Abstract
A novel tellurite-resistant photosynthetic bacterium, Rhodopseudomonas palustris strain TX618, was isolated from wastewater and reduction of tellurite by this strain was investigated. The results showed that Rhodopseudomonas palustris strain TX618 could reduce tellurite to elemental tellurium, both anaerobically and aerobically. During anaerobic and illuminated growth, strain TX618 possessed a high-level resistance and removal efficiency to tellurite, that it could resist up to 180 mg/L Na2TeO3 in the medium and removed 91.9% of 90 mg/L Na2TeO3 over 8 days. The high efficiency in the removal of tellurite could sustain wide variations in pH (5.0–9.0), temperature (20–40 °C), light intensity (1500–3000 lx), and initial tellurium concentration (30–180 mg/L Na2TeO3). It could be observed by scanning electron micrograph (SEM), transmission electron micrograph (TEM), and X-ray diffraction (XRD) analysis that the cells suffered serious deformation due to the toxicity of tellurite, and the less toxic black precipaite (Te0) generated by bioreduction of tellurite mostly located in the central cytoplasm. This is the first study to observe that Rhodopseudomonas palustris can reduce tellurite to elemental tellurium, which will provide a new microbial species for bioremediation and biotransformation of toxic tellurite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tellurium (Te) is a scarce and valuable metalloid belonging to the chalcogen family, along with oxygen (O), sulfur (S), selenium (Se), and polonium (Po). Despite its low abundance of around 0.027 ppm in the earth’s crust (Ba et al. 2010), Te has widely used in various industries including metallurgy, electronics, and applied chemical industries, such as improving optoelectronic and thermal properties of steel and glass and developing new materials (e.g., CdTe thin-film solar panels) as well as producing fluorescent probes (Belzile and Chen 2015; Ba et al. 2010; Tang et al. 2006; Deng et al. 2007). On the one hand, the expanded use of Te can lead to environmental pollution and induce acute and chronic Te poisoning to human (e.g., the degeneracy of the liver and kidney) (Ba et al. 2010; Issa et al. 2016). The Occupational Safety and Health Administration (OSHA) have set the permissible exposure limit for workers to Te compounds in the workplace as 0.1 mg/m3 over an 8-h workday (Qin et al. 2017; Issa et al. 2016). On the other hand, with its increasing applications, Te has been regarded as a critical element because its shortage might compromise the development of energy- and defense-related advanced technologies (de Boer and Lammertsma 2013).
In natural environment, Te can exist in tellurate (Te(VI)), tellurite (Te(IV)), elemental Te(0), and telluride (Te(-II)) (Zannoni et al. 2007). Oxyanion tellurate and tellurite are the predominant soluble form in wastewater but the toxicity of tellurite is 10-fold higher than that of tellurate. Among these, tellurite is the most toxic form for both prokaryotes and eukaryotes (Turner 2001) at concentrations as low as 1 μg/mL (Presentato et al. 2016). However, the toxic tellurite can be found highly concentrated in soil and water near waste discharge sites of manufacturing and processing facilities (Jobling and Ritchie 1987), as a hazardous and toxic pollutant (Taylor 1999). Therefore, the development of new technologies for the recovery of Te from the soluble tellurite in waste streams is imperative to overcome Te supply risk and mitigate toxicity concern.
Microbial reduction of toxic oxyanions has been known as the cost-effective and green technology, and several microorganisms have shown to be able to reduce tellurite to crystal particles of elemental Te both inside andr outside the cells (Borghese et al. 2014; Chasteen et al. 2009; Ramos-Ruiz et al. 2016; Kim et al. 2013). Elemental Te is insoluble in water and has less mobility and toxicity. This microbial process is considered to be useful for recovering Te from waste streams. However, it is assuredly a first important work to screen suitable strains with the characteristic in bioremediation for toxic metal-contaminated environments. Rhodopseudomonas palustris, a purple non-sulfur photosynthetic bacterium, which is a suitable species for treatment of wastes due to its remarkable capacity to metabolize a wide range of compounds (Larimer et al. 2004; Zhang et al. 2009; Austin et al. 2015; Selimoğlu et al. 2011). Moreover, this species has also proven to be able to tolerate toxic selenite, a kind of chalcogen oxyanion, by reduction of selenite to elemental selenium (Li et al. 2014). However, to date, there are no reports on its metabolic ability towards toxic tellurite.
