Abstract
Microbiological activities are essential in the bioremediation of polluted soils. The enzymatic activities of microorganisms are usually used as a biological indicator of soil health. The aim of this work was to observe the catalase, acid phosphatase (AcP), and alkaline phosphatase (AlP) activities in soil that was amended with agro-industrial by-products and macronutrients during the process of total petroleum hydrocarbon (TPH) removal. To this end, microcosm tests were performed with soil and agro-industrial by-products ratios of 100:2:2, for soil:sugarcane bagasse pith:filter cake mud (SSF); 100:2, for both soil:sugarcane bagasse pith (SS); and for soil filter cake mud (SF). The macronutrients—carbon, nitrogen, and phosphorus—in the experimental treatments were adjusted to 100:10:1 with a solution of NH4NO3 and K2HPO4. The best TPH removal (51.4%) was obtained with SSF at 15 days. In addition, a significant correlation was observed between TPH removal and AlP as well as AcP (r = 0.74, p < 0.0001; r = 0.70, p < 0.0107, respectively). Fungi growth was also correlated with both AlP (r = 0.97, p < 0.0001) and AcP (r = 0.95, p < 0.0001) activities. Besides, bacterial and fungi growth showed a correlation with TPH (r = 0.86, p < 0.001; r = 0.77, p < 0.0034, respectively). It could be said that the agro-industrial by-products and macronutrients contributed to pollutant removal from the oil-polluted soil at relatively short amount of time. In addition, the enzymatic activities were increased after the treatment; in this study, the high sensitivity enzyme was AlP, and it could be used as an indirect indicator of oil pollutant removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, Mexico has been subject to a range of soil, air, and water pollution problems across different ecosystems. This pollution could have been related to either organic or inorganic compounds. Various industrial activities involve the use or processing of petrochemicals, which has caused soil pollution problems due to accidental spills worldwide. Such problems also occur in the state of Veracruz, located in southeastern Mexico (PEMEX 2006). Oil pollution modifies the physicochemical and biological characteristics of the soil (Wyszkowska et al. 2002).
Veracruz is an important producer of agro-industrial products including 16,674,866 t year−1 of sugarcane (Saccharum officinarum), 1,707,170 t year−1 of orange (Citrus sinensis L.), and 304,231 t year−1 of coffee cherries (Coffea arabica L.) (INEGI 1998). However, these industrial activities also generate large amounts of waste that have a potential use within those industries. Every ton of sugarcane processed produces 340 kg of sugarcane bagasse pith and 35 kg of filter cake mud. A small portion of sugarcane bagasse pith has been used for energy production (as boiler fuel) and to improve the soil agro-industrial characteristics (Serratia et al. 1990; Benedicto-Valdés et al. 2005). These studies showed that the agro-industrial by-products are useful as amendments and texturizers in oil-polluted soil (soils that have received chronic contamination) to enhance pollutant removal.
Enzyme activities in soil have been used as a biological indicator of contamination from metals, pesticides, and hydrocarbons (Karaca et al. 2011). Studies have shown the potential of soil enzymes in evaluating the impact of hydrocarbons and fertilizers on soil microorganisms, indicating that soil enzymes represent a useful tool for the early detection and monitoring of soil contamination (Margesin et al. 2000a; Singh et al. 2009; Megharaj et al. 2011; Singh and Ghoshal 2013). While enzymes are primarily of microbial origin, they may also originate from animal or vegetable remains. They may be found under diverse conditions ranging from those associated with living organisms (endoenzymes) to enzyme complexes immobilized by clay and humic substances (exoenzymes or abiotic components) (Paolini 2003; Šarapatka 2003; Burns et al. 2013). These activities are closely related to parameters such as carbon (C) and nitrogen (N) contents, C and N from the microbial biomass, and N mineralization. Metabolic activity in soil is responsible for important processes such as mineralization and humidification of organic matter (OM), which in turn will affect a series of processes involving fundamental elements such as C, N, phosphorus (P), and sulfur (S) (García et al. 2003; García-Ruiz et al. 2008). All these reactions can be measured by enzymes that are specific to each substrate. Soil enzymes that have been more widely studied include oxidoreductases (particularly dehydrogenase, peroxidase, and catalase) and hydrolases (phosphatase, protease, and urease). These play an important role in the soil environment and the C, N, P, and S cycles (García et al. 2003; Cadwell 2005). In this study, the enzyme phosphatase (phosphomonoesterases: acid (EC 3.1.3.2) and alkaline (EC 3.13.1)) and catalase (EC 1.11.1.6) activities in soil were measured to evaluate the improvement of soil health as a result of biostimulation treatment. Phosphatases are good indicators of soil fertility, and these enzymes play critical roles in P cycles because they have been correlated to P stress (Bakshi and Varma 2011). Catalase is an intracellular enzyme in all aerobic bacteria and in most facultative aerobes, and is absent in obligate anaerobic bacteria, facilitating its use as an indicator of the microbial activity in soil and the soil fertility (García et al. 2003). The objective of this study was to remove total petroleum hydrocarbons (TPH) from oil-contaminated soil amended with macronutrients and agro-industrial by-products (sugarcane bagasse pith and filter cake mud) and to monitor phosphatase and catalase activities as a biological indicator of enhanced pollutant removal throughout the experimental period.
