Abstract
Activated carbon (AC) amendment has been shown to reduce bioavailability of hydrophobic contaminants in the bioactive layer of sediment. Unwanted secondary effects of AC amendment could be particularly undesirable for ecologically important seagrass meadows, but so far, only a few studies have been conducted on effects on submerged plants. The purpose of this study was to investigate effects on growth and cover of submerged macrophytes in situ after AC amendment. Test sites were established within a seagrass meadow in the severely contaminated Norwegian fjord Gunneklevfjorden. Here we show that AC amendment does not influence neither cover nor length of plants. Our study might indicate a positive effect on growth from AC in powdered form. Hence, our findings are in support of AC amendment as a low-impact sediment remediation technique within seagrass meadows. However, we recommend further studies in situ on the effects of AC on submerged vegetation and biota. Factors influencing seasonal and annual variation in plant species composition, growth and cover should be taken into consideration.

The effects of activated carbon amendment to growth and cover of submerged macrophytes were tested in an in situ experiment
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Activated carbon (AC) amendment to contaminated sediments has been introduced as a low-impact approach for sediment remediation (Ghosh et al. 2011) and an alternative to removal or isolation of contaminated sediments. Several in situ and ex situ studies have reported on significant reduction in pore water concentration and bioavailability of hydrophobic contaminants in the bioactive layer of sediments after AC amendment (Zimmermann et al. 2005; Zimmermann et al. 2004; Millward et al. 2005; Cornelissen et al. 2011; Josefsson et al. 2012). However, recently, there has been an awareness on the potential harmful secondary effects of AC amendment to benthic organisms and submerged vegetation (Beckingham et al. 2013; Janssen and Beckingham 2013), though only a few studies have been conducted on secondary effects of AC amendment on submerged vegetation(Beckingham et al. 2013; Janssen and Beckingham 2013; Kupryianchyk et al. 2012). Laboratory studies have indicated reduced growth after amendment with AC (Beckingham et al. 2013). However, in a long-term study on recovery of benthic communities after amendment with different AC concentrations (0–10%), no significant effects were found in macrophyte densities between different AC treatments (Kupryianchyk et al. 2012). Lehmann et al. (2011) have evaluated the growth of terrestrial plants in soil amended with different types of manufactured black carbon and found that biochar can greatly improve plant growth, while AC has shown somewhat diverging effects on growth of terrestrial plants (Lau et al. 2008; Jakob et al. 2012). However, it is unclear whether observations in terrestrial systems can be translated to aquatic environments (Beckingham et al. 2013).
Secondary effects would be particularly undesirable for submerged meadows that already are experiencing a global decline (Orth et al. 2006; Waycott et al. 2009), as they are offering several important aquatic ecosystem services; providing foraging, shelter and breeding grounds to organisms (Neckles et al. 1993; Fredriksen et al. 2005; Lee et al. 2001); as well as functioning as carbon sinks (Duarte et al. 2013). Seagrass meadows are known to trap particles from the water column (Agawin and Duarte 2002; Hendriks et al. 2008), thus enhancing sediment deposition and reducing resuspension (Gacia and Duarte 2001) and are therefore suspect to high concentrations of contaminants within polluted areas. Accordingly, submerged meadows may be important exposure sites for contaminants to inhabiting organisms, and recent studies have shown enhanced bioavailability of sediment Hg within vegetated areas(Canário et al. 2010; Windham-Myers et al. 2014; Olsen et al. 2018), which may initiate a transfer of contaminants through food webs, with a potential to biomagnify at each trophic level. Thus, ecologically important submerged meadows within polluted areas potentially face the duality of being suspect to both remediation and conservation, which actualises the need to develop low-impact risk-reducing remediation strategies.
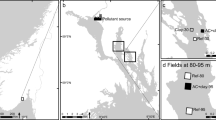
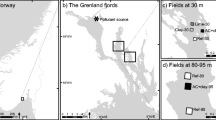
The purpose of the experiment was to investigate in situ whether amendment with powdered or granulated AC has effects on growth or cover of submerged macrophytes, prior to recommend it as a low-impact approach for remediation of contaminated sediments. To test the hypothesis of no variation between different treatments, test sites were established in situ within the submerged seagrass meadow found in the Norwegian brackish fjord Gunneklevfjorden (Fig. 1).