The objective of the present study was to isolate Rhodopseudomonas palustris strain TX618 from wastewater and investigate the ability of this strain to reduce tellurite to elemental Te. In this study, the effect of tellurite on microbial growth was firstly carried out. Then, we investigated the effects of various culture parameters on tellurite removal and also conducted the reducing behaviors of tellurite though microscopic observation and XRD analysis.
2 Materials and Methods
2.1 Strain Isolation and Identification
The tellurite-reducing bacterium was isolated from sewage wastewater of a metal refinery plant (29.25 N, 102.41 E) in Shimian County, Sichuan Province of China. Enrichment and pure culture procedure were carried out in tellurite-supplemented RCVBN medium as follows: dl-malic acid 4 g/L, (NH4)2SO4 1 g/L, KH2PO4 0.9 g/L, K2HPO4 0.6 g/L, MgSO4·7H2O 0.12 g/L, CaCl2·2H2O 0.075 g/L, EDTA 0.02 g/L, vitamin B1 0.001 g/L, niacin 0.001 g/L, biotin 0.015 g/L, H3BO3 2.8 mg/L, MnSO4·H2O 1.59 mg/L, CuSO4·3H2O 40 μg/L, Na2MoO4·2H2O 750 μg/L, ZnSO4·7H2O 240 μg/L and supplemented with 100 mg/l Na2TeO3. A pure strain TX618 was obtained by selecting one typical black colony formed on the same medium solidified by addition of 1.5% (w/v) Bacto agar (BD Diagnostics) at 30 °C.
The isolate was identified by 16S rRNA gene sequence analysis. Bacterial genomic DNA was extracted using the Genelute DNA Extraction Kit. The 16S rRNA gene of strain TX618 was amplified with bacterial universal primers: forward primer 27F, 5′-GAGTTTGATCCTGGCTCAG-3′ and reverse primer 1492R, 5′-GGTTACCTTGTTACGACTT-3′ as described (Wong et al. 2014). The purified PCR product was sequenced and compared with bacterial 16S rRNA sequences in the NCBI databases. The phylogenetic analysis based on 16S rRNA gene sequences of strain TX618 and related taxa was constructed by the neighbor-joining method included in Lasergene software (MEGA).
2.2 Effect of Tellurite on Bacterial Growth
Rhodopseudomonas palustris strain TX618 was cultured in RCVBN medium until the culture reached the late logarithmic phase. Then, the culture of this strain was used for inoculation for further experiment. The strain was grown in glass bottles containing 100 mL of RCVBN medium supplemented with various concentrations of Na2TeO3 (50–250 mg/L) to evaluate the tolerance capacity and maximum resistance concentrations of tellurite. After sealed with a plastic cap, the cultured bottles were anaerobically and statically cultivated at 30 °C in the presence of incandescent light. Cell concentration was determined periodically by hemocytometer to assess the growth. In addition, the culture conditions maintained unchanged for else investigation unless otherwise states.
2.3 Tellurite Removal Experiments
Strain TX618 was cultured in RCVBN medium with 50 mg/L or 100 mg/L Na2TeO3. Then, the culture was harvested by removing 1 mL sample and centrifuging at 14,000×g for 5 min. The tellurite content was measured by using hydride generation atomic fluorescence spectrometry method (Chen et al. 2017). The removal efficiency was evaluated by the ratio of residual tellurite to added tellurite in the medium. The efficiency of tellurite reduction by strain TX618 were investigated under four different culture conditions including anaerobic illuminated, anaerobic unilluminated, aerobic illuminated, and aerobic unilluminated conditions. All experiments were carried out at 30 °C for 15 days. Furthermore, the anaerobic groups were statically cultured in an incubator and the aerobic groups in a shaker at 120 rpm.
