Abstract
Groundwater composition may have a pronounced impact on long-term performance of permeable reactive barriers (PRBs). Here, batch and column experiments were conducted to investigate the effects of humic acid (HA) on Cr(VI) removal by pyrite in systems containing cations such as Ca2+ and Mg2+. HA was observed to have inhibitory effect on Cr(VI) uptake by pyrite under the experimental conditions studied (e.g., pH 3 to 8). HA sorbed onto pyrite surface and thus (1) competed against Cr(VI) for pyritic surface sites and/or (2) increased electrostatic repulsion between Cr(VI) and pyrite. In systems with HA and Ca2+/Mg2+, the Cr(VI) uptake by pyrite decreased drastically relative to HA alone due to the aggregation of HA with Ca2+/Mg2+. The formation of such HA aggregates/precipitates blocked Cr(VI) ions to reach its binding sites, thereby resulting in a substantial decrease in Cr(VI) uptake. Overall, the results have major implications for proper design and operation of PRBs with pyrite as the reactive material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Permeable reactive barriers (PRB) using Fe-containing minerals such as pyrite can offer a great potential for removing contaminants in subsurface environment (e.g., Blowes et al. 1997; Kantar et al. 2015a, b; Liu et al. 2015). However, the efficiency of PRBs to remove contaminants from aqueous systems is highly influenced by groundwater composition (e.g., pH). Subsurface systems are very complex and may contain multiple different inorganic and organic ions (Kantar 2007; Bulbul et al. 2016). Natural organic matter (NOM), for instance, is commonly found in most subsurface systems. NOM may significantly influence interactions between mineral surfaces and ions (Deng and Stone 1996). NOM contains multiple different reactive groups (e.g., carboxylic groups) for complexing with cations (e.g., Ca2+, Mg2+) in groundwater (Kantar 2007). The interactions between metal ions and NOM dominate metal ion speciation under a wide pH range, and thus affect their solubility and bioavailability. Natural organic ligands can adsorb onto mineral surfaces, and thus compete against inorganic ions for surface sites, and modify particle surface properties by changing particle surface charge and potential (Kantar 2007).
NOM has been shown to reduce redox sensitive metal ions such as Cr(VI) (Kantar et al. 2008). However, the reaction rates are very slow and may require hours to days to complete (Deng and Stone 1996; Sun et al. 2009). Fe(II)-bearing minerals can act as a catalyzer to increase Cr(VI) removal rates by organic substances (Buerge and Hug 1998; Gaberell et al. 2003). In addition, humic substances may minimize surface passivation of Fe-containing minerals (e.g., Fe0) due to the formation of soluble Fe-humic acid (HA) species (Liu et al. 2008, 2009). However, several studies, primarily, conducted with zero valent iron show that humic substances played an inhibitory role on Cr(VI) reduction by Fe0 by strongly binding with Fe0 sites, and thus blocking the Fe0 sites where processes such as chemical reduction of contaminants occur (Liu et al. 2009; Liu and Lo 2011). Liu et al. (2008) demonstrated that the Cr(VI) uptake rates by Fe0 were slightly reduced by humic acid adsorption on Fe0 surface. Although the effects of NOM on metal ion interaction with metal oxides have been heavily studied in the literature (e.g., Kantar et al. 2008), the information on the effects of NOM in Cr(VI) reduction with sulfide minerals such as pyrite is scarce in the literature. The goal of our study was to study the effect of HA on Cr(VI) removal with pyrite in systems containing major groundwater ions such as HCO3 −, Ca2+, and Mg2+. Humic acid was used as a surrogate to represent NOM in our experiments. Electrophoretic mobility and X-ray photoelectron spectroscopy (XPS) measurements were performed to better understand the role of HA on pyrite surface speciation.
