Abstract
The presence of synthetic dyes in effluents leads to an environmental imbalance characterized by a decrease in photosynthetic activity and, therefore, a reduction of available oxygen, which affects all living aquatic species. To reduce this problem, a combination adsorption and biodegradation treatment strategy is proposed. In this work, Red 40 dye was adsorbed onto a low-cost waste product, followed by degradation by Trametes versicolor under solid state fermentation conditions. The principal aim of this research was to establish the best fermentation conditions using a kinetic evaluation of both degradation and laccase enzyme activity. The process was scaled-up using a rotating drum bioreactor. The best process conditions were a carbon:nitrogen ratio of 30:1, a moisture percentage of 75%, and an inductor concentration of 0.5 mM; the maximum dye degradation was 96.04%. Under these optimized conditions, the highest enzymatic activity was 8.49 U/gdm after 14 days of culture at the flask scale. Using a rotating drum bioreactor, 630 mg of azo dye was degraded after 30 days of culture. Red 40 dye degradation was confirmed using infrared spectroscopy Fourier transform infrared spectrometer and HPLC-MS techniques. The results show that the degradation percentage has a direct relation with laccase activity, and the obtained efficiency in the rotating drum bioreactor confirms the potential of this methodology for implementation at the industrial level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over 7 × 105 t of synthetic dyes is annually produced worldwide and is mainly used in dyeing operations in the textile industry and in products manufactured by the food industry (Hunger and Herbst 2004). In the textile industry, it was recently estimated that 200,000 t of dyes produced annually is lost during dyeing and finishing operations because of inefficient dyeing processes. Unfortunately, most of this dye usually receives little or no treatment before being discharged directly into waterways (Slater 2003). The presence of these pollutant molecules impacts the dynamic balance of the ecosystem by reducing sunlight penetration in natural water bodies that in turn decreases both photosynthetic activity of aquatic life and the level of dissolved oxygen (De Ventura-Camargo and Marin-Morales 2013; Ohe et al. 2004).
Red 40 (R40), also known as Allura Red, is an azo dye used in the textile dyeing processes and as a feed additive (Pourreza et al. 2011). In general, synthetic dyes containing the azo group and aromatic ring structures are harmful to human health (De Ventura-Camargo and Marin-Morales 2013). In particular, it has been found that R40 is potentially toxic and pathogenic at the maximum doses recommended by the OECD guideline (Honma 2015; Jabeen et al. 2013). The currently available physical and chemical methods for the treatment of dye effluents, such as ozonation, flocculation-coagulation, and adsorption onto activated carbon, offer satisfactory decolorization percentages but can be expensive and can lead to molecule fragmentation, sludge generation, and toxic compound production, such as aromatic amines (Forgacs et al. 2004; Soon and Hameed 2011; Verma et al. 2012; Arslan et al. 2016). In recent years, there has been increased interest in biological processes because of its minimal environmental impact. Some microorganisms can integrate into the metabolic pathway the dye molecules, reaching a progressive degradation without toxic compound generation (Stolz 2001; Solís et al. 2012). Nevertheless, the main difficulty associated with this method is how to scale-up the design (Saratale et al. 2011). Thus, the disadvantages of physical, chemical, and biological methods stimulated interest in the development of combined methodologies in order to achieve a maximum elimination of dyes from contaminated water and to avoid secondary effects.
Moreover, considering the high volume of the effluents generated from dyeing units, it is important to implement an effective method that allows such large quantities of water to be treated in a short time. The adsorption process is a quick alternative for removing dyes from large volumes of effluents; the dye is concentrated onto an adsorbent and the residual product can be subsequently treated. In this research, an integral method to R40 dye treatment was carried out. This methodology results from the synergy between physical adsorption and a biological process called solid-state fermentation (SSF). In the first stage, the dye’s adsorbent capacity of agro-industrial wastes is used, and subsequently, in the SSF process, dye degradation is performed by taking advantage of the enzymatic production of white-rot fungi (Bello et al. 2015; Kaushik and Malik 2009).
Highly satisfactory results for adsorption onto agro-industrial waste with greater than 95% dye removal have been reported (Moreno et al. 2012). Thus, adsorption onto low-cost materials represents an alternative for dye removal. However, using only this technique, dye would be removed from wastewater to the adsorbent material. Pollution problems will persist and the concern then becomes how to dispose of dyed, solid waste. This is the reason why, in the method described above, the SSF process is subsequently applied to reach complete dye bioremediation.
In the SSF process, a solid substrate on which the microorganism grows in the absence of free water is used (Ali and Zulkali 2011). High degradation percentages have been reported for different dyes, both cationic and anionic, with white rot fungi (Si et al. 2013). These microorganisms are characterized by the production of ligninolytic enzymes, which have the capacity to oxidize a wide range of recalcitrant compounds, including synthetic dyes (Jebapriya and Gnanadoss 2013). Under SSF conditions, this type of fungus produces a greater quantity of laccase enzymes compared with submerged fermentation (Viniegra-Gonzalez et al. 2003). The literature reports that the enzymatic activity of a white rot fungus, such as T. versicolor, is able to degrade approximately 80% of dyes such as Remazol Brilliant blue and Remazol Red (Ashraf and Legge 2008), which have similar structural characteristics to R40. These results indicate that the SSF biological method, based on the production of diverse ligninolytic enzymes, constitutes a promising complementary alternative to the described mixed strategy.
In this study, R40 adsorption was carried out using corncob waste (CW). This agricultural waste is widely available in Colombia and has a high adsorption capacity, allowing for the removal of different types of dye (Moreno et al. 2012; Robinson et al. 2002a, 2002b). Additionally, CW is a potential substrate for mycelial fungus growth. Specifically, it was reported that, under the best conditions, R40 had an adsorption of 99% using CW as the adsorbent (Moreno et al. 2012). To complete the degradation process, a subsequent treatment of R40 adsorbed onto CW was performed using the fungus Trametes versicolor by SSF. Culture parameters such as the carbon:nitrogen ratio, moisture percentage, and inductor concentration were optimized through a full factorial design, 23. With the best culture conditions, a kinetic study of the process was performed. Additionally, an analysis of the relationship between the degradation percentage and laccase enzyme activity was conducted. Finally, the SSF process was carried out in a rotary drum reactor, and its degradation ability was evaluated using Fourier transform infrared spectrometer (FT-IR) and HPLC-MS techniques.