Various factors including pH value, temperature, light intensity, and initial content of tellurite were selected to value the influence on removal of tellurite in anaerobic illuminated environment. The efficiency of tellurite removal was calculated at different pH values (5.0–9.0), temperatures (20–40 °C), and light intensities (500–2500 lx) in RCVBN medium supplemented with 100 mg/l Na2TeO3. To evaluate the effect of initial tellurite concentration, tellurite reduction was investigated in RCVBN medium supplemented with varying concentration of Na2TeO3 (30, 60, 90, 120, 150, and 180 mg/L). All experiments were done in triplicate.
2.4 Scanning Electron Microscopy Observation
Strain TX618 was cultivated in RCVBN containing 60 mg/L Na2TeO3 for 6 days. The liquid culture was centrifuged at 3000 rpm for 3 min and rinsed three times in phosphate buffer. The precipitate was dealt with 4% glutaraldehyde at 4 °C for 1 h. Next, the strains were fixed in 2% glutaraldehyde for 1 h, washed three times with phosphate buffer, and dehydrated in an ethanol series (20, 50, 80, and 100%). A drop of bacterial suspension was taken to disperse in a silver paper (1 cm × 1 cm) and was freeze dried in vacuum. Finally, samples were observed under a S-4800 scanning electron microscope (Hitachi Limited, Tokyo, Japan).
2.5 Transmission Electron Microscopy Observation
The strain was cultured in the RCVBN medium containing 60 mg/L tellurite; then, the liquid medium was centrifuged and bacteria were harvested when the bacterial cultures became black. The precipitate was collected and fixed in 3% precooling glutaraldehyde at 4 °C for 2 h, washed three times with phosphate buffer for10 min every time. Then, the sample was fixed in 1% OsO4 at 4 °C for 2 h, dehydrated in an ethanol series (30, 50, 70, 80, 90, 100, and 100%) for 10 min every time. Samples finally treated with embedding solution overnight. Subsequently, ultrathin sections were prepared by a diamond cutter and dyed by lead citrate for 10 min. Specimens were examined under a H-600IV transmission electron microscope (Hitachi Limited, Tokyo, Japan).
2.6 X-ray Diffraction Analysis
The liquid bacterial culture was centrifuged at 3000 rpm for 3 min. The precipitate was collected and rinsed three times in phosphate buffer. The volume of the 1/5 original bacteria of the lysate was used to resuspend the bacteria. The component of the lysate was described as follows: 50 mM Tris-HCl (pH 8.0), 2 mM EDTA, 100 mM NaCl, 100 μg/mL lysozyme, and 0.1% Triton X-100. The bacteria were disrupted with ultrasonic (300 W, frequency of 10 s/10 s) in ice bath for 20 min. Finally, the sample was analyzed by a DX-2700 X-ray diffractometer (Dandong Haoyuan Instrument Co., Ltd., China).
2.7 Data Analysis
Experimental data presented were expressed as the mean of three independent trials. Differences analysis was conducted using one-way ANOVA.
3 Results
3.1 Strain Isolation and Identification
In this study, we firstly collected the samples from sewage wastewater of a metal refinery plant (29.25 N, 102.41 E) in Shimian County, Sichuan Province, China, and then isolated and identified tellurite-reducing bacteria from the water samples.
The enrichment isolation technique was used to isolate tellurite-reducing bacteria. One typical black colony was picked up from plates and purified by subculturing onto fresh nutrient-tellurite agar plates using the streak-plate technique. The isolated strain TX618 exhibited resistance to Na2TeO3 of concentration 100 mg/l, which was associated with reduction of tellurite to elemental Te and the appearance of black colonies in the medium (Fig. 1(a)). The colonies in nutrient agar without tellurite appeared round and smooth and formed a reddish-brown pigment (Fig. 1(b)).
Strain TX618 was shown to be a Gram-negative, rod-shaped, facultative anaerobic bacterium with polar flagella and is identified as Rhodopseudomonas palustris on the basis of 16S rRNA gene sequence analysis. The phylogenetic tree (Fig. 2) constructed by the neighbor-joining method indicated that the isolated strain TX618 was part of the cluster within the genus Rhodopseudomonas. Among the described species, the closest relative of strain TX618 was Rhodopseudomonas palustris.
