Abstract
The aim of this study was to evaluate the redox mediating capacity of anthraquinone-2,6-disulfonate (AQDS) immobilized on granular activated carbon (GAC) during the reductive decolorization of direct blue 71 (DB71) under microbial and chemical conditions. The immobilization of AQDS on GAC was conducted by adsorption, and it has obtained an uptake capacity of 0.227 mmol g−1. The anchorage of AQDS on GAC improved its electron transfer capacity (ETC) up to 2.05 times higher than the raw material. Similarly, the addition of GAC-AQDS increased up to 1.75- and 1.16-fold the rate of decolorization (k d ) of DB71 under microbial and chemical conditions, respectively, in comparison to the unmodified GAC. Surprisingly, a higher k d value was achieved in incubations without either GAC or GAC-AQDS because of the generation of aromatic amines, from the reduction DB71, taking into account that these species may act as a catalyst in the DB71 reduction process. In contrast, adsorption of aromatic amines on either GAC or GAC-AQDS decreased its redox mediating capacity as evidenced by spectrophotometric screenings of the decolorized solution and the supporting material. The development of materials with enhanced both redox and adsorption properties, as the GAC used in this study, offers a promising way to increase the redox conversion of recalcitrant pollutants commonly found in industrial wastewaters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It has been extensively documented that humic substances and quinone analogs play an important role catalyzing the transformation of several electron-accepting contaminants by acting as redox mediators (RM) in both microbial and chemical schemes. The addition of RM in the selected systems promotes the reductive (bio)transformation of pollutants and accelerates the process by several orders of magnitudes (Van der Zee and Cervantes 2009). A number of pollutants such as polyhalogenated compounds (aromatics and aliphatic), nitroaromatics, azo dyes, oxidized metalloids are susceptible to be reduced mediated with humus and quinones as RM. These pollutants are mainly present in effluents of the chemical, petrochemical, and textile industries (Martinez et al. 2013; van der Zee et al. 2001). Because of its electrophilic nature, these types of contaminants are not susceptible to be treated by convectional aerobic wastewater treatment systems. In contrast, these pollutants are susceptible to suffer reductive (bio)transformations under anaerobic conditions (Field et al. 1995). Nevertheless, the reductive transformation of several recalcitrant compounds occurs very slowly due to electron transfer limitations and to toxicity effects leading to poor performance or collapse of anaerobic bioreactors (Rodgers and Bunce 2001; van der Zee et al. 2001). Moreover, it has been extensively documented that these limitations may be lessened with the usage of RM (van der Zee et al. 2001).
Despite the advantages of using RM for the treatment of electrophilic pollutants, the application at full scale in wastewater treatment systems is limited due to its high solubility that implies the continuous addition in anaerobic reactors, making the process expensive and environmentally non-viable (Van der Zee and Cervantes 2009). As a response to overcome the continuous addition of RM, it has been documented the use of different support materials to immobilize RM and its application in reductive processes of pollutants (Martinez et al. 2013). Several immobilizing mechanisms have been tried for supporting RM on several materials, for instance entrapment (Guo et al. 2007, 2010a), ion exchange (Cervantes et al. 2010, 2011), covalent binding (Lu et al. 2010), electropolymerization (Li et al. 2008, 2009), and adsorption (Alvarez et al. 2010, 2012; Alvarez and Cervantes 2012). Furthermore, some carbonaceous materials such as fibers, graphite, and granular activated carbon (GAC) have been chemically modified under acidic or thermal conditions (Alvarez et al. 2013) or through the immobilization of quinones (Amezquita et al. 2014) with their subsequent usage as RM during the microbial or chemical reductive transformation of several contaminants. In this study, the anthraquinone-2,6-disulfonate (AQDS) immobilized on GAC was used as solid-phase RM to reduce direct blue 71 (DB71) under microbial and chemical conditions.
