Abstract
Electric arc furnace dust (EAFD) is a toxic waste which is mainly rich in iron oxide, zinc, and lead. Hydrometallurgical extraction of zinc from Jordanian EAFD in alkaline medium was investigated; NaOH, NaHCO3, and Na2CO3 were used as leaching agents. The pH values for the prepared solutions were 8.3, 8.2, and 12.55 for NaHCO3, Na2CO3, and NaOH, respectively. The effect of NaOH concentration (1, 3, 5, 7, and 9 M), contact time (5 min to 3 h), temperature (20, 40, and 60), and solid-to-liquid ratio (SLR; 20, 40, 80, and 120 mg/ml) on the leachability of zinc from EAFD were tested. The initial EAFD and the resulting leach residues were characterized using X-ray diffraction (XRD) and X-ray fluorescence (XRF). EAFD contained 25.9% Zn, 18.0% Fe, and 3.2% Pb. A maximum zinc recovery of 92.9% was achieved using 6 M NaOH at 60 °C with solid loading of 20 g/L and 3 h leaching time. NaHCO3 and Na2CO3 were not efficient leaching agents for Zn extraction since the recoveries were only 2.6 and 4.5%, respectively. Zn and Pb were depleted in the residues with an E-factor of 0.5–0.6 and 0.1–0.25, respectively. Iron was enriched in the residues; the E-factor was around 2. The EAFD contained mainly zincite, franklinite, and magnetite. After 3 h leaching, only traces of zincite exist in the residues, while sylvite and halite were completely dissolved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electric arc furnace dust (EAFD) is one of the most intensely growing waste streams on global scale. It is generated at a rate of 10–20 kg/ton of steel implying 5–7 million tons of EAFD generation each year worldwide (Kelebek et al. 2004). The U.S. EPA considered EAFD one of the most hazardous waste since it contains heavy metals (e.g., Zn, Fe, Cr, Cd, and Pb), releases potential amount of heavy metals into the ground, and contaminates ground water and sewage systems (Kavouras et al. 2007; Niubo et al. 2009).
Electric arc furnace produces about 20 kg of flue dust for each ton of steel, which forms as a result of coal and scrap mixing at an approximate temperature of 1500 °C (Kavouras et al. 2007; Oustadakis et al. 2010). The composition of EAFD varies greatly depending on scrap composition and furnace additives (Sofilić et al. 2004; Stegemann et al. 2000). The EAFD shows a complex heterogeneous mineralogy with spinel mineral group predominance (de Araujo and Schalch 2014).
Zinc is the most abundant metal among the non-ferrous metals existing in the EAFD. Its content varies between 7 and 40%, based on the ratio of galvanized scrap used (Youcai and Stanforth 2000). Zinc occurs mainly as zincite (ZnO) and franklinite (ZnFe2O4) (Abkhoshk et al. 2014). Due to the environmental legislation that restricts dumping of hazardous metals and the interest in secondary zinc, zinc recycling interest has been increased dramatically (Vieira et al. 2013). Today, approximately 30% of global zinc production arises from recycled zinc. The quantity of EAFD generated per year around the world represents a possible recovery of about 0.86 to 1.14 million tons/year of zinc (Leclerc et al. 2003).
Numerous pyrometallurgical and hydrometallurgical processes have been developed for the extraction of zinc from EAFD. Most of the commercially available processes for the treatment of EAFD are pyrometallurgical (e.g., rotary kilns, plasma, and flame reactor processes). These methods are used to recover zinc from the dust by fuming and condensing metals in pure form or in the form of zinc oxide (Kekki et al. 2012). The use of pyrometallurgical methods generates emission of many toxic gases that pollute the environment (Havlík et al. 2006). They are energy-consuming methods and require various gas cleaning and dust collection systems (Suetens et al. 2014). In contrast, hydrometallurgical methods are more advantageous in terms of process economy and environment compared with pyrometallurgy (Oishi et al. 2008). Pure metals are obtained directly from leach solution or recovered from impure leach solution (Al-Harahsheh and Kingman 2004). The main drawback of hydrometallurgical methods lies in the presence of 50% of the total EAFD zinc in the form of ferrite ZnFe2O4, which affect the beneficial utilization of zinc (Leclerc et al. 2003). Hydrometallurgical methods are limited on an industrial scale due to the accumulations of iron species in acidic solutions (Al-Harahsheh et al. 2015).
