Abstract
The dynamics of phosphorus (P) reactions in stream water are important because of their potential to trigger eutrophication. This study aimed to explore the nature of P in sediments associated with Walnut Creek, Jasper County, Iowa. The Walnut Creek watershed supports row crop production, grazing, and riparian buffer zones. The alluvial cross section is composed of a sequence of sediments that contribute differentially to the amounts and forms of P entering the stream. Twenty-five sediment samples collected near Walnut Creek (classified as bank, in-stream, and floodplain deposits) were sequentially extracted for P. Across all 25 samples, the inorganic P (Pi) fractions followed the order Fe-bound Pi > Ca-bound Pi > reductant-soluble Pi > Al-bound Pi > soluble and loosely bound Pi. For the organic (Po) fractions, the order was nonlabile Po > fulvic acid-bound Po > humic acid-bound Po > labile Po > moderately labile Po. The ranges of total P (TP), Mehlich-3-extractable P (P-M3), and ammonium oxalate-extractable P (Pox) were 386 to 1134, 5 to 85, and 60 to 823 mg kg−1, respectively. Among the sample groups, the highest concentrations of TP, P-M3, and Pox were measured in in-stream deposits. Total P was significantly correlated with Fe oxides, clay, and soil organic matter, especially in the bank and floodplain deposits. Because of the potential release of P from these sediments, we can speculate that changes in land use within the riparian areas may, at least initially, have little direct effect on soluble or particulate P loads in Walnut Creek.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Past studies have linked nutrients discharged through the Mississippi River in the Midwestern United States to extensive hypoxia in the Gulf of Mexico (Bianchi et al. 2010; Royer et al. 2006). Nitrogen is widely accepted as a major contributing nutrient to the hypoxia (Rabalais et al. 2002); however, Sylvan et al. (2006) suggested that phosphorus (P) is also important in promoting the hypoxic zone in the Gulf of Mexico. Phosphorus loads in the Mississippi River have been associated with intensive agricultural activities in tributary watersheds. Jacobson et al. (2011) found that the fraction of land in row crops and the use of fertilizer P were correlated with total and dissolved P loads, respectively, in the Mississippi River basin. A model developed by Alexander et al. (2008) estimated the contribution to P loads of pasture and rangeland where manure from grazing animals was present, row crop land where fertilizers had been applied, and other crop land was 37, 25, and 18%, respectively. The contribution of each of these agricultural sources was higher than that from urban sources (12%).
Besides direct runoff from agriculturally managed lands, another pathway for P to enter surface waters is through stream bed and bank erosion (Schilling et al. 2011; Wilson et al. 2008). In Iowa, streambank erosion is considered the main source of suspended sediments in stream water (Schilling and Wolter 2000). Certainly, transport of dissolved P occurs (Sinaj et al. 2002; Turner and Haygarth 2000), but the quantity (load) is commonly less than P transported with eroded sediments. The bioavailability of sediment-bound P varies with the physicochemical properties of the sediments, which reflect sediment origin and land-use history (McDowell et al. 2003). The processes that control sediment transport are influenced by stream velocity and the aggregate stability of the sediments. Quite often, these processes favor transport of finer particles that have higher P sorption capacity than coarser sediments (McDaniel et al. 2009).
Eroded sediments carry both inorganic P (Pi) and organic P (Po) forms. Inorganic P consists of water-soluble or loosely bound fractions, as well as fractions that are precipitated with or adsorbed to Al, Fe, and Ca components or occur as hydroxyapatite (Hedley et al. 1982; Tiessen and Moir 2008; Zhang and Kovar 2009). For Po fractions, the dominant compounds in soils are inositol phosphates. Phospholipids, nucleic acids, phosphoproteins, and sugar phosphates usually occur in smaller concentrations (Dalal 1977). McLaren et al. (2015) found that a substantial proportion of Po in soils is present in large molecular weight phosphomonoesters. Organic phosphorus in chemically and physically stable forms can represent more than 90% of the total Po (Hedley et al. 1982). These forms slowly degrade, contributing to the labile Po pool. The environmental behavior of sediment P depends on the form in which it appears in the sediment (Hund et al. 2013), and to predict the potential release of P from eroded sediments, reliance only on measurements of total P content is not likely to be accurate (Kisand 2005). Therefore, characterizing Pi and Po fractions can be of value in predicting the fate of P in eroded materials entering the stream.
The aim of the present study was to characterize Pi and Po fractions associated with eroded and erodible sediments in the Walnut Creek watershed and to investigate the relationship of various P fractions with other sediment properties. We hypothesized that an analysis of stream sediments can provide insight into how P inputs and sediment P losses may impact water quality within the watershed. The characterization and differentiation of near-stream and in-stream sediments is essential for robust modeling efforts to predict the fate of P in this and other Midwestern streams.