2 Materials and Methods
2.1 Microorganism
T. versicolor (CBS100.29) was maintained at 4 °C on malt agar plates and subcultured every 3 months.
2.2 Dye Adsorption
Agro-industrial CW was obtained from a local market and was processed using the standard pretreatment process that included washing, grinding, and sieving. The batch adsorption process was carried out under the optimal conditions reported in preliminary studies (Moreno et al. 2012). The dye solution at a concentration of 44 g/L at pH 2.0 was mixed with dried CW at ratio of 10 g of CW per liter of solution and kept at 25 °C with continuous stirring at 150 rpm for 1 h. The dye concentration was measured in a UV-Vis spectrophotometer Lambda PerkinElmer at the R40 maximum adsorption wavelength (λ max = 502 nm). The removal percentage (R) was calculated using Eq. (1).
where C 0 and C f are the initial and final dye concentrations in the solution, respectively.
2.3 Full Factorial Design
To establish the optimal operational conditions for the degradation of adsorbed R40 onto CW through SSF, a full design factorial, 23, with a central point was performed using the Statgraphics Centurion XV software (version 16.1.18). The three factors studied were moisture percent (H), carbon:nitrogen ratio (C:N), and copper sulfate concentration as an inductor (IC). The design levels are shown in Table 1.
2.4 Culture Conditions
The SSF process was performed in a 50-mL Erlenmeyer flask containing 500 mg of dyed CW. Two agar plugs (diameter, 1 cm) from an actively growing fungus on wheat bran agar (Jaramillo et al. 2014) per Erlenmeyer were used as an inoculum. CW adsorbent has a composition of 57.13% acid detergent fiber, 85.34% neutral detergent fiber, 7.39% lignin, and 0.6% nitrogen. The C:N ratio was determined to be 68:1. The following was added to each Erlenmeyer flask: 0.4 mL of malt extract at 6 g/L and 1 mL of nutrient solution composed of 28 g/L of yeast extract and 0.5 mM of CuSO4. Then, 5 H2O was added to adjust the C:N ratio and the initial substrate moisture. The Erlenmeyer flasks were incubated statically under an aerobic atmosphere at 25 °C for 20 days.
2.5 Determination of Enzyme Activity
After the incubation period, the content of each flask was suspended in 10 mL of citrate with 0.05 M phosphate and 0.1 M buffer (pH 4.0) for 1 h in an ice bath. Solids were separated by centrifugation. The supernatant solution was used for enzyme activity measurements.
2,20-Azino-di-[3-ethyl-benzo-thiazolin-sulpho-nate] (ABTS) was used as a substrate for spectrophotometric determination of laccase activity and veratryl alcohol for lignin peroxidase activity. Manganese peroxidase activity was determined by the oxidation change of Mn2+ to Mn3+. One activity unit was defined as the amount of enzyme that oxidized 1 μmol of substrate per min. The activity was expressed in units per gram of dry matter (U/gdm).
2.6 Dye Degradation
To determine the degradation percentage of the azo dye, the residual R40 retained on the CW must be removed. For this, 34 mL of 0.01 M KOH solution was added to each flask during 2 h of continuous agitation. Solids were separated by centrifugation, and the supernatant was used for the measurement of the dye concentration. The dye degradation percentage, D, was determined according to Eq. (2).
where m a is the initial mass of the adsorbed dye onto CW and m d is the mass of the desorbed dye.
2.7 Kinetic Curves
The dye degradation percentage and laccase activity in SSF, as described above, were measured daily for 20 days to determine the profiles and kinetic behavior. Simultaneously, a control culture under the same conditions was performed with native CW in the absence of R40 dye in order to test the influence of the dye on laccase enzyme production during fermentation.
2.8 Fermentation in a Rotating Drum Bioreactor
The fermentation process was carried out in a rotating drum bioreactor containing 200 g of dyed CW. A total of 140 mL of malt extract (0.014 g/L) was added to 400 mL of a nutrients solution composed of 28 g/L yeast extract and 0.5 mM CuSO4. 5 H2O were added to adjust the C:N ratio to 30:1 and the initial substrate moisture to 75%. Pre-culturing of fungus was performed in Fernbach flasks containing dyed CW as a support and maintained in a chamber at 25 °C. After 10 days, the colonized support was transferred to the bioreactor and was incubated under an aerobic atmosphere at 25 °C for 30 days.
The culture was maintained without stirring for the first 5 days, and beginning at day 6, intermittent stirring at 60 rpm was applied for 10 min daily. Aeration was applied with both wet and dry air with a stream of 300 L/h. The drum bioreactor dimensions were 30.5 cm and 16.0 cm in inner diameter. Figure 1 displays a diagram of the process.
2.9 Infrared Spectroscopy FT-IR
FT-IR analysis in solid phase in KBr was performed using a Fourier transform infrared spectrometer (Nicolet 6700) with the purpose of determining changes between native CW, dyed CW, and CW after the treatment process. The spectra were measured from 400 to 450 cm−1 with a resolution of 4.0 cm−1.
2.10 Degradation Product Identification
HPLC-MS was used in order to identify the main degradation products formed by the R40 dye degradation. HPLC analysis was carried out using an Agilent 1100 on a C18 column (symmetry, 4.6 × 250 mm) using the isocratic method with a 60-min run time. The injection volumes were 20 and 40 μL of the same supernatant solution used for the enzyme activity measurements. The oven temperature was set at 25 °C. The mobile phase was a mixture of 10 mM ammonium acetate in ultrapure water and methanol eluted at a flow rate of 0.4 mL/min in gradient conditions. Compound detection was performed using ESI, operated in negative ions with an m/z between 150 and 800.
3 Results and Discussion
3.1 R40 Adsorption on CW
As previously mentioned, this strategy is a combination of two processes: adsorption and biodegradation by SSF. Preliminary tests were performed with the aim of evaluating the adsorption capacity of the CW, following the conditions determined in a previous study. Usually, the concentration of R40 used in the industry varies highly depending on the manufacturing of final products (10–200 mg/L) (Chanlon et al. 2005). It is known that around 10–14% of the total dye used in the dyeing process remain unfixed and end up in the wastewater. For this reason, an initial concentration of R40 of 44 g/L was selected to perform these experiments.
