Abstract
The intensive pig production has been causing huge amounts of pig slurry with high content of potential pollutants. However, there is a lack of information on the efficiency of combined techniques applied to pig slurry purification. The objective of this research was to assess the pollutant removal efficiency and pathogenic microorganism decrease using mechanical treatments, phytoextraction, and microalgae bioremediation. The purification system was located in the southeast of Spain. Physico-chemical and microbiological parameters were studied in each module of treatment. We observed significant declines for total suspended solids (89 %), settleable solids (100 %), chemical oxygen demand (91 %), biochemical oxygen demand (90 %), total phosphorus (97 %), copper (96 %), zinc (92 %), total nitrogen (89 %), total coliforms (78 %), fecal coliforms (70 %), fecal streptococcus (75 %), Salmonella, Shigella, and Escherichia coli (100 %) in the final effluent of the combined purification system. This survey pointed out the effectiveness of phytoextraction and bioremediation treatments. The results indicated the high efficiency of the purification system, minimizing environmental and human risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pig slurry is considered one of the most polluting agroindustrial wastewaters worldwide. Nitrogen, phosphorous, organic matter, and salts in high concentrations can trigger serious environmental problems such as eutrophication of water bodies (Carpenter et al. 1998), groundwater contamination (Krapaca et al. 2002), emission of greenhouse gases, and soil degradation due to over-fertilization and unsustainable use (Plaza et al. 2004; Gómez-Garrido et al. 2014).
Numerous studies show efficient technologies to treat municipal wastewaters (Melse and Verdoes 2005; Burton 2007). Some of these technologies could be applied in pig slurry purification. However, there is a lack of information on operational practicalities and efficiency regarding the combination and application of different physico-chemical and microbiological techniques applied to treat pig slurry (Lorimor et al. 2006).
Various types of mechanical screen separators have been tested with dissimilar slurries (Reimann and Potsdam 1991; Zhang and Westerman 1997; Møller et al. 2000). These studies assessed that both sedimentation and mechanical screen separation are simple, effective, and low-cost techniques. Conversely, other technologies such as some biological treatments and other physico-chemical ones such as coagulation-flocculation, evaporation, ultrafiltration, and reverse osmosis are complex and expensive (Burton 1997; Lema et al. 2014). The constructed wetlands (CWs) have been widely used to treat urban wastewater. The combination of biological, physical, and chemical interactions among plants (commonly macrophytes such as Phragmites australis), substrate, and influent improves the purification (Hill et al. 1999; Kadlec and Knight 1996). Moreover, P. australis can phytoextract and phytoaccumulate pollutants in its tissues and favors the microorganisms growing (Vymazal 2002; Lee and Scholz 2007; Lema et al. 2014). Even though the plants are not harvested, the use of plant species in constructed wetlands is highly recommended (Vymazal 2011). They increase both the microcosm and nitrification processes dealing with high NH4 +-N concentration as in swine wastewaters (Knops et al. 2002; Ge et al. 2015). Constructed wetlands are promoted as low-cost technology for the wastewater treatment, thanks to the minimum operational and management requirements (Kadlec and Knight 1996).
CWs have been previously evaluated for pig slurry purification (Hunt et al. 2002; Knight et al. 2000). These studies showed the CW effectiveness for the main potential pollutant removal, minimizing the risk of environmental pollution or human health effects. Likewise, CWs could provide suitable effluent to be used for crops and pastureland irrigation.
Recent surveys conclude that microalgae systems perform as bioremediators in open ponds, potential pollutant contents in the pig slurry (Kebede-Wheshead et al. 2003; Mulbry et al. 2005). Some researchers attributed ammonium removal mainly to the algae assimilation (de la Noue and Basseres 1989) and reported the total NH4-N exhaustion at 20 °C with three different species of microalgae (two Chlorophyceae, Chlorella sp., and Scenedesmus obliquus, and a cyanobacterium, Phormidium bohneri). Other surveys pointed out the oxidation of organic matter and ammonium due to bacteria activity (Muñoz and Guieysse 2006; Hoffman 1998). Authors such as de Godos et al. (2009) described high efficiencies of total Kjeldhal nitrogen removal while NH4-N was almost completely removed in algae ponds with pig slurry. This study indicated nitrification-denitrification as the main mechanisms to achieve the N removal in accordance with Zimno et al. (2003).
With the aim to enhance the knowledge about the combination of different treatments to purify pig slurry, the objectives of this study were as follows:
-
1.
To assess the variation of physico-chemical parameters and pathogenic microorganisms in the pig slurry using a sequential combination of treatments: (a) fan press screw separator, (b) intermittent aeration process, (c) sludge thickener with a rotor screen, (d) sedimentation tank, (e) CWs with macrophytes, and (f) open storage pond with microalgae
-
2.
To assess the pollutant removal efficiency of the purification system combining mechanical elimination, phytoextraction, and bioremediation
2 Materials and Methods
2.1 Study Area
This study was conducted in The Integrated Centre of Training and Agricultural Experiences (CIFEA), which has an experimental intensive pig farm, located in the municipality of Lorca. This zone is the most intensive pig production area in Murcia region (southeast of Spain, 37° 39′ N, 1° 41′ W). The climate of the area is semiarid Mediterranean, with a mean annual temperature of 17 °C and a mean annual rainfall of 307 mm. The potential evapotranspiration rate surpasses 1246 mm/year (AETMET 2012).
The combination of physico-chemical and microbiological modules