3.2 Effect of Tellurite on Bacterial Growth
Rhodopseudomonas palustris strain TX618 was able to grow anaerobically in the RCVBN medium supplemented with Na2TeO3 up to 200 mg/L. There is apparent difference in generation times for strain TX618 grown in the medium with or without tellurite (Fig. 3) and the growth curve of TX618 was shown in Fig. 4. At the initial stage of culture, the culture with and without tellurite had the same color. In the control group, the strain began to proliferation at the 2 day after inoculated and showed a red color of general growth all the time. However, in the treated group, it became deep red color and showed a different color with the control group at the second day (Fig. 3b). Finally, the treated group exhibited a dark color at the eighth day (Fig. 3c).
Anaerobic culture of Rhodopseudomonas palustris strain TX618 in RCVBN medium containing tellurite. a At the initial stage of cultivation, b at the second day of cultivation, c at the 8 day of cultivation, and d at the fifteenth day of cultivation. Treated: strain cultured in RCVBN medium containing tellurite; control: strain cultured in RCVBN medium without tellurite
The strain was cultivated in RCVBN medium containing tellurite and reached the end of the logarithmic growth at 4 days, while the medium without tellurite at 3 days (Fig. 4). The growth kinetics data showed that the maximum attainable cell concentration was reduced and the growth of bacteria was inhibited with the increasing of tellurite concentration. High concentration of tellurite had an obvious inhibitory effect on the growth of this bacterium.
3.3 Tellurite Removal by Strain TX618
Due to the versatile metallic modes of photosynthetic bacterium Rhodopseudomonas palustris (Larimer et al. 2004), tellurite removal experiments by strain TX618 were conducted under four different culture conditions including anaerobic illuminated, anaerobic unilluminated, aerobic illuminated, and aerobic unilluminated conditions. The results presented in Fig. 5 showed that strain TX618 could remove the tellurite in the medium under four conditions above. Moreover, when cultured in anaerobic illuminated environment, this strain acquired more than 80% removal efficiency, greatly higher than that in other environments. This probably means that Rhodopseudomonas palustris strain TX618 adopted a suitable metallic mode of photoheterotrophic nutritional type under anaerobic illuminated conditions. So, in the next step, we optimized the removal parameters of tellurite under anaerobic illuminated conditions.
The tellurite removal test indicated that almost more than 70% efficiency could sustain wide variations in pH (5.0–9.0), temperature (20–40 °C), light intensity (1500–3000 lx), and initial tellurium concentration (30–180 mg/L Na2TeO3). As shown in Fig. 6a, b, pH and temperature had significant effect on tellurite removal. The best tellurite removal was reached 90.1–91.0% when the range of pH was 6.5–7.5. The optimum cultivated temperature was observed at 30 °C with 91.9% removal efficiency. The light intensity also affected the removal of tellurite. With the increase of light intensity, the removal of tellurite by Rhodopseudomonas palustris showed a rapidly increasing trend (Fig. 6c). When the light intensity changed in the range of 1500–3000 lx, the removal rate of tellurite was relatively stable. So we selected pH 6.5–7.5, temperature 30 °C, and light intensity 1500 lx as the optimum environmental parameter.
The maximum tellurite removal in the RCVBN medium was determined to be 91.9% with an initial concentration of 90 mg/L tellurite (Fig. 6d). When the concentration of initial tellurite was above 90 mg/L, the removal rate of Te decreased gradually; the activity of the bacteria was inhibited by the higher toxicity.
3.4 Cell Morphologic Observation and XRD Analysis
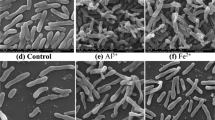
Figure 7 portrays scanning electron microscopy (SEM) observation results for the culture of strain TX618. The bacteria were smooth and grew well when strain TX618 was cultivated in the RCVBN medium (Fig. 7b). The surface of the bacteria suffered serious deformation and cracked in the presence of tellurite. Cells of strain TX618 became shortening contrasting to the cells cultivating in RCVBN medium (Fig. 7a).
