Abstract
Carbon-rich biomass products from thermal pyrolysis have been considered as an appropriate alternative for the remediation of contaminated lands. However, the impacts of the physico-chemical properties of biochar on adsorption, desorption, and leaching processes are not fully understood. In this study, adsorption, desorption, and leaching of fomesafen in a soil amended with six biochars were investigated. The highest fomesafen adsorption coefficient (kfads = 20.67) was observed when 2% of hardwood biochar (B4) was added onto the soil due to its highest specific surface area (SSA) (331.70 m2/g) and lowest dissolved organic carbon (DOC) content (0.43%) relative to the other tested biochars. By contrast, the lowest adsorption coefficient (kfads = 16.64) was observed in the soil amended with 2% rice straw biochar (B1) with the lowest SSA (63.10 m2/g) and highest DOC content (3.67%). Nevertheless, during desorption process, the lowest coefficients were observed in the soil amended with softwood (B2) and walnut (B5) biochars, which possessed higher SSA and lower pH than B1, most likely due to their lower micro-pore volume/total pore volume ratios (MPV/TPV). Moreover, fomesafen adsorption in the soils amended with B2 and B5 was highly reversible. The outcomes of the leaching experiment also showed that fomesafen leaching from the soil column followed the same trend as desorption. These results suggested that although the adsorption capacity of biochar is most likely controlled by SSA and DOC, desorption and leaching processes are mainly affected by MPV/TPV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides as the most popular applied chemicals are still considered as a key tool for higher crop yields, especially in less-developed countries (Jin et al. 2014). Therefore, environmental contamination caused by pesticides overuse has been raising concerns about long-term side effects of pesticides on non-target living organisms in soil and groundwater resources (Arivalagan et al. 2014; Liu et al. 2016). Adsorption-desorption of pesticides in soil environment is probably the most important process affecting their mobility, bioavailability, and degradation because the soil is usually the first medium to receive the sprayed pesticides (Ahmadi et al. 2016). Furthermore, adsorption-desorption behaviors could be more important for specific groups of pesticides like herbicides, because the adsorption rate of herbicides directly determine negative effects of the adsorbed herbicide on succession crops (Liu et al. 2016).
Fomesafen (5-[2-chloro-4-(trifluoromethyl) phenoxy]-N-[methylsul-fonyl]-2-nitrobenzamide) is one of the widely used diphenyl ether herbicides after its introduction. It is believed that fomesafen is among the most effective herbicide against broad-leaf weeds, especially in soybean fields in China (Guo et al. 2003). Although one of the advantages of this herbicide is acceptable herbicidal activity at low concentrations, overuse of fomesafen is increasing the risk of water pollution, especially when it takes into account that fomesafen is categorized as a pesticide with high leachability and medium runoff potential (Khorram et al. 2015). Moreover, since fomesafen is considered as a relatively persistent herbicide for its long soil half-life (60–240 days) (Guo et al. 2003), its successive application possibly raises the risk of irreversible injuries to non-target living organisms.
Biochar is a carbon-rich by-product obtained from the heated biomass in the absence of oxygen or in an environment with limited oxygen in a process known as “pyrolysis.” In recent years, increasing attention has been paid to biochar due to its multi-functional capabilities, such as carbon sequestration, soil fertilization, and microbial growth stimulation (Khorram et al. 2017; Sohi 2012). Moreover, biochar performs well as a soil amendment due to its high-cation exchange capacity, SSA, negative surface charge, and surface charge density (Pignatello et al. 2006), which enhance the adsorption of organic contaminants (Khorram et al. 2016; Martin et al. 2012; Li et al. 2013), decrease contaminants bioavailability (Khorram et al. 2017), and improve soil quality (Awad et al. 2012).
Physical and chemical properties of biochar, which affect their adsorption capacities, are mainly influenced by two factors: (1) feedstock composition and (2) pyrolytic temperature (Zimmerman et al. 2011). When subjected to high-pyrolysis temperatures (500–700 °C), wheat biochars are well carbonized and have relatively high-surface areas and low-oxygen contents (Pignatello et al. 2006). Brewer et al. (2011) reported that biochars from switchgrass and corn stover have lower aromatic carbon and higher ash contents than biochars produced from woody materials. Exposing biochars to steam and CO2 at temperatures higher than 700 °C also increases SSA and porosity of the biochar, which improves the adsorption capacity of biochars (Sun and Lu 2014). There are extensive reports in which higher adsorption capacity of biochars has been attributed to higher SSA, carbon content, and greater binding capacity (Cabrera et al. 2011; Khorram et al. 2015, 2016). However, the effects of TPV and MPV on desorption behavior and adsorption reversibility have received limited attention (Dechene et al. 2014), despite the fact that environmental fates and impacts of contaminants are strongly influenced by their desorption behavior as well (Kuppusamy et al. 2016). Therefore, the objective of this study was to determine the effects of biochar physico-chemical properties on adsorption, desorption, and leaching behaviors of fomesafen. In addition, we evaluated the efficacy of tested biochars for reducing the potential environmental pollution associated with pesticide use through increasing pesticides sorption and reducing their mobility.