2 Materials and Methods
2.1 Materials
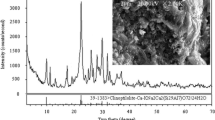
The experiments used natural pyrite samples obtained from Kastamonu, Turkey. The pyrite particles were crushed, and ground to sizes <45 μm. The BET measurements suggested that the ground pyrite samples had a surface area of 0.91 m2/g, and the XRD analysis indicated that the ground samples contained high purity pyrite (Supplementary Material, page S2; Fig. S1). All stock solutions were made by dissolving high purity chemicals (as supplied by Merck) (K2Cr2O7, MgCl2, CaCl2, NaHCO3) in ultrapure water (Millipore, Direct Q3 UV System). HA sodium salt as obtained from Aldrich was used without further purification. HA used in the experiments has been previously used to investigate the binding of natural organic ligands with mineral surfaces (Liu et al. 2008). The stock solution of HA was prepared in ultrapure water, and the solution was then filtered through 0.45-μm Millipore membrane and stored in amber bottle at 4°C. In all experiments, 0.01 M NaCl was used as the background electrolyte.
2.2 Batch Experiments
All batch experiments used 250-mL Erlenmeyer wrapped with aluminum foil to diminish photocatalytic Cr(VI) reduction. The experiments were carried out at room temperature (24°C). The total suspension volume was 200 mL and contained 10 g/L pyrite (<45 μm in size), 20 to 52 mg/L Cr(VI) and 9.5 to 95 mg C/L HA. The experiments were carried out at different pH values ranging from pH 3 to 8. In all experiments involving cations such as Ca2+ or Mg2+, the pyrite suspensions also contained 500 mg/L Ca2+or Mg2+ as CaCO3. For batch tests performed at pH 8, 103 mg/L NaHCO3 (as CaCO3) was used to determine the effects of carbonate on chromium uptake with pyrite in systems containing HA. The pyrite suspensions were continuously shaken at 175 rpm. The samples taken at predetermined reaction times from the suspensions were first centrifuged at 5600 rpm, and then analyzed for their hexavalent chromium and total metal (Cr, Fe) contents. Concentrations of dissolved Cr(VI) and Fe(II) were determined using 1,5 diphenylcarbazide and 1.10-phenanthroline colorimetric procedures, respectively (APHA 1995). The concentration of humic acid (HA), expressed as dissolved organic carbon (mg/L DOC), was analyzed by a TOC-TN analyzer (Hach 550 IL). The total concentrations of Cr and Fe were determined with atomic absorption spectroscopy (AAS) (Thermo ICE 3000 Series). Solution pH was measured with Orion Ross 5-Star (Thermo). All batch experiments were performed in duplicate.
2.3 XPS and Electrophoretic Measurements
The pyrite samples collected at the end of batch experiments were analyzed with XPS to determine pyrite surface speciation. Electrophoretic measurements were carried out with Brookhaven ZetaPals zeta meter. Experimental conditions for these measurements were given in detail in Supplementary Material (Page S3).
2.4 Column Experiments
A liquid chromatography column (I:D 2.2 cm) was used to investigate the effects of HA on Cr(VI) mobility in columns packed with pyrite under continuous flow conditions in systems containing major groundwater constituents (Ca2+, Mg2+, and HCO3 −) at room temperature (24°C). The columns were packed with 40 g pyrite (<45 μm in size) to a bulk density of 2.84 g/cm3 and a porosity of 0.44. An HPLC pump was used to inject solutions upward into columns at a rate of 0.05 mL/min. Prior to experiments, the columns were preconditioned with 0.01 M NaCl electrolyte solution containing 103 mg/L HCO3 − as CaCO3. Synthetic groundwater containing HA and/or Cr(VI) was then fed into the columns, and the effluent samples collected at desired intervals were analyzed for Fe(II), Cr(VI), HA, and total metal (Fe, Cr) contents. The input solution contained Cr(VI) (CT = 20–104 mg/L) and/or HA (CT = 9.5–95 mg C/L). These concentrations used in the experiments lie within the range reported for Cr(VI) contaminated sites (Loyaux-Lawniczak et al. 2001). In experiments involving Ca2+ or Mg2+, the synthetic groundwater also contained 500 mg/L (as CaCO3) Ca2+/Mg2+. In all experiments, the electrolyte solution and artificial groundwater had an initial pH of 8 and contained HCO3 − (103 mg/L HCO3 −as CaCO3) to minimize pH drift. The column experiments are explained in detail in Supplementary Material, pages S4-S6 (Fig. S2 and Fig.S3).