2 Materials and Methods
2.1 Watershed Characteristics
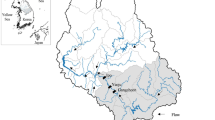
Walnut Creek is in Jasper County, Iowa, and it drains an area of approximately 7951 ha (Fig. 1). The creek discharges into the Des Moines River, a tributary of the Mississippi River, at the upper end of the Red Rock Reservoir (Schilling et al. 2006). The watershed supports a variety of land uses, including row crop production, grazing, and riparian buffer zones within the Neal Smith National Wildlife Refuge, a large-scale prairie restoration area (3502 ha) in central Iowa. Alluvium in the watershed is composed of a characteristic sequence of sediments with potential to contribute differentially to the amounts and forms of P entering the stream.
Map indicating location of the Walnut Creek watershed in Jasper County Iowa adapted from Schilling et al. (2006)
2.2 Sediment Sampling and Characterization
Twenty-five sediment samples were collected from the channel and floodplain along an 8-km reach of Walnut Creek. The samples were grouped into three categories: bank sediments, in-stream deposits, and floodplain soils (Table 1). Samples from the streambanks came from the major stratigraphic units in Walnut Creek, namely the Camp Creek, Roberts Creek, and Gunder Members of the Holocene DeForest Formation, with dates of deposition from the last 380 years, 4000 to 400 years BP, and 11,000 to 4000 years BP, respectively. The fourth material sampled, Pre-Illinoian Till, is associated with glaciation that occurred between 2,000,000 and 500,000 years BP (Baker et al. 1996; Schilling et al. 2004). In-stream deposit samples consisted of materials found in a debris dam, a sand bar, in-stream slumps, a beaver dam, and stream bottom sediment (Table 1). Floodplain samples were collected from the surface horizon (0–20 cm depth) at sites in row-crop production (maize (Zea mays L.) and soybean (Glycine max L. Merr.)), pasture (the majority being beef cattle (Bos taurus L.) operations that rely on grassland for grazing, with little or no confinement of livestock), and riparian buffer zones (forest). After collection, samples were transported to the lab, air dried, and sieved to pass a 2-mm screen. Coarse mineral fragments and organic materials were removed.
Following initial processing, selected physical and chemical properties were determined. Total carbon and total nitrogen were analyzed using high-temperature dry combustion (Nelson and Sommers 1996), particle size distribution was determined gravimetrically (Kettler et al. 2001), and pH was determined at a soil to water ratio of 1:1. Organic matter content was determined with the loss-on-ignition (LOI) method (Konen et al. 2002). A perchloric acid digestion method (Kuo 1996) was used to extract the total phosphorus (TP), and the P concentration in the digest was determined colorimetrically with the molybdate blue-ascorbic acid method (Watanabe and Olsen 1965). The molybdate blue-ascorbic method was also used to determine P in Mehlich-3 (Mehlich 1984) extracts (PM3), while Ca in extracts (CaM3) was determined with inductively coupled plasma-atomic emission spectrometry (ICP-AES). Citrate-bicarbonate-dithionite extractable Fe (FeCBD) was determined by atomic absorption spectroscopy (Shang and Zelazny 2008). Ammonium oxalate extractable Fe, Al, and Mn (Feox, Alox, and Mnox) were determined using ICP-AES, while P in oxalate extracts (Pox) was determined colorimetrically with a malachite green method (D’Angelo et al. 2001).
2.3 Phosphorus Fractionation
Inorganic P and Po were sequentially extracted following the methods outlined by Zhang and Kovar (2009) (Figs. 1 and 2, respectively, of Online Resource 1). For Pi fractionation, 0.5 g of air-dried sediment (< 2 mm) was sequentially extracted with 1 M NH4Cl, 0.5 M NH4F, 0.1 M NaOH, citrate-bicarbonate-dithionite (CBD), and 0.25 M H2SO4. A different scheme was used for Po fractionation: 0.5 g of air-dried sediment (< 2 mm) was sequentially extracted with 0.5 M NaHCO3, 1.0 M HCl, and 0.5 M NaOH, after which the samples were ignited at 550 °C and then extracted with 1.0 M H2SO4. To determine Pt, a modification from the original method was applied, in which ammonium persulfate was used for the digestion in an autoclave instead of potassium persulfate on a hot plate. Phosphorus concentration in the extracts was determined colorimetrically with the molybdate blue-ascorbic acid method (Watanabe and Olsen 1965). Absorbance was read at a wavelength of 880 nm, using a spectrophotometer. Inorganic P and Pt were determined directly from the extracts, whereas Po fractions were determined by subtracting Pi from Pt.