Figure 8a, b shows electron micrograph of strain TX618 in the RCVBN medium with and without tellurite. Regular periphery and no black particle were observed in the control medium without tellurite. In the medium treated with tellurite, a large number of black particles or needle-like substances accumulated in the surface and cytoplasm of bacterial cells.
The X-ray diffraction (XRD) pattern was obtained from the extracellular and intracellular black substance with three intense peaks the whole spectrum of 2 h values ranging from 10 to 80 by using software Jade 5.0 (Fig. 9). By comparing the XRD pattern of reducing product with the standard pattern of the Pdf2 Card (2004 Edition), it can be concluded that the large amount of black matter accumulating on the surface and cytoplasm of the bacterial cell is the elemental Te.
4 Discussions
Tellurite is toxic to microbial growth and proliferation, even if Te and its soluble salts are both consider rare in the nature (Yurkov et al. 1996; Jobling and Ritchie 1987; Rathgeber et al. 2002). However, high concentrations of Te compounds still is found in industrial waste discharge (Perkins 2011). Bacteria residing in these environments contaminated by tellurite often reduce highly toxic tellurite to a less toxic form, which accumulate as black deposits in the cells (Moscoso et al. 1998; Pérez et al. 2007). The resistant and reductive phenomenon of tellurite by bacteria has been reported in many studies (Ramos-Ruiz et al. 2016; Kim et al. 2013; Chien et al. 2011; Kagami et al. 2012). In this study, we isolated a tellurite-resistant and reduced bacteria strain TX618 from the sewage wastewater of metal refinery plant. Based on the 16S rDNA sequence, strain TX618 was closely related to Rhodopseudomonas palustris.
Rhodopseudomonas palustris is well-known for its ability to utilize a wide range of growth substrates (Harwood and Gibson 1988; Imhoff 1992; Khanna et al. 1992). It also has extraordinary metabolic versatility as energy from light and carbon from carbon dioxide for photoautotrophic, energy from light and carbon from organic compounds for photoheterotrophic, energy from inorganic compounds and carbon from carbon dioxide for chemoautotrophic, and carbon and energy from organic compounds for chemoheterotrophic (Larimer et al. 2004). Furthermore, aerobic and anaerobic reduction has been reported to resist tellurite for some species (Pearion and Jablonski 1999; Yurkov et al. 1996; Rathgeber et al. 2002; Borsetti et al. 2003; Baesman et al. 2007; Csotonyi et al. 2006; Lloyd et al. 2001). Herein, we designed a series experiments to simulate the four modes of metabolism and explore the difference of removal tellurite. The static groups obtained more tellurite removal from the culture medium compared to the cells group with aerobic shaking, and the results are similar to the removal of tellurite by Paenibacillus sp. (Chihching and Han 2010). Finally, the removal of Te in the photoheterotrophic is far greater than that of other metabolic types.
Rhodopseudomonas sp. strain TX618 can live in the tellurite-containing medium more than 180 mg/L, which is a comparable result to the discovered tellurite-resistant bacterium like E. coli, Rhodobacter sp., and Paenibacillus sp. (Walter and Taylor 1992; Moore and Kaplan 1992; Chihching and Han 2010). Moreover, the growth patterns of Rhodopseudomonas sp. strain TX618 were the same regardless of the cells which grow in the medium with or without the tellurite. These results and other experiments, such as apparent change of culture method (Fig. 3), X-ray diffraction studies (Fig. 9), scanning electron microscope (Fig. 7), and transmission electron micrograph (Fig. 8) analysis, confirmed reduction of tellurite to black precipitate (Te0) by the strain TX618.