The preliminary adsorption tests confirmed that after 1 h of contact time, the final concentration of the R40 solution was 2 mg/L with a removal percentage of 95.5% of the dye adsorbed onto 4.2 mg/g of CW.
3.2 Optimization of SSF at the Flask Scale
SSF holds enormous potential for the production of secondary metabolites of industrial relevance, such as laccase enzymes, by the cultivation of the fungus T. versicolor. Therefore, laccase can be produced by means of the SSF technique, especially operating with nutritive supports from low-cost agro-industrial polluted wastes, such as dyed CW. In addition, the produced laccases can degrade the dye adsorbed on the CW, leading to a dye-free biosorbent that can be used in other adsorption processes. For this reason, in this study, the potential of CW and dyed CW as support substrates for laccase production by T. versicolor under SSF was investigated.
It is known that enzyme production by SSF is influenced by several parameters, such as selection of microorganisms, C:N ratio, temperature, pH, moisture, carbon and nitrogen source, the solid substrate employed, and its particle size (Thomas et al. 2013). Numerous studies demonstrate that fungal enzyme synthesis is strongly influenced by the carbon:nitrogen ratio (D’Agostini et al. 2011). The amount of nitrogen in the substrate may be a limiting factor for fungus growth. A lack or excess of nitrogen may have an influence on substrate degradation (Bellettini et al. 2016). C:N ratios in the cultivation substrate vary for different fungus species. A C:N ratio of 20:1 is suitable for mycelial growth of most fungi (Mantovani et al. 2007). In the same way, the initial moisture content is a significant factor in SSF processes. The water serves as a solvent for nutrients and metabolites, influences the diffusion of biochemicals and gases, and provides stability for biological compounds and cell structures (Gervais and Molin 2003). Usually, the moisture percentage is fixed at values between 60 and 80% in the SSF process for enzyme production, but it heavily depends on other variables such as temperature (Rani et al. 2009).
Several aromatic compounds such as ferulic acid, guaiacol, veratryl alcohol, 1-hydroxybenzotriazole, and Tween 80 have been successfully used as ligninolytic enzymes inducers (Vrsanska et al. 2015). Choosing one of these compounds depends on the nature of the dyes to be degraded and on the fungi strain. It has been reported that the addition of veratryl alcohol and Tween 80 improves the degradation of dyes by fungi strains with high production of manganese peroxidase (Gassara et al. 2010). However, the major drawback to the use of this kind of aromatic compounds is their cost and can generate toxic derivatives (Christopher et al. 2014). Alternatively, there are low molecular weight compounds and metals that increase the activity of the enzymes involved in decolorization reactions. The effect of a metal inducer of laccase enzyme has been evaluated to maintain an eco-friendly and low-cost methodology for dye treatment.
It has been described that copper sulfate participates in the regulation of ligninolytic enzyme-encoding genes, increasing the laccase production in white rot fungi (Vasina et al. 2015). For T. versicolor, optimal CuSO4 concentrations of approximately 0.1 and 1 mM (Shah et al. 2010; Li et al. 2014; Kapoor et al. 2015) have been reported. The partial and complete inhibition phenomenon occurs at 2 and 80 mM, respectively (Lorenzo et al. 2005). Thus, a range of inductor concentrations between 0 and 1 mM were selected.
Although research on the SSF process is extensive and advanced for several fungus species, there are few basic studies related to the degradation of in situ dyes by white rot fungi. For this reason, after the adsorption of dye by CW, it is necessary to optimize the dye degradation by SSF. Based on the parameters that have the greatest influence on the SSF process, such as the C:N ratio, H, and IC, a 23 full factorial design was carried out. The results are shown in Table 2.
The Pareto chart of effects is shown in Fig. 2. From the chart, statistically significant parameters can be selected. As can be seen, all the parameters were statistically significant for R40 dye degradation. The IC is the most significant factor with a positive influence over the response variable. The IC-C:N interaction, C:N alone, and the interaction between the three evaluated factors have almost the same effect. This result validates the use of a statistical experimental design because it gives the relative importance of the individual components and the effects of their interactions. This information would not be acquired in a univariate evaluation.
The 23-full factorial design allowed for the optimization of the culture conditions to R40 degradation by the SSF process. By analyzing treatments 1 to 4, it can be observed that the highest degradation percentages were recorded in assays with a C:N of 40:1, whereas the lowest degradation occurred in treatments with a C:N ratio of 20:1. Last, only one half of the total dye amount was degraded. These results suggest that in the absence of an inductor, low nitrogen quantities enhance dye degradation. This result can be confirmed in Fig. 2, where the interaction between IC-C:N has a significant and negative effect over the degradation. Regarding the optimal C:N ratio, there are considerable variations reported in the literature for ligninolytic enzyme production. Values between 5:1 and 30:1 were reported as optimal (Kurt and Buyukalaca 2010; D’Agostini et al. 2011; Shi et al. 2012; Asgher and Irshad 2012; Dhakar and Pandey 2013; Nadeem et al. 2014). This finding suggests that variations in culture medium for white rot fungi and the addition of supplemental compounds, such as inductors or salts, can influence dye degradation according to the carbon and nitrogen concentrations in culture.
On the other hand, treatments 5 to 8 show (Table 2) that inductor addition has a significant influence on the degradation percentage, as can be observed in the Pareto chart where IC has the most significant and positive effect. It is noteworthy that in this case, other factors were not significant. These results suggest that the used inductor concentration (1 mM) does not perform a synergistic effect with other factors for R40 degradation. This finding shows the importance of simultaneous parameter evaluation because it is difficult to determine the interaction between factors in univariate experimentation.
By comparing assay 1 with assay 5 as well as assay 2 with assay 6, it was observed that the degradation level increased approximately twofold when the inductor was added to the culture medium. Similarly, in treatments 7 and 8, degradation increased approximately 10% in comparison with treatments 3 and 4. These results confirm that CuSO4 induces laccase activity (Shah et al. 2010; Li et al. 2014; Kapoor et al. 2015) and thereby R40 dye degradation by T. versicolor.