2 Materials and Methods
2.1 Soil Sampling and Obtaining Agricultural By-products
The sampling site was located in Nuevo Teapa in the state of Veracruz, Mexico (18°04′ N and 94°19′ W) at 80 m elevation. The weather is wet with mean annual temperature of 27 °C and an annual average precipitation of 2456 mm. The types of soil are acrisol and vertisol, characterized by the accumulation of clay in the subsoil and its susceptibility to erosion (INAFED 2013). Samples of oil-polluted soil (Fig. 1) were collected at 13 random points in the A1 horizon at depths of 0–30 cm (Mason 1992), and the distance to the contamination source (Industrial activity) was approximately 20–30 m. The filter cake mud and sugarcane bagasse pith were provided by the Cuatotolapan Sugar Plant located in Hueyapan of Ocampo, Veracruz.
2.2 Soil and Agricultural By-product Analysis
The pH was electronically determined using an Orion 420A potentiometer, USA, using epoxy electrodes; humidity was determined by the gravimetric method as established by NOM-021 (DOF 2002); and OM was determined by the method developed by Walkley and Black in 1949 (Muñoz-Iniestra et al. 2000; DOF 2002). Total N determinations were performed with the Micro-Kjeldahl method, as adapted by Bremner in 1965 (Muñoz-Iniestra et al. 2000; DOF 2002). Available P was determined using the Bray method, as reported in NOM-021 (DOF 2002). All these tests were performed in triplicate and the reagents blank and standards (ACS reagent of Sigma-Aldrich (Sigma, St. Louis, MO, USA)) were used for calibration.
2.3 Microbial Analysis
The soil was crushed with a mortar and pestle, passed through a standard sieve of 2-mm mesh size, and kept at 4 °C in sterile amber glass bottles until its characterization and analysis (Paetz and Wilke 2005). Bacterial growth was evaluated using the plate counting method (Clark 1965) in triplicate. Bacterial groups were analyzed through plating on nutritive agar at 37 °C for 48 h. The Parkinson method (Parkinson et al. 1971) was used to determinate the fungi groups; a 10−2–10−4 dilution was performed, and samples were plated on potato-dextrose-agar at 28 °C for 4 days, with rose bengal as the indicator and chloramphenicol as the bactericide. The microorganisms were reported as colony-forming units (cfu) per gram of dry soil (g dm−1). All chemicals (ACS reagent) were purchased from Sigma-Aldrich.
2.4 Hydrocarbon Extraction and Quantification
The micro Soxhlet extraction method (USEPA 2002) was employed to obtain the TPH concentrations. The test was conducted in triplicate for each sample. In brief, 1 g of soil with 2 g of Na2SO4 was placed in a cartridge and dichloromethane was used as a solvent (50 mL), at a constant reflux (60 °C) for 4 h. After that, the solvent was evaporated to near dryness in a rotary evaporator, the organic extraction was kept in a weighed amber flask with a Teflon lid, and then the solvent was evaporated until dryness using a nitrogen stream. The final weight of the flask represented the weight fraction of TPH concentrations (DOF 2006). All chemicals (ACS reagent) were purchased from Sigma-Aldrich.