Mean total P (TP), oxalate-extractable P (P ox ), Mehlich 3-extractable P (P M3 ), Mehlich 3-extractable Ca (Ca M3 ), oxalate-extractable Fe (Fe ox ), oxalate-extractable Al (Al ox ), and oxalate-extractable Mn (Mn ox ) concentrations in the sample groups of bank (n = 9) in-stream deposit (n = 10) and floodplain (n = 6) sediments. Error bars represent standard error
2.4 Data Analyses and Comparisons
Statistical analyses were performed with SAS version 9.4 (SAS Institute 2012). These analyses included simple regression, paired t tests, and ANOVA, and they were used to evaluate differences in physicochemical properties among sediment types. The PROC REG procedure in SAS was used to determine correlation of sediment properties with selected P fractions.
3 Results and Discussion
3.1 Sediment Characteristics
Sediment characteristics varied greatly across all 25 samples. Organic matter content ranged from 1.51 to 7.53% (Table 1). Over all, the physical composition of the in-stream deposits tended to be coarser than that of the bank sediments, which may reflect downstream transport of finer material with stream flow (McDowell and Sharpley 2001). The pH values ranged from 5.5 to 8.1, indicating a wide range of sediment acidity.
Total P in the sediments ranged from 370 to 1134 mg kg−1 (Table 1), which is comparable to the TP concentrations in stream bed sediments (389–964 mg P kg−1) collected by Zaimes et al. (2008) in several watersheds in southeast Iowa. Mehlich-3-extractable P ranged from 5 to 85 mg kg−1 (Table 1), and ammonium oxalate-extractable P concentrations ranged from 60 to 823 mg kg−1 (Table 2). Among the three sample groups, the in-stream deposits had the highest average TP, PM3, and Pox values (Fig. 2), indicating their potential to contribute to P loss from the watershed when bed sediments are re-suspended during high-flow events. Although use of PM3 as an environmental threshold to predict the likelihood of P loss from soils or sediments has not yet been defined in Iowa, the environmental threshold for PM3 has been set at 150 mg kg−1 in Arkansas (Sharpley et al. 2003). According to this standard, floodplain soils and sediments in the Walnut Creek watershed have a low risk of P loss.
Simple correlations between total P and oxalate-extractable P and sediment properties are summarized in Table 3, and several details may be inferred. First, across all sediment types, TP was not significantly correlated with clay or organic matter (OM). This observation does not agree with other reports that total P concentrations are correlated with soil clay because clay contributes to Pi retention, or that total P in soils may be strongly influenced by Po in OM (e.g., Dalal 1977; Deng and Dixon 2002; Holford 1997; Kirkby et al. 2011; Quintero et al. 1999). However, when the analysis included only data from the bank and the floodplain deposits (n = 15), i.e., excluding the in-stream deposits (which had more heterogeneous soil characteristics), TP was significantly correlated with clay and OM (Table 3). Second, TP was not significantly correlated with total Fe oxides (FeCBD), but it was positively correlated with poorly crystalline Fe oxides (Feox), which have a high capacity for P binding (Kleinman and Sharpley 2002; van Rotterdam et al. 2012).
Iron oxides are not the only potential source of P binding capacity. For example, phosphorus adsorption by manganese oxides (MnO2) is possible when MnO2 occurs in sufficient concentrations (Yao and Millero 1996). While TP was statistically associated with the sum of oxalate-extractable Fe, Al, and Mn across all sediments, the much stronger correlation of TP with poorly crystalline oxides in only the bank and floodplain deposits (0.76, p = 0.0029) reflects the importance of those minerals in retaining P in stream materials that are only occasionally subject to flooding or submergence. These relationships were largely mirrored in the correlations between oxalate-extractable P (Pox) and oxalate-extractable metals (Table 3).
The higher correlation coefficients obtained by excluding data from the in-stream deposits indicated that processes of P adsorption and desorption, precipitation and dissolution of P-containing minerals, and mineralization and immobilization of organic P would be less predictable for the in-stream deposits compared to the other groups. Regular contact with flowing stream water may have had an impact on the P-retention characteristics of the in-stream deposits compared with the bank sediments and floodplain soil samples (McDaniel et al. 2009).
3.2 Phosphorus Fractionation
3.2.1 Comparison of Total P and Sum of all Fractions
Total P (TP) extracted with perchloric and nitric acid was highly correlated with the sum of all individual P fractions (TPsum) (i.e., TPsum = 0.78 TP + 86.4, R2 = 0.83; Fig. 3 of Online Resource 1). Thus, we conclude that the fractionation methodology used in this study reasonably recovered total P in the individual Pi and Po fractions.
3.2.2 Inorganic P Fractions
Following the method described by Zhang and Kovar (2009), inorganic P fractions were classified as soluble and loosely bound Pi (SL-Pi), Al-bound Pi (Al-Pi), Fe-bound Pi (Fe-Pi), reductant-soluble Pi (RS-Pi), and Ca-bound Pi (Ca-Pi). Results for each of the 25 samples are shown in Fig. 3. The total of all individual Pi fractions (Pisum) ranged from 125 to 856 mg kg−1 or 35 to 90% of TPsum. As expected for these soils and sediments, SL-Pi ranged from 4 to 11 mg kg−1 or only 0.7 to 3.5% of Pisum.