Treatment 9, the central point of the 23-full factorial design, exhibited the highest degradation percentage. In this case, a greater amount of nitrogen (30:1) and a lower concentration of inductor (0.5 mM) are more efficient for R40 dye degradation using T. versicolor compared to treatment 8. The results achieved in the present work support the additive interaction between CuSO4 and the C:N ratio, which explains the wide variation of reported C:N ratio values as mentioned above.
Therefore, it has been concluded that the best culture conditions were a C:N ratio of 30:1, a moisture percentage of 75%, and an inductor concentration of 0.5 mM, resulting in a maximum dye degradation of 96.9%. These conditions were implemented for all the subsequent experiments.
3.3 Degradation and Laccase Profiles
After determining the optimal conditions to obtain maximum dye degradation and laccase activity, profiles were evaluated in order to determine the kinetic behavior of the SSF process. This kind of study is an important aspect of the evaluation of biological process efficiency (Rodriguez and Sanromán 2005). Thus, in this study, a daily measurement of R40 dye degradation percentage was taken. Figure 3 presents a profile of the R40 dye degradation percentage over 20 days. As can be observed, the degradation percentage increases progressively until 9 day (73.48%), after which the increase is lower. The maximum degradation percentage reached was 96.04% after 16 days, and this value remains approximately constant in the following days. A proper characterization of the SSF process requires the performance of a kinetic analysis of degradation by fitting the data to suitable mathematical equations. In this case, a logistic model was applied to describe the degradation (D) at each time (t), as indicated by Deive et al. (2010) and as described in Eq. (3).
where D 0 and D max are the initial and maximum dye degradation and μ D is the specific degradation rate.
The values of the optimized parameters are given in Table 3, and it can be stated that the logistic model properly characterized the experimental data with a regression coefficient of 0.984.
These results are satisfactory based on the complex structure of the R40 dye and its recalcitrant nature due to the azo linkage (Kobylewski and Jacobson 2010). Ademakinwa and Agboola (2014) reached a 52.23% for Allura red degradation using laccase enzyme extracted from the fungi Auerobasidium pullulans. In further studies done by the same authors, crude laccase from the same fungi was able to decolorize 57.2% of R40 (Ademakinwa and Agboola 2015). Other studies showed a 53% degradation of several adsorbed dyes onto barley husks after 21 days of fermentation by Bjerkandera adusta (Robinson and Nigam 2008). On the other hand, Bedekar et al. (2015) reports a 62.11% degradation of methylene blue adsorbed on water hyacinth.
The activities of the enzymes laccase, manganese peroxidase (MnP), and lignin peroxidase (LiP) were measured to a crude extract collected after 16 days of fermentation (time in which maximum degradation was reached). Lacasse and MnP activities were 7.3 and 1.4 U/gdm, respectively; LiP activity was not detected. Similar results were found by Iandolo et al. (2011), who report a laccase activity of 36 U/gdm produced by WRF growing on tomato pomace; MnP and LiP activities were not detected. In the same way, other studies showed the predominance of laccase activity in T. versicolor cultures. Using wheat straw as solid substrate, Valásková and Baldrian (2006) obtained a laccase activity of 1.8 U/gdm and a MnP activity of 0.65 U/gdm. Schlosser et al. (1997) found that the most abundant extracellular enzymes of T. versicolor growing on wheat straw and beech wood were laccase and MnP, LiP was not present, and laccase was the only intracellular enzyme detected.
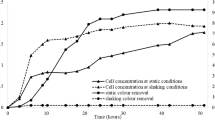
Monitoring laccase (most abundant enzyme founded) activity during fermentation processes was performed in order to establish a possible relation between R40 degradation and laccase activity (Fig. 4). Laccase activity started to increase after 2 days and achieved a maximum value of 8.49 U/gdm after 14 days, followed by detection of a drastic decay in activity. Modeling of the experimental data using logistic Eq. (4) for the first stage (until the maximum value) was performed, and the parameter values obtained are compiled in Table 3 together with the regression coefficients. The regression coefficients obtained are higher at 0.98, which points to the reliability of the proposed model (Eq. (4)).
where L 0 and L max are the initial and maximum laccase activities, respectively, and μ L is the specific enzyme production rate.
The abrupt decrease of laccase activity can be explained by the fact that the fungus could reach cell death after approximately 15 days of culture due to nutrient depletion from the culture medium or the total consumption of available energy sources. After the maximum laccase activity was reached, the degradation percentage was also stabilized (Fig. 4). These results suggest a relationship between R40 degradation and laccase activity.
A control culture of T. versicolor growing on native CW showed similar behavior. However, the maximum levels of activity recorded in this culture of dyed CW were more than 2.5 times higher than those reached in control cultures. In addition, there was a similar effect detected in relation to the specific laccase production rate, with values of 0.75 and 0.55 U/gdm for the SSF cultures of dyed and native CW, respectively. These results suggest that the R40 dye presence stimulated laccase generation, and therefore, this enzyme actively participates in the dye degradation as reported previously (Akkaya et al. 2016). These results are in accordance with those reported by Robinson and Nigam (2008). They found a similar relation between laccase activity and the degradation of a dye adsorbed onto husks of barley. They obtained a degradation percentage of 53% after 21 days of this process and a maximum laccase activity of 2 U/gdm after 12 days. In addition, a gradual increase of laccase activity followed by a subsequent reduction was observed. Additionally, when the maximum laccase activity was achieved, dye degradation was stabilized.
3.4 Fermentation in a Rotating Drum Bioreactor
The last step of the present work included the validation of the proposed strategy at a reactor scale by using a rotating drum bioreactor. This step included the placement of 200 g of dyed CW in the rotating drum bioreactor (Fig. 1). This bioreactor was closed, and it was therefore not possible to take samples during the fermentation process. For this reason, after 30 days, all dyed CW was recollected and homogenized, and a representative sample was taken for analysis and comparison to the initial sample.