2.5 Enzymatic Assays
2.5.1 Catalase Activity (EC 1.11.1.6)
The Johnson and Temple (1964) method (García et al. 2003) was used to determinate the catalase activity in the soil. This involved adding 40 mL of distilled water to 1 g of soil (2-mm mesh size) and shaking for 30 min, then 5 mL of a 1:100 dilution of H2O2 in distilled water was added and shaken for 30 min at 20 °C, followed by the addition of 5 mL of 1.5 M H2SO4 to stop the enzymatic activity. The solution was filtered and a 25 mL aliquot was evaluated with 0.01 M KMnO4. Control tests were processed in the same manner as the samples but the 5 mL of H2O2 was replaced by distilled water. A blank was conducted with a mix of 40 mL of distilled water, 5 mL of H2O2, and 5 mL of 1.5 M H2SO4, and 25 mL of this mixture was evaluated with KMnO4. The assay was carried out in triplicate for each sample. All substrates (ReagentPlus®) and chemicals (ACS reagents) were purchased from Sigma-Aldrich.
2.5.2 Phosphatase Activity: Alkaline (EC 3.13.1) and Acid (EC 3.1.3.2)
Phosphatase activity was determined using the Tabatabai and Bremner (1969) method. In brief, 1 g of soil (2-mm mesh size) was added to MUBFootnote 1 (4 mL) buffer solution (with a pH 6.5 or pH 11, for acid phosphatase or alkaline phosphatase, respectively) and p-nitrophenyl phosphate (p-NP; 1 mL) 0.025 M, and shaken for 1 min at 120 rpm. Next, the sample was incubated for 1 h at 37 °C and the reaction was stopped by placing the sample in a cool bath at 4 °C for 5 min. Following this, 1 mL of CaCl2 0.5 M and 4 mL of NaOH 0.5 M were added and this was shaken at high speed for 1 min. The sample was then promptly filtered with Whatman no. 42 filter paper. Finally, the absorbance was measured with a UV-vis spectrophotometer at 400 nm. A calibration curve was constructed with p-NP between 0 and 250 μg mL−1; 1 mL of each dilution was mixed with 4 mL of distilled water, 1 mL of CaCl2 0.5 M, and 4 mL of NaOH 0.5 M; then filtered and the absorbance was measured. Control tests were processed with the same procedure as samples but the p-NP was added after the addition of CaCl2 0.5 M and NaOH 0.5 M. This assay was performed in triplicate for each sample. All substrates (ReagentPlus®) and chemicals (ACS reagent) were purchased from Sigma-Aldrich.
2.6 Experimental Procedure
A microcosm test was performed with 10 g of soil and agro-industrial by-products were added at a soil:by-product ratio of 100:2 for soil:sugarcane bagasse pith (SS) and soil:filter cake mud (SF) and 100:2:2 for soil:sugarcane bagasse pith:filter cake mud (SSF). The moisture was adjusted to 60% of holding capacity. Next, the physicochemical analyses were conducted and the nutrients were adjusted with a solution of NH4NO3 and K2HPO4 to obtain a C:N:P ratio of 100:10:1. All the treatments were conducted in triplicate. These conditions were established according to previous reports of the optimal conditions to remove hydrocarbons from oil-contaminated soil using macronutrients and agro-industrial by-products (García-Torres et al. 2011; Antonio-Ordaz et al. 2011). The four experimental treatments are described in Table 1. The study was performed for 15 days at 28 °C and samples were taken on days 5, 10, and 15. Aeration was conducted every 72 h under sterile conditions. Moreover, experimental controls were soil that was sterilized with N3Na at 5% (sterile soil (S)) and soil without treatment (control soil (C)). All chemicals (ACS reagent) were purchased from Sigma-Aldrich.
2.7 Statistical Analyses of Data
A one-way analysis of variance and Pearson’s correlation with a 0.05 significance level and three replicates (n = 3) were performed using Statistical Analysis Systems (SAS) software for Windows version 9.