2 Material and Methods
2.1 Chemicals
Analytical-grade fomesafen (99.5%) and HPLC gradient-grade methanol were purchased from Dr. Ehrenstorfer; GmbH and all other chemicals and solvents were analytical grade. Deionized water was prepared using a lab water purification system.
2.2 Soil and Biochars
The soil samples used in this study were collected at a depth of 0–15 cm from Xixi campus (S) at Zhejiang University in Hangzhou, China. Air-dried soil passed through a 2-mm sieve and was stored at room temperature prior to use. The physico-chemical properties of the collected soil, measured by previously described method (Liu 1996), were as follows: sand (15.25%), silt (73.21%), clay (11.55%), pH (7.10), organic carbon (OC) (2.11%), water holding capacity (WHC) (31%), and cation exchange capacity (CEC) (12.42 cmol kg−1).
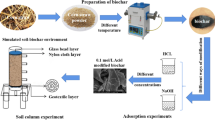
Rice straw (B1), softwood (B2), hardwood (B4), and nut shell (B5) biochars were obtained from the Environmental Science and Engineering Department at Zhejiang University, Hangzhou, China. Coconut shell (B3) and bamboo (B6) biochars were provided by Prof. Hailong Wang, Zhejiang Agriculture and Forestry University, Hangzhou, China. Table 1 summarizes the feedstocks, production processes, and physico-chemical properties of studied biochars. The moisture content was determined by drying an aliquot of the material at 105 °C for 48 h. pH was measured in 10 mmol L−1 CaCl2 solution (solid/solution = 1:2.5 (w/v)) using a glass electrode of a corning pH 10 portable pH meter (Acton, MA). The pH meter was calibrated with standard pH 4 and pH 7 buffers. Dissolved organic carbon (DOC) was determined using the method explained by Venegas et al. (2015). Briefly, carbonate content of biochar was removed with 2 mol L−1 HCl; 50 mL of a 0.01 mol L−1 CaCl2 solution was added to 2 g of tested biochars, and the resulting suspensions were shaken vigorously for 10 min. Then, the supernatants were filtered, acidified to pH 2, and finally, DOC content was measured with TOC analyzer Shimatzu TOC-50000. The particle size distribution, SSA, TPV, and MPV were all characterized according to Yu et al. (2006). Before use, biochars were soaked with deionized water (biochar/water = 1:50 (g ml−1)), and the mixture was stirred at 250 rpm for approximately 10 min at room temperature. Then, the washed biochars were recovered by a centrifuge and dried at 105 °C for 24 h (Khorram et al. 2015). Soil was amended with biochars by thoroughly mixing 1 kg of the original soil with 5, 10, or 20 g of each biochar to obtain soil amended with 0.5, 1, and 2% (w/w) biochar, respectively.
2.3 Adsorption and Desorption Test
Adsorption experiments were performed according to OECD guidelines (OECD 106 2000). Samples of air-dried soil (5.0 g) amended with six biochars at three levels (0.5, 1, and 2% of soil weight) were placed in 50-mL polypropylene centrifuge tubes and vortexed to ensure the homogeneity. Appropriate amounts of a 1000-mg L−1 fomesafen stock solution were added separately to a 1.1-g L−1 CaCl2 solution to produce five different initial aqueous solution concentrations. Then, 10 mL of the solutions containing five initial concentrations of the herbicide (0.5, 1, 2, 4, and 8 mg L−1) were added to each tube. Samples were shaken horizontally at 25 ± 1 °C and 150 rpm for 36 h before centrifuging at 6000 g rpm for 10 min (Beckman Coulter Allegra® 25R centrifuge, USA) in order to separate the sediment and aqueous phases. Previously, it was determined that equilibrium was reached in < 36 h with no measurable degradation during this period (Khorram et al. 2015). The supernatant was filtered through a 0.2-μm nylon syringe filter, and the concentration of fomesafen was analyzed by high-performance liquid chromatography (HPLC) and diode-array detector (DAD). The amount of fomesafen adsorbed by the soil was calculated from the difference between the initial and final fomesafen concentrations in solution.
Herbicide desorption was performed immediately after the adsorption experiment using successive 36-h equilibrations of soil with the same amount of 1.1 g L−1 CaCl2 without fomesafen. The suspension was collected after centrifugation to analyze the fomesafen concentration in the aqueous phase, as described above.
