 and 4-methoxy-6-methyl-1,3,5-triazin-2-amine (TM2) with characteristic ion m/z 223. Besides these two organic products, CO2 formed during TFSM degradation. These types of degradation and formation of the degradation products were also confirmed by TOC analysis, showing the degree of mineralization after degradation of NS and TFSM. The degree of mineralization should be higher for TFSM than for NS, because of the CO2 released during degradation of TFSM.
3.4 Results of Toxicity Tests
Nicosulfuron and thifensulfuron methyl and their degradation products were also tested for acute toxicity on D. magna. Degradation products resulting from the highest degradation efficiency reactions were used, i.e., nicosulfuron treated with 10 mg/L ClO2 at рН 3.00 after 24 h and thifensulfuron methyl treated with 10 mg/L ClO2 after 24 h without pH adjustment. The results obtained are presented in Fig. 6 and Table 2. No mortality was observed in the control, so the test acceptability criterion (90% or higher survival rate) for 24 h was fulfilled. The data obtained indicate that degradation products of both NS and TFSM were of lower toxicity than the parent herbicides and could be classified as category III (toxic unit (TU) = 1–10) (Table 2), i.e., acutely toxic on a scale from I to V (Persoone et al. 2003). The same LC50 values were obtained for both herbicides after 24 h (data not shown) and 48-h test periods.
Dugandžić et al. (2017) investigated phytotoxicity before and after the photocatalytic degradation (with TiO2 as a catalyst under UV light) of nicosulfuron using mung bean seeds. They found that phytotoxicity was reduced from 56.8% before the degradation to 14.8% after 90 min of photocatalysis, indicating that photocatalytic degradation of nicosulfuron using TiO2 is a sustainable and environmentally friendly method of herbicide degradation. Some bacterial and fungal strains able to biotransform nicosulfuron have been isolated, but only by using an additional source of carbon (e.g., glucose-rich medium) (co-metabolism) (Lu et al. 2012; Song et al. 2013; Wang et al. 2016; Zhang et al. 2012; Zhao et al. 2015). Carles et al. (2017) assessed the microbial toxicity of nicosulfuron and its metabolites, alone or in mixtures, with the standardized Microtox®test. They found a bacterial strain, Pseudomonas fluorescens SG-1, able to biotransform nicosulfuron efficiently. While the classic hydrolytic cleavage of the sulfonylurea bridge was observed, other more unusual pathways were also identified, such as contraction/rearrangement of the sulfonylurea bond, demethylation, and cleavage of the NH-triazinic ring or opening of the triazinic ring according to the sulfonylurea structure, showing the great potential of this bacterium. The toxicity of metabolites showed a 20-fold higher toxicity of 2-amino-4,6-dimethoxypyrimidine than nicosulfuron. They showed increased toxicity during the transformation of nicosulfuron from an inhibitory concentration 50% (IC50) > 45% on day 0 to an IC50 of 3.9% on day 14. This increased toxicity could be caused by the production of nicosulfuron metabolites that are more toxic than the parent compound. Some studies also showed toxic effects of nicosulfuron on various aquatic plants, such as macrophytes, phytoplankton, diatoms, and freshwater microalgae (Carles et al. 2017; Leboulanger et al. 2001; Seguin et al. 2001).
3.5 TOC Analysis
The extent of mineralization is indicative of the efficiency of ClO2-driven degradation processes, and higher levels of mineralization imply fewer negative effects of contaminated wastewater on the environment. Mineralization was studied using TOC analysis. TOC was determined for the parent herbicides as well as for the degradation products after the most efficient degradation processes as determined by HPLC. Results of TOC analyses, presented in Table 2, did not coincide with results obtained by HPLC analyses. The difference between these results likely originates partially in the methods themselves. HPLC analysis monitors herbicide concentration during degradation, while TOC analysis monitors the content of organic carbon and so can tell us if some of the organic carbon has been mineralized. This difference in our observed results indicates that the parent herbicides were successfully degraded to some extent, but some organic degradation products remained. Low levels of mineralization, and thus, the presence of organic degradation products, can influence the toxicity of contaminated water after ClO2 degradation of herbicides. Organic degradation products can have adverse effects but are not necessarily harmful to the environment. The best way to determine this is by toxicity analysis using D. magna. Comparison of all three methods is presented in Fig. 6.
Figure 6 shows that nicosulfuron had much better degradation efficiency than TFSM (monitored by HPLC), which means that the parent herbicide was efficiently degraded using ClO2. On the other hand, the percent of degradation (monitored by TOC) was very similar between these two herbicides. In order to conclude which ClO2 degradation produced better results (i.e., degradation of NS or of TFSM), we must take a look at the third method used. Toxicity analysis performed using D. magna showed the degradation of nicosulfuron should have a lower impact on the environment than TFSM degradation because the LC50 value determined for nicosulfuron degradation products was higher. Comparison of HPLC and TOC degradation also showed that even if the percent of mineralization was similar for these two herbicides, more organic degradation products were produced in nicosulfuron degradation but with lower adverse effects, while TFSM degradation produced a lower amount of organic degradation products and more mineralization.