3 Results
3.1 Soil and By-product Characteristics
Table 2 lists the physicochemical characteristics of the soil, the agricultural by-products, and the initial enzymatic activity. The initial pH (8.02) of the soil was good for the bioremediation process (Gómez-Romero et al. 2008). Nevertheless, the initial OM of the soil was less than 3%, also, initial N and P were 0.39% and 0.76 mg kg−1, respectively, and these conditions limited the microbial activity in the soil (5.8 Ln cfu g dm−1of bacteria and 7.0 Ln cfu g dm−1of fungi, both of them at initial conditions). The initial enzymatic activities in the soil were as follows: catalase 0.19 μmol H2O2 g−1 h−1, acid phosphatase (AcP) 0.39 μmol p-NP g−1 h−1, and alkaline phosphatase (AlP) 0.17 μmol p-NP g−1 h−1. Humidity measurements remained constant throughout the experimental period in all the experimental units, and this condition supported microbial activity in the soil. The pH ranged from 6.60–7.35 in all experimental treatments with these conditions favoring the bacterial growth.
3.2 TPH Removal, Microbial Progress, and Enzymatic Activities
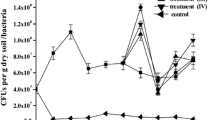
The results of fungal growth, bacterial growth, pH, OM, TPH, N, P, and enzymatic activities of the experiments are presented in Table 3. The initial concentration of the TPH in the soil was 55,954 mg kg−1; the SSF treatment showed that the best TPH removal was at 51.4% (Fig. 2) and the second-best result (46.4%) was obtained with the SS treatment and the lowest TPH removal (34.2%) was obtained with the SF treatment. Bacteria played an important role in the bioremediation process, indicating that they were more reactive than the fungi population. Nevertheless, the bacterial and fungal growth were observed to have similar significant correlations with TPH removal (Table 4): for SSF treatment, the bacteria and fungi correlations were r = 0.86, p < 0.0003 and r = 0.77, p < 0.0034, respectively; for SS, the correlations were r = 0.80, p < 0.0017 and r = 0.75, p < 0.005, respectively; and for SF were r = 0.82, p < 0.001 and r = 0.81, p < 0.0013, respectively. For treatments with SSF and SS, the TPH had a negative correlation with N content (r = −0.94, p < 0.0001 and r = −0.97, p < 0.0001 for SSF and SS, respectively). For all the treatments, a maximum bacterial growth was presented on the tenth day, at the same time catalase activity decreased, while AcP and AlP activities remained constant. Higher catalase, AcP, and AlP activities were present on day 5 for all treatments: the day on which hydrocarbon removal began to increase. The maximum catalase activity observed was with the SSF treatment (0.49 μmol H2O2 g−1 h−1) on day 5 (Table 3), but there was no significant difference between this result and that obtained with SS. The lowest activity occurred under the SF treatment. In the same experimental period, with regard to AcP activity, the highest was observed with the SF treatment (1.80 μmol p-NP g−1 h−1), followed by SS (1.33 μmol p-NP g−1 h−1), and finally SF (1.33 μmol p-NP g−1 h−1). The AlP activity for the SSF treatment was 1.83 μmol p-NP g−1 h−1 on day 5 and no significant differences were be observed between this result and those for the SS treatment; the lower value was for the SF treatment (1.12 μmol p-NP g−1 h−1). The Pearson’s correlation tests for the SSF treatment showed that P consumption had a significant correlation with bacterial and fungal growth (r = 0.81, p < 0.0014 and r = 0.86, p < 0.0003, respectively), as well as with AlP, AcP, and catalase activities (r = 0.92, p < 0.0001; r = 0.94, p < 0.0001; and r = 0.97, p < 0.0001, respectively), as shown in Fig. 3. The best results were observed on day 15 under the SSF treatment: 7.98 × 109 cfu g dm−1 for bacterial growth, 1.05 × 107 cfu g dm−1 for fungal growth, 0.35 μmol H2O2 g−1 h−1 for catalase activity, 1.30 μmol p-NP g−1 h−1 for AcP activity, and 1.80 μmol p-NP g−1 h−1 for AlP activity. Finally, with this treatment, the AcP was observed to have a significant correlation with bacterial growth (r = 0.95, p < 0.0001) and TPH removal (r = 0.70, p < 0.0107).