2.4 Leaching Test
Leaching experiments (OECD 312 2004) were carried out using 45 cm (length) × 8 cm (internal diameter) glass columns with a spongy layer at the bottom. The columns were packed with 1300 g of air-dried soil to a height of 35 cm, over-saturated with 1.1 g L−1 CaCl2, and allowed to drain freely for 24 h. Fomesafen was applied by adding 10 mL of 650 mg L−1 fomesafen stock solution to top of the columns to achieve an initial concentration of 5 mg kg−1 in the soil body. Then, after 24 h, the columns were thoroughly washed by continuously pumping 1800 mL of 1.1 g L−1 CaCl2 at a rate of 25 mL h−1 using a peristaltic pump. Fractions of the collected leachate were sampled at 3-h intervals, filtered through a syringe filter, and immediately analyzed by HPLC.
After fomesafen leaching, the columns were cut into five segments (7 cm each) and the soil contained in each segment was dried at room temperature. Soil samples of 5 g were taken from each segment, ultrasonically extracted for 2 h (frequency 25–40 Hz; Khorram et al. 2015) and shaken in a rotatory shaker overnight in 50 mL of a methanol/hydrochloric acid mixture 95:5 (v/v). Then, the filtered solution from the mixture was extracted three times using 50 mL CH2Cl2 and dehydrated by anhydrous sodium sulfate. Subsequently, the collected filtrate was concentrated on a rotary evaporator (37 °C), dried under a gentle stream of nitrogen, and dissolved in 10 mL of HPLC-grade methanol.
2.5 HPLC Analysis
The fomesafen concentration was determined using a 1200 series HPLC (Agilent Technologies, USA) equipped with a diode array detector (DAD). A Hewlett Packard stainless steel analytical column (Eclipse XDB-C18, 15 cm × 4.6 mm × 5 μm) was used for chromatographic separation, with a mobile phase of acetonitrile and 0.1% phosphoric acid (65:35, v/v) at a flow rate of 1 mL min−1. The extract (10 μL) was injected into the HPLC system and recorded at 290 nm.
2.6 Data Analysis
Adsorption and desorption data were fit using the Freundlich equation, Cs = Kf Ce1/nf, where Cs (mg kg−1) is the amount of fomesafen adsorbed; Ce (mg L−1) is the equilibrium concentration in solution; and Kf and 1/nf are empirical indicators for the adsorption capacity and the amount of linearity between adsorbed pesticide and solution concentrations, respectively. Since Kf data can be difficult to interpret in cases of different 1/nf values, the value of Kd-4 was determined as Cs Ce −1, where Ce is equal to 4 mg L−1, and was used to compare the Kf values with Kd. Kd-4 was used in the adsorption process because its concentration was close to the conventional recommended field application dose for spring soybean crops in China (375 g active ingredient ha−1 assuming a soil bulk density of 1 g cm−1 and an effective soil depth of 1 cm; Wu et al. 2014). The hysteresis coefficient, calculated as H = (1/nfdes)/(1/nfads) (Cabrera et al. 2011, 2014; Khorram et al. 2015), also provides information regarding the reversibility of the adsorption process.
All of the data were presented as the means of triplicate samples from three independently performed experiments. Statistical significance was determined using ANOVA and a t test, and a p value ≤ 0.05 was considered significant.
3 Results and Discussion
3.1 Validation of Fomesafen Extraction
The recoveries of fomesafen from the soil and water phases ranged from 87.4–98.8% and 91.3–102.7%, respectively, with a relative standard deviation (RSD) of ≤ 4.27%. Detection limit for fomesafen was 0.02 mg kg−1.
3.2 Effects of Biochars on Adsorption Capacity
Adsorption isotherms of fomesafen on soil with and without biochars are shown in Fig. 1. The adsorption isotherms for unamended and biochar-amended soil were all nonlinear, L-shaped, and fit the Freundlich equation (R2 > 0.95) (Fig. 1a, Table 2).
Considering the effects of biochar application rate on adsorption capacity, fomesafen adsorption significantly enhanced from 0.69 in unamended soil to 2.37–2.72, 7.52–9.23, and 16.64–20.67 in the soil amended with biochars at 0.5, 1, and 2%, respectively (Table 2). In addition, the enhanced adsorption was positively correlated with the amount of biochar added (r > 0.97**, p < 0.01) for the tested biochars. Similarly, Kd-4, which was 0.52 in the control soil, increased progressively to 28.18–134.95 when the soil was amended with 2% of the biochars (Table 2). Since Kd (distribution coefficient) is calculated by the content of fomesafen adsorbed into the soil divided by the mass concentration of fomesafen in aqueous phase, progressive increase of this value clearly shows that the biochar amendment increased the immobilization of fomesafen in soil profile through the adherence of pesticide molecules into the biochar particles.