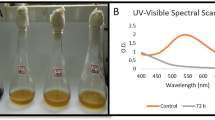
As can be seen in Fig. 5, a large amount of dye was degraded after SSF treatment. The desorption evaluation revealed a degradation level of 75.74% and a residual laccase activity of 3.03 U/gdm. Although the R40 dye degradation obtained in Erlenmeyer flasks was higher than degradation achieved in the bioreactor, the fermentation efficiency in the bioreactor is satisfactory considering that 400 times the amount of material was treated in comparison to the initial degradation, where 0.5 g of dyed CW was treated. At the bioreactor scale, a longer fermentation time is necessary due to the fungi colonizing more substrate. The amount of dye degraded in this study corresponds to 630 L of an industrial effluent (dye concentration 1 mg/L), which shows the potential of SSF as an efficient alternative methodology for implementation in the large-scale treatment process of colored effluents.
The type of bioreactor implemented in this study exhibits certain advantages over other described systems. For example, the use of internal paddles for homogenizing the material allowed for intermittent agitation. The intermittent agitation promotes mycelial growth of the fungus, which is obstructed in continuously stirred reactors. A high level of colonization of white rot fungi on a solid residue is critical in the production of enzymes because growing hyphae can better utilize nutrients in the substrate (Huang et al. 2010). In the rotating drum bioreactor, it was necessary to install only one air flux channel for appropriate distribution of oxygen. In this way, the energetic consumption was lower compared to tray bioreactors in which there is more than one channel for adequate air distribution (Mitchell et al. 1999). Bedekar et al. (2015) performed the degradation of methylene blue adsorbed on water hyacinth in a semi-continuous anaerobic sludge bioreactor with an aerobic trickling bed and achieved a highest degradation of 62.11%.
It is important to highlight that the design used optimizes the workspace. The rotating drum bioreactor occupies an area of 445.4 cm2, while treatment of the same volume of material in a tray bioreactor would require an area of 699.47 cm2. All the above reasons demonstrate that the selected bioreactor model and operating conditions were adequate to achieve an efficient degradation process of the R40 dye adsorbed onto CW.
In addition, as shown in Fig. 5, the used CW shows satisfactory physicochemical properties and can be used as a biosorbent, reducing adsorption process costs.
3.5 Infrared Spectroscopy FT-IR
By infrared spectroscopy (FT-IR) measurement of the samples, it was possible to check for eventual chemical shifts of functional group bands. Figure 6 displays the spectra of native CW, dyed CW, and dyed CW after treatment.
In the native CW, the following bands can be observed: 3.396, 2.920, 1.734, 1.654, 1.637, 1.516, 1.428, 1.384, 1.252, and 1.038 cm−1. The broad and strong band at 3.396 cm−1 is attributed to the stretching vibration from overlapping O–H groups due to the intermolecular hydrogen bond. The peak at 2.920 cm−1 is assigned to the stretching vibration of the aliphatic C–H bond. The strong bands at 1.734, 1.654, and 1.637 cm−1 can be assigned to the vibration of the carbonyl group, which is present in the polymer structure, and includes the carbonyl group of the acid and ester. These peaks appear shifted to low wave numbers because of the formation of intermolecular hydrogen bonds (Sangiumsak and Punrattanasin 2014).
The peak at 1.516 cm−1 is attributed to the stretching vibration of the olefinic bond, C = C, of the aromatic rings. The bands at 1.428 and 1.384 cm−1 are assigned to the vibration of methoxy groups (−OCH3) present in lignin and hemicellulose. Finally, the peak at 1.252 cm−1 is assigned to the stretching vibration of C–O, and the strong band at 1.038 cm−1 is associated with the asymmetric stretching vibration of O–Si–O (Martinez and Ruiz 2002).
The observed bands in the initial sample (dyed CW) and the final sample (CW after treatment) display a minimum shift compared to native CW. All spectra show the same functional groups. For instance, the first band of associated OH appears at 3.395 cm−1 in native CW, at 3.396 cm−1 in the dyed CW, and at 3.996 cm−1 in the CW after treatment.
This trend is observed for the other peaks and can be seen in Fig. 6, which shows the overlapping spectra. The main difference between the samples is the band intensities. The initial sample shows the highest intensity due to the absorbed dye over the surface compared with the native CW. After the treatment process, it can be observed that the band of the initial sample is close to the native CW, showing that the degradation of R40 has occurred through the fermentation process.
3.6 Identification of R40 Dye Degradation Products
In the degradation of R40 by T. versicolor under optimized SSF, the main products formed were identified using the HPLC-MS technique. This technique has been widely used for R40 dye determination (Kirschbaum et al. 2003; Dinc et al. 2006; Pourreza et al. 2011; Ji et al. 2011). This technique is a powerful tool since it is suitable for the identification and determination of a wide molecular weight range of polar, semi-volatile, and thermally labile compounds (Gosetti et al. 2012; Gosetti et al. 2013). In Fig. 7, the chromatograms of the initial (1 day) and the final (20 days) samples are shown. The samples are complex mixtures of diverse kinds of compounds, such as the nutrient solution added and the products of fungus metabolism. Due to this heterogeneity, the chromatograms report a considerable number of molecules. In this study, the presence of R40 in the first sample, its decreasing intensity in the final sample, and the products formed as a result of R40 degradation were identified. The peaks appearing in both chromatograms with the same intensity, retention time, and m/z relation were not considered. Table 4 summarizes the degradation products, accurate mass, elemental composition, and retention time.
In the initial sample, the I16 peak was identified as R40 dye (M = 496 u.m.a). The most intense signal observed for I16 was m/z 225.00758 corresponding to the [M − 2Na] − 2 species, and the another signal with less intensity, m/z 451.02504, was considered to be the [M − 2Na + H] − species.
In the final sample (20 days of fermentation), the formation of four peaks, F10, F12, F16, and F17, can be observed. Peak F13 shows the same retention time and mass spectrum as I16, but with a considerable reduction in intensity. It is concluded that F13 also corresponds to the R40 dye. The decrease of R40 in the final sample is in accordance with the degradation results reported previously.
The F12 signal has a considerable intensity and mass spectra, with a unique m/z peak of 201.02147. For this compound, the structure shown in Fig. 8 is proposed. The chemical structure of the unknown species formed in the degradation process was proposed on the basis of the molecular mass identified in the mass spectrum and the similarity with the structure of the R40 dye. This chemical structure is supported by a Gosetti et al. (2013) study, in which a similar signal was detected during a photodegradation process of R40 dye in a beverage.