3 Results and Discussion
3.1 Batch Studies
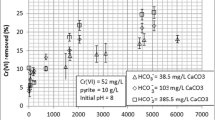
The influence of HA and pH on Cr(VI) uptake by pyrite is presented in Fig. 1a. Hereafter, the term “uptake” will be used in the text to explain chromium removal from solution since multiple different mechanisms (e.g., sorption, reduction and precipitation) are known to participate in Cr(VI) removal by pyrite. Note that, in all cases studied, the Cr(VI) uptake with pyrite decreased with increasing solution pH. This result coincides well with the results of other researchers (e.g., Lin and Huang 2008; Kantar and Bulbul 2016). Cr(VI) reduction may occur directly on pyrite surface and/or in solution with the oxidation of disulfide and Fe(II) to sulfate and Fe(III), respectively (Lin and Huang 2008; Kantar et al. 2015a). Subsequently, the reaction products such as Cr(III) and Fe(III) precipitate as Cr3+/Fe3+ hydroxides and/or mixed Fe3+/Cr3+ (oxy)hydroxides depending on solution pH (Lin and Huang 2008).
As shown in Fig. 1a, humic acid had a significant inhibitory effect on Cr(VI) uptake by pyrite relative to non-HA containing systems (Fig. 1a) at pH values ranging from pH 3 to 8. This is in contradiction with the results obtained for low molecular weight organic acids such as citrate. For instance, Kantar et al. (2015a) found that while low molecular weight organics had stimulatory effect on Cr(VI) removal by pyrite under acidic to slightly alkaline pH conditions, the inhibitory effect was observed only at pH > 8. This is not surprising since HA is a complex mixture of multiple different functional groups (e.g., carboxylic, phenolic, hydroxylic, amine, and phosphate groups) with different sorptive characteristics whereas low molecular weight organic acids contain only a few reactive functional groups such as carboxylic groups. The decrease in Cr(VI) uptake by pyrite in the presence of HA may be explained through the binding of HA onto pyrite surface, which then led to an increase in the intensity of surface charges on pyrite surface (Fig. 1b). HA exhibited ligand-like binding behavior with HA uptake decreasing with increasing solution pH (Fig. 1b). The decrease in HA uptake by pyrite with increasing solution pH occurred due to an increase in electrostatic repulsion between negatively charged pyrite surface and deprotonated HA. The electrophoretic measurements for pyrite are given in Fig. 2. While the pyrite surface was negatively charged in systems containing pyrite only under a wide pH range, the addition of Cr(VI) significantly changed surface zeta potential from negative to positive under acidic to slightly neutral conditions due to oxidation and formation of Fe/Cr (oxy) hydroxides on pyrite surface (Kantar et al. 2015a). As given in Fig. 2, the pyrite reacted with Cr(VI) had a pHpzc value of ∼5.5, which lies in between the pHpzc values observed for pyrite and Fe (III) (hydr)oxides. Note that while the pHpzc value of unreacted pyrite ranges from 1.5 to 2.5, that reported for Fe (III) (hydr)oxides lies between 6 and 9 (Bonnissel-Gissinger et al. 1998; Kantar 2016). On the other hand, the addition of HA led to charge reversal due to the binding of HA onto Fe(II) reactive sites associated with pyrite. As HA binds with pyrite, it competes against Cr(VI) for Fe(II) sites on pyrite. HA has been shown to bind more strongly onto metal oxides under acidic conditions due to an increase in proton neutralization, electrostatic attraction, and specific interaction (Liu et al. 2008; Wang et al. 2011). Negative surface charge on HA arises from deprotonation of carboxylic groups at pH > 3 (Kantar 2007; Cetin et al. 2009).
Reaction kinetics for the uptake of Cr(VI) with pyrite are presented in Fig. 3. Except for experiments involving Ca2+ or Mg2+, the Cr(VI) uptake with pyrite showed an initial fast uptake rate, followed by much slower Cr(VI) uptake at both pH 4 and 8. According to Kantar et al. (2015a, b), the initial rapid Cr(VI) uptake occurred due to presence of higher number of fresh reactive sites on pyrite in the initial stage. However, the reaction rates became smaller as the reactive sites were consumed or covered with surface precipitates over time. The addition of HA significantly decreased Cr(VI) uptake at pH 4 (Fig. 3a). As described above, the decrease observed in Cr(VI) uptake in systems containing HA under acidic to alkaline pH conditions was caused by the modification of surface electrostatic properties of pyrite due to sorption of HA on pyrite surface. The binding of HA onto pyrite increased surface negativity, and thus the electrostatic repulsion between pyrite surface and deprotonated Cr(VI) species (e.g., HCrO4 −, CrO4 2−) (Fig. 2). Note that while the dominant Cr(VI) species under acidic to neutral pH condition is HCrO4 −, CrO4 2− becomes the primary Cr(VI) species at pH > 7 (Kantar et al. 2015a).