Regarding the influences of the biochar properties on the adsorption capacity of fomesafen, as shown in Table 2, the highest fomesafen adsorption coefficient (kfads) and distribution coefficient (Kd-4) were observed when B4 with the highest SSA (331.7 m2 g−1) and lowest DOC (0.43%) (Table 1) was added into the soil. In this case, kfads and Kd-4 increased from 0.69 and 0.52 in the control to 20.67 and 134.95 in the soil amended with B4 biochar at 2%, respectively. Furthermore, the lowest adsorption index (1/nf = 0.60) occurred in this treatment. By contrast, the addition of 2% rice straw biochar (B1), which showed the lowest SSA (63.1 m2 g−1) and highest DOC (3.67%) among the tested biochars, resulted in the lowest fomesafen adsorption capacity (kfads = 16.64; Kd-4 = 28.18) and highest adsorption index (1/nf = 0.74) among 2% amended biochar treatments (Tables 1 and 2). These results suggested that the order of adsorption for biochars decreased as follows: B4 > B6 > B3 > B5 > B2 > B1. This decreasing order was the same as that observed for SSA and DOC in biochars. In addition, this result implies that the enhancement of fomesafen adsorption was positively correlated with biochar SSA (Y = 166.43X–1190.64; R2 = 0.96) and negatively correlated with biochar DOC (Y = − 0.82X + 17.45; R2 = 0.97). SEM images (Fig. 2) also presented that B4 with the highest adsorption capacity was highly macroporous followed by B3 and B6. On the other hand, B5, B2, and B1 showed highly smooth appearance. Nevertheless, it is noteworthy that the appearance and pore structure in SEM probably do not show the overall SSA of biochars since B5, B2, and B1 with glossy surface showed totally different SSA.
3.3 Effects of Biochars on Desorption Capacity
The desorption isotherms of fomesafen from unamended and biochar-amended soils were modeled using the Freundlich equation in all cases (R2 > 0.93) (Fig. 1b, Table 3).
Biochar amendment reduced fomesafen desorption significantly (Fig. 1b), which was conversely correlated with biochar amendment rate (r > 0.96*, p < 0.05). Furthermore, the desorption index (1/nf) values progressively decreased as the rate of added biochar increased (Table 3).
Regarding the effects of biochar properties on fomesafen desorption, the highest fomesafen desorption coefficient (kfdes) was obtained when B4 was used as an amendment (Table 4). It can be observed that kfdes increased from 0.42 for unamended soil to 5.10, 12.13, and 30.73 when B4 was applied at rates of 0.5, 1, and 2%, respectively. However, the lowest desorption coefficients were obtained when soil was amended with B2 and B5 (Table 3) with higher SSA and lower DOC than B1 (Table 1). The desorption coefficients for the soil amended with 2% B2 and B5 were 19.73 and 19.24, respectively (Table 3). Furthermore, the hysteresis coefficient (H = (1/nf des )/(1/nf ads )), which decreased from unamended soils to the soils amended with 2% B1, B3, B4, and B6, increased when B2 and B5 were used as soil amendments. In these cases, the H value increased from 0.83 in the unamended soil to 0.86 and 0.92 in the soils amended with 2% B2 and B5, respectively. The higher H value in these cases was associated with increase in fomesafen desorption, which were higher than expected based on the adsorption capacity (Tatarkova et al. 2013). According to SEM images (Fig. 2), the glossy appearance of B5, B2, and B1 with no visible macropores most likely resulted in easier detachment of fomesafen from biochar surface. In addition, higher SSA of B5 and B2 than B1 provided more adsorption sites for weak binds of fomesafen molecules during adsorption and consequently higher probability for the detachment of higher percentage of adsorbed fomesafen. These results showed that the desorption coefficients of fomesafen in the tested biochars followed the same trend as for MPV/TPV (B4 > B6 > B3 > B1 > B2 > B5). Furthermore, a strong positive correlation was observed between the desorption enhancement of fomesafen with MPV/TPV (Y = 0.041X–0.77; R2 = 0.83).
3.4 Effects of Biochar Amendment on Fomesafen Leaching in Soil
Leaching experiments were also conducted with unamended and biochar amended soil to clarify the possible effects of biochar amendment on fomesafen mobility in soil columns (Fig. 3). The leaching and fomesafen adsorbed ratios onto the soil are summarized in Table 4.