2 Materials and Methods
2.1 Chemicals, Basal Medium, and Inoculum
AQDS (98% of purity) and DB71 (85% of purity) were purchased from Sigma Aldrich. The GAC (Carboactive 8 × 30, Clarimex), was firstly sieved to recover particles in a range between 0.5 and 1 mm. The anaerobic granular sludge was collected from an up-flow anaerobic reactor (UASB reactor) installed in a brewery industry (Sonora, Mexico), and it contains 9.6% of volatile suspended solids (VSS). The sludge, with a proven capacity to reduce humic substances and quinones, was washed with distilled water and disintegrated with a sieve of 0.4 mm previous to be inoculated. The basal medium (pH 7.0) for decolorization assays of DB71 was prepared as follows (mg L−1): NaHCO3 (5000), NH4Cl (300), K2HPO4 (200), MgCl2•6H2O (30), CaCl2 (10), and 1 mL L−1 of trace elements solution containing (mg L−1) of FeCl2•4H2O (2000), H3BO3 (50), ZnCl2 (50), CuCl2•2H2O (38), MnCl2•4H2O (500), (NH4)6Mo7O24•4H2O (50), AlCl3•6H2O (90), CoCl2•6H2O (2000), NiCl2•6H2O (92), Na2SeO•5H2O (162), EDTA (1000), and 1 mL L−1 of HCl (36%).
2.2 Anchorage of AQDS on GAC
To improve the electron transfer capacity (ETC) of GAC, AQDS was adsorbed on the material. The adsorption step was carried out mixing a solution of 1.5 g AQDS L−1 and 2 g GAC L−1 at pH 4.0. The vial was kept in a shaker at 30°C and 150 rpm until the equilibrium was achieved, and the adsorption capacity of AQDS was calculated as follows:
where q e is the adsorption capacity (mg g−1), Co and Ce are the initial and equilibrium concentration of AQDS (mg L−1), respectively, V is the volume of solution (L), and m is the mass of GAC (g). Then, AQDS-load GAC was dried at 100°C during 24 h. After that period, the cooled and modified GAC (GAC-AQDS) was exposed six times to the basal medium at pH 7, 30°C, and 150 rpm to verify a possible desorption of AQDS. In all cases, AQDS in solution was monitored spectrophotometrically at 330 nm. The remaining adsorption capacity of AQDS on GAC after the desorption cycles (q d ) was calculated with the following equation:
where C e is equilibrium concentration of AQDS (mg L−1), V d is the volume of desorption solution (L), and m is the mass of GAC (g), q e is the initial adsorption capacity value (mg g−1), and the subsequent q d values were calculated with the previous q d obtained for each desorption cycle.
On the other hand, the ETC was determined microbiologically in both GAC-AQDS and GAC. Serum bottles of 60 mL (by triplicate) were prepared as follows: 10 mL of the basal medium previously described, 1 g VSS L−1 of anaerobic sludge, 1 g L−1 of GAC-AQDS or GAC, 2 g L−1 of glucose as electron donor, and N2/CO2 (80%/20%) to establish anaerobic conditions. All vials were incubated in a shaker at 30°C and 150 rpm during 2 days. Then, the ETC was determined following the ferrozine method as previously described (Lovley et al. 1996). Briefly, 10 mL of ferric citrate (20 mmol L−1) were added to the vials to obtain a final concentration of 10 mmol L−1 and allowed to react during 30 min. Then, 20 mL of 0.5 mmol L−1 HCl were added to the vial and an aliquot was mixed with the ferrozine solution for spectrophotometric (562 nm) determination of Fe(II). The Fe(II) concentration was used to calculate the ETC from GAC-AQDS or GAC to Fe(III). All ETC measurements were corrected for intrinsic capacity of anaerobic sludge to reduce Fe(III) by using a control without any type of GAC. Thus, interference of redox-active molecules from the sludge on the ETC measurements can be diminished.