The effects of different environmental and chemical parameters were used to calculate the reduction of tellurite in photoheterotrophic culture by strain TX618. The results indicated that a maximum removal efficiency of sodium tellurite would be obtained as pH 6.5–7.5, temperature 30 °C, light intensity 1500–3000 lx, and initial concentration of Na2TeO3 90 mg/L. The pH and temperature of the growth medium strongly influenced the removal of tellurite by strain TX618. The results clearly indicated that a suitable environment is crucial for the growth of Rhodopseudomonas palustris (Butow and Dan 1991; Kuo et al. 2012; Rey and Harwood 2010) and the removal of tellurite.
More and more studies focus on the removal of toxic compounds in polluted environment by bacterium instead of chemical methods. Here, we isolated a strain that could reduce the enrichment of tellurite in environment. This strain may be a good candidate for bioremediation of polluted effluents from the industry. Microbial reduction of toxic oxyanions known as the cost-effective and green technology, so the discovery of reducing tellurite by Rhodopseudomonas palustris enriches the selection of biological treatment of polluted environment. The mechanism and process involved in the tellurite reduction in Rhodopseudomonas palustris require further study.
5 Conclusions
We isolated and characterized a tellurite-reducing Rhodopseudomonas palustris strain TX618 which can resist up to 180 mg/L Na2TeO3 and remove more than 90 mg/L Na2TeO3 over 8 days. Moreover, through electron microscopy and X-ray diffraction analysis, we demonstrated bioreduction of tellurite to black elemental Te, which accumulated in the cells. Although Rhodopseudomonas palustris is capable of metabolizing many compounds, this is the first report on its reduction ability towards tellurite. It not only expands the knowledge on the range of bacteria known for the tellurite bioremediation but also shows a biotechnological potential that is gainfully utilized in waste treatment.
References
Austin, S., Kontur, W. S., Ulbrich, A., Oshlag, Z., Zhang, W., Higbee, A., Zhang, Y., Coon, J., Hodge, D. B., Donohue, T. J., & Noguera, D. R. (2015). Metabolism of multiple aromatic compounds in corn stover hydrolysate by Rhodopseudomonas palustris. Environmental Science & Technology, 49(14), 8914–8922.
Ba, L. A., Döring, M., Jamier, V., & Jacob, C. (2010). Tellurium: an element with great biological potency and potential. Organic & Biomolecular Chemistry, 8(19), 4203–4216.
Baesman, S., Bullen, T., Dewald, J., Zhang, D., Curran, S., Islam, F. S., Beveridge, T. J., & Oremland, R. S. (2007). Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Applied & Environmental Microbiology, 73(7), 2135–2143.
Belzile, N., & Chen, Y. W. (2015). Tellurium in the environment: a critical review focused on natural waters, soils, sediments and airborne particles. Applied Geochemistry, 63, 83–92.
Borghese, R., Baccolini, C., Francia, F., Sabatino, P., Turner, R. J., & Zannoni, D. (2014). Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus. Journal of Hazardous Materials, 269(7), 24–30.
Borsetti, F., Borghese, R., Francia, F., Randi, M. R., Fedi, S., & Zannoni, D. (2003). Reduction of potassium tellurite to elemental tellurium and its effect on the plasma membrane redox components of the facultative phototroph Rhodobacter capsulatus. Protoplasma, 221(1–2), 153–161.
Butow, B., & Dan, B. B. (1991). Effects of growth conditions on acetate utilization by Rhodopseudomonas palustris isolated from a freshwater lake. Microbial Ecology, 22(1), 317–328.
Chasteen, T. G., Fuentes, D. E., Tantaleán, J. C., & Vásquez, C. C. (2009). Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiology Reviews, 33(4), 820–832.
Chen, M., Wu, L. C., Yi, X., Yang, K. Z., & Xie, H. G. (2017). Tellurium speciation in a bioleaching solution by hydride generation atomic fluorescence spectrometry. Analytical Methods, 9(20), 3061–3066.
Chien, C. C., Jiang, M. H., Tsai, M. R., & Chien, C. C. (2011). Isolation and characterization of an environmental cadmium- and tellurite- resistant Pseudomonas strain. Environmental Toxicology & Chemistry, 30(10), 2202–2207.