Distribution of inorganic P (Pi) fractions in 25 sediment samples collected from the Walnut Creek watershed. Bars represent Pi concentrations in five fractions, including soluble and loosely bound Pi (SL-Pi), Al-bound Pi (Al-Pi), Fe-bound Pi (Fe-Pi), reductant-soluble Pi (RS-Pi), and Ca-bound Pi (Ca-Pi). Line chart represents percentage of Pisum relative to TPsum
For each group of sediments, SL-Pi averaged 6 mg kg−1 in the bank sediments and 7 mg kg−1 in the floodplain soils and the in-stream deposits (Table 4). The small concentrations of SL-Pi in this study agreed with those reported by Wang et al. (2010) for the surface sediments of a river system in China. The amounts, however, were much less than the SL-Pi values reported by Kisand (2005) for sediment in Lake Verevi, a eutrophic temperate lake in South Estonia. Although the loosely bound P fraction was almost negligible compared with Pisum, SL-Pi is probably the solid-phase P which may be released to solution first when sediments are re-suspended during the high-flow events. Hence, its impact on causing impairment of water quality may be more direct than that of other P fractions.
Among the other fractions, Al-Pi and Fe-Pi values ranged from 0 to 99 mg kg−1 and from 1 to 571 mg kg−1, respectively (Fig. 3). The Fe-Pi fraction has potential to elevate soluble P levels in the water column under anaerobic conditions when Fe3+ can be reduced to Fe2+, and P-adsorbing Fe oxides are dissolved (Pettersson 1998). The lowest values of Al-Pi and Fe-Pi were found in the stream bank sample of Pre-Illinoian Till (sample B-20, Fig. 3). This sample had some characteristics that could promote P retention (e.g., large values for FeCBD and Mehlich 3-extractable Ca), yet there were also other characteristics that would be unlikely to lead to P accumulation (e.g., high sand content, low oxalate-extractable Fe, Al, and Mn) (Table 2). It is likely that the low concentrations of Fe-Pi in the till are because it was relatively unweathered and had not been exposed to biological activity that would promote P cycling (Hongthanat et al. 2011).
Reductant-soluble Pi ranged from 7 to 223 mg kg−1 (Fig. 3). Both the lowest and the highest RS-Pi values were found in the in-stream deposit samples. Of the three sample groups, the in-stream deposits had the highest average Al-Pi, Fe-Pi, and RS-Pi. Calcium-bound Pi ranged from 26 to 288 mg kg−1 (Fig. 3). Unlike other Pi fractions, the highest average Ca-Pi concentration was found in the bank sediments. Further, Ca-Pi concentrations in the bank sediments followed the order Camp Creek < Roberts Creek < till < Gunder, indicating that Ca-Pi concentrations were lowest in the youngest sediments.
Averaged over all 25 sediments in this study, the Pi fractions followed the order Fe-Pi > Ca-Pi > RS-Pi > Al-Pi > SL-Pi. This order was similar to the distribution of P fractions reported by Huanxin et al. (1997) for Washington tidal river sediments, which followed the order Fe-P > Ca-P > Al-P. The order for the in-stream deposits was similar to the composite average order (Table 4). However, the orders of Pi fractions in the floodplain soils and the bank deposits were different. The Pi fractions followed the order Fe-Pi > RS-Pi > Ca-Pi > Al-Pi > SL-Pi in the floodplain soils and Ca-Pi > Fe-Pi > RS-Pi > Al-Pi > SL-Pi in the bank samples, reflecting the diversity of P forms in watershed sediments that might contribute to P in the stream.
3.2.3 Organic P Fractions
Following the sequential extraction procedures outlined by Zhang and Kovar (2009), Po fractions in this study were classified as labile (Lab-Po), moderately labile (MLab-Po), associated with humic acid (HA-Po), associated with fulvic acid (FA-Po), and nonlabile (NLab-Po). Results are shown in Fig. 4. Labile Po ranged from 2 to 47 mg kg−1 or 0.5 to 8.5% of the total of all individual Po fractions (Posum). Averaged over each sample group, Lab-Po concentrations were 20, 14, and 32 mg kg−1 in the bank deposits, in-stream deposits, and floodplain soils, respectively (Table 5). Especially in the bank sediments, Lab-Po concentrations in Camp Creek and Roberts Creek samples were higher than those in the Gunder (sample B-14; Fig. 4) and till (samples B-7 and B-20; Fig. 4). As reported by Xu et al. (2013), the decrease of Lab-Po with increasing depth likely indicates a transformation of the labile Po fraction into nonlabile Po pools over time.