2.3 Microbial and Chemical Decolorization of DB71
Decolorization assays were performed in 120 mL serum glass bottles with the basal medium (50 mL) previously described. The bottles were inoculated with 0.1 g VSS L−1 of anaerobic sludge and provided with 1 g L−1 of glucose as a sole electron donor, 150 mg L−1 of DB71, and 1 mmol L−1 of AQDS immobilized or soluble. After sealing the bottles with rubber stoppers and aluminum caps, the anaerobic conditions were established with a mixture of N2/CO2 (80%/20%). The included control experiments were sludge with GAC (in all cases equivalent to the mass used with GAC-AQDS) to evaluate the role of the unmodified material as RM, sterile with GAC-AQDS or GAC to evaluate a possible adsorption of DB71, endogenous with GAC, sludge without AQDS nor GAC, and finally a control with sludge including GAC with soluble AQDS (AQDSsol). All conditions were carried out by triplicate and incubated at 30°C and 150 rpm. The chemical decolorization of DB71 was conducted with 2.5 mmol L−1 of sodium sulfide (Na2S) and 2 mmol L−1 of AQDS immobilized and the conditions previously described.
3 Results and Discussion
3.1 Anchorage of AQDS on GAC
Figure 1 shows the adsorption capacity of ADQS on GAC in the first cycle as well as the remaining adsorption capacity when the AQDS-loaded GAC was desorbed with basal medium. The adsorption capacity of AQDS on GAC initially achieved 0.276 mmol g−1, and it decreased to 0.227 mmol g−1 after to expose the AC-AQDS to six desorption cycles; it represents a decrement of about 18% with respect to the initial adsorption capacity. The anchorage mechanism of AQDS on GAC could be associated with electrostatic interactions between the sulfonate groups (−SO3 −) of AQDS and the positive functional groups in GAC prevailing at pH 4.0 (according to a previous surface charge distribution obtained). Another physical interactions between GAC and AQDS may related to π–π stacking or π donor–acceptor mechanisms (Zhu and Pignatello 2005). The chemical modification of GAC with AQDS significantly improved the redox capacity of this material, evidenced by an increment of 2.05-fold in the ETC with respect to the unmodified GAC. The ETC values obtained were 0.408 and 0.199 meq g−1 for GAC-AQDS and GAC, respectively (Table 1).
3.2 Decolorization of DB71 by AQDS Immobilized on GAC
The catalytic effect of GAC-AQDS was evaluated during the microbial and chemical decolorization of DB71 by using anaerobic sludge and sodium sulfide, respectively. Figures 2 and 3 depict decolorization profiles of DB71 using GAC-AQDS under microbial and chemical systems. Decolorization of DB71 followed first-order kinetics, and the first-order rate constants (k d ) of decolorization were calculated according to the following equation:
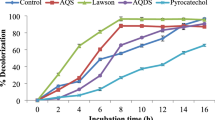
where C is the concentration at a given time (mg L−1); C0 is the initial concentration (mg L−1); k d is the first-order rate constant of decolorization (h−1), and t is the reaction time (h). Decolorization of DB71 could be mainly attributable to a biotransformation process induced by the electron equivalents produced during the anaerobic oxidation of substrate. Certainly, sterile controls supplemented with either GAC-AQDS or GAC showed a negligible decolorization (1%) after 27 h of incubation. This issue can also indicate that an adsorption process was not responsible of DB71 decolorization in active assays. Similarly, the incubations under endogenous conditions only reached 4% of decolorization in the same period. In contrast, the active incubations supplemented with anaerobic sludge showed different decolorization efficiencies depending on the tested conditions as follows: AQDSsol (82.6%), GAC with AQDSsol (56.1%), GAC-AQDS (46.3%), and GAC (15.1%) (Table 1). Moreover, the decolorization efficiencies achieved under chemical conditions were sodium sulfide (63%), sodium sulfide with GAC-AQDS (52.3%), and sodium sulfide with GAC (46.6%) (Table 1). The catalytic effect of GAC-AQDS was confirmed by the k d values observed in both microbial (0.028 h−1) and chemical (0.085 h−1) conditions; values up to 1.75 and 1.16 times higher, respectively, as compared to the control with unmodified GAC. The highest k d (0.12 h−1) value and decolorization efficiency (82.6%) were achieved by the incubation with AQDSsol under microbial conditions.