2 Materials and Methods
2.1 Study Site
The semi-enclosed brackish fjord Gunneklevfjord covers an area of approximately 0.7 km2 and is connected to the river Skienselva to the north and to the fjord Frierfjorden to the south (Fig. 1). There are sills in both outlets, with the shallowest parts reaching only 2 m depth. The main area in the southern part of the Gunneklevfjord is reaching 4–5 m depth, while the northern part reaches down to 11 m depth (Molvær 1989). The salinity of surface waters in the Gunneklevfjord is typically in the range of 0.5–6‰. Periodically, a halocline is found at 2–3 m depth and stagnant deep waters have been found with salinity in the range of 10–20‰ (Molvær 1989). The fjord is hosting a large seagrass meadow in the south-eastern part of the fjord, covering approximately 70,000 m2 and reaching from 0.5 to 2.5 m depth. The seagrass meadow is classified as very important (of national value) due to its size and quality, according to the Norwegian Environment Agency (DN Kartlegging av marint biologisk mangfold 2007). In 2014, a survey identified 13 aquatic macrophytes in the Gunneklevfjord (Mjelde 2014), with dominating species being the vascular plants Elodea canadensis and Potamogeton crispus, in addition to the charophyte Chara virgata. Brackish waters and varying salinity are a challenge to both marine and estuarine organisms, limiting the biological diversity of the fjord. Nevertheless, recent sampling of benthos and fish within the meadow has revealed high abundance of organisms and demonstrated the ecological importance of seagrass meadows(Olsen et al. 2015). Most of the species found in the fjord are freshwater species that have a tolerance for low and stable salinity. Since early 1900, the fjord has received substantial amounts of Hg and chlorinated compounds like dioxins/furans (PCDD/PCDF), octachlorstyren (OCS) and hexachlorobenzene (HCB) due to discharges from nearby industrial activities (Skei et al. 1989). Recent investigations have revealed sediment surface concentrations reaching 15.5 mg Tot-Hg kg−1 and 3.2 μg MeHg kg−1 (Olsen et al. 2018).
Our in situ test sites for AC amendment were established within the seagrass meadow (Fig. 1). Sediment was treated with thin layers (< 3 cm) of powdered or granulated activated carbon, approximately 2 kg AC m−2. The limestone was included in the experiment as an alternative non-active capping material, which is traditionally placed on the sediments in much ticker layers than AC (> 30 cm). In this experiment, limestone was added in a 3–5-cm layer, which is not as thick as a realistic treatment. Cover of plants was documented over a period of 3 months during the growing season in 2014 and then once in August 2015. Length of plants was measured once in 2014.
2.2 Placement of Frames on Seabed
Two in situ test sites (GT and GM) were established within the seagrass meadow with a distance of approximately 200 m (Fig. 1). The sites differed slightly in plant species composition at the initiation of the test. Site GT was dominated by Chara virgata while site GM was equally dominated by Chara virgata and Potamogeton crispus. In each site, 12 frames (80 × 120 cm) were placed on the seabed at 2–2.5 m depth, and with a distance of 5–10 m between the frames, giving triplicate frames for each of three different treatments in addition to three untreated frames in both the test sites (controls). The frames were constructed by cutting off the bottom of bricklayer buckets, leaving a 10-cm high edge. To weigh down the frames, heavy chains were attached to the outside of each frame. Each frame was marked with a rope and a buoy to the surface.
2.3 Capping of Sediment within Frames
The three treatments were distributed randomly to the frames within the two test sites GM and GT (Fig. 2).
At each test site, approximately 2 kg m−2 of powdered or granulated AC was added to three replicate frames each (named treatment ACP and ACG, respectively), without any pre-treatment. First, 1 kg m−2 of AC was added (8th July 2014), and the placement of the capping material within the frames was visually observed by the use of a subsea GoPro Hero3+ action camera after the capping material had settled, approximately 1 h after application. Another 1 kg m−2 was added 1 week later. Limestone (Norstone, 0–8 mm; treatment LIM) was added in a 3–5-cm-thick layer to three replicate frames at each site (8th July 2014). All capping materials were brought down to the seabed by the use of a pipe. A silt curtain was surrounding the pipe from the edge of the frame up to the water surface to limit loss of material outside the frames. Photos taken after capping revealed insignificant loss of capping materials outside the frames.