Distribution of organic P (Po) fractions in 25 sediment samples collected from the Walnut Creek watershed. Bars represent Po concentrations in five fractions, including labile (Lab-Po), moderately labile (MLab-Po), associated with humic acid (HA-Po), associated with fulvic acid (FA-Po), and nonlabile (NLab-Po). Line chart represents percentage of Posum relative to TPsum
In general, Lab-Po was greater than MLab-Po, likely because of differences in the pH of the extracting solution for each fraction. As demonstrated by You et al. (2006), soil organic matter (SOM) fractions are more readily extractable from soil/sediment samples with a higher pH extracting solution. Labile Po was fractionated by extraction with 0.5 M NaHCO3 at pH 8.5, while MLab-Po was extracted with 1 M HCl at pH 0. Fulvic acid-Po and HA-Po are two fractions that are extracted with alkaline extracting solution (0.5 M NaOH) and are thought to be associated with humic substances. Across all 25 sediments, the ranges of FA-Po and HA-Po were 8 to 127 and 2 to 105 mg kg−1, respectively. On average, FA-Po was 29% of total Po (Posum), and HA-Po 24% of Posum. Paing et al. (1999) reported FA-Po and HA-Po values of 58 to 60 and 19 to 28%, respectively, for sediments in two French Mediterranean coastal lagoons, a very different ecological setting. Hong and Yamane (1980) reported that about 60% of FA-Po was inositol hexakisphosphate, and 40% was other forms of Po. Further fractionation of FA-Po or HA-Po, however, was beyond the scope of the present study.
The final Po fraction estimated with sequential extraction in this study was NLab-Po. These Po forms are thought to be associated with nonhumic substances. Nonlabile Po for the samples ranged from 27 to 163 mg kg−1 (Fig. 4). Nonlabile Po comprised the greatest proportion of Posum, especially for the bank and the in-stream deposit samples (Fig. 4; Table 5).
Across all samples, the Po fractions followed the order NLab-Po > FA-Po > HA-Po > Lab-Po > MLab-Po. The overall order was similar in the bank and the in-stream deposits, but in the floodplain soils, Po fractions followed a different order: FA-Po > HA-Po > NLab-Po > Lab-Po > MLab-Po. Interestingly, whether in the bank sediments, the in-stream deposits, or the floodplain soils, the ratio of (NLab-Po + FA-Po + HA-Po) to (Lab-Po + MLab-Po) was ~ 9:1. This indicates that the ratio of labile organic P to Posum remained stable across the sample groups, although the reason is unclear.
The sum of all individual Po fractions (Posum) in this study ranged from ~ 10 to 65% of TPsum. The Posum was strongly correlated with organic matter content determined by the LOI method (Fig. 4 of Online Resource 1). For samples in the floodplain, it was reasonable that about half of TPsum would be organic P, since all samples in this group were collected from soil surface horizons where organic matter generally accumulates. Differences in the values of Posum among the major stratigraphic units in the stream banks of Walnut Creek were consistent with sediment age. The OM content and the fraction of Posum in the younger materials in the upper part of the stratigraphic column, i.e., Camp Creek (samples B-2, B-17, and B-21) and Roberts Creek (samples B-3, B-18, and B-22) sediments, were greater than those in older Gunder sediments (sample B-19) and Pre-IllinoianTill (samples B-7 and B-20) deposits (Fig. 4).
The values of Posum and total C in the 25 samples were highly correlated (Fig. 5a), and this agrees with previous research by Kirkby et al. (2011) for Australian and other soils. However, a stronger correlation was obtained when the data from the in-stream deposits were excluded from the analysis (Fig. 5b). The average C:Posum ratio in the bank sediments and floodplain soils was similar (~ 68:1), while the ratio in the in-stream deposits was ~ 88:1. As noted earlier, characteristics of the in-stream deposits that are relevant to the fate and transport of P may have been affected by continuous contact with the flowing stream. We speculate that the relative physical stability of the bank sediments and floodplain deposits led to a more constant C:P ratio than in the in-stream deposits. This is based on assumption that organic matter in stream banks and floodplains could be related to consistent vegetation cycling, while organic matter in the in-stream deposits was affected by a continuous process of transport, sorting, and deposition by stream flow. While the extraction scheme did not differentiate P in microbial biomass, some variation in organic P could also be related to variations in microbial communities in the stream banks and floodplains versus the in-stream deposits. The C:P ratio of soil microbial biomass can vary greatly in freshwater ecosystems. According to Cross et al. (2005), the C:P ratio for fungi in a freshwater benthic system ranged from 5 to 370; for bacteria, it ranged from 300 to 1190. Further research is needed to explore the reasons that these ratios differ among the Walnut Creek watershed soils and sediments.