Profile of DB71 decolorization by anaerobic sludge. Symbols: (white circle) sterile with GAC-AQDS, (white square) sterile with GAC, (white triangle) endogenous, ( ) sludge + GAC, (
) sludge + GAC, ( ) sludge + GAC-AQDS, (
) sludge + GAC-AQDS, ( ) sludge + GAC with AQDSsol, (
) sludge + GAC with AQDSsol, ( ) sludge AQDS nor GAC, (
) sludge AQDS nor GAC, ( ) sludge + AQDSsol. In all cases, the error was lower than 5%
) sludge + AQDSsol. In all cases, the error was lower than 5%
Previously, different authors have reported the use of immobilized AQDS during the microbial decolorization of azo dyes by using calcium alginate (Guo et al. 2007, 2010b) and ion exchange resins (Cervantes et al. 2010, 2011). The use of GAC has also been tested as RM for microbial decolorization processes because of its redox functional groups (Mezohegyi et al. 2007; Van Der Zee et al. 2003). Nevertheless, some disadvantages of these immobilizing methods include the gradual loss of the redox mediating capacity due to either wash-out of the RM from bioreactors (Van Der Zee et al. 2003) or disintegration of the immobilizing material as well as the mass transfer limitations since the RM remained entrapped within the immobilizing material (Guo et al. 2007). In other studies, AC felt (modified with AQDS by the electropolymerization method) was used to reduce azo dyes (Li et al. 2009; Wang et al. 2009) and nitroaromatics (Li et al. 2008). The increment in the k d values varied from 1 to 5.7 times higher than the controls without AQDS, and they were similar to the increments observed in the present study.
Moreover, the significance of the chemical decolorization assays is related to the presence of sulfate in textile effluents that is microbially reduced to sulfide in anaerobic digesters and is capable to act as reducing agent of azo dyes (Cervantes et al. 2007). Pereira et al. (2010) reported that the presence of sulfide and AC as RM influenced the decolorization of acid orange 7, reactive red 2, and mordant yellow, obtaining k d values of 0.091, 0.054, and 0.079 h−1, respectively. These results are similar to the obtained in the present study, reaching 0.073 and 0.085 h−1 for GAC and GAC-AQDS, respectively (Table 1).
The results also indicate that the incubations in the absence of AQDS or GAC showed an important catalytic activity (Figs. 2 and 3). For instance, the k d values in incubations lacking AQDS or GAC were 0.069 and 0.101 h−1 for microbial and chemical conditions, respectively. These values are higher than the values obtained in all experiments except to the incubations with AQDSsol (Table 1). These results could be associated with the autocatalytic capacity of aromatic amines generated by the reduction of DB71. The autocatalytic capacity of the reduced compounds was previously documented in experiments for methyl orange reduction supplemented with small doses of a decolorized solution of DB71 (Encinas-Yocupicio et al. 2006). These authors found an increment up to 2 times on the rate of decolorization of methyl orange with respect to the control lacking the supplement of decolorized DB71. Moreover, incubations containing GAC (with or without AQDS) showed extents of decolorization lower than incubations without adsorbent. This behavior is presumably due to an adsorption phenomenon of the aromatic amines on GAC, which limited its autocatalytic capacity. Further adsorption experiments on GAC revealed that the intensity of signal at 250 nm of a solution containing decolorized DB71 decreased over time (measured by UV–Vis spectrophotometry), indicating a possible adsorption (Fig. 4). This experiment was conducted using a decolorized (≥98%) solution of DB71 obtained from a microbial incubation with similar conditions previously described. Then, the solution was transferred to bottles (under anaerobic conditions) containing GAC by using a syringe provided with a filter (0.2 μm). The UV–Vis spectrophotometric screening (200–800 nm) revealed a decrement in the intensity of the signal of the decolorized solution up to ∼26 and 61% compared to the initial signal observed at 250 nm, after 2 and 30 h of incubation, respectively.