Enzymatic activities and phosphorus consumption during the treatment of soil/sugarcane bagasse pith/filter cake mud (n = 3). White triangle alkaline phosphatase, AIP; white circle acid phosphatase, AcP; white diamond phosphorus, P; and black square catalase, average values. Error bars show the standard deviation
4 Discussion
The pH ranges in the soil (6.60–7.35) did not significantly change throughout the experimental period in all the treatments. According to some authors (Gómez-Romero et al. 2008; Montejo et al. 2008), it is important to maintain a pH range between 6 and 8 to obtain good results for removal of pollutants from soil. The pH values in the present study resulted in more bacterial growth than fungal growth. The humidity in the soil was another factor that facilitated microbial development, and in our experimental treatments, the constancy of this parameter facilitated heterotrophic microbial growth. Geisseler et al. (2011) reported that moisture was enhanced by the addition of residues, which enables the enzymatic activities in the soil to increase. In the current study, the OM in the experimental treatment increased following the amendments, thereby resulting in the progression of the microbial and enzymatic activities according to the reports of Burns (1982) and Shahsavari et al. (2013). There was a lower consumption of total N than of P; the latter nutrient is important in the mineralization process as well as many metabolic pathways and certain enzymatic activities in soil (Sylvia et al. 1999; Margesin et al. 2000b). The enzyme activities were also low in the soil during the experimental period compared with the activities observed in other healthy soils (alkaline phosphatase, 6.76–27.34 μmol p-NP g−1 h−1; acid phosphatase, 0.05–86.33 μmol p-NP g−1 h−1; and catalase, 61.20–73.90 μmol H2O2 g−1 h−1) (Nannipieri et al. 2002). The alkaline phosphatase activity was higher than that of acid phosphatase because the pH remained neutral during experimental period; it was suggested that these proteases are more affected for soil pH than for OM or level of disturbance (Acosta-Martínez and Tabatabai 2011). For the SSF treatment, P concentration was positively correlated with AcP and AlP activities, as well as with fungal growth. Therefore, P availability for this condition was important to reactivate the native micro-flora in order to remove the pollutants, which is consistent with the findings of Margesin et al. (2000b). An inverse relationship was observed between inorganic P availability and phosphatase activity, although this depends on the initial bioavailability of P (DeForest et al. 2012). An increase in enzyme activity as a response to demand of resources results in the release excess of products, which may be due to a positive relationship between enzyme activity and resource availability (Singh and Ghoshal 2013). Nevertheless, the N content was related to TPH removal for the SSF and SF treatments. This suggests that the N content in the filter cake mud is more available to the microorganisms (waste degradation) and allows the pollutant removal. Similar results were obtained by Adesodum and Mbagwu (2008), Lee et al. (2008), and Ros et al. (2010) in hydrocarbon-contaminated soils amended with poultry pig manure compost and fresh sewage sludge, respectively. The higher hydrolysis rates for organic P with respect to proteins and the increasing ratio of phosphatase to aminopeptidase activity indicated that N cycling was slower than P cycling (Singh and Ghoshal 2013). Although low, fungal growth was sufficient to promote a synergism with the bacterial population to achieve TPH removal. In a similar report by Shahsavari et al. (2013), 85% removal of TPH from soil amended with pea straw was observed, as compared to 51% for the control case. They also observed a change in the bacterial population in soils amended with plant residues and a weak correlation (r = 0.68) between these residues and TPH removal. This confirms that the residues provided a substrate for microorganism growth. The constancy in the enzymatic activity in this study may be due to a protective effect against degradation promoted by the OM from the by-products. Similar results were reported by Pascual et al. (2002). It has also been reported (Chaudhary et al. 2012) that AlP increases more than AcP in soil polluted by kerosene.
The decrease in catalase activity occurred when the TPH began to get established, as reported by Achuba and Peretiemo (2008). This activity was altered as a result of the environmental changes to soil properties such as pH, nutrient availability, hypoxia, and microorganism populations. The mechanisms by which the enzymes are immobilized and consequently become established in soil have not been entirely explained (Gianfreda and Ruggiero 2006). In addition, it has been reported that enzymatic activities could be affected by factors such as sampling methods, storage time, and changes in pollutants (García et al. 2003). For all treatments, it was difficult to attribute the AlP activity to only bacterial or fungal growth because it had a significant correlation with both groups, although the correlation was slightly higher with bacterial growth. Moreover, AlP was correlated with TPH removal in the SS treatment, while AcP was correlated with TPH removal for the SSF treatment. Therefore, phosphatase activities played an important role in the removal of the pollutants. These results are consistent with those obtained by Wyszkowska et al. (2002, 2006).