4 Summary and Conclusions
Sediment characteristics, as well as P forms associated with eroded sediments in the Walnut Creek watershed, varied greatly across all 25 sediments we studied. Among the sediment types, the highest concentrations of TP, PM3, and Pox were measured in the in-stream deposits, indicating that they are potentially major contributors to P leaving the watershed from bed sediment re-suspension during high-flow events. We found strong correlations between extractable P forms and other sediment properties when data from bank sediments and floodplain soils were pooled. Across all 25 sediments, the relative amounts of extractable inorganic P followed the order Fe-bound Pi > Ca-bound Pi > reductant-soluble Pi > Al-bound Pi > soluble and loosely bound Pi. For the organic P fractions, the relative order was nonlabile Po > fulvic acid-bound Po > humic acid-bound Po > labile Po > moderately labile Po. Further research is needed to relate these P fractions directly to P loads in Walnut Creek.
Phosphorus mobility in streams is regulated by a number of interacting biotic and abiotic processes that make both prediction and control challenging (Hongthanat et al. 2016). In some cases, sediments alone are thought to exert significant control on water-column P, while in others, sediments in combination with microbes and macrophytes act to buffer stream P loads (Haggard et al. 2007; McDaniel et al. 2009). Sediment P content, readiness to release P to the stream (that is, to act as a source of P), and ability to retain soluble P (that is, act as a sink for P) may vary significantly. As pointed out by Jarvie et al. (2013) and Sharpley et al. (2013), the release of “legacy” P from stream sediments can buffer against rapid water quality improvement after nutrient management measures are introduced. Still, if P inputs from eroded bank sediments are reduced, whether by riparian management, stream bank stabilization, or some other practice, it is likely that P loads in Walnut Creek can be gradually reduced as well.
References
Alexander, R. B., Smith, R. A., Schwarz, G. E., Boyer, E. W., Nolan, J. V., & Brakebill, J. W. (2008). Differences in phosphorus and nitrogen delivery to the Gulf of Mexico from the Mississippi River basin. Environmental Science and Technology, 42, 822–830.
Baker, R. G., Bettis 3rd, E. A., Schwert, D. P., Horton, D. G., Chumbley, C. A., Gonzalez, L. A., & Reagan, M. K. (1996). Holocene paleoenvironments of northeast Iowa. Ecological Monographs, 66, 203–234.
Bianchi, T. S., DiMarco, S. F., Cowan Jr., J. H., Hetland, R. D., Chapman, P., Day, J. W., & Allison, M. A. (2010). The science of hypoxia in the northern Gulf of Mexico: a review. Science of the Total Environment, 408, 1471–1484.
Cross, W. F., Benstead, J. P., Frost, P. C., & Thomas, S. A. (2005). Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Freshwater Biology, 50, 1895–1912.
D’Angelo, E., Crutchfield, J., & Vandiviere, M. (2001). Rapid, sensitive, microscale determination of phosphate in water and soil. Journal of Environmental Quality, 30, 2206–2209.
Dalal, R. C. (1977). Soil organic phosphorus. Advances in Agronomy, 29, 85–117.
Deng, Y., & Dixon, J. B. (2002). Soil organic matter and organic–mineral interactions. In J. B. Dixon & D. G. Schulze (Eds.), Soil mineralogy with environmental applications (pp. 69–107). Madison: Soil Science Society of America.
Haggard, B. E., Smith, D. R., & Brye, K. R. (2007). Variations in stream water and sediment phosphorus among select Ozark catchments. Journal of Environmental Quality, 36, 1725–1734.
Hedley, M. J., Stewart, J. W. B., & Chauhan, B. S. (1982). Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Science Society of America Journal, 46, 970–976.
Holford, I. C. R. (1997). Soil phosphorus: its measurement, and its uptake by plants. Australian Journal of Soil Research, 35, 227–239.
Hong, J. K., & Yamane, I. (1980). Inositol phosphate and inositol humic acid and fulvic acid fractions extracted by three methods. Soil Science and Plant Nutrition, 26, 491–496.
Hongthanat, N., Kovar, J. L., & Thompson, M. L. (2011). Sorption indices to estimate risk of soil phosphorus loss in the Rathbun Lake watershed, Iowa. Soil Science, 176, 237–244.
Hongthanat, N., Kovar, J. L., Thompson, M. L., Russell, J. R., & Isenhart, T. M. (2016). Phosphorus source–sink relationships of stream sediments in the Rathbun Lake watershed in southern Iowa, USA. Environmental Monitoring and Assessment, 188, 453–467. https://doi.org/10.1007/s10661-016-5437-6
Huanxin, W., Presley, B. J., & Velinsky, D. J. (1997). Distribution and sources of phosphorus in tidal river sediments in the Washington, DC, area. Environmental Geology, 30, 224–230.