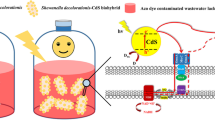
Figure 5 shows the reduction mechanisms involved during the decolorization of DB71 mediated by both AQDS and the produced aromatic amines. The initial step of DB71 decolorization is the microbial or chemical reduction of AQDS (electron acceptor) conducted by quinone-reducing microorganisms (from the oxidation of glucose) or sulfide, respectively. The reduced AQDS (acting as RM) donates electrons to promote the chemical-reductive decolorization of DB71 and produce aromatic amines. Then, the aromatic amines can act as RM similarly to AQDS and work as catalyst during the decolorization of DB71 (Fig. 5). In a previous study, the autocatalytic capacity of an aromatic amine (1-amino-2-naphthol), with similar structure to the amines produced from DB71, was the responsible of the decolorization of acid orange 7 (Van der Zee et al. 2000). The microbial decolorization of DB71 mediated by AQDSsol (k d = 0.120 h−1) occurred faster than obtained in incubations without the mediating capacity of either AQDS or GAC (k d = 0.069 h−1). This response was promoted by the redox mediating capacity of both AQDS and the aromatic amines produced in the system. Furthermore, the cultures with GAC-AQDS and GAC showed k d values of 0.028 and 0.016 h−1, respectively; these values are much lower than the values obtained in the assays non-supplemented with GAC-AQDS or GAC. This result indicates that the adsorption of aromatic amines (Figs. 4 and 5) is the main responsible for the loss of the redox mediating capacity due to electron transfer limitations. Certainly, the adsorption of aromatic amines produced from the decolorization process could be attributable to the sulfonate groups (−SO3 −), negatively charged species at pH 7.0 (pKa1 = 1.76 and pKa2 = 7.2, (Tartar and Garretson 1941)) and the basic sites available on GAC.
Proposed mechanisms involved during the decolorization of DB71 by quinone-reducing microorganisms and sulfide, mediated with GAC-AQDS (dashed arrows) and by autocatalytic aromatic amines (solid arrows). The immobilized AQDS is reduced by quinone-reducing microorganisms or sulfide. Reduced AQDS can transfer the reducing electron equivalents to DB71. The aromatic amines with autocatalytic capacity are suceptible to be reduced and then they act as RM-like AQDS. Consequently, the reductive decolorization of DB71 is enhanced due to these processes. In addition, the aromatic amines are suceptible to be adsorbed on GAC (double line arrows), limiting the redox mediating capacity of the compounds
There are advantages of using GAC as immobilizing support of quinoid RM for the reductive decolorization of azo dyes and other electron-accepting contaminants, for example, 1) it is a low-cost available material; 2) it is a long-lasting material, capable of being used in bioreactors by extended periods; 3) the diversity of functional groups on GAC surface offers the possibility to immobilize redox molecules with different structures, including by covalent binding; and 4) it can promote a dual function, support of RM to improve the process and adsorbent of contaminants or by-products of biotransformation to reduce the inhibitory and toxicological effects on microorganisms.
4 Conclusion
The present study provides evidence related to the role of GAC modified with AQDS during the reductive decolorization of DB71 under microbial and chemical conditions. The presence of immobilized AQDS increases the rate of decolorization of DB71 in relation to the assays with GAC lacking AQDS. The assays in absence of GAC showed to be more efficient to decolorize the tested dye due to the production of aromatic amines with redox capacity. Nevertheless, the addition of GAC, either modified or not, promotes the adsorption of the amines and diminishes DB71 reduction, due to mass transfer limitations. The development of materials with enhanced redox properties as the GAC here used is proposed for accelerating the redox conversion of recalcitrant pollutants commonly found in industrial wastewaters.
References
Alvarez, L. H., & Cervantes, F. J. (2012). Assessing the impact of alumina nanoparticles in an anaerobic consortium: methanogenic and humus reducing activity. Applied Microbiology and Biotechnology, 95(5), 1323–1331. doi:10.1007/s00253-011-3759-4.