The mass spectrum of the F10 m/z = 217 represents a low molecular weight compared with the R40 dye. This signal can be attributed to a compound produced by the solid substrate degradation used during the SSF process. The F16 m/z = 331.23373 and F17 m/z = 329.23187 peaks could be representative of chemical modifications of 4-hydroxy-2-naphthalenesulfonic acid. Formation of hydroxyl groups during azo dye degradation using ligninolytic enzymes has been reported (Ali and Zulkali 2011). Several studies have reported the break of the azo bond, which gives rise to the subsequent oxidation of the aromatic rings that form the dye (López et al. 2004; Wong 2009). This research indicates that the proposed method is suitable for the degradation of R40 dye without the formation of toxic compounds, such as aromatic amines, that is indicative of the less toxicity of the final product in comparison to the parent compound.
4 Conclusions
A full factorial statistical design allowed for the establishment of the best culture medium composition for R40 dye degradation under SSF conditions by T. versicolor. The maximum dye degradation of 96.04% was obtained using a C:N ratio of 30:1, a moisture percentage of 75%, and a CuSO4 concentration of 0.5 mM as the inductor.
The fermentation process kinetics showed a maximum degradation of 96.04% after 16 days. This result was satisfactory given the required biological transformations for pollutant degradation. A maximum laccase activity of 8.44 U/gdm after 16 days was obtained. Monitoring the laccase activity produced in dyed and native CW supports a direct relationship between laccase and R40 degradation, attributing this decolorization to the action of this enzyme.
A rotating drum bioreactor allowed for the treatment of 200 g of dyed CW and degradation of a total of 630 mg of R40 dye, with an efficiency of 75.74% after 30 days of culture, which represents a 400-fold increase compared to the first treated amount. This result shows the potential of this combined methodology (adsorption and SSF) for the treatment of recalcitrant industrial dyes.
The results obtained with FT-IR and HPLC-MS techniques confirm Red 40 dye degradation. Ligninolytic enzyme activity patterns and the compounds identified allow for the conclusion that the degradation products are derived from oxidation reactions.
References
Ademakinwa, N. A., & Agboola, F. K. (2014). Production of laccase by Auerobasidium pullulans and Cladosporium werneckii under optimized conditions: applications in decolourization of textile dyes. Research & Reviews: Journal of Microbiology and Biotechnology, 3(2), 32–40.
Ademakinwa, A. N., & Agboola, F. K. (2015). Bioremediation of textile dye solutions, textile dye mixtures and textile effluents by laccase from Aureobasidium pullulans (de Bary) G. Arnaud (1918) (fungi:Ascomycota). Brazilian Journal of Biology, 2(4), 253–262.
Akkaya, A., Ozseker, E. E., & Akdogan, H. A. (2016). Degradation of dyes by laccase. Analytical Letters. doi:10.1080/00032719.2015.1081916.
Ali, H. K. Q., & Zulkali, M. M. D. (2011). Utilization of agro-residual ligno-cellulosic substances by using solid state fermentation: a review. Croatian Journal of Food Technology Biotechnology and Nutrition, 6(1–2), 5–12.
Arslan, S., Eyvaz, M., Gürbulak, E., & Yüksel, E. (2016). A review of state-of-the-art technologies in dye-containing wastewater treatment—the textile industry case. Croatia: InTech. doi:10.5772/64140.
Asgher, M., & Irshad, M. (2012). Purification and characterization of LiP produced by schyzophyllum commune IBL-06 using banana stalk in solid state cultures. BioResources. doi:10.5897/AJB11.2242.
Ashraf, M., & Legge, A. R. L. (2008). Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation. doi:10.1007/s10532-008-9185-3.
Bedekar, P. A., Kshirsagar, S. D., Gholave, A. R., & Govindwar, S. P. (2015). Degradation and detoxification of methylene blue dye adsorbed on water hyacinth in semi continuous anaerobic—aerobic bioreactors by novel microbial consortium-SB. RSC Advances. doi:10.1039/C5RA17345K.
Bellettini, M. B., Fiorda, F. A., Maieves, H. A., Teixeira, G. L., Ávila, S., Hornung, P. S., Júnior, A. M., & Ribani, R. H. (2016). Factors affecting mushroom Pleurotus spp. Saudi Journal of Biological Sciences. doi:10.1016/j.sjbs.2016.12.005.
Bello, O. S., Adegoke, K. A., Olaniyan, A. A., & Abdulazeez, H. (2015). Dye adsorption using biomass wastes and natural adsorbents: overview and future prospects. Desalination and Water Treatment. doi:10.1080/19443994.2013.862028.
Chanlon, S., Joly-Pottuz, L., Chatelut, M., Vittori, O., & Cretier, J. L. (2005). Determination of Carmoisine, Allura red and Ponceau 4R in sweets and soft drinks by differential pulse polarography. Journal of Food Composition and Analysis. doi:10.1016/j.jfca.2004.05.005.
Christopher, L. P., Yao, B., & Ji, Y. (2014). Lignin biodegradation with laccase-mediator systems. Frontiers in Energy Research. doi:10.3389/fenrg.2014.00012.
D’Agostini, É. C., Rafaele, T., Mantovani, D. A., Silveira, J., Paccola-meirelles, D., Colauto, N. B., & Linde, G. A. (2011). Low carbon/nitrogen ratio increases laccase production from basidiomycetes in solid substrate. Scientia Agricola. doi:10.1590/S0103-90162011000300004.
De Ventura-Camargo, B. C., & Marin-Morales, M. A. (2013). Azo dyes: characterization and toxicity—a review. Textiles and Light Industrial Science Technology, 2(2), 85–103.
Deive, F. J., Domínguez, A., Barrio, T., Moscoso, F., Morán, P., Longo, M. A., & Sanromán, M. A. (2010). Decolorization of dye reactive black 5 by newly isolated thermophilic microorganisms from geothermal sites in Galicia (Spain). Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2010.06.096.