Hund, S. V., Brown, S., Lavkulich, L. M., & Oswald, S. E. (2013). Relating P lability in stream sediments to watershed land use via an effective sequential extraction scheme. Water, Air, & Soil Pollution, 224, 1643. https://doi.org/10.1007/s11270-013-1643-9
Jacobson, L. M., David, M. B., & Drinkwater, L. E. (2011). A spatial analysis of phosphorus in the Mississippi River basin. Journal of Environmental Quality, 40, 931–941.
Jarvie, H. P., Sharpley, A. N., Withers, P. J. A., Scott, J. T., Haggard, B. E., & Neal, C. (2013). Phosphorus mitigation to control river eutrophication: murky waters, inconvenient truths and ‘post-normal’ science. Journal of Environmental Quality, 42, 295–304.
Kettler, T. A., Doran, J. W., & Gilbert, T. L. (2001). Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Science Society of America Journal, 65, 849–852.
Kirkby, C. A., Kirkegaard, J. A., Richardson, A. E., Wade, L. J., Blanchard, C., & Batten, G. (2011). Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma, 163, 197–208.
Kisand, A. (2005). Distribution of sediment phosphorus fractions in hypertrophic strongly stratified Lake Verevi. Hydrobiologia, 547, 33–39.
Kleinman, P. J. A., & Sharpley, A. N. (2002). Estimating soil phosphorus sorption saturation from Mehlich-3 data. Communications in Soil Science and Plant Analysis, 33, 1825–1839.
Konen, M. E., Jacobs, P. M., Burras, C. L., Talaga, B. J., & Mason, J. A. (2002). Equations for predicting soil organic carbon using loss-on-ignition for north central U.S. soils. Soil Science Society of America Journal, 66, 1878–1881.
Kuo, S. (1996). Phosphorus. In D. L. Sparks (Ed.), Methods of soil analysis. Part 3. Chemical methods (pp. 869–919). Madison: Soil Science Society of America.
McDaniel, M. D., David, M. B., & Royer, T. V. (2009). Relationships between benthic sediments and water column phosphorus in Illinois streams. Journal of Environmental Quality, 38, 607–617.
McDowell, R. W., & Sharpley, A. N. (2001). A comparison of fluvial sediment phosphorus (P) chemistry in relation to location and potential to influence stream P concentrations. Aquatic Geochemistry, 7, 255–265.
McDowell, R. W., Sharpley, A. N., & Folmar, G. (2003). Modification of phosphorus export from an eastern USA catchment by fluvial sediment and phosphorus inputs. Agriculture Ecosystems and Environment, 99, 187–199.
McLaren, T. I., Smernik, R. J., McLaughlin, M. J., McBeath, T. M., Kirby, J. K., Simpson, R. J., Guppy, C. N., Doolette, A. L., & Richardson, A. E. (2015). Complex forms of soil organic phosphorus—a major component of soil phosphorus. Environmental Science & Technology, 49, 13238–13245.
Mehlich, A. (1984). Mehlich 3 soil extractant: a modification of Mehlich 2 extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416.
Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. In D. L. Sparks (Ed.), Methods of soil analysis, part 3, Soil Science Society of America Book Series 5 (pp. 961–1010). Madison: SSSA.
Paing, J., Gomez, E., & Picot, B. (1999). Humic substances interactions with sedimentary phosphorus. Analusis, 27, 436–438.
Pettersson, K. (1998). Mechanisms for internal loading of phosphorus in lakes. Hydrobiologia, 373(374), 21–25.
Quintero, C. E., Boschetti, G. N., & Benavidez, R. A. (1999). Phosphorus retention in some soils of the Argentinean Mesopotamia. Communications in Soil Science and Plant Analysis, 30, 1449–1461.
Rabalais, N. N., Turner, R. E., & Scavia, D. (2002). Beyond science into policy: Gulf of Mexico hypoxia and the Mississippi River. Bioscience, 52, 129–142.
Royer, T. V., David, M. B., & Gentry, L. E. (2006). Timing of riverine export of nitrate and phosphorus from agricultural watersheds in Illinois: Implications for reducing nutrient loading to the Mississippi river. Environmental Science and Technology, 40, 4126–4131.
SAS Institute. (2012). SAS/STAT® 9.4 user’s guide (2nd ed.). Cary: SAS Institute.
Schilling, K. E., & Wolter, C. F. (2000). Application of GPS and GIS to map channel features in Walnut Creek, Iowa. Journal of the American Water Resources Association, 36, 1423–1434.
Schilling, K. E., Zhang, Y. K., & Drobney, P. (2004). Water table fluctuations near an incised stream, Walnut Creek, Iowa. Journal of Hydrology, 286, 236–248.
Schilling, K. E., Hubbard, T., Luzier, J., & Spooner, J. (2006). Walnut Creek watershed restoration and water quality monitoring project: final report. Iowa City: Iowa Department of Natural Resources.