Alvarez, L. H., Perez-Cruz, M. A., Rangel-Mendez, J. R., & Cervantes, F. J. (2010). Immobilized redox mediator on metal-oxides nanoparticles and its catalytic effect in a reductive decolorization process. Journal of Hazardous Materials. doi:10.1016/j.jhazmat.2010.08.032.
Alvarez, L. H., Jimenez-Bermudez, L., Hernandez-Montoya, V., & Cervantes, F. J. (2012). Enhanced dechlorination of carbon tetrachloride by immobilized fulvic acids on alumina particles. Water, Air, & Soil Pollution, 223(4), 1911–1920. doi:10.1007/s11270-011-0994-3.
Alvarez, L. H., Cervantes, F. J., & Gortares, P. (2013). Avances en la aplicación de mediadores redox durante la (bio)transformación de contaminantes recalcitrantes. BioTecnologia, 17(3), 43–65.
Amezquita, G. H. J., Razo-Flores, E., Cervantes, F. J., & Rangel-Mendez, J. R. (2014). Anchorage of anthraquinone molecules onto activated carbon fibers to enhance the reduction of 4-nitrophenol. Journal of Chemical Technology and Biotechnology, 90(9), 1685–1691.
Cervantes, F. J., Enriquez, J. E., Galindo-Petatán, E., Arvayo, H., Razo-Flores, E., & Field, J. A. (2007). Biogenic sulphide plays a major role on the riboflavin-mediated decolourisation of azo dyes under sulphate-reducing conditions. Chemosphere, 68(6), 1082–1089.
Cervantes, F. J., Garcia-Espinosa, A., Moreno-Reynosa, M. A., & Rangel-Mendez, J. R. (2010). Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes. Environmental Science & Technology, 44(5), 1747–1753. doi:10.1021/es9027919.
Cervantes, F. J., Gonzalez-Estrella, J., Marquez, A., Alvarez, L. H., & Arriaga, S. (2011). Immobilized humic substances on an anion exchange resin and their role on the redox biotransformation of contaminants. Bioresource Technology, 102(2), 2097–2100. doi:10.1016/j.biortech.2010.08.021.
Encinas-Yocupicio, A. A., Razo-Flores, E., Sanchez-Diaz, F., dos Santos, A. B., Field, J. A., & Cervantes, F. J. (2006). Catalytic effects of different redox mediators on the reductive decolorization of azo dyes. Water Science and Technology, 54(2), 165–170. doi:10.2166/wst.2006.500.
Field, J. A., Stams, A. J. M., Kato, M., & Schraa, G. (1995). Enhanced biodegradation of aromatic pollutants in cocultures of anaerobic and aerobic bacterial consortia. Antonie van Leeuwenhoek, 67(1), 47–77. doi:10.1007/bf00872195.
Guo, J., Zhou, J., Wang, D., Tian, C., Wang, P., Salah Uddin, M., & Yu, H. (2007). Biocalalyst effects of immobilized anthraquinone on the anaerobic reduction of azo dyes by the salt-tolerant bacteria. Water Research, 41(2), 426–432. doi:10.1016/j.watres.2006.10.022.
Guo, J., Kang, L., Lian, J., Yang, J., Yan, B., Li, Z., et al. (2010a). The accelerating effect and mechanism of a newly functional bio-carrier modified by redox mediators for the azo dyes decolorization. Biodegradation, 21(6), 1049–1056. doi:10.1007/s10532-010-9365-9.
Guo, J., Kang, L., Yang, J., Wang, X., Lian, J., Li, H., et al. (2010b). Study on a novel non-dissolved redox mediator catalyzing biological denitrification (RMBDN) technology. Bioresource Technology, 101(11), 4238–4241. doi:10.1016/j.biortech.2010.01.029.
Li, L., Wang, J., Zhou, J., Yang, F., Jin, C., Qu, Y., et al. (2008). Enhancement of nitroaromatic compounds anaerobic biotransformation using a novel immobilized redox mediator prepared by electropolymerization. Bioresource Technology, 99, 6908–6916.