Although approximately 84% of the applied fomesafen was leached from the column containing unamended biochar, biochar amendment significantly decreased the amount of fomesafen leaching in all cases. As shown in Fig. 3a, the addition of 0.5% biochar decreased the total amount of leached fomesafen to 66.25–59.5% for the soils amended with different biochars (Table 4). Nevertheless, the leaching trend of fomesafen from the soil amended with 0.5% biochar followed a similar trend to that of unamended soil column. By contrast, increasing the amount of biochar introduced into the column resulted in significantly lower fomesafen leaching from the columns (Fig. 3) because the retention volumes (the water volume required to elute a substance from the soil column) were 645–870 and 495–685 mL in the columns containing 1 and 2% biochar amended soils, respectively. In addition, the maximum leaching rates of 15.6% in the unamended soil decreased remarkably to 7.5%–9.2% and 2.6%–4.8%, with total leaching rates of 34.5%–45.15% and 12.4%–18.7%, in the soils amended with 1% and 2% biochar, respectively. Total amount of fomesafen retained in the columns significantly increased from 13.6% in unamended soil to 79.45–85.32% in the column filled with the 2% biochar amended soils (Table 4), and more than 60% of the herbicide was retained in top 14 cm of the columns (Fig. 3b), when 1 and 2% biochar were added to the soil.
The lowest fomesafen leaching ratio among the columns was observed when the soil was amended with B4 (Table 4). In this case, fomesafen leaching ratio of 84% in unamended soil decreased to 55.82, 34.51, and 12.43% when the soil was amended with 0.5, 1, and 2% B4, respectively (Table 4). Consequently, the highest amount of fomesafen immobilization occurred under this treatment as well. However, when B2 and B5 were added to the soil columns, the highest total leached ratio (18.72–66.25% and 17.64–63.5%) and lowest retained fomesafen ratio (30.73–79.45% and 33.52–80.45%) were obtained (Table 4). These results indicated fomesafen leaching from the soil columns in the presence of different biochars followed the same trend as desorption: B5 > B2 > B1 > B3 > B6 > B4. Therefore, as it appears, fomesafen leaching is most likely influenced by desorption behavior rather than adsorption.
4 Discussion
4.1 Effects of Biochars on Adsorption Capacity
Higher pesticide adsorption by biochar amendment has been previously reported by several authors and has been attributed to high SSA and greater microporous structures of biochars (Li et al. 2013; Si et al. 2011; Tatarkova et al. 2013). Si et al. (2011) indicated that increasing the adsorption coefficient of the isoproturon herbicide from 0.9–1.8 to 11.2–17.1 after the introduction of 10, 30, and 50 g kg−1 of charcoal onto three soils is due to high-carbon content and SSA of the tested charcoal compared with the soil organic matter. Similarly, the adsorption of MCPA (4-chloro-2- methylphenoxy acetic acid) was three times higher in an agricultural soil amended with 1% wheat straw biochar than in an unamended soil due to microporous structure and high SSA of the added biochar (Tatarkova et al. 2013).
The importance of biochar physical and chemical properties and their effects on pesticide adsorption has been addressed in previous studies (Cabrera et al. 2011, 2014; Srinivasan and Sarmah 2015). Similar to our results, Srinivasan and Sarmah (2015) showed that the addition of biochars with greater macroporous structures increased the adsorption capacity of biochar amended more since micro- and macropores are the main sites for binding and entrapment of contaminants molecules. Yu et al. (2006) studied the effects of two woodchip biochars produced at different temperatures on the adsorption-desorption behavior of diuron and demonstrated that higher herbicide adsorption occurred when the tested soil was amended with biochar produced at 850 °C due to its higher surface area (566 m2 g−1) and greater micropore volume. The lower adsorption capacities of the biochars produced at 450 °C in that study were attributed to the presence of fewer micropores and also lower SSA (27 m2 g−1). Cabrera et al. (2011) reported that the adsorption of fluometuron on six tested biochars was correlated with not only biochar SSA, but also the DOC content of the biochars. In this study, the highest adsorption of the herbicide was observed in the soil amended with two wood pellet biochars possessing the highest SSA (16.2 m2 g−1), while the lowest fluometuron adsorption was seen in the soil amended with biochar from macadamia nut shells with the lowest SSA (3.29 m2 g−1) and highest DOC content (352 mg L−1). Predominant roles of SSA and DOC in pesticide adsorption have also been illustrated in studies of bentazone and aminocyclopyrachlor adsorption in soils amended with five biochars (Cabrera et al. 2014), where the highest adsorption coefficient (kfads = 0.92 mg1−1/n L1/n kg−1 for bentazone and kfads = 2.54 mg1−1/n L1/n kg−1 for aminocyclopyrachlor) was observed when the soil was amended with wood chip pellets biochar which had SSA of 17.8 m2 g−1 and a DOC content of 14 mg L−1. In both studies, the lower adsorption of the tested pesticides in the biochar-amended soils was attributed to the competition between the DOC and herbicide molecules for adsorption sites.
Although all tested biochars were produced from different feedstocks using different techniques and pyrolysis temperatures, the results confirmed that the effects of SSA and DOC on the adsorption capacity of biochar for fomesafen were significantly greater than other factors. In addition, tested biochars with higher TPV were those that possessed higher SSA, except for B6. This association may be a result of the effects of pyrolysis temperature or feedstock composition (Yu et al. 2006; Pignatello et al. 2006). However, additional studies considering other types of biochars produced at different pyrolysis temperatures should be conducted to understand what the possible effects of these factors on biochar TPV are.