Alkaline and acid leaching technologies are being used to eliminate zinc from EAFD (Jha et al. 2001). Jha et al. (2001) reviewed the most recent hydrometallurgical processes employed for extraction of valuable metals from EAFD. The industrial scale utilization of acidic leaching processes (i.e., using H2SO4, HCl, HNO3, etc.) has been impeded due to the low solubility of zinc ferrite in acidic solutions and the necessity of eliminating iron from the leachate when the conditions break down the ferrite structure (Barrett et al. 1992; Jha et al. 2001; Xia and Picklesi 2000; Zhang et al. 2010). Sodium hydroxide dissolves zinc selectively, but it needs further development for the metal to recover from the sodium zincate solution by electrolysis. Under atmospheric pressure and room temperature, Caravaca et al. (1994) showed that zinc and iron were completely dissolved in acidic leaching process. While, sodium hydroxide and ammonium salts were partially dissolved. Dreisinger (1990) found that caustic soda is an efficient solvent for zinc dissolution on a large scale.
Many attempts were made in order to improve the process and increase the recovery rate. Frenay et al. (1986) developed a process called the Cebedeau process for the recovery of metal from EAFD. They leached zinc and lead using hot (95 °C) concentrated NaOH (6–12 M) solution for 1–2 h. The process was economical in recovering very fine zinc powder from dust containing 20% zinc (Frenay et al. 1986; Jha et al. 2001). Youcai and Stanforth (2000) obtained 38% Zn efficiency through direct alkaline leaching, as compared with a 95% Zn efficiency by caustic roasting of hydrolyzed EAFD at 350 °C, followed by a two-step alkaline leaching. To recycle the valuable metallic fraction embedded in EAFD and treat its halogen content, co-pyrolysis is the mainstream strategy. Ahmed et al. (2017) investigated chemical interplay between HCl/HBr and zincite surfaces as a representative model for structures of zinc oxides in EAFD by using different sets of functionals, unit cell size, and energy cut-off.
Alkaline leaching offers the potential advantage that iron remains largely insoluble and efforts to develop alkaline leaching technologies for EAFD continue. Therefore, the purpose of this work was to investigate the optimum conditions for the maximum Zn extraction using alkaline hydrometallurgical technique.
2 Experimental Work
2.1 Characterization of Jordanian EAFD
Representative samples of EAFD were analyzed for their mineral composition using X-ray diffraction (XRD) (LabX XRD-6100, Shimadzu). A computer-controlled Hiltonbrooks® generator with a Philips® PW 1050 diffractometer with an automatic divergence slit, and Cu anode producing X-rays of wavelength λ = 1.54056 Å was used. The diffractometer was operating at 40 kV and 20 mA, and automatic routines allowed scanning for values 2θ from 5° to 95° using a step size of 0.05° and scan speed of 2°/min. Also, the raw sample and some of the residues were characterized for their chemical composition using X-ray fluorescence (XRF) (EXD-7000, Shimadzu).
2.2 Experimental Set-up
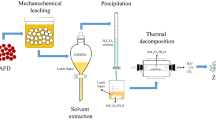
The experimental set-up is shown in Fig. 1. All batch experiments were conducted in a 500-ml five-necked, round-bottomed split reactor, which was fitted with a mechanical stirrer and a thermometer. The reactor was immersed in a water bath with a temperature accuracy of about ± 2 °C and covered to maintain a constant solid-to-liquid ratio (SLR). The leaching was carried out under atmospheric pressure. To ensure suspension of particles, a stirrer with a stirring speed of 500 rpm was used. Heating was provided by water bath, and the liquid temperature was controlled. A condenser was used as the temperature of test was more than 40 °C.