Li, L., Zhou, J., Wang, J., Yang, F., Jin, C., & Zhang, G. (2009). Anaerobic biotransformation of azo dye using polypyrrole/anthraquinonedisulphonate modified active carbon felt as a novel immobilized redox mediator. Separation and Purification Technology, 66(2), 375–382. doi:10.1016/j.seppur.2008.12.019.
Lovley, D. R., Coates, J. D., Blunt-Harris, E. L., Phillips, E. J. P., & Woodward, J. C. (1996). Humic substances as electron acceptors for microbial respiration. Nature, 382(6590), 445–448.
Lu, H., Zhou, J., Wang, J., Si, W., Teng, H., & Liu, G. (2010). Enhanced biodecolorization of azo dyes by anthraquinone-2-sulfonate immobilized covalently in polyurethane foam. Bioresource Technology, 101(18), 7185–7188. doi:10.1016/j.biortech.2010.04.007.
Martinez, C. M., Alvarez, L. H., Celis, L. B., & Cervantes, F. J. (2013). Humus-reducing microorganisms and their valuable contribution in environmental processes. Applied Microbiology and Biotechnology, 97(24), 10293–10308.
Mezohegyi, G., Kolodkin, A., Castro, U. I., Bengoa, C., Stuber, F., Font, J., et al. (2007). Effective anaerobic decolorization of azo dye Acid Orange 7 in continuous upflow packed-bed reactor using biological activated carbon system. Industrial & Engineering Chemistry Research, 46(21), 6788–6792.
Pereira, L., Pereira, R., Pereira, M. F. R., Van der Zee, F. P., Cervantes, F. J., & Alves, M. M. (2010). Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. Journal of Hazardous Materials, 183(1), 931–939.
Rodgers, J. D., & Bunce, N. J. (2001). Treatment methods for the remediation of nitroaromatic explosives. Water Research, 35(9), 2101–2111. doi:10.1016/S0043-1354(00)00505-4.
Tartar, H. V., & Garretson, H. H. (1941). The thermodynamic ionization constants of sulfurous acid at 25 1. Journal of the American Chemical Society, 63(3), 808–816.
Van der Zee, F. P., & Cervantes, F. J. (2009). Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnology Advances, 27(3), 256–277. doi:10.1016/j.biotechadv.2009.01.004.
Van der Zee, F. P., Lettinga, G., & Field, J. A. (2000). The role of (auto) catalysis in the mechanism of an anaerobic azo reduction. Water Science and Technology, 42(5–6), 301–308.
van der Zee, F. P., Bouwman, R. H. M., Strik, D. P. B. T. B., Lettinga, G., & Field, J. A. (2001). Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnology and Bioengineering, 75(6), 691–701. doi:10.1002/bit.10073.
Van Der Zee, F. P., Bisschops, I. A. E., Lettinga, G., & Field, J. A. (2003). Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environmental Science & Technology, 37(2), 402–408.
Wang, J., Li, L., Zhou, J., Lu, H., Liu, G., Jin, R., & Yang, F. (2009). Enhanced biodecolorization of azo dyes by electropolymerization-immobilized redox mediator. Journal of Hazardous Materials, 168, 1098.
Zhu, D., & Pignatello, J. J. (2005). Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environmental Science & Technology, 39(7), 2033–2041.
Acknowledgements
This research was financially supported by the Mexican Council of Science and Technology (Conacyt) thought the grant SEP-Conacyt No. 236129 and the Programa para el Desarrollo Profesional Docente (PRODEP). Yair A. Del Angel acknowledges Conacyt for the scholarship granted during graduate studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez, L.H., Del Angel, Y.A. & García-Reyes, B. Improved Microbial and Chemical Reduction of Direct Blue 71 Using Anthraquinone-2,6-disulfonate Immobilized on Granular Activated Carbon. Water Air Soil Pollut 228, 38 (2017). https://doi.org/10.1007/s11270-016-3212-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3212-5






 ) GAC, (
) GAC, ( ) GAC-AQDS, (
) GAC-AQDS, ( ) only sulfide. In all cases, the error was lower than 5%
) only sulfide. In all cases, the error was lower than 5%