2.4 Monitoring Cover
Documentation of cover of plants within the frames was done by photographing each frame from above with a waterproof GoPro Hero3+ Black edition camera. The camera body was attached to a rod and subsequently lowered into the water to about 30 cm over the seabed, consequently shooting one photo/2 s. Photography was completed on three occasions during the growing season in 2014 (time 1 = 6th of August 2014; time 2 = 27th of August 2014; time 3 = 29th of September 2014) and again 1 year later on one occasion in August 2015 (time 4 = 21th August 2015). The first round of photography (time 1) was carried out 4 weeks after placement of capping material in the frames. At time 3, one frame of AC granulate (ACG) amendment in site GT and one untreated frame (NON) in site GM had been lost, giving a total of 22 frames photographed. At time 4 (August 2015), one more frame of AC granulate (ACG) and one of limestone (LIM) amendment had been lost from GT, giving 20 frames for both sites. The images were analysed by estimating the percentage cover of vegetation within each frame. The percentage cover was estimated manually using a 10 × 10 grid placed over the image. Percentage cover of plants in an identically sized area just outside each frame was similarly quantified as a non-treated reference for each frame. It was assumed that the area just outside each frame gave a better reference than the non-treated frames assigned as controls, given the natural patchiness of cover within the meadow. The ratio of the percentage cover outside (Co) and within the frames (Ci) was used as a measure for the effect of treatment, expressed as the cover ratio (Cr).
The Cr calculated for the non-treated frames was used as a measure for effect of the frame itself.
2.5 Measuring Length of Plants
Plant material from inside the frames was collected 3 months after amendment using divers (at time 3). Divers cut plants from a square approximately 10 × 10 cm within each frame and as close to the sediment surface as possible, for the measurement of plant length. Cut plants were put directly into plastic zipper bags under water. Immediately after sampling, the plants were brought ashore and determined to species. For comparison of length of plants between treatments, only the most abundant species Potamogeton crispus in site GM was measured. All sampled plants were measured and the median plant length for each frame was used for comparison between treatments.
2.6 Statistical Analysis
All statistical analyses were done using the computing program RStudio version 0.98.1056 running on R version 3.1.0 (Team 2014). Correlation between percentage cover within and outside the frames was calculated using both parametric and non-parametric correlation coefficients and tests, as the data violated parametric assumptions being non-normally distributed. Differences in cover ratio (Cr) between treatments were tested using both parametric methods (ANOVA) and the non-parametric Kruskal-Wallis multiple comparison test. Differences in length of plants between treatments were tested using ANOVA and multiple regressions.
3 Results and Discussion
The central question of this study was whether amendment with powdered or granulated AC affects length or cover of macrophytes in a submerged meadow in the contaminated sediment site Gunneklevfjorden in Norway. The experiment revealed no significant effects of activated carbon whatsoever to the macrophytes, neither acute nor after 1 year. However, amendment with the non-active material limestone did reduce cover the first weeks after treatment. The results are presented and discussed below.
3.1 Effect of Study Design (Frames) on Percentage Cover
To check for possible effects on percentage cover of plants from the frames themselves, the percentage cover observed outside and within the non-treated frames (treatment NON) were compared (Fig. 3). There was no difference in cover ratio (Cr) between the two test sites for the untreated frames; hence, data from both sites were merged when testing for effect of frames. Testing was done first for all sampling events merged (times 1, 2, 3 and 4) and then for the last sampling event in 2014 (time 3) separately.
Correlation of percentage cover outside and within NON frames for all sampling events and both sites merged by Pearson’s correlation coefficient and Spearman’s rho was r = 0.87 and r = 0.85, respectively, with p < 0.05. Welch two sample t test and Wilcoxon rank sum test were used for testing for difference in percentage cover between outside and within the frames. Neither of the tests showed significant difference between outside and within NON frames.
Checking for correlation in percentage cover and for difference between inside and within frames for the last sampling event in 2014 did also give significant correlation and no significant difference (p > 0.05). Based on the results for the untreated frames, it was assumed that the placement of the frames on the seabed did not have any significant effect on the percentage cover of plants within the frames. Hence, effect of frames was not taken into consideration when testing for effect of treatments.
3.2 Effect on Cover Ratio (C r)
Cover ratio (Cr) for each frame was calculated to look for effects of different treatments, and differences between treatments were tested using both parametric test (ANOVA and pairwise comparison using t test) and non-parametric test (Kruskal-Wallis rank sum test and post hoc multiple comparison test after Kruskal-Wallis). There was a significant difference between the treatments (p < 0.05) when all sampling events (times 1, 2, 3 and 4) were merged (Fig. 4). The difference was caused by limestone (LIM), which was found to be significantly different from all other treatments, including the untreated frames (NON). No significant effects on Cr could be found for either powdered AC (ACP) or granulated AC (ACG).