As shown in Fig. 3b, the addition of HA to systems containing HCO3 − with Ca2+ or Mg2+ significantly reduced Cr(VI) uptake rates compared to systems containing HCO3 − only at pH 8. Note that, unlike systems without Ca2+ or Mg2+, the Cr(VI) uptake followed a linear trend with time. This discrepancy in reaction kinetics may be explained through competitive effect observed between HA and CrO4 2− for sorption sites as well as significant accumulation of surface precipitates/aggregates (e.g., CaCO3(S)) which led to a decrease in the number of sorption sites (Tinnacher et al. 2013; Kantar and Bulbul 2016; Bulbul et al. 2016). Liu et al. (2008, 2009) and Liu and Lo (2011) showed that the co-existence of Ca2+ or Mg2+ with humic acid led to co-aggregation and precipitation of HA with Ca/Mg and Fe (hydr)oxides on Fe0 surface during Cr(VI) uptake by Fe0. Similarly, Liu et al. (2008) found that a minor decrease in Cr(VI) uptake by Fe0 occurred due to the fact that both Ca2+ and Mg2+ ions were able to promote HA adsorption onto Fe oxides via the formation of type A ternary Fe0/Ca (or Mg)/HA surface complex. Humic substances are known to strongly complex with cations (e.g., Fe2+, Ca2+, and Mg2+) through multiple different reactive groups (e.g., carboxylic reactive sites) found in their structures (Kantar 2007; Liu et al. 2008). The formation of such HA complexes with Ca2+ or Mg2+ can reduce electrostatic repulsion between negatively charged mineral surfaces and HA, which, in turn, may lead to enhanced HA adsorption and aggregation (Kantar 2007). This was clearly evidenced by the accumulation of visible large aggregates in solution in our experimental systems. According to Liu et al. (2008, 2009), the binding of HA with Ca2+ and Mg2+ may lead to a more compact orientation and aggregation of HA molecules due to charge neutralization, thereby resulting in a decrease in intramolecular electrostatic repulsive forces. The addition of Ca2+ or Mg2+ may induce a bridging effect for co-aggregation and co-precipitation of Fe (oxy)hydroxide colloids and humic acid (Liu et al. 2009).
3.2 Column Studies
The influence of HA on Cr(VI) transport in pyrite packed-columns is presented in Fig. 4. Figure 4a shows that, in systems containing 20 mg/L Cr(VI), while the initial breakthrough occurred at 163 pore volumes in the absence of HA, the addition of 9.5 mg C/L HA significantly enhanced Cr(VI) mobility with an initial breakthrough occurring at 73 pore volumes. The enhanced Cr(VI) mobility in the presence of HA may be explained through the sorption of HA onto pyrite surface, which, in turn, led to the blockage of pyritic reactive sites for the access of Cr(VI). Figure 5 shows the corresponding effluent pH and total Fe concentrations in the absence or presence of HA. While the effluent pH exhibited an increasing trend in the absence of HA due to proton consuming nature of Cr(VI) uptake by pyrite, the effluent pH remained stable throughout the experiment in systems containing HA (Fig. 5a), indicating that HA had a pronounced impact on pH buffering capacity. As shown in Fig. 1b, HA interacts more strongly with pyrite surface under more acidic conditions, and thus can modify pyrite surface properties. Despite the fact that a small portion of Fe was mobilized in the presence of HA relative to non-HA system (Fig. 5b), apparently, it was not adequate enough to prevent surface passivation of pyrite surface. This is contrary to the results obtained for low molecular weight organic acids such as oxalate (Kantar 2016). A number of recent work showed that low molecular weight organic acids significantly increased the dissolution of surface oxidation products during Cr(VI) removal by pyrite, thereby creating new surface sites for enhanced Cr(VI) removal (Kantar et al. 2015b; Kantar and Bulbul 2016; Kantar 2016). Similarly, the analysis of effluent for Cr species suggested that 100% of Cr in the effluent was in hexavalent form, implying that Cr(VI) sorbed and/or precipitated within the column (data not shown).