A typical run was carried out by placing a leaching solution volume of 250 cm3 in the reactor and heating into the desired temperature (± 2 °C), adjusting the overhead stirrer, adding a weight of 5 g EAFD under constant agitation (500 rpm), and withdrawing about 2 ml solution at convenient time intervals. Samples were centrifuged at 8000 rpm for 2 min and then filtered. The supernatant was analyzed for Zn concentration using atomic absorption spectrometry (AAS) (novAA 300, Analytik-Jena, Germany). At the end of each run, the content of the reactor was filtered and the resulting leached residue was washed with water, dried at 105 °C, and weighed. Zn recovery was calculated as:
where C S is the concentration of Zn in supernant at each condition (ppm), and C D is the concentration of Zn if all Zn content was dissolved (ppm).
2.3 Experimental Parameters Using Caustic Soda
2.3.1 Kinetic Study
The effect of time was carried out by reacting 5 ± 0.0005 g of EAFD with 250 ml of NaOH at 20 °C. The stirring speed was fixed at 500 rpm. Samples of 2 ml were taken at the following time periods: 5, 10, 20, 30, and 45 min and 1, 2, and 3 h.
2.3.2 Effect of Sodium Hydroxide Concentration
The effect of NaOH concentration was studied using 1, 3, 5, and 7 M at 20 °C for 2 h leaching time.
2.3.3 Effect of Temperature
The effect of temperature was studied using NaOH concentration of 3 and 6 M. The SLR was 20 mg/ml, and the stirring speed was 500 rpm. The studied temperatures were 20, 40, 50, and 60 °C. A condenser was used when the temperature was raised to 40 °C and higher to keep the SLR.
2.3.4 Effect of SLR
The effect of SLR was studied at different loadings ranging from 20 to 120 mg/ml solid concentration using 3 M sodium hydroxide solution at 20 °C. The stirring speed was fixed at 500 rpm.
2.4 Experimental Parameters Using Sodium Carbonate and Sodium Bicarbobate
Sodium carbonate and sodium bicarbonate were used as leaching reagents. The effect of time was examined by reacting 5 ± 0.0005 g of EAFD with 250 ml of 2 M sodium bicarbonate and sodium carbonate at 70 °C. The stirring speed was fixed at 500 rpm. Samples of 2 ml were withdrawn at the following time periods: 5, 10, 20, 30, and 45 min and 1, 2, 3, and 4 h.
3 Results and Discussion
3.1 Characterization of Jordanian EAFD
The chemical composition of the EAFD depends mainly on the quality of steel scrap processed and the type of steel being produced. Table 1 lists the chemical composition of the used Jordanian EAFD. The major components of the dust are Zn and Fe, while minor components include Ca, Pb, Na, Si, K, and Mn.
The XRD pattern analysis for the as-received Jordanian EAFD revealed that Jordanian EAFD consists mainly of zincite, franklinite and magnetite. Salts such as halite and sylvite exist in substantial amount. Zinc exists in the form of zinc oxide (zincite (ZnO)) and zinc ferrite (franklinite (ZnFe2O4)), whereas iron exists in the form of franklinite, magnetite, and hematite.
3.2 Results of Alkaline Leaching by AAS
3.2.1 Feasibility of Sodium Carbonate/Bicarbonate Leaching
We studied the feasibility of using sodium bicarbonate and sodium carbonate as leaching reagent for Zn extraction. The results were compared with that found using NaOH at the same conditions. Figure 2 shows zinc recovery using all leaching reagents with a concentration of 2 M at 70 °C and using 5 g of EAFD. NaOH is the best reagent—after 3 h, zinc recovery increased to 56.6%, but in the case of using Na2CO3 and NaHCO3, the recovery increased only to 2.6 and 4.5%, respectively.