4.2 Effects of Biochars on Desorption Capacity
It has been reported that biochar amendment decreases the desorption capacities of contaminants probably due to higher adsorption capacity of the biochar (Martin et al. 2012).
Regarding the effects of biochar properties on fomesafen desorption, a recent study by Eibisch et al. (2015) showed that the presence of more micropores in pyrochars than hydrochars facilitated isoproturon diffusion and binding in micropores, resulting in irreversible adsorption of a larger amount of isoproturon in the pyrochar-amended soils. Micropore occlusion and, consequently, less reversible immobilization of pyrimethanil in biochars with greater micropores were also reported by Yu et al. (2010). Similarly, Tian et al. (2010) showed that the pronounced adsorption-desorption hysteresis of isoproturon in charcoal-amended soils originates from the strong physical adsorption of isoproturon within the microporous network of technical charcoal. Physical adsorption mechanisms in small micropores mainly involve pore filling because the pore wall potentials overlap and result in stronger binding of the adsorbate due to the fact that adsorption selectivity or molecular sieve ability is relatively high in micropores and decreases with increasing pore size (Mamchenko et al. 1982). Nevertheless, Cabrera et al. (2014), who used the same index as the hysteresis coefficient (H = (1/nfdes)/(1/nfads)), indicated that lower reversible adsorption of bentazone was attributed to the competition between biochar DOC and herbicide molecules for sorption sites, as previously reported for diuron (Cox et al. 2004) and fluometuron (Cabrera et al. 2011).
To the best of our knowledge, this is the first study in which the correlations between different biochar properties and desorption capacities have been investigated. The reversible adsorption and higher desorption of fomesafen in the soils amended by B2 and B5 than in the soil amended with B1, despite the higher SSA and lower DOC, were potentially influenced by lower MPV/TPV of these two biochars. The only explanation could be that although higher SSA and lower DOC of B2 and B5 provide greater binding areas for fomesafen and DOC molecules can occupy fewer adsorptive sites on biochar particles during the adsorption process, greater fraction of weakly attached fomesafen molecules on outer biochar surface or macropore walls could be detached or displaced by bioavailable DOC during the desorption phase, most likely due to the lower MPV/TPV of these biochars.
4.3 Effects of Biochar Amendment on Fomesafen Leaching in Soil Column
In soil column study, 84% leaching of the applied fomesafen from the column containing unamended biochar indicated that fomesafen is an herbicide with relatively low adsorption and large leachability (Khorram et al. 2015) which was in agreement with previous reports, considering fomesafen as a moderately mobile herbicide (Weber 1993). Biochar amendment reduced the amount of fomesafen leaching as it was reported earlier for other contaminants (Si et al. 2011; Khorram et al. 2015; Delwiche et al. 2014; Yu et al. 2006; Pignatello et al. 2006). For example, Si et al. (2011) showed that significant decrease in isoproturon leaching form three soils amended with charcoal resulted from the larger adsorption capacity of charcoal for isoproturon due to higher organic carbon content and adsorption capacity relative to soil organic matter. Higher adsorption capacity of tested biochars in the present study with higher total carbon contents (48.7–79.3%), SSAs (63.10–331.70 m2 g−1) and TPVs (0.052–0.291 cm3 g−1) than rice hull biochar (total carbon contents, 44.85%; SSA, 10.66 m2 g−1; and TPV, 0.049 cm3 g−1) in our previous study (Khorram et al. 2015) also resulted in greater fomesafen immobilization in the soil columns.
Nevertheless, there are few studies in which the effects of macro- and micropore volume on leaching process have been investigated (Delwiche et al. 2014; Yu et al. 2006; Sander and Pignatello 2007). For instance, Delwiche et al. (2014) reported that lower atrazine leaching from the columns with homogenized soil amended by biochar was mainly due to the presence of more macropore structures, which played a significant role in entrapping and accumulating more pesticide molecules around the biochar particles. Additionally, macropore deformation during adsorption-desorption likely results in higher adsorption and lower pesticide leaching in experiments with homogenized soils (Yu et al. 2006; Sander and Pignatello 2007). However, because the soils amended by B2 and B5 with higher TPV than B1 retained less fomesafen, the main explanation for higher fomesafen leaching from B2 and B5 may be the lower MPV/TPV ratio, which results in (1) the availability of strong binding sites for lower ratio of adsorbed fomesafen molecules and (2) easier detachment of the more weakly attached fomesafen molecules from non-deformed macropores. However, the mechanisms underlying this phenomenon should be further investigated.