Chihching, C., & Han, C. T. (2010). Tellurite resistance and reduction by a Paenibacillus sp. isolated from heavy metal-contaminated sediment. Environmental Toxicology & Chemistry, 28(8), 1627–1632.
Csotonyi, J. T., Stackebrandt, E., & Yurkov, V. (2006). Anaerobic respiration on tellurate and other metalloids in bacteria from hydrothermal vent fields in the eastern Pacific Ocean. Applied & Environmental Microbiology, 72(7), 4950–4956.
de Boer, M. A., & Lammertsma, K. (2013). Scarcity of rare earth elements. ChemSusChem, 6(11), 2045–2055.
Deng, Z., Zhang, Y., Yue, J., Tang, F., & Wei, Q. (2007). Green and orange CdTe quantum dots as effective pH-sensitive fluorescent probes for dual simultaneous and independent detection of viruses. Journal of Physical Chemistry B, 111(41), 12024.
Harwood, C. S., & Gibson, J. (1988). Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris. Applied & Environmental Microbiology, 54(3), 712–717.
Imhoff, J. F. (1992). The genus rhodospirillum and related genera. The Prokaryotes, 2141–2155.
Issa, Y. M., Abdel-Fattah, H. M., Shehab, O. R., & Abdel-Moniem, N. B. (2016). Determination and speciation of tellurium hazardous species in real and environmental samples. International Journal of Electrochemical Science, 11, 7475−7498.
Jobling, M. G., & Ritchie, D. A. (1987). Genetic and physical analysis of plasmid genes expressing inducible resistance of tellurite in Escherichia coli. Molecular & General Genetics, 208(1–2), 288–293.
Kagami, T., Fudemoto, A., Fujimoto, N., Notaguchi, E., Kanzaki, M., Kuroda, M., Kuroda, M., Soda, S., Ike, M., & Yamashita, M. (2012). Isolation and characterization of bacteria capable of reducing tellurium oxyanions to insoluble elemental tellurium for tellurium recovery from wastewater. Waste & Biomass Valorization, 3(4), 409–418.
Khanna, P., Rajkumar, B., & Jothikumar, N. (1992). Anoxygenic degradation of aromatic substances by Rhodopseudomonas palustris. Current Microbiology, 25(2), 63–67.
Kim, D. H., Kim, M. G., Jiang, S., Lee, J. H., & Hur, H. G. (2013). Promoted reduction of tellurite and formation of extracellular tellurium nanorods by concerted reaction between iron and Shewanella oneidensis MR-1. Environmental Science & Technology, 47(15), 8709–8715.
Kuo, F. S., Chien, Y. H., & Chen, C. J. (2012). Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresource Technology, 113(4), 315–318.
Larimer, F. W., Chain, P., Hauser, L., Lamerdin, J., Malfatti, S., Do, L., Land, M. L., Pelletier, D. A., Beatty, J. T., Lang, A. S., Tabita, F. R., Gibson, J. L., Hanson, T. E., Bobst, C., Torres, J. L. T., Peres, C., Harrison, F. H., Gibson, J., & Harwood, C. S. (2004). Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nature Biotechnology, 22(1), 55–61.
Li, B., Liu, N., Li, Y., Jing, W., Fan, J., Li, D., et al. (2014). Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris strain N. PLoS One, 9(4), e95955.
Lloyd, J. R., Mabbett, A. N., Williams, D. R., & Macaskie, L. E. (2001). Metal reduction by sulphate-reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy, 59(2–3), 327–337.
Moore, M. D., & Kaplan, S. (1992). Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. Journal of Bacteriology, 174(5), 1505–1514.
Moscoso, H., Saavedra, C., Loyola, C., Pichuantes, S., & Vásquez, C. (1998). Biochemical characterization of tellurite-reducing activities of Bacillus stearothermophilus V. Research in Microbiology, 149(6), 389–397.
Pearion, C. T., & Jablonski, P. E. (1999). High level, intrinsic resistance of Natronococcus occultus, to potassium tellurite. FEMS Microbiology Letters, 174(1), 19–23.
Pérez, J. M., Calderón, I. L., Arenas, F. A., Fuentes, D. E., Pradenas, G. A., & Fuentes, E. L. (2007). Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS One, 2(2), e211.