5 Conclusions
The results of this study showed that agricultural by-products could be a good option for the bioremediation of oil-polluted soil. Furthermore, a mix of by-products may be optimal to obtain substantial results over a relatively short time, as they serve not only to support microorganisms but also to supply nutrients and OM to immobilize the enzymes. The enzymatic activities for all the treatments increased during the experimental period and could thus be used as an indicator of pollutant removal. Alkaline phosphatase activity was the more representative enzyme in this study, which had a significant correlation with both microbial growth and THP removal. It is necessary to develop site-specific evaluation criteria for the more accurate use of soil enzyme activities as a soil quality parameter.
Notes
Modified universal buffer (MUB) consisted of 3.025 g trishydroxymethylaminomethane, 2.9 g maleic acid, 3.5 g citric acid, 1.57 g boric acid, 1 M sodium hydroxide (NaOH) solution (122 mL), and distilled water added to give a final volume of 250 mL (Skujins et al. 1962).
References
Achuba, F. I., & Peretiemo, B. O. (2008). Effect of spent engine oil on soil catalase and dehydrogenase activities. International Agrophysics, 22, 1–4.
Acosta-Martínez, V., & Tabatabai, M. A. (2011). Phosphorous cycle enzymes. In R. P. Dick (Ed.), Methods of soil enzymology (pp 163–165). SSSA book series 9. USA: Soil Science Society of America.
Adesodum, J. K., & Mbagwu, J. S. C. (2008). Distribution of heavy metals and hydrocarbon contents in an alfisol contaminated with waste-lubricating oil amended with organic wastes. Bioresource Technology, 99, 3195–3204.
Antonio-Ordaz, J., Martínez-Toledo, A., Ramos-Morales, F. R., Sánchez-Díaz, L. P., Martínez, A. J., Tenorio-López, J. A., & Cuevas-Díaz, M. C. (2011). Biorremediación de un suelo contaminado con petróleo mediante el empleo de bagazo de caña con diferentes tamaños de partícula. Multiciencias, 11(2), 136–145.
Bakshi, M., & Varma, A. (2011). Soil enzyme: State-of- art. In G. Shukla & A. Varma (Eds.), Soil Biology. Soil enzymology (pp. 11–18). New York: Springer.
Benedicto-Valdés, G. S., Hidalgo-Moreno, C., Ordaz-Chaparro, V., Sánchez-Hernández, R., & López-David, J. P. (2005). Cambios en las propiedades físicas de un suelo arcilloso por aportes de lombricompuesto de cachaza y estiércol. Interciencia, 30(12), 775–779.
Burns, R. G. (1982). Enzyme activity in soil. Location and possible role in microbial ecology. Soil Biology Biochemistry, 14, 423–427.
Burns, R. G., DeForest, J. L., Marxsen, J., Sinsabaugh, R. L., Stromberger, M. E., Wallenstein, M. D., Weintraub, M. N., & Zoppini, A. (2013). Soil enzymes in a changing environment: current knowledge and future directions. Soil Biology Biochemistry, 58, 216–234.
Cadwell, B. A. (2005). Enzyme activities as a component of soil biodiversity: a review. Pedobiologia, 49, 637–644.
Chaudhary, P., Singh, S. B., Chaudhary, S., & Nain, L. (2012). Impact of PAH on biological health parameters of soils of an Indian refinery and adjoining agricultural area—a case study. Environmental Monitoring Assessment, 184, 1145–1156.
Clark, F. E. (1965). Agar-plate method for total microbial count. In: C. A. Black, D. D. Evans, L. E. Ensminger, J. L. White, & F. E. Clark (Eds.), Methods for soil analysis. Part 2 chemical and microbiological properties (pp. 1461–1465).