5 Conclusion
The results of this study showed that the addition of biochars as an agriculture soil amendment generally increases the adsorption of fomesafen and, subsequently, decreases fomesafen desorption and leaching. In addition, SSA, DOC, and MPV/TPV are the biochar properties with the main influence on adsorption-desorption behavior of pesticides. SSA and DOC are most likely the key factors affecting the adsorption process because soils amended with biochars possessing higher SSA and lower DOC showed higher adsorptions. However, during the desorption process, MPV/TPV plays a vital role since using biochars with lower MPV/TPV as an amendment showed significant adsorption reversibility. This reversibility is probably due to the presence of less suitable sites for strong and irreversible binding of pesticide molecules and, consequently, facilitate the detachment of adsorbed pesticide molecules during desorption. Moreover, the addition of the two biochars (B2 and B5) with the lowest MPV/TPV to the soil resulted in higher fomesafen leaching from the soil columns. Therefore, it is important to note that although SSA and DOC are most likely appropriate characteristics for predicting adsorption behavior of biochars, desorption process is most likely controlled by a different factor, which should be carefully considered. This information is important because desorption directly affects the leaching, degradation, and bioavailability of pesticides in soils.
References
Ahmadi, A. R., Shahbazi, S., & Diyanat, M. (2016). Efficacy of five herbicides for weed control in rain-fed lentil (Lens culinaris Medik.) Weed Technology, 30, 448–455.
Arivalagan, P., Singaraj, D., Haridass, V., & Kaliannan, T. (2014). Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecological Engineering, 71, 728–735.
Awad, Y. M., Blagodatskaya, E., Ok, Y. S., & Kuzyakov, Y. (2012). Effects of polyacrylamide, biopolymer and biochar on decomposition of soil organic matter and plants residues as determined by 14C and enzyme activities. European Journal of Soil Biology, 48, 1–10.
Brewer, C. E., Unger, R., Schmidt-Rohr, K., & Brown, R. C. (2011). Criteria to select biochars for field studies based on biochar chemical properties. Bioenergy Research, 4(4), 312–323.
Cabrera, A., Cox, L., Hermosin, M. C., Cornejo, J., & Koskinen, W. (2014). Influence of biochar amendments on the sorption-desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Science of the Total Environment, 470-471, 438–443.
Cabrera, A., Cox, L., Spokas, K. A., Celis, R., Hermosín, M. C., Cornejo, J., & Koskinen, W. C. (2011). Comparative sorption and leaching study of the herbicides fluometuron and 4-Chloro-2-methylphenoxyacetic acid (MCPA) in a soil amended with biochars and other sorbents. Journal of Agricultural and Food Chemistry, 59(23), 12550–12560.
Cox, L., Fernandes, M. C., Zsolnay, A., Hermosin, M. C., & Cornejo, J. (2004). Changes in dissolved organic carbon of soil amendments with aging: effect of pesticide adsorption behavior. Journal of Agricultural and Food Chemistry, 52(18), 5635–5642.
Dechene, A., Rosendahl, I., Laabs, V., & Amelung, W. (2014). Sorption of polar herbicides and herbicide metabolites by biochar-amended soil. Chemosphere, 109, 180–186.
Delwiche, K. B., Lehmann, J., & Walter, M. T. (2014). Atrazine leaching from biochar-amended soils. Chemosphere, 95, 346–352.
Eibisch, N., Schroll, R., FuB, R., Mikutta, R., Helfrich, W., & Flessa, H. (2015). Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere, 119, 155–162.
Guo, J. F., Zhu, J. N., Shi, J. J., & Sun, J. H. (2003). Adsorption, desorption and mobility of fomesafen in Chinese soils. Water, Air, & Soil Pollution, 148, 77–85.
Jin, X. X., Cui, N., Zhou, W., Safaei Khorram, M., Wang, D. H., & Yu, Y. L. (2014). Soil genotixicity induced by successive applications of chlorothalonil under greenhouse conditions. Environmental Toxicology and Chemistry, 33, 1043–1047.
Khorram, M. S., Lin, D., Zhang, Q., Zheng, Y., Fang, H., & Yu, Y. (2017). Effects of aging process on adsorption–desorption and bioavailability of fomesafen in an agricultural soil amended with rice hull biochar. Journal of Environmental Sciences, 56, 180–191.
Khorram, M. S., Wang, Y., Jin, X., Fang, H., & Yu, Y. (2015). Reduced mobility of fomesafen through enhanced adsorption in biochar amended soil. Environmental Toxicology and Chemistry, 34(6), 1258–1266.
Khorram, M. S., Zhang, Q., Lin, D., Zheng, Y., Fang, H., & Yu, Y. L. (2016). Biochar: a review of its impact on pesticide behavior in soil environments and its potential applications. Journal of Environmental Sciences, 44, 269–279.