The same tests were carried out separately for difference in Cr between treatments at each time of sampling (Fig. 5). Significant variation in Cr between the treatments was found at all times of sampling during the first year (times 1, 2, 3), but not the second year (time 4). At times 1, 2 and 3, treatment LIM was found to be different from ACP (p < 0.05), but none of the other treatments differed from each other in Cr.
Reduced cover of plants within frames amended with limestone the first year may be caused by the mechanical disturbance of the plants by limestone. Limestone was added in a thicker layer (3–5 cm) and with larger grain size than AC. Also, limestone (CaCO3) may have an influence on the water chemistry. Earlier studies have shown that addition of CaCO3 have reduced or eliminated macrophyte biomass in hardwater lakes(Chamber et al. 2001). In addition, it is known that limestone (CaCO3) may slowly dissolve and change the pH locally, subsequently reducing the CO2 content of water. A local decrease in [CO2] compared to [HCO3] may be one reason for the negative effect on cover. However, Potamogeton crispus can assimilate HCO3 for growth, but it seems to prefer CO2 as a carbon source (Sand-Jensen 1983). However, also AC may lower water pH with a potential for influencing water chemistry. Since water chemistry effects from addition of capping materials were not within the scope of this study, no measurements of [CO2] or pH in water were carried out. The plant species in our study seem to senesces early in the season compared to similar species(Chamber et al. 2001). This may have an effect on the results.
During the study period, there was a marked change in the general cover of plants within the entire vegetation area. In August 2014 (time 3), the mean cover outside the frames was 88%, while in August 2015 (time 4), the mean cover was 99%. The species composition in the study sites also made a change from the first to the second year of study. In the first year the Chara virgata and Potamogeton crispus was the dominating species in the study area, while in 2015, Potamogeton crispus was barley seen. Our study reveals the cause neither of the general increase in cover of plants from 2014 to 2015 nor of the dominance of Chara over Potamogeton crispus observed in 2015. The change in cover and in species composition was observed not only within the frames but across the entire meadow. Therefore, we find it not likely that the changes were initiated by our treatments. The changes might rather be due to external factors, such as light, nutrients or salinity, and to annual variation in competition between species. Salinity is recognised as the most important factor controlling species composition in brackish areas (Haller et al. 1974). Occasional inflow of high salinity waters between sampling in August 2014 and September 2015 cannot be foreclosed.
3.3 Check of Possible Covariates Influencing Length of Plants
To check whether site or number of different species within the frames had an influence on the length of plants, ANOVA was used to compare the median length of plants between the two sites GM and GT and between groups of plants defined by numbers of species found when sampling (1, 2 or 3 species). Neither site nor number of species were found to give significant differences in length of plants, even though somewhat longer plants were found at site GT compared to GM (mean 30.5 and 26.3 cm, respectively) (Fig. 6). Hence, site and number of species were not included as covariates when fitting models for length of plants.
3.4 Possible Correlation Between Cover Ratio and Length
Correlation between percentage cover of plants and median length of plants within each of the non-treated frames (treatment NON) was found not to be significant (p > 0.05 by Pearson’s product-moment correlation). Also, a simple linear regression model fitted for length of plants showed that percentage cover was not a significant predictor. Hence, length of plants was not normalised to percentage cover before testing for effect of treatments.
3.5 No Effects from Treatments on Length of Plants
Variation in median length of plants between treatments was tested using ANOVA and pairwise comparison using t test (Fig. 6). Testing of differences in length was done within each site and for the sites merged. There were no significant differences in length of plants between the treatments.
Our results do not support earlier findings that AC in powdered form reduces plant growth (Beckingham et al. 2013; Jakob et al. 2012) and that AC in granulate form increases plant growth (Jakob et al. 2012). No significant effect was found after AC amendment on neither length nor cover of the plants within the study area in the Gunneklevfjord. The results are in support of AC amendment as a low-impact remediation method in areas of submerged vegetation. Still, since studies on secondary effects of AC amendment are few, knowledge is scarce and results are diverging, there is a need of more studies in situ to understand the effects of activated carbon on submerged vegetation. Factors influencing seasonal and annual variation in plant species composition and cover should be taken into consideration when carrying out in situ studies.
References
Agawin, N. S. R., & Duarte, C. M. (2002). Evidence of direct particle trapping by tropical seagrass meadow. Estuaries and Coasts, 25, 1205–1209.