The effect of different leaching reagents plays an important role in the process of extracting zinc due to the pH of the solution; it affects Zn solubility in solutions. The pH values for the prepared solutions were 8.3, 8.2, and 12.55 for NaHCO3, Na2CO3, and NaOH, respectively. Zinc oxide is insoluble in water when the pH is fairly neutral and its solubility increases at pH above 11 (Lenntech 2017), which explains the higher zinc recovery using NaOH as a solvent. It was difficult to prepare higher concentrations of NaHCO3 and Na2CO3 solutions due to their solubility limits in water (i.e., supersaturating solution). Thus, NaHCO3 and Na2CO3 were not effective for Zn extraction under the studied conditions. Hence, the optimum parameters for Zn extraction were studied only using NaOH.
3.2.2 Effect of NaOH Concentration
We studied the effect of NaOH concentration on zinc extraction from EAFD at 20 °C. The NaOH solution concentrations were 1, 3, 5, 7, and 9 M. The results of Zn recovery as a function of time and concentration is shown in Fig. 3. After 20 min of leaching, more than 90% of the recoverable zinc was dissolved for all investigated solvent concentrations. The extraction process was rapid due to the high solubility of ZnO and the small size of EFAD particles (Nagib and Inoue 2000). The recovery of Zn using different NaOH concentrations at 20 °C is shown in Fig. 4. Zinc extraction increased with increasing NaOH concentration up to 7 M. Zn recovery increased from 17.1% at 1 M to 86.3% at 7 M NaOH. These results were in a good agreement with Dutra et al. (2006) who reported that NaOH concentration is directly proportional to zinc recovery. But as the NaOH concentration increased to 9 M, the recovery of Zn decreased to 72.8% due to the very high viscosity of the solvent which decreased the solubility of Zn. These results were in a good agreement with Orhan (2005).
3.2.3 Effect of Temperature
Temperature had an obvious effect on the extraction process, since the solubility of Zn increased with increasing temperature (Fig. 4). When 3 M NaOH solvent was used, Zn recoveries were 61.1, 71.6, and 80.6% at 20, 40, and 60 °C, respectively. While using 6 M NaOH, Zn recoveries were 76.7, 83.0, and 92.9% at 20, 40, and 60 °C, respectively. Thus, a maximum Zn recovery of 92.9% was obtained at 60 °C using 6 M NaOH.
Zn recovery relation with the leaching temperature for 3 and 6 M NaOH concentrations is shown in Fig. 5. The data were fitted linearly with high correlation coefficients, indicating linear increase in Zn recovery as the temperature increases in the range of studied temperatures. These results were in agreement with Dutra et al. (2006) and Mordogan et al. (1999) who studied the influence of temperature on dissolving zinc present in EAFD using different alkaline leaching techniques, and who reported that the solubility of Zn increased with increasing temperature.
The activation energy for the dissolution of Zn from the EAFD was calculated assuming that the dust particle containing zinc is spherical and shrinks uniformly during the dissolution process. For this purpose, the following kinetic equation was used:
where t is time (min), k is the constant containing rate constant (min−1), and xZn is fraction of Zn reacted.
The relation between k and the activation energy is governed by Arrhenius equation:
where A is the frequency factor, E is the activation energy (J/mol), R is the gas constant (J/(K mol)), and T is the reaction temperature (K).
The apparent activation energy for the dissolution of Zn in NaOH where found to be 14.6 and 15.4 kJ/mol using 3 and 6 M NaOH, respectively; the dissolution reaction is chemically controlled. These values are in close agreement with those reported by Li et al. (2010) who found that the activation energy of zinc dissolution from EAFD by NaOH solution is 15.7 kJ/mol.
3.2.4 Effect of Solid-to-Liquid Ratio
The effect of SLR on Zn extraction rate using 3 M NaOH concentration at 20 °C and after 3 h leaching is shown in Fig. 6. Zn removal was very fast at the early stages of leaching and then increased steadily. As the SLR decreased, the rate of zinc leaching increased. Zn recoveries were 59.2 and 21.9% at SLR of 20 and 120 mg/ml, respectively. Orhan reported that zinc recovery is inversely proportional to SLR (Orhan 2005).