Additional column experiments were preformed to investigate the effects of major groundwater ions such as Ca2+ and Mg2+ on Cr(VI) mobility in systems containing HA (Fig. 4b). Note that the co-injection of HA with Ca2+ or Mg2+ further increased Cr(VI) mobility compared to systems containing HA alone. This indicates that HA in systems with Ca2+ and Mg2+ would have more severe adverse effects on long-term performance of PRBs. In a column study with Fe0, the co-injection of Ca2+ with HA significantly reduced Cr(VI) removal capacity of Fe0 (Liu and Lo 2011). Especially, in systems with humic acid, a significant pressure build up occurred within the column, indicating co-precipitation and aggregation of humic acid with Ca2+/Mg2+ and Fe oxides, which not only adversely affected surface reactivity of pyrite grains but also significantly lowered permeability of pyrite-packed columns. For instance, the Cr(VI) breakthrough curve for HA and HCO3 − system demonstrated a sharp increase in C/Co values at 20 pore volumes due to consumption of all reactive sites and clogging of pore spaces with precipitation products (Fig. 4b). In our previous study, we observed a similar effect of low molecular weight organic acids (e.g., tartrate) on breakthrough curves of Cr(VI) in pyrite-packed columns (Kantar and Bulbul 2016). While, in systems with Ca2+, the Cr(VI) breakthrough curve exhibited a self-sharpening front, a diffuse front was observed in the presence of Mg2+. According to Liu et al.(2008), the aggregation/sorption of HA onto Fe0 occurred earlier in systems with Ca2+ than Mg2+ due to much higher affinity of Ca2+ for binding with HA. Wall and Choppin (2003) reported that HA coagulation was much higher in the presence of Ca2+ relative to systems containing Mg2+ due to its much smaller size of the hydrated ions. In addition, the accumulation of HA aggregates on pyrite surface inhibited electron transfer between pyrite and Cr(VI), which had an inhibitory effect on Cr(VI) removal capacity of pyrite.
3.3 XPS Analysis
The effects of HA on C 1s, O 1s, S 2p, and Fe 2p XPS spectra of pyrite reacted with 52 mg/L Cr(VI) at pH 3 are presented in Fig. 6. The binding energies of the C1 s line exhibited peaks at 283.65 eV for C, 285.08 for C-S, and 287.59 eV for carboxyl carbon, indicating the binding of HA onto pyrite surface (Fig. 6a). Similarly, the O 1s spectra showed peaks at the binding energies of 530.5, 531.55, and 528.6 eV, which corresponded to O–H, O–S, and O–Fe surface species, respectively (Fig. 6b), indicating presence of Fe- and Cr-hydrolyzed surface species. The S 2p spectra (Fig. 6c) are associated with peaks at 161.34, 162.65, and 167.77 eV, suggesting a partial oxidation of disulfide to sulfate on pyrite surface during Cr(VI) removal. The binding energies of Fe 2p lines centering at 705.9, 718.74, 709.75, and 723.29 eV suggested the existence of Fe(II)-S and hydrolyzed Fe(III) species, implying partial oxidation and precipitation of Fe(III)-(hydr)oxides on pyrite surface. This is not surprising since the pyritic Fe(II) sites were oxidized to Fe(III), while reducing Cr(VI) to Cr(III) on pyrite surface. While the analysis of Cr 2p spectra showed peaks at 576.45 and 585.04 eV for reduced chromium species (e.g., Cr(III)), no peaks for hexavalent chromium were observed on pyrite surface (Fig. S4, Supplementary Material). Considering the low surface area of pyrite samples used in the experiments, the direct sorption of Cr(VI) onto pyrite surface was negligible, and the main mechanism responsible for Cr(VI) removal with pyrite was Cr(VI) reduction to Cr(III), followed by precipitation and/or sorption of Cr(III) onto pyrite surface (Kantar et al. 2015a).