Beckingham, B., Buys, D., Vandewalker, H., & Ghosh, U. (2013). Observations of limited secondary effects to benthic invertebrates and macrophytes with activated carbon amendment in river sediments. Environmental Toxicology and Chemistry, 32(7), 1504–1515.
Canário, J., Vale, C., Poissant, L., Nogueira, M., Pilote, M., & Branco, V. (2010). Mercury in sediments and vegetation in a moderately contaminated salt marsh (Tagus Estuary, Portugal). Journal of Environmental Sciences, 22(8), 1151–1157.
Chamber, P. A., Prepas, E. E., Ferguson, M. E., Serediak, M., Guy, M., & Holst, M. (2001). The effects of lime addition on aquatic macrophytes in hard water: in situ and microcosm experiments. Freshwater Biology, 46, 1121–1138.
Cornelissen, G., Elmquist Kruså, M., Breedveld, G. D., Eek, E., Oen, A. M. P., Arp, H. P. H., Raymond, C., Samuelsson, G., Hedman, J. E., Stokland, Ø., & Gunnarsson, J. S. (2011). Remediation of contaminated marine sediment using thin-layer capping with activated carbon—a field experiment in Trondheim Harbor, Norway. Environmental Science & Technology, 45(14), 6110–6116.
DN Kartlegging av marint biologisk mangfold. DN håndbok 19:2001. Revidert 2007. Direktoratet for naturforvaltning.; 2007.
Duarte, C. M., Sintes, T., & Marbà, N. (2013). Assessing the CO2 capture potential of seagrass restoration projects. Journal of Applied Ecology, 50(6), 1341–1349.
Fredriksen, S., Christie, H., & Sæthre, B. A. (2005). Species richness in macroalgae and macrofauna assemblages on Fucus serratus L. (Phaeophyceae) and Zostera marina L. (Angiospermae) in Skagerrak, Norway. Marine Biology Research, 1, 2–19.
Gacia, E., & Duarte, C. M. (2001). Sediment retention by a mediterranean Poseidonia oceanica meadow: the balance between deposition and resuspension. Estuarine, Coastal and Shelf Science, 52(4), 505–514.
Ghosh, U., Luthy, R. G., Cornelissen, G., Werner, D., & Menzie, C. A. (2011). In-situ sorbent amendments: a new direction in contaminated sediment management. Environmental Science & Technology, 45(4), 1163–1168.
Haller, W. T., Sutton, D. L., & Barlowe, W. C. (1974). Effects of salinity on growth of several aquatic macrophytes. Ecology, 55(4), 891–894.
Hendriks, I. E., Sintes, T., Bouma, T. J., & Duarte, C. M. (2008). Experimental assessment and modeling evaluation of the effects of seagrass (Posidonia oceanica) on flow and particle trapping. Marine Ecology Progress Series, 356, 163–173.
Jakob, L., Hartnik, T., Henriksen, T., Elmquist, M., Brändli, R. C., Hale, S. E., & Cornelissen, G. (2012). PAH-sequestration capacity of granular and powder activated carbon amendments in soil, and their effects on earthworms and plants. Chemosphere, 88(6), 699–705.
Janssen, E. M. L., & Beckingham, B. A. (2013). Biological responses to activated carbon amendments in sediment remediation. Environmental Science & Technology, 47(14), 7595–7607.
Josefsson, S., Schaanning, M., Samuelsson, G. S., Gunnarsson, J. S., Olofsson, I., Eek, E., & Wiberg, K. (2012). Capping efficiency of various carbonaceous and mineral materials for in situ remediation of polychlorinated dibenzo-p-dioxin and dibenzofuran contaminated marine sediments: sediment-to-water fluxes and bioaccumulation in Boxcosm tests. Environmental Science & Technology, 46(6), 3343–3351.
Kupryianchyk, D., Peeters, E. T. H. M., Rakowska, M. I., Reichman, E. P., Grotenhuis, J. T. C., & Koelmans, A. A. (2012). Long-term recovery of benthic communities in sediments amended with activated carbon. Environmental Science & Technology, 46(19), 10735–10742.
Lau, J. A., Puliafico, K. P., Kopshever, J. A., Steltzer, H., Jarvis, E. P., Schwarzländer, M., Strauss, S. Y., & Hufbauer, R. A. (2008). Inference of allelopathy is complicated by effects of activated carbon on plant growth. New Phytologist, 178(2), 412–423.