3.3 Analysis of Leaching Residue
3.3.1 XRF Analysis
We analyzed the chemical composition of the collected residues using XRF; the main components of the collected residues were Fe and Zn. The chemical composition of the collected residues was compared with the raw EAFD by using an E-factor (i.e., the ratio of the element in the residue to that in the original EAFD) as shown in Fig. 7. Na and K were depleted completely due to the complete dissolution of potassium and sodium chlorides. For Zn and Pb, the E-factors were around 0.5–0.6 and 0.1–0.25, respectively. Depletion was noticed also for Si with an E-factor range of 0.6–0.8. Fe, Ca, and Mn were enriched in the residues (E-factor > 1). Zn and Pb were selectively dissolved in sodium hydroxide; rejecting iron in the residue. According to Jha et al. (2001), Youcai and Stanforth (2000), Xia and Picklesi (2000), and Orhan (2005), the following reactions are involved during leaching process using caustic soda:
Zn content in the residue decreased as the temperature and molarity of the leaching reagent increased. The XRF analysis results of the residues were in a good agreement with those results obtained by using AAS analysis for the leaching solution.
3.3.2 XRD Analysis
We carried out phase identification for leaching residue (dried at 105 °C) using XRD analysis. The XRD patterns for the raw EAFD and for the residue were compared as shown in Fig. 8. All sylvite and halite were dissolved completely. Whereas, some zincite still exist as evident from the presence of its major peaks at 31.72° and 36.20°. When leaching was carried out at 20 °C and 1 M NaOH concentration, two new major peaks appeared at 14.28° (peak 1) and at 28.70° (peak 2). These two peaks could be related to some calcium silicate. At more harsh leaching conditions (i.e., 3 and 6 M NaOH and 60 °C), zincite peaks disappeared completely, whereas, peaks related to magnetite/franklinite still exist. It should be pointed out that both franklinite and magnetite has almost similar XRD pattern. Therefore, it is very difficult to differentiate between them. However, the reduced intensity of their peaks may suggest a partial leaching of zinc from franklinite, although it is reported that zinc ferrite is not reactive at such conditions (Elgersma et al. 1992; Youcai and Stanforth 2000). Additionally, it is known that franklinite is considered one of the most refractory zinc minerals in alkaline solutions. To increase Zn recovery, decomposition of ferrites is required (Xia and Picklesi 2000; Youcai and Stanforth 2000). Furthermore, harsher leaching conditions (6 M, 60 °C) revealed new phases such as calcium iron titanium oxide hydroxide that is existed in the raw dust, but hydrated and became concentrated in the leaching residue.
The XRD patterns for the load effect residues with 3 M NaOH and 20 °C are shown in Fig. 9. They confirm those results obtained from the AAS analysis; traces of zincite still exist at 20 mg/ml (5 g of EAFD) loading, while at 80 and 120 mg/ml load (20 and 30 g), there was a low dissolution of zincite as evident from the presence of its peaks. In addition, sylvite peaks (28.30° and 40.48°) as well as halite peaks disappear at all studied loading while new peaks at 14.28° and 28.7° appeared at SRL of 80 and 120 mg/ml.
4 Conclusions
Zn extraction from Jordanian EAFD using alkaline solvents (NaOH, NaHCO2, and Na2CO3) was investigated successfully in this study. Experiments were performed under different conditions, such as NaOH concentration (1, 3, 5, 7, and 9 M), contact time from 5 min to 3 h, temperature (20, 40, and 60 °C), and SLR (20–120 mg/l). Using 2 M and at 70 °C, NaHCO2 and Na2CO3 solvents were ineffective for Zn extraction due to their low recoveries (2.6% for NaHCO2 and 4.5% for Na2CO3) compared with NaOH that was very efficient with a recovery of 56.6%.