4 Conclusion
Degradation of the sulfonylurea herbicides, nicosulfuron and thifensulfuron methyl (10 mg/L), was studied in deionized water under light and dark conditions with different dosages of ClO2, different degradation periods, and at different pH values. Degradation of the herbicides with ClO2 occurred in the dark, in the light, and at all pH values. Higher degradation efficiency was achieved for NS compared to TFSM. For both herbicides, the most efficient degradation occurred in the light at a concentration of 10 mg/L of ClO2 (93% for NS and 73% for TFSM), while degradation of NS was conducted at pH 3.00 in order to achieve the best efficiency.
GC-QQQ identified three main NS and two main TFSM degradation products. In synergy with GC-QQQ, TOC analysis confirmed that mineralization occurred to a greater extent during TFSM degradation than during NS degradation and that TFSM degradation produced CO2, thus confirming the degradation mechanisms for both herbicides as suggested by GC-QQQ analysis.
Toxicological analysis showed that the resultant degradation products of both herbicides had lower toxicity than the parent herbicides and were classified as category III, i.e., acutely toxic on a scale of I to V. Further research will be required to reduce toxicity levels of degraded herbicides. Accurate estimates of the overall effects of sulfonylurea herbicides on environmental ecosystems are needed, which will also contribute to the development of improved removal processes for these compounds. The results obtained can be applied to the treatment of industrial wastewaters and other waters contaminated with these herbicides.
Change history
03 September 2018
During typesetting, the image of figure 4 was also used in figure 5. The mistake was discovered after the original article was published online.
References
Ahmed, S., Rasul, M. G., Brown, R., & Hashib, M. A. (2011). Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. Journal of Environmental Management, 92(3), 310–330.
APHA (American Public Health Association). (1998). American Water Works Association, and water environment federation, standard methods for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association.
Battaglin, W. A., Furlong, E. T., Burkhardt, M. R., & Peter, C. J. (2000). Occurrence of sulfonylurea, sulfonamide, Imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Science of the Total Environment, 248(2–3), 123–133.
Ben, W., Shi, Y., Li, W., Zhang, Y., & Qiang, Z. (2017). Oxidation of sulfonamide antibiotics by chlorine dioxide in water: Kinetics and reaction pathways. Chemical Engineering Journal, 327(11), 743–750.
Benzi, M., Robotti, E., & Gianotti, V. (2011). HPLC-DAD-MSn to investigate the Photodegradation pathway of Nicosulfuron in aqueous solution. Analytical and Bioanalytical Chemistry, 399(4), 1705–1714.
Berger, B. M., & Wolfe, N. (1996). Hydrolysis and biodegradation of sulfonylurea herbicides in aqueous buffers and anaerobic water sediment systems: Assessing fate pathways using molecular descriptors. Environmental Toxicology and Chemistry, 15(9), 1500–1507.
Carles, L., Joly, M., Bonnemoy, F., Leremboure, M., Batisson, I., & Besse-Hoggan, P. (2017). Identification of sulfonylurea biodegradation pathways enabled by a novel Nicosulfuron-transforming strain Pseudomonas fluorescens SG-1: Toxicity assessment and effect of formulation. Journal of Hazardous Materials, 324(2), 184–193.
Chamberlain, E., Shi, H., Wang, T., Ma, Y., Fulmer, A., & Adams, C. (2012). Comprehensive screening study of pesticide degradation via oxidation and hydrolysis. Journal of Agricultural and Food Chemistry, 60(6), 354–363.
Chen, Q., Wang, Y., Chen, F., Zhang, Y., & Liao, X. (2014). Chlorine dioxide treatment for the removal of pesticide residues on fresh lettuce and in aqueous solution. Food Control, 40(6), 106–112.
Djorđević, J. S., Vladisavljević, G. T., & Trtić-Petrović, T. M. (2014). Removal of the selected pesticides from a water solution by applying hollow Fiber liquid−liquid membrane extraction. Industrial and Engineering Chemistry Research, 53(12), 4861–4870.
Dugandžić, A. M., Tomašević, A. V., Radišić, M. M., Šekuljić, N. Ž., Mijin, D. Ž., & Petrović, S. D. (2017). Effect of inorganic ions, Photosensitisers and scavengers on the photocatalytic degradation of Nicosulfuron. Journal of Photochemistry and Photobiology A: Chemistry. A, 336(3), 146–155.