Schilling, K. E., Isenhart, T. M., Palmer, J. A., Wolter, C. F., & Spooner, J. (2011). Impacts of land-cover change on suspended sediment transport in two agricultural watersheds. Journal of the American Water Resources Association, 47, 672–686.
Shang, C., & Zelazny, L. W. (2008). Selective dissolution techniques for mineral analysis of soils and sediments. In A. L. Ulery & L. R. Dress (Eds.), Methods of soil analysis, part 5—mineralogical methods (pp. 33–80). Madison: Soil Science Society of America.
Sharpley, A. N., Daniel, T., Sims, T., Lemunyon, J., Stevens, R., & Parry, R. (2003). Agricultural phosphorus and eutrophication, 2 nd edition. USDA-ARS report 149. Washington, D.C.: U.S. Government Printing Office.
Sharpley, A., Jarvie, H. P., Buda, A., May, L., Spears, B., & Kleinman, P. (2013). Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. Journal of Environmental Quality, 42, 1308–1326.
Sinaj, S., Stamm, C., Toor, G. S., Condron, L. M., Hendry, T., Di, H. J., Cameron, K. C., & Frossard, E. (2002). Phosphorus exchangeability and leaching losses from two grassland soils. Journal of Environmental Quality, 31, 319–330.
Sylvan, J. B., Dortch, Q., Nelson, D. M., Brown, A. F. M., Morrison, W., & Ammerman, J. W. (2006). Phosphorus limits phytoplankton growth on the Louisiana shelf during the period of hypoxia formation. Environmental Science and Technology, 40, 7548–7553.
Tiessen, H., & Moir, J. O. (2008). Characterization of available P by sequential extraction. In M. R. Carter (Ed.), Soil sampling and analysis (2nd ed., pp. 293–306). New York: Canadian Society of Soil Science.
Turner, B. L., & Haygarth, P. M. (2000). Phosphorus forms and concentrations in leachate under four grassland soil types. Soil Science Society of America Journal, 64, 1090–1099.
van Rotterdam, A. M. D., Busssink, D. W., Temminghoff, E. J. M., & van Riemsdijk, W. H. (2012). Predicting the potential of soils to supply phosphorus by integrating soil chemical processes and standard soil tests. Geoderma, 189, 617–626.
Wang, Z., Lin, C., He, M., Quan, X., & Yang, Z. (2010). Phosphorus content and fractionation of phosphate in the surface sediments of the Dalio river system in China. Environmental Earth Science, 59, 1349–1357.
Watanabe, F. S., & Olsen, S. R. (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society of America Proceedings, 29, 677–678.
Wilson, C. G., Kuhnle, R. A., Bosch, D. D., Steiner, J. L., Starks, P. J., Tomer, M. D., & Wilson, G. V. (2008). Quantifying relative contributions from sediment sources in conservation effects assessment project watersheds. Journal of Soil and Water Conservation, 63, 523–532.
Xu, D., Ding, S., Li, B., Bai, X., Fan, C., & Zhang, C. (2013). Speciation of organic phosphorus in a sediment profile of Lake Taihu I: chemical forms and their transformation. Journal of Environmental Sciences, 25, 637–644.
Yao, W., & Millero, F. J. (1996). Adsorption of phosphate on manganese dioxide in seawater. Environmental Science and Technology, 30, 536–541.
You, S. J., Thakali, S., & Allen, H. E. (2006). Characteristics of soil organic matter (SOM) extracted using base with subsequent pH lowering and sequential pH extraction. Environment International, 36, 101–105.
Zaimes, G. N., Schultz, R. C., & Isenhart, T. M. (2008). Streambank soil and phosphorus losses under different riparian land-uses in Iowa. Journal of American Water Resources Association, 44, 935–947.
Zhang, H., & Kovar, J. L. (2009). Fractionation of soil phosphorus. In J. L. Kovar & G. M. Pierzynski (Eds.), Methods of phosphorus analysis for soils, sediments, residuals, and waters (2nd ed., pp. 29–32). Southern Cooperative Series Bulletin 408). Blacksburg: Virginia Polytechnic Institute and State University.
Acknowledgements
The senior author’s present address is Indonesian Oil Palm Research Institute, Medan, North Sumatra, Indonesia. The authors are grateful to Teresita Chua and Jay Berkey and for technical support. This research was supported by Agriculture & Food Research Initiative Competitive Grant no. 2013-67019-21393 from the USDA National Institute of Food & Agriculture. Mention of trade names does not imply recommendation or endorsement by Iowa State University or the USDA Agricultural Research Service.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 205 kb)
Rights and permissions
About this article
Cite this article
Rahutomo, S., Kovar, J.L. & Thompson, M.L. Inorganic and Organic Phosphorus in Sediments in the Walnut Creek Watershed of Central Iowa, USA. Water Air Soil Pollut 229, 72 (2018). https://doi.org/10.1007/s11270-018-3721-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3721-5