During leaching using NaOH, the extraction process was fast; 30 min was enough to reach the optimum Zn recovery at each condition. As NaOH concentration increased from 1 to 7 M, Zn recovery increased from 17.1% using 1 M NaOH to 86.3% using 7 M NaOH at 20 °C and 3 h leaching. As the temperature increased Zn removal increased; using 3 M NaOH and 3 h leaching, Zn recoveries were 61.1, 71.6, and 80.6% at 20, 40, and 60 °C, respectively. When NaOH concentration was 6 M, Zn recoveries after 3 h were 76.7, 83.0, and 92.9% at 20, 40, and 60 °C, respectively. Zn recovery was inversely affected by SLR. At 20 °C and using 3 M NaOH, it decreased from 59.2 to 21.9% as SLR load increased from 20 to 120 mg/ml. The optimum conditions for zinc extraction from the Jordanian EAFD via alkaline leaching technique were 6 M NaOH, SLR of 20 mg/ml, 60 °C and 3 h leaching time with Zn recovery of 92.9%. Acidic solutions (e.g., HCl, H2SO4, and HNO3) could also be used for Zn extraction as a future work.
References
Abkhoshk, E., Jorjani, E., Al-Harahsheh, M. S., Rashchi, F., & Naazeri, M. (2014). Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy, 149, 153–167.
Ahmed, O. H., Altarawneh, M., Al-Harahsheh, M., Jiang, Z.-T., & Dlugogorski, B. Z. (2017). Recycling of zincite (ZnO) via uptake of hydrogen halides. Physical Chemistry Chemical Physics.
Al-Harahsheh, M., & Kingman, S. W. (2004). Microwave-assisted leaching—a review. Hydrometallurgy, 73, 189–203.
Al-Harahsheh, M., Al-Otoom, A., Al-Makhadmah, L., Hamilton, I. E., Kingman, S., Al-Asheh, S., & Hararah, M. (2015). Pyrolysis of poly(vinyl chloride) and-electric arc furnacedust mixtures. Journal of Hazardous Materials, 299, 425–436.
de Araujo, J. A., & Schalch, V. (2014). Recycling of electric arc furnace (EAF) dust for use in steel making process. Journal of Materials Research and Technology, 3, 274–279.
Barrett, E. C., Nenniger, E. H., & Dziewinski, J. (1992). A hydrometallurgical process to treat carbon steel electric arc furnace dust. Hydrometallurgy, 30, 59–68.
Caravaca, C., Cobo, A., & Alguacil, F. J. (1994). Considerations about the recycling of EAF flue dusts as source for the recovery of valuable metals by hydrometallurgical processes. Resources, Conservation and Recycling, 10, 35–41.
Dreisinger, D. (1990). A challenge for the 1990s: the hydrometallurgical treatment of wastes and residues. JOM, 42, 27–27.
Dutra, A. J. B., Paiva, P. R. P., & Tavares, L. M. (2006). Alkaline leaching of zinc from electric arc furnace steel dust. Minerals Engineering, 19, 478–485.
Elgersma, F., Kamst, G. F., Witkamp, G. J., & van Rosmalen, G. M. (1992). Acidic dissolution of zinc ferrite. Hydrometallurgy, 29, 173–189.
Frenay, J., Ferlay, S. & Hissel, J.: 1986, Zinc and lead recovery from EAF dusts by caustic soda process. In: Electric furnace proceedings, treatment options for carbon steel electric arc furnace dust, Iron Steel Society, pp. 171–175.
Havlík, T., Vidor e Souza, B., Bernardes, A. M., Schneider, I. A., & Miškufová, A. (2006). Hydrometallurgical processing of carbon steel EAF dust. Journal of Hazardous Materials, 135, 311–318.
Jha, M. K., Kumar, V., & Singh, R. J. (2001). Review of hydrometallurgical recovery of zinc from industrial wastes. Resources, Conservation and Recycling, 33, 1–22.