Lee, S. Y., Fong, C. W., & Wu, R. S. S. (2001). The effects of seagrass (Zostera japonica) canpoy structure on associated fauna: a study using artificial seagrass units and sampling of natural beds. Journal of Experimental Marine Biology and Ecology, 259, 23–50.
Lehmann, J., Rillig, M., Thies, J., Masiello, C., Hackaday, W., & Crowley, D. (2011). Biochar effects on soil biota—a review. Soil Biology and Biochemistry, 43, 1812–1836.
Millward, R. N., Bridges, T. S., Ghosh, U., Zimmermann, J. R., & Luthy, R. G. (2005). Addition of activated carbon to sediments to reduce PCB bioaccumulation by a polychaete (Neanthes arenaceodentata) and in amphipod (Leptocheirus plumulosus). Environmental Science & Technology, 39, 2880–2887.
Mjelde, M., Faktaark: Brakkvannssjø. Revidert veileder for kartlegging, verdisetting og forvaltning av naturtyper på land og i ferskvann. Utkast pr. 30.11.2014. In 2014.
Molvær, J. (1989). Miljøgifter i Gunnekleivfjorden. Delrapport 2: Miljøgifter i vannmassene. Transport av miljøgifter gjennom kanalene.; O-88068; NIVA: 31.01.1989; p 68.
Neckles, H. A., Wetzel, R. R., & Orth, R. J. (1993). Relative effects of nutrient enrichment and grazing on epiphyte-macrophyte (Zostera marina) dynamics. Oecologia, 93, 285–295.
Olsen, M.; Beylich, B. A.; Braaten, H. F. V. (2015) Næringsnett og miljøgifter i Gunneklevfjorden. Beslutningsgrunnlag og tiltaksplan for forurensede sedimenter i Gunneklevfjorden. Delrapport akivitet 2. NIVA-rapport 6795–2015.
Olsen, M., Schaanning, M. T., Braaten, H. F. V., Eek, E., Moy, F. E., & Lydersen, E. (2018). The influence of permanently submerged macrophytes on sediment mercury distribution, mobility and methylation potential in a brackish Norwegian fjord. Science of the Total Environment, 610–611, 1364–1374.
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., & Duarte, C. M. (2006). A global crisis for seagrass ecosystems. BioScience, 56, 987–996.
Sand-Jensen, K. (1983). Photosynthetic carbon sources of stream macrophytes. Journal of Experimental Botany, 34(139), 198–210.
Skei, J., Pedersen, A., Bakke, T., Berge, J. A. (1989). Miljøgifter i Gunnekleivfjorden. Delrapport 4: Utlekking av kvikksølv og klororganiske forbindelser fra sedimentene, bioturbasjon og biotilgjengelighet.; O-8806804; NIVA: 31.01.1989; p 114.
Team, R. C. R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria: 2014.
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., & Olyarnik, S. (2009). Accelerating loss of seagrass across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America, 106, 12377–12381.
Windham-Myers, L., Marvin-DiPasquale, M., Stricker, A., C., Agee, J. L., Kieu, H., L., & Kakouros, E. (2014). Mercury cycling in agricultural and managed wetlands of California, USA: Experimental evidence of vegetation-driven changes in sediment biogeochemistry and methylmercury production. Science of the Total Environment, 484(0), 300–307.
Zimmermann, J. R., Ghosh, U., Millward, R. N., Bridges, T. S., & Luthy, R. G. (2004). Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments; physiochemical tests. Environmental Science & Technology, 38, 5458–5464.
Zimmermann, J., Werner, D., Ghosh, U., Milward, R., Bridges, T., & Luthy, R. (2005). Effects of dose and particle size on activated carbon treatment to sequester polychlorinated biphenyls and polycyclic aromatic hydrocarbons in marine sediments. Environmental Toxicology & Chemistry, 24, 1594–1601.
Acknowledgements
We thank Lise Ann Tveiten, Janne Kim Gitmark, Maia Røst Kile and Vetle B. Fredheim for assistance during sampling. This research was funded through a joint PhD by University College of Southeastern Norway, University of Agder, Institute of Marine Research and Norwegian Institute for Marine Research to Marianne Olsen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olsen, M., Moy, F.E., Mjelde, M. et al. An In Situ Experimental Study of Effects on Submerged Vegetation After Activated Carbon Amendment of Legacy Contaminated Sediments. Water Air Soil Pollut 229, 263 (2018). https://doi.org/10.1007/s11270-018-3918-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3918-7