Fan, J., Wei, Z., Ming, Z. Z., Xiang, X., Jun, W. J., Zhen, Q., Ke, L., & Ming, L. L. (2008). Desorption character of Nicosulfuron and effect of pH on adsorption of Nicosulfuron in soils. Journal of Agricultural Science, 21, 702–708.
Fenoll, J., Hellín, P., Flores, P., Martínez, C. M., & Navarro, S. (2012). Photocatalytic degradation of five sulfonylurea herbicides in aqueous semiconductor suspensions under natural sunlight. Chemosphere, 87(8), 954–961.
Fenoll, J., Sabater, P., Navarro, G., Vela, N., Pérez-Lucas, G., & Navarro, S. (2013). Abatement kinetics of 30 sulfonylurea herbicide residues in water by photocatalytic treatment with semiconductor materials. Journal of Environmental Management, 130(11), 361–368.
George, J., & Shukla, Y. (2011). Pesticides and Cancer: Insights into Toxicoproteomic-based findings. Journal of Proteomics, 74(12), 2713–2722.
Gonzalez, J. M., & Ukrainczyk, L. (1996). Adsorption and desorption of Nicosulfuron in soils. Journal of Environmental Quality, 25(6), 1186–1192.
Halle, A., Lavieille, D., & Richard, C. (2010). The effect of mixing two herbicides Mesotrione and Nicosulfuron on their photochemical reactivity on Cuticular wax film. Chemosphere, 79(4), 482–487.
Hwang, E., Cash, J. N., & Zabik, M. J. (2002). Chlorine and chlorine dioxide treatment to reduce or remove EBDCs and ETU residues in a solution. Journal of Agricultural and Food Chemistry, 50(16), 4734–4742.
Jia, X.-H., Feng, L., Liu, Y.-Z., Zhang, L.-Q., Jia, X.-H., Feng, L., Liu, Y.-Z., & Zhang, L.-Q. (2017). Oxidation of Antipyrine by chlorine dioxide: Reaction kinetics and degradation pathway. Chemical Engineering Journal, 309(2), 646–654.
Jović, M., Manojlović, D., Stanković, D., Dojčinović, B., Obradović, B., Gašić, U., & Roglić, G. (2013). Degradation of Triketone herbicides, Mesotrione and Sulcotrione, using advanced oxidation processes. Journal of Hazardous Materials, 260(9), 1092–1099.
Klüttgen, B., Dülmer, U., Engels, M., & Ratte, T. H. (1994). ADaM, an artificial freshwater for the culture of zooplankton. Water Research, 28(3), 743–746.
Lanchote, V. L., Bonato, P. S., Cerdeira, A. L., Santos, N. A. G., de Carvalho, D., & Gomes, M. A. (2000). HPLC screening and GC-MS confirmation of Triazine herbicides residues in drinking water from sugar cane area in Brazil. Water, Air, and Soil Pollution, 118(3–4), 329–337.
Leboulanger, C., Rimet, F., de Lacotte, M. H., & Bérard, A. (2001). Effects of atrazine and Nicosulfuron on freshwater microalgae. Environmental International, 26(3), 131–135.
Lopez, A., Mascolo, G., Tiravanti, G., & Passino, R. (1997). Degradation of herbicides (Ametryn and Isoproturon) during water disinfection by means of two oxidants (hypochlorite and chlorine dioxide). Water Science and Technology, 35(4), 129–130 132-136.
Lu, X. H., Kang, Z. H., Tao, B., Wang, Y. N., Dong, J. G., & Zhang, J. L. (2012). Degradation of Nicosulfuron by Bacillus subtilis YB1 and aspergillus Niger YF1. Applied Biochemistry and Microbiology, 48(5), 460–466.
Ma, X. L., Yu, P. B., Gao, H. N., & Zhang, H. (2011). Bioremediation of exogenous degrading Bacteria to the Nicosulfuron-contaminated soil. Journal of Safety and Environment, 11(4), 44–47.
Maurino, V., Minero, C., Pelizzetti, E., & Vincenti, M. (1999). Photocatalytic transformation of sulfonylurea herbicides over irradiated titanium dioxide particles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 151(1–2), 329–338.
OECD Guideline for testing of chemicals (2004) Daphnia sp. Acute Immobilisation Test. OECD Guideline 202.
Otero, R., Fernandez, J. M., Gonzalez, M. A., Pavlovic, I., & Ulibarri, M. A. (2013). Pesticides adsorption–desorption on mg–Al mixed oxides. Kinetic modeling, Competing Factors and Recyclability. Chemical Engineering Journal, 221(4), 214–221.
Persoone, G., Marsalek, B., Blinova, I., Törökne, A., Zarina, D., Manusadzianas, L., Nalecz-Jawecki, G., Tofan, L., Stepanova, N., Tothova, L., & Kolar, B. (2003). A practical and user-friendly toxicity classification system with Microbiotests for natural waters and wastewaters. Environmental Toxicology, 18(6), 395–402.