Dhakar, K., & Pandey, A. (2013). Laccase production from a temperature and pH tolerant fungal strain of Trametes hirsuta (MTCC 11397). Enzyme Research. doi:10.1155/2013/869062.
Dinc, E., Aktas, A. H., Baleanu, D., & Üstündağ, Ö. (2006). Simultaneous determination of tartrazine and Allura red in commercial preparation by chemometric HPLC method. Journal of Food and Drug Analysis. doi:10.1515/REVAC.2010.29.2.107.
Forgacs, E., Cserháti, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: a review. Environment International. doi:10.1016/j.envint.2004.02.001.
Gassara, F., Brar, S. K., Tyagi, R. D., Verma, M., & Surampalli, R. Y. (2010). Screening of agro-industrial wastes to produce ligninolytic enzymes by Phanerochaete chrysosporium. Biochemical Engineering Journal. doi:10.1016/j.bej.2010.01.015.
Gervais, P., & Molin, P. (2003). The role of water in solid-state fermentation. Biochemical Engineering Journal. doi:10.1016/S1369-703X(02)00122-5.
Gosetti, F., Chiuminatto, U., Mazzucco, E., Calabrese, G., Gennaro, M. C., & Marengo, E. (2012). Identification of photodegradation products of Allura red AC (E129) in a beverage by ultra high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Analytica Chimica Acta. doi:10.1016/j.aca.2012.08.020.
Gosetti, F., Chiuminatto, U., Mazzucco, E., Calabrese, G., Gennaro, M. C., & Marengo, E. (2013). Non-target screening of Allura red AC photodegradation products in a beverage through ultra high performance liquid chromatography coupled with hybrid triple quadrupole/linear ion trap mass spectrometry. Food Chemistry. doi:10.1016/j.foodchem.2012.08.019.
Honma, M. (2015). Evaluation of the in vivo genotoxicity of Allura red AC (food red no. 40). Food and Chemical Toxicology. doi:10.1016/j.fct.2015.09.007.
Huang, D., Zeng, G., Feng, C., Hu, S., & Zhao, M. (2010). Mycelial growth and solid-state fermentation of lignocellulosic waste by white-rot fungus Phanerochaete chrysosporium under lead stress. Chemosphere. doi:10.1016/j.chemosphere.2010.09.029.
Hunger, W., & Herbst, K. (2004). Industrial organic pigments production, properties, applications. Frankfurt: Wiley-VHC.
Iandolo, D., Piscitelli, A., Sannia, G., & Faraco, V. (2011). Enzyme production by solid substrate fermentation of Pleurotus ostreatus and Trametes versicolor on tomato pomace. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-010-9014-0.
Jabeen, H. S., Rahman, S., Mahmood, S., & Anwer, S. (2013). Genotoxicity assessment of amaranth and allura red using Saccharomyces cerevisiae. Bulletin of Environmental Contamination and Toxicology. doi:10.1007/s00128-012-0870-x.
Jaramillo, A., Jiménez, S., Merino, A., & Hormaza, A. (2014). Obtención de un inóculo fúngico para la degradación de un colorante azo por fermentación en estado sólido. Revista UDCA Actualidad & Divulgación Científica, 17, 577–586.
Jebapriya, G., & Gnanadoss, J. (2013). Bioremediation of textile dye using white rot fungi: a review. International Journal of Current Research and Review, 5(3), 01–13.
Ji, C., Feng, F., Chen, Z., & Chu, X. (2011). Highly sensitive determination of 10 dyes in food with complex matrices using SPE followed by UPLC-DAD-tandem mass spectrometry. Journal of Liquid Chromatography & Related Technologies. doi:10.1080/10826076.2010.526876.
Kapoor, R. K., Rajan, K., & Carrier, D. J. (2015). Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresource Technology. doi:10.1016/j.biortech.2015.03.100.
Kaushik, P., & Malik, A. (2009). Fungal dye decolourization: recent advances and future potential. Environment International. doi:10.1016/j.envint.2008.05.010.
Kirschbaum, J., Krause, C., Pfalzgraf, S., & Brückner, H. (2003). Development and evaluation of an HPLC-DAD method for determination of synthetic food colorants. Chromatographia. doi:10.1007/BF02492092.
Kobylewski, S., & Jacobson, M. (2010). Food dyes: a rainbow of risks. Resource document. Center for Science in the Public Interest. https://cspinet.org/sites/default/files/attachment/food-dyes-rainbow-of-risks.pdf
Kurt, S., & Buyukalaca, S. (2010). Yield performances and changes in enzyme activities of Pleurotus spp. (P. ostreatus and P. sajor-caju) cultivated on different agricultural wastes. Bioresource Technology. doi:10.1016/j.biortech.2009.12.011.
Li, Q., Pei, J., Zhao, L., Xie, J., Cao, F., & Wang, G. (2014). Overexpression and characterization of laccase from Trametes versicolor in Pichia pastoris. Applied Biochemistry and Microbiology. doi:10.1134/S0003683814020124.
López, C., Valade, A. G., Combourieu, B., Mielgo, I., Bouchon, B., & Lema, J. M. (2004). Mechanism of enzymatic degradation of the azo dye Orange II determined by ex situ 1H nuclear magnetic resonance and electrospray ionization-ion trap mass spectrometry. Analytical Biochemistry. doi:10.1016/j.ab.2004.08.037.
Lorenzo, M., Moldes, D., Rodríguez, C. S., & Sanromán, M. (2005). Inhibition of laccase activity from Trametes versicolor by heavy metals and organic compounds. Chemosphere. doi:10.1016/j.chemosphere.2004.12.051.
Mantovani, T. R. D., Linde, G. A., & Colauto, N. B. (2007). Effect of the addition of nitrogen sources to cassava fiber and carbon-to-nitrogen ratios on Agaricus brasiliensis growth. Canadian Journal of Microbiology. doi:10.1139/W06-112.
Martinez, J. R., & Ruiz, F. (2002). Mapeo estructural de sílica xerogel utilizando espectroscopía infrarroja. Revista Mexicana de Física, 48(2), 142–149.
Mitchell, D. A., Pandey, A., Sangsurasak, P., & Krieger, N. (1999). Scale-up strategies for packed-bed bioreactors for solid-state fermentation. Process Biochemistry. doi:10.1016/S0032-9592(99)00048-5.