Kavouras, P., Kehagias, T., Tsilika, I., Kaimakamis, G., Chrissafis, K., Kokkou, S., Papadopoulos, D., & Karakostas, T. (2007). Glass-ceramic materials from electric arc furnace dust. Journal of Hazardous Materials, 139, 424–429.
Kekki, A., Aromaa, J., & Forcen, O. (2012). Leaching characteristics of EAF and AOF stainless steel production dusts. Physicochemical Problems of Mineral Processing, 48, 599–606.
Kelebek, S., Yörük, S., & Davis, B. (2004). Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Minerals Engineering, 17, 285–291.
Leclerc, N., Meux, E., & Lecuire, J.-M. (2003). Hydrometallurgical extraction of zinc from zinc ferrites. Hydrometallurgy, 70, 175–183.
Lenntech, B. V.: 2017, Zinc and water: reaction mechanisms, environmental impact and health effects. Distributieweg 3, EG Delfgauw: Lenntech.
Li, H.-X., Wang, Y., & Cang, D.-Q. (2010). Zinc leaching from electric arc furnace dust in alkaline medium. Journal of Central South University of Technology, 17, 967–971.
Mordogan, H., Cicek, T., & Isik, A. (1999). Caustic soda leach of electric arc furnace dust. J. Eng. Environ. Sci., 23, 199–207.
Nagib, S., & Inoue, K. (2000). Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy, 56, 269–292.
Niubo, M., Fernandez, A. I., Chimenos, J. M., & Haurie, L. (2009). A possible recycling method for high grade steels EAFD in polymer composites. Journal of Hazardous Materials, 171, 1139–1144.
Oishi, T., Yaguchi, M., Koyama, K., Tanaka, M., & Lee, J. C. (2008). Hydrometallurgical process for the recycling of copper using anodic oxidation of cuprous ammine complexes and flow-through electrolysis. Electrochimica Acta, 53, 2585–2592.
Orhan, G. (2005). Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy, 78, 236–245.
Oustadakis, P., Tsakiridis, P. E., Katsiapi, A., & Agatzini-Leonardou, S. (2010). Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I. Characterization and leaching by diluted sulphuric acid. Journal of Hazardous Materials, 179, 1–7.
Sofilić, T., Rastovčan-Mioč, A., Cerjan-Stefanović, Š., Novosel-Radović, V., & Jenko, M. (2004). Characterization of steel mill electric-arc furnace dust. Journal of Hazardous Materials, 109, 59–70.
Stegemann, J. A., Roy, A., Caldwell, R. J., Schilling, P. J., & Tittsworth, R. (2000). Understanding environmental leachability of electric arc furnace dust. Journal of Environmental Engineering, 126, 112–120.
Suetens, T., Klaasen, B., Van Acker, K., & Blanpain, B. (2014). Comparison of electric arc furnace dust treatment technologies using exergy efficiency. Journal of Cleaner Production, 65, 152–167.
Vieira, C. M. F., Sanchez, R., Monteiro, S. N., Lalla, N., & Quaranta, N. (2013). Recycling of electric arc furnace dust into red ceramic. Journal of Materials Research and Technology, 2, 88–92.
Xia, D. K., & Picklesi, C. A. (2000). Microwave caustic leaching of electric arc furnace dust. Minerals Engineering, 13, 79–94.
Youcai, Z., & Stanforth, R. (2000). Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium. Journal of Hazardous Materials, 80, 223–240.
Zhang, Y., Li, X., Pan, L., Wei, Y., & Liang, X. (2010). Effect of mechanical activation on the kinetics of extracting indium from indium-bearing zinc ferrite. Hydrometallurgy, 102, 95–100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Makhadmeh, L.A., Batiha, M.A., Al-Harahsheh, M.S. et al. The Effectiveness of Zn Leaching from EAFD Using Caustic Soda. Water Air Soil Pollut 229, 33 (2018). https://doi.org/10.1007/s11270-018-3694-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3694-4