DeForest, J. L., Smemo, K. A., Burke, D. J., Elliott, H. L., & Becker, J. C. (2012). Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry, 109, 189–202.
Diario Oficial de la Federación (DOF). (2002). Norma oficial Mexicana NOM-021-SEMARNAT-2000, que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreos y análisis. D. F. México.
Diario Oficial de la Federación (DOF). (2006). Norma Mexicana. NMX-AA-134-SCFI-2006, Suelo. Hidrocarburos Fracción pesada por Extracción y Gravimetría-Método de Prueba. D. F. México
Environmental Protection Agency (USEPA). (2002). SW 846 Method 3570. Microscale solvent extraction (MSE). http://www.epa.gov/SW-846/pdfs/3570.pdf. Accessed 28 Feb 2007.
García, C., Gil, F., Hernández, T., & Trasar, C. (2003). Técnicas de Análisis de Parámetros Bioquímicos en suelos. Medida de Actividades Enzimáticas y Biomasa Microbiana. España: Mundi-Prensa.
García-Ruiz, R., Ochoa, V., Hinojosa, M. B., & Carreira, J. A. (2008). Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biology Biochemistry, 40, 2137–2145.
García-Torres, R., Ríos-Leal, E., Martínez-Toledo, A., Ramos-Morales, F. R., Cruz-Sánchez, J. S., & Cuevas-Díaz, M. C. (2011). Uso de cachaza y bagazo de caña de azúcar en la remoción de hidrocarburos en suelo contaminado. Rev. Int. Contam. Ambie., 27(1), 31–39.
Geisseler, D., Horwath, W. R., & Scow, K. M. (2011). Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity. Pedobiologia, 54, 71–78.
Gianfreda, L., & Ruggiero, P. (2006). Enzyme activities in soil. In P. Nannipieri & K. Smalla (Eds.), Soil biology, volume 8 nucleic acids and proteins in soil. Berlin Heidelberg: Springer-Verlag.
Gómez-Romero, S. E., Gutiérrez-Bustos, D. C., Hernández-Marín, A. M., Hernández-Rodríguez, C. Z., Losada-Casallas, M., & Mantilla-Vargas, P. C. (2008). Factores bióticos y abióticos que condicionan la biorremediación por Pseudomonas en suelos contaminados por hidrocarburos. NOVA, 6, 76–84.
Instituto Nacional de Estadística Geografía e Informática (INEGI). (1998). Análisis a nivel nacional de la producción de caña de Azúcar. México.
Instituto Nacional para el Federalismo y el Desarrollo Municipal (INAFED). (2013). Enciclopedia de los municipios y Delegaciones de México. http://www.inafed.gob.mx/work/enciclopedia/index.html. Accessed 5 May 2013.
Johnson, J. L., & Temple K. L. (1964). Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Science Society of America Processes, 28, 207–209.
Karaca, A., Cetin, S.-C., Turgay, O.-C., & Kizilkaya, R. (2011). Soil enzymes as indication of soil quality. In G. SG & A. Varma (Eds.), Soil biology. Soil enzymology (pp. 125–139). New York: Springer.
Lee, S., Oh, B., & Kim, J. (2008). Effect of various amendments on heavy mineral oil bioremediation and soil microbial activity. Bioresource Technology, 99, 2578–2587.
Margesin, R., Zimmerbauer, A., & Schinner, F. (2000a). Monitoring of bioremediation by soil biological activities. Chemosphere, 40, 339–346.
Margesin, R., Walder, G., & Schinner, F. (2000b). The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities and microbial properties of soil. Acta Biotechnologica, 20, 313–333.
Mason, B. (1992). Preparation of soil sampling protocols: sampling techniques and strategies. USEPA, EPA/600/R-921/128.69.
Megharaj, M., Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., & Naidu, R. (2011). Bioremediation approaches for organic pollutants: a critical perspective. Environment International, 37, 1362–1375.
Montejo, M. M., Torres, L. C., Cales, M. G., Roa, L. M., & Cuevas, D. M. C. (2008). Comportamiento de la ureasa en suelo contaminado con hidrocarburos y tratado con cascarilla de café y bagazo/cachaza de caña. Xalapa: I Congreso de Biotecnología Ambiental.