Khorram, M. S., Zheng, Y., Lin, D., Zhang, Q., Fang, H., & Yu, Y. (2016). Dissipation of fomesafen in biochar-amended soil and its availability to corn (Zea mays L.) and earthworm (Eisenia fetida). Journal of Soils and Sediments, 16, 2439–2448.
Kuppusamy, S., Thavamani, P., Megharaj, M., Venkateswarlu, K., & Naidu, R. (2016). Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environment International, 87, 1–12.
Li, J., Li, Y., Wu, M., Zhang, Z., & Lu, J. (2013). Effectiveness of low-temperature biochar in controlling the release and leaching of herbicides in soil. Plant and Soil, 370(1), 333–344.
Liu, G. S. (1996). Physical and chemical analysis of soils and profile description. Beijing: China Standard Publishing House.
Liu, N., Zhu, M., Wang, H., & Ma, H. (2016). Adsorption characteristics of direct red 23 from aqueous solution by biochar. Journal of Molecular Liquids, 223, 335–342.
Mamchenko, A. V., Yakimova, T. I., & Koganovskii, A. M. (1982). The mechanism of the filling of the micropores in activated charcoals during the adsorption of organic substances dissolved in water. Russian Journal of Physical Chemistry A, 56, 741–743.
Martin, S. M., Kookana, R. S., Van Zwieten, L., & Krull, E. (2012). Marked changes in herbicide sorption-desorption upon aging of biochars in soil. Journal of Hazardous Materials, 231–232, 70–78.
OECD. (2000). OECD guideline for the testing of chemicals 106, adsorption-desorption using a batch equilibrium method. Paris: OECD.
OECD. (2004). OECD guidelines for the testing of chemicals 312, leaching in soil columns. Paris: OECD.
Pignatello, J. J., Kwon, S., & Lu, Y. F. (2006). Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environmental Science and Technology, 40(24), 7757–7763.
Sander, M., & Pignatello, J. J. (2007). On the reversibility of sorption to black carbon: distinguishing true hysteresis from artificial hysteresis caused by dilution of a competing adsorbate. Environmental Science and Technology, 41(3), 843–849.
Si, Y. B., Wang, M., Tian, C., Zhou, J., & Zhou, D. (2011). Effect of charcoal amendment on adsorption, leaching and degradation of isoproturon in soils. Journal of Contaminant Hydrology, 123(1–2), 75–81.
Sohi, S. P. (2012). Carbon storage with benefits. Science, 338(6110), 1034–1035.
Srinivasan, P., & Sarmah, A. K. (2015). Characterization of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: a spectroscopic investigation. Science of the Total Environment, 502, 471–480.
Sun, F., & Lu, S. (2014). Biochars improve aggregate stability, water retention, and pore space properties of clayey soil. Journal of Plant Nutrition and Soil Science, 177(1), 26–33.
Tatarkova, V., Hiller, E., & Vaculik, M. (2013). Impact of wheat straw biochar addition to soil on the sorption, leaching, dissipation of the herbicide (4-chloro-2-methylphenoxy) acetic acid and the growth of sunflower (Helianthus annuus L.) Ecotoxicology and Environmental Safety, 92, 215–221.
Tian, C., Wang, M. D., & Si, Y. B. (2010). Influences of charcoal amendment on adsorption-desorption of isoproturon in soils. Agricultural Sciences in China, 9(2), 257–265.
Venegas, A., Rigol, A., & Vidal, M. (2015). Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere, 119, 190–198.
Weber, J. B. (1993). Ionization and sorption of fomesafen and atrazine by soils and soil constituents. Pesticide Science, 39, 31–38.
Wu, X., Xu, J., Dong, F., Liu, X., & Zheng, Y. (2014). Responses of microbial community to different concentration of fomesafen. Journal of Hazardous Materials, 273, 155–164.
Yu, X. Y., Ying, G. G., & Kookana, R. S. (2006). Sorption and desorption behaviors of diuron in soils amended with charcoal. Journal of Agricultural and Food Chemistry, 54(22), 8545–8550.
Yu, X. Y., Pan, L. G., Ying, G. G., & Kookana, R. S. (2010). Enhanced and irreversible sorption of pesticide pyrimethanil by soil amended with biochars. Journal of Environmental Sciences, 22(4), 615–620.
Zimmerman, A. R., Gao, B., & Ahn, M. (2011). Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biology and Biochemistry, 43(6), 1169–1179.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation (LZ13D010001), the National Natural Science Foundation of China (41271489 and 21477112), and the Specialized Research Fund for the Doctoral Program of Higher Education (20120101110073).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khorram, M.S., Sarmah, A.K. & Yu, Y. The Effects of Biochar Properties on Fomesafen Adsorption-Desorption Capacity of Biochar-Amended Soil. Water Air Soil Pollut 229, 60 (2018). https://doi.org/10.1007/s11270-017-3603-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3603-2