Perkins, W. T. (2011). Extreme selenium and tellurium contamination in soils—an eighty year-old industrial legacy surrounding a Ni refinery in the Swansea Valley. Science of the Total Environment, 412-413, 162–169.
Presentato, A., Piacenza, E., Anikovskiy, M., Cappelletti, M., Zannoni, D., & Turner, R. J. (2016). Rhodococcus aetherivorans, BCP1 as cell factory for the production of intracellular tellurium nanorods under aerobic conditions. Microbial Cell Factories, 15(1), 204.
Qin, H. B., Takeichi, Y., Nitani, H., Terada, Y., & Takahashi, Y. (2017). Tellurium distribution and speciation in contaminated soils from abandoned mine tailings: comparison with selenium. Environmental Science & Technology, 51(11), 6027–6035.
Ramos-Ruiz, A., Field, J. A., Wilkening, J. V., & Sierraalvarez, R. (2016). Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environmental Science & Technology, 50(3), 1492–1500.
Rathgeber, C., Yurkova, N., Stackebrandt, E., Beatty, J. T., & Yurkov, V. (2002). Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Applied and Environmental Microbiology, 68(9), 4613–4622.
Rey, F. E., & Harwood, C. S. (2010). Fixk, a global regulator of microaerobic growth, controls photosynthesis in Rhodopseudomonas palustris. Molecular Microbiology, 75(4), 1007–1020.
Selimoğlu, H., Öztürk, A., Arısoy, M., & Abdullah, M. I. (2011). Biosorption of dichlorvos by the anaerobic photosynthetic bacterium Rhodopseudomonas palustris NU51. Fresenius Environmental Bulletin, 20(5), 1183–1189.
Tang, Z., Zhang, Z., Wang, Y., Glotzer, S. C., & Kotov, N. A. (2006). Self-assembly of cdte nanocrystals into free-floating sheets. Science, 314(5797), 274–278.
Taylor, D. E. (1999). Bacterial tellurite resistance. Trends in Microbiology, 7(3), 111–115.
Turner, R. J. (2001). Tellurite toxicity and resistance in Gram-negative bacteria. Recent Research Developments in Microbiology, 5, 69–77.
Walter, E. G., & Taylor, D. E. (1992). Plasmid-mediated resistance to tellurite: expressed and cryptic. Plasmid, 27(1), 52–64.
Wong, W. T., Tseng, C. H., Hsu, S. H., Lur, H. S., Mo, C. W., Huang, C. N., Hsu, S. C., Lee, K. T., & Liu, C. T. (2014). Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microbes & Environments, 29(3), 303–313.
Yurkov, V., Jappe, J., & Vermeglio, A. (1996). Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Applied & Environmental Microbiology, 62(11), 4195–4198.
Zannoni, D., Borsetti, F., Harrison, J. J., & Turner, R. J. (2007). The bacterial response to the chalcogen metalloids Se and Te. Advances in Microbial Physiology, 53, 1–72.
Zhang, S. B., Zhang, D. Y., Yong, L., Luo, X. W., Cheng, F. X., Liu, Y., Cheng, J. E., Ma, X. M., & Luo, Y. H. (2009). Degradation characteristics and pathway of fenpropathrin by Rhodopseudomonas sp. strain PSB07-6. Fresenius Environmental Bulletin, 18(11), 2060–2065.
Acknowledgments
This study received financial support from the Sichuan Provincial Key Research and Development Projects, China (Nos. 2017GZ0293 and 2017GZ0387), the Key Project of Education Department of Sichuan, China (No. 18ZA0046) and the Cultivating Program of Young and Middle-aged Backbone Teachers of Chengdu University of Technology, China (No. JXGG201515).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, H.G., Xia, W., Chen, M. et al. Isolation and Characterization of the Tellurite-Reducing Photosynthetic Bacterium, Rhodopseudomonas palustris Strain TX618. Water Air Soil Pollut 229, 158 (2018). https://doi.org/10.1007/s11270-018-3817-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3817-y