The XPS analysis of pyrite reacted with 52 mg/L Cr(VI) in system containing 103 mg/L HCO3 − as CaCO3, 500 mg/L Ca2+ as CaCO3, and 200 mg/L HA at pH 8 is shown in Fig. 7a, b. The binding energies of C1 s and Ca 2p centered at 287.37 and 346.19 eV, respectively, indicating the existence of carboxyl carbon and carbonates (e.g., CaCO3) due to adsorption and/or co-precipitation/co-aggregation of HA with Ca2+ and hydrolyzed metal species onto pyrite surface (Liu et al. 2008). Similarly, Fig. 7c, d shows the effects of HA on pyrite surface speciation in the presence of 52 mg/L Cr(VI), 103 mg/L HCO3 − as CaCO3, 500 mg/L Mg2+ as CaCO3, and 200 mg/L HA at pH 8. The C 1s and Mg 2p spectra show peaks for carboxyl carbon at 287.59 eV and Mg-O at 49.23 eV, implying the sorption and accumulation of HA aggregates with hydrolyzed metal species containing Mg-(hydr) oxides on pyrite surface.
Fitted C 1s (a) and Ca 2p (c) XPS spectra of pyrite (10 g/L) for experiments conducted at pH 8 in 0.01 M NaCl solution containing an initial Cr(VI) concentration of 52 mg/L, an initial humic acid concentration of 200 mg/L, a Ca2+ concentration of 500 mg/L as CaCO3, and a HCO3 − concentration of 103 mg/L as CaCO3. Fitted C 1s (b) and Mg 2p (d) XPS spectra of pyrite (10 g/L) exposed to 52 mg/L Cr(VI) at pH 8 in 0.01 M NaCl containing 200 mg/L humic acid, 500 mg/L Mg2+ as CaCO3, and 103 mg/L HCO3 − as CaCO3
4 Conclusion
The role of HA on Cr(VI) uptake by pyrite in system containing major groundwater cations such as Ca2+ and Mg2+ was investigated in a series of batch and column experiments. Our batch experiments showed that HA had inhibitory effects on Cr(VI) uptake under the experimental conditions investigated (e.g., pH 3 to 8). In experiments involving Ca2+ or Mg2+, HA was shown to significantly lower Cr(VI) uptake compared to systems containing HA alone. Column results showed that, in systems with HA, a significant pressure build occurred within the column due to co-precipitation and aggregation of humic acid with Ca2+/Mg2+, which not only adversely affected the reactivity of pyrite surface but also column hydraulic properties. Electrophoretic measurements proved that HA modified pyrite surface electrostatic properties by binding with pyritic reactive sites, which led to an in increase in electrostatic repulsion between reactive sites and CrO4 2−. In addition, the XPS data also provided further evidence for the deposition of precipitates and/or aggregates containing HA with Mg (hydr) oxides/Ca-carbonates on pyrite surface, which eventually blocked electron transfer between pyrite and Cr(VI). Overall, it is clear that the effects of HA and cations such as Ca2+ and Mg2+ on Cr(VI) removal must be taken into consideration for the proper design and operation of PRBs containing pyrite as the reactive material.
References
APHA. (1995). Standard methods for the examinations of water and wastewater (19th ed.). Washington, DC: American Public Health Association.
Blowes, D. W., Ptacek, C. J., & Jambor, J. L. (1997). In-situ remediation of Cr(VI)-contaminated groundwater using permeable reactive walls: laboratory studies. Environmental Science & Technology, 31(12), 3348–3356.
Bonnissel-Gissinger, P., Alnot, M., Ehrhardt, J.-J., & Behra, P. (1998). Surface oxidation of pyrite as a function of pH. Environmental Science & Technology, 32, 2839–2845.
Buerge, I. J., & Hug, S. J. (1998). Influence of organic ligands on chromium (VI) reduction by iron (II). Environmental Science & Technology, 32, 2092–2099.
Bulbul, M. S., Kantar, C., & Keskin, S. (2016). Role of major groundwater ions on reductive Cr(VI) immobilization in subsurface systems with pyrite. Water, Air, & Soil Pollution, 227(3), 1–11.
Cetin, Z., Kantar, C., & Alpaslan, M. (2009). Interactions between uronic acids and Cr(III). Environmental Toxicology and Chemistry, 28, 1599–1608.
Deng, B., & Stone, A. T. (1996). Surface catalyzed chromium reduction: reactivity comparisons of different organic reductants and different oxide surfaces. Environmental Science & Technology, 30, 2484–2494.