Moreno, A., Figueroa, D., & Hormaza, A. (2012). Diseño estadístico para la remoción eficiente del colorante rojo 40 sobre tusa de maíz. Producción más Limpia, 7(2), 9–19.
Nadeem, A., Baig, S., Iqbal, K., & Sheikh, N. (2014). Impact of laccase enzyme inducers on solid waste compost maturity and stability. Environmental Technology. doi:10.1080/09593330.2014.932439.
Ohe, T., Watanabe, T., & Wakabayashi, K. (2004). Mutagens in surface waters: a review. Mutation Research. doi:10.1016/j.mrrev.2004.08.003.
Pourreza, N., Rastegarzadeh, S., & Larki, A. (2011). Determination of Allura red in food samples after cloud point extraction using mixed micelles. Food Chemistry. doi:10.1016/j.foodchem.2010.11.158.
Rani, R., Kumar, A., Soccol, C. R., & Pandey, A. (2009). Recent advances in solid-state fermentation. Biochemical Engineering Journal. doi:10.1016/j.bej.2008.10.019.
Robinson, T., & Nigam, P. (2008). Remediation of textile dye waste water using a white-rot fungus Bjerkandera adusta through solid-state fermentation (SSF). Applied Biochemistry and Biotechnology. doi:10.1007/s12010-008-8272-6.
Robinson, T., Chandran, B., & Nigam, P. (2002a). Removal of dyes from an artificial textile dye effluent by two agricultural waste residues, corncob and barley husk. Environment International. doi:10.1016/S0160-4120(01)00131-3.
Robinson, T., Chandran, B., & Nigam, P. (2002b). Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresource Technology. doi:10.1016/S0960-8524(02)00099-8.
Rodriguez, C. S., & Sanromán, M. (2005). Coconut flesh: a novel raw material for laccase production by Trametes hirsuta under solid-state conditions. Journal of Food Engineering. doi:10.1016/j.jfoodeng.2004.10.035.
Sangiumsak, N., & Punrattanasin, P. (2014). Adsorption behavior of heavy metals on biomaterials. Polish Journal of Environmental Studies. doi:10.1021/jf0496402.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: a review. Journal of the Taiwan Institute of Chemical Engineers. doi:10.1016/j.jtice.2010.06.006.
Schlosser, D., Grey, R., & Fritsche, W. (1997). Patterns of ligninolytic enzymes in Trametes versicolor. Distribution of extra- and intracellular enzyme activities during cultivation on glucose, wheat straw and beech wood. Applied Microbiology and Biotechnology. doi:10.1007/s002530050949.
Shah, V., Dobiášová, P., Baldrian, P., Nerud, F., Kumar, A., & Seal, S. (2010). Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2010.01.141.
Shi, M., Yang, Y., Guan, D., Wang, Y., & Zhang, Z. (2012). Evaluation of solid-state fermentation by Ganoderma lucidum using soybean curd residue. Food and Bioprocess Technology. doi:10.1007/s11947-012-0857-y.
Si, J., Cui, B. K., & Dai, Y. C. (2013). Decolorization of chemically different dyes by white-rot fungi in submerged cultures. Annals of Microbiology. doi:10.1007/s13213-012-0567-8.
Slater, K. (2003). Environmental impact of textiles: production, processes and protection. England: Woodhead publishing limited.
Solís, M., Solís, A., Pérez, H. I., Manjarrez, N., & Flores, M. (2012). Microbial decolouration of azo dyes: a review. Process Biochemistry. doi:10.1016/j.procbio.2012.08.014.
Soon, A. N., & Hameed, B. H. (2011). Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination. doi:10.1016/j.desal.2010.11.002.
Stolz, A. (2001). Basic and applied aspects in the microbial degradation of azo dyes. Applied Microbiology and Biotechnology. doi:10.1007/s002530100686.
Thomas, L., Larroche, C., & Pandey, A. (2013). Current developments in solid-state fermentation. Biochemical Engineering Journal. doi:10.1016/j.bej.2013.10.013.
Valásková, V., & Baldrian, P. (2006). Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Research in Microbiology. doi:10.1016/j.resmic.2005.06.004.
Vasina, D. V., Mustafaev, O. N., Moiseenko, K. V., Sadovskaya, N. S., Glazunova, O. A., Tyurin, A. A., Fedorova, T. V., Pavlov, A. R., Tyazhelova, T. V., Goldenkova-Pavlova, I. V., & Koroleva, O. V. (2015). The Trametes hirsuta 072 laccase multigene family: genes identification and transcriptional analysis under copper ions induction. Biochimie. doi:10.1016/j.biochi.2015.07.015.
Verma, K. A., Dash, R., & Bhunia, P. (2012). A review on chemical coagulation-flocculation technologies for removal of colour. Journal of Environmental Management. doi:10.1016/j.jenvman.2011.09.012.
Viniegra-Gonzalez, G., Favela-Torres, E., Aguilar, C. N., Rómero-Gomez, S. D. J., & Diaz-Godinez, G., Augur, C. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering Journal. doi:10.1016/S1369-703X(02)00128-6.
Vrsanska, M., Buresova, A., Damborsky, P., & Adam, V. (2015). Influence of different inducers on ligninolytic enzyme activities. Journal of Metallomics and Nanotechnologies, 3, 64–70.
Wong, D. W. S. (2009). Structure and action mechanism of ligninolytic enzymes. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-008-8279-z.
Acknowledgments
The authors gratefully acknowledge the Universidad Nacional de Colombia–Sede Medellín for the support by providing the Laboratory of Experimental Chemistry infrastructure as well as Colciencias (Departamento Administrativo de Ciencia Tecnología e Innovación de Colombia) for funding of the project code 111871250685. The support of the Bioengineering and Sustainable Process Group of University of Vigo, Spain, is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaramillo, A.C., Cobas, M., Hormaza, A. et al. Degradation of Adsorbed Azo Dye by Solid-State Fermentation: Improvement of Culture Conditions, a Kinetic Study, and Rotating Drum Bioreactor Performance. Water Air Soil Pollut 228, 205 (2017). https://doi.org/10.1007/s11270-017-3389-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3389-2