Pileggi, M., Pileggi, S. A. V., Olchanheski, L. R., da Silva, P. A. G., Gonzalez, A. M. M., Koskinen, W. C., Barber, B., & Sadowsky, M. J. (2012). Isolation of Mesotrione-degrading Bacteria from aquatic environments in Brazil. Chemosphere, 86(11), 1127–1132.
Sabadie, J. (2002). Nicosulfuron: Alcoholysis, chemical hydrolysis, and degradation on various minerals. Journal of Agricultural and Food Chemistry, 50(3), 526–531.
Sarmah, A. K., & Sabadie, J. (2002). Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. Journal of Agricultural and Food Chemistry, 50(22), 6253–6265.
Seguin, F., Leboulanger, C., Rimet, F., Druart, J. C., & Bérard, A. (2001). Effects of atrazine and Nicosulfuron on phytoplankton in Systems of Increasing Complexity. Archives of Environmental Contamination and Toxicology, 40(2), 198–208.
Sharma, V. K. (2008). Oxidative transformations of environmental pharmaceuticals by Cl2, ClO2, O3, and Fe (VI): Kinetics assessment. Chemosphere, 73(9), 1379–1386.
Shukla, G., Kumar, A., Bhanti, M., Joseph, P. E., & Taneja, A. (2006). Organochlorine pesticide contamination of ground water in the City of Hyderabad. Environmental International, 32(2), 244–247.
Song, J., Gu, J., Zhai, Y., Wu, W., Wang, H., Ruan, Z., Shi, Y., & Yan, Y. (2013). Biodegradation of Nicosulfuron by a Talaromyces Flavus LZM1. Bioresource Technology, 140(3), 243–248.
Tian, F., Qiang, Z., Liu, C., Zhang, T., & Dong, B. (2010). Kinetics and mechanism for Methiocarb degradation by chlorine dioxide in aqueous solution. Chemosphere, 79(6), 646–651.
Tian, F.-X., Xu, B., Zhang, T.-Y., & Gao, N.-Y. (2014). Degradation of Phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent Chlor(am)ination. Chemical Engineering Journal, 258(12), 210–217.
Ukrainczyk, L., & Rashid, N. (1995). Irreversible sorption of Nicosulfuron on clay minerals. Journal of Agricultural and Food Chemistry, 43(4), 855–857.
Vela, N., Navarro, G., Giménez, M. J., & Navarro, S. (2004). Gradual fall of s-Triazine herbicides in drinking and wastewater samples as influenced by light and temperature. Water, Air, and Soil Pollution, 158(1), 3–19.
Wang, Y., Liu, H., Xie, Y., Ni, T., & Liu, G. (2015). Oxidative removal of diclofenac by chlorine dioxide: Reaction kinetics and mechanism. Chemical Engineering Journal, 279(11), 409–415.
Wang, L., Zhang, X., & Li, Y. (2016). Degradation of Nicosulfuron by a novel Isolatedbacterial strain Klebsiella sp. Y1: Condition optimization, Kinetics and Degradation Pathway. Water Science and Technology, 73(12), 2896–2903.
Zhang, H., Mu, W., Hou, Z., Wu, X., Zhao, W., Zhang, X., Pan, H., & Zhang, S. (2012). Biodegradation of Nicosulfuron by the bacterium Serratia Marcescens N80. Journal of Environmental Science and Health, Part B, 47(3), 153–160.
Zhao, W., Wang, C., Xu, L., Zhao, C., Liang, H., & Qiu, L. (2015). Biodegradation of Nicosulfuron by a novel Alcaligenes Faecalis strain ZWS11. Journal of Environmental Science, 35(9), 151–162.
Acknowledgements
The authors would like to thank TwinOxide-RS d.o.o. for providing components for the preparation of chlorine dioxide (“TWINS” preparation).
Funding
Financial support for this study was granted by the Ministry of Science and Technological Development of the Republic of Serbia, Project Number 172030. DMS was supported by Magbiovin project (FP7-ERAChairs-Pilot Call-2013, Grant agreement: 621375).
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised:During typesetting, the image of figure 4 was also used in figure 5. Figure 5 was updated with the correct image.
Electronic supplementary material
HPLC chromatograms of initial nicosulfuron (10 mg/L), initial thifensulfuron methyl (10 mg/L), their degradation products, and GC-QQQ chromatograms and mass spectra for degradation products of the sulfonylurea herbicides.
ESM 1
(DOCX 406 kb)
Rights and permissions
About this article
Cite this article
Pergal, M.V., Kodranov, I.D., Pergal, M.M. et al. Assessment of Degradation of Sulfonylurea Herbicides in Water by Chlorine Dioxide. Water Air Soil Pollut 229, 287 (2018). https://doi.org/10.1007/s11270-018-3947-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3947-2