Gaberell, M., Chin, Y.-P., Hug, S. J., & Sulzberger, B. (2003). Role of dissolved organic matter composition on the photoreduction of Cr(VI) to Cr(III) in the presence of iron. Environmental Science & Technology, 37, 4403–4409.
Kantar, C. (2007). Heterogeneous processes affecting metal ion transport in the presence of organic ligands: Reactive transport modeling. Earth-Science Reviews, 81, 175–198.
Kantar, C. (2016). Role of low molecular weight organic acids on pyrite dissolution in aqueous systems: Implications for catalytic chromium (VI) treatment. Water Science and Technology, 74(1), 99–109.
Kantar, C., & Bulbul, M. S. (2016). Effect of pH-buffering on Cr(VI) reduction with pyrite in the presence of various organic acids: Continuous-flow experiments. Chemical Engineering Journal, 287, 173–180.
Kantar, C., Cetin, Z., & Demiray, H. (2008). In situ stabilization of chromium(VI) in polluted soils using organic ligands: The role of galacturonic, glucuronic and alginic acids. Journal of Hazardous Materials, 159, 287–293.
Kantar, C. A., Keskin, S., Dagaroglu, Z. G., Karadeniz, A., & Alten, A. (2015a). Cr(VI) removal from aqueous systems using pyrite as the reducing agent: batch, spectroscopic and column experiments. Journal of Contaminant Hydrology, 174, 28–38.
Kantar, C., Ari, C., & Keskin, S. (2015b). Comparison of different chelating agents to enhance reductive Cr(VI) removal by pyrite treatment procedure. Water Research, 76, 66–75.
Lin, Y.-T., & Huang, C.-P. (2008). Reduction of chromium (VI) by pyrite in dilute aqueous solutions. Separation and Purification Technology, 63, 191–199.
Liu, T., & Lo, I. M. C. (2011). Influences of humic acid on Cr(VI) removal by zero-valent iron from groundwater with various constituents: implication for long-term PRB performance. Water, Air, and Soil Pollution, 216, 473–483.
Liu, T., Tsang, D. C. W., & Lo, I. M. C. (2008). Chromium (VI) reduction kinetics by zero-valent iron in moderately hard water with humic acid: iron dissolution and humic acid. Environmental Science & Technology, 42, 2092–2098.
Liu, T., Rao, P., Shi, J., & Lo, I. M. C. (2009). Influences of humic acid, bicarbonate and calcium on Cr(VI) reductive removal by zero-valent iron. Science of the Total Environment, 407, 3407–3414.
Liu, Y., Mou, H., Chen, L., Mirza, Z. A., & Liu, L. (2015). Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: environmental factors and effectiveness. Journal of Hazardous Materials, 298, 83–90.
Loyaux-Lawniczak, S., Lecomte, P., & Ehrhardt, J. J. (2001). Behavior of hexavalent chromium in a polluted groundwater: redox processes and immobilization in soils. Environmental Science & Technology, 35(7), 1350–1357.
Sun, J., Mao, J., Gong, H., & Lan, Y. (2009). Fe(III) photocatalytic reduction of Cr(VI) by low-molecular weight organic acids with α-OH. Journal of Hazardous Materials, 168, 1569–1574.
Tinnacher, R. M., Nico, P. S., Davis, J. A., & Honeyman, B. D. (2013). Effects of fulvic acid on uranium (VI) sorption kinetics. Environmental Science & Technology, 47, 6214–6222.
Wall, N. A., & Choppin, G. R. (2003). Humic acids coagulation: influence of divalent cations. Applied Geochemistry, 18, 1573–1582.
Wang, Q., Cissoko, N., Zhou, M., & Xu, X. (2011). Effects and mechanisms of humic acid on chromium (VI) removal by zero-valent iron (Fe0) nanoparticles. Physics and Chemistry of the Earth, 36, 442–446.
Acknowledgements
The study was fully funded by the Scientific and Technological Research Council of Turkey (TUBITAK) under a contract number of 114Y024.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 195 kb)
Rights and permissions
About this article
Cite this article
Kantar, C., Bulbul, M.S. & Keskin, S. Role of Humic Substances on Cr(VI) Removal from Groundwater with Pyrite. Water Air Soil Pollut 228, 48 (2017). https://doi.org/10.1007/s11270-016-3233-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3233-0