Muñoz-Iniestra, D. J., Mendoza-Cantu, A., López-Galindo, F., Soler-Aburto, A., & Hernández-Moreno, M. M. (2000). Manual de métodos de análisis de suelos. México: UNAM.
Nannipieri, P., Kandeler, E., & Ruggiero, P. (2002). Enzyme activities and microbiological and biochemical processes in soil. In R. G. Burns & R. P. Dick (Eds.), Enzymes in the environment: activity, ecology and applications (pp. 7–8). New York: Marcel Dekker, Inc..
Paetz, A., & Wilke, B.-M. (2005). Soil sampling and storage. In R. Margesin & F. Schinner (Eds.), Manual for soil analysis—monitoring and assessing (pp. 37–44). New York: Springer.
Paolini, J. E. (2003). Actividades enzimáticas en suelos de los altos llanos centrales (estado Guárico). Venesuelos, 11(1, 2), 39–46.
Parkinson, D., Gray, T. R. G., & Williams, S. T. (1971). Methods for studying the ecology of soil microorganism. I.B.P. Handbook no. 19. Oxford: Blackwell.
Pascual, J. A., Moreno, J. L., Hernández, T., & García, C. (2002). Persistence of immobilized and total urease and phosphatase activities in soil amended with organic wastes. Bioresource Technology, 82, 73–78.
Petróleos Mexicanos (PEMEX). (2006). Informe Desarrollo Sustentable 2006. México: Desempeño Ambiental: Derrames y Fugas.
Ros, M., Rodriguez, I., García, C., & Hernández, T. (2010). Microbial communities involved in the bioremediation of an aged recalcitrant hydrocarbon polluted soil by using organic amendments. Bioresource Technology, 101, 6916–6923.
Šarapatka, B. (2003). Phosphatase activities (ACP, ALP) in agroecosystem soils. Doctoral thesis. Swedish University of Agricultural Sciences. Uppsala.
Serratia, P., Solano, A., & Preston, T.R. (1990). Utilización de jugo de caña y cachaza panelera en la alimentación de cerdos. Livestock research for rural development http://www.cipav.org.co/lrrd/lrrd2/2/sarria.htm. Accessed 26 Aug 2007.
Shahsavari, E., Adetutu, E. M., Anderson, P. A., & Ball, A. S. (2013). Plant residues—a low cost, effective bioremediation treatment for petrogenic hydrocarbon-polluted soil. Science Total Environment, 443, 766–774.
Singh, A., & Ghoshal, N. (2013). Impact of herbicide and various soil amendments on soil enzymes activities in a tropical rainfed agroecosystem. European Journal of Soil Biology, 54, 56–62.
Singh, A., Kuhad, R. C., & Ward, O. P. (2009). Biological remediation of soil: an overview of global market and available technologies. In A. Singh, R. C. Kuhad, & O. P. Ward (Eds.), Advances in applied bioremediation. Dordrecht: Springer.
Skujins, J. J., Braal, L., & McLaren, A. D. (1962). Characterization of phosphatase in a terrestrial soil sterilized with an electron beam. Enzymologia, 25, 125–133.
Sylvia, M. D., Fuhrmann, J. J., Hartel, P. G., & Zuberer, D. A. (1999). Principles and applications of soil microbiology. Upper Saddle River: Prentice Hall.
Tabatabai, M. A., & Bremner, J. A. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology Biochemistry, 1, 301–307.
Wyszkowska, J., Kucharski, J., & Waldoska, E. (2002). The influence of diesel oil contamination on soil enzymes activity. Rost Vyroba, 48(2), 58–62.
Wyszkowska, J., Kucharski, M., & Kucharski, J. (2006). Application of the activity of soil enzymes in the evaluation of soil contamination by diesel oil. Polish Journal of Environmental Studies, 15(3), 501–506.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
del Carmen Cuevas-Díaz, M., Martínez-Toledo, Á., Guzmán-López, O. et al. Catalase and Phosphatase Activities During Hydrocarbon Removal from Oil-Contaminated Soil Amended with Agro-Industrial By-products and Macronutrients. Water Air Soil Pollut 228, 159 (2017). https://doi.org/10.1007/s11270-017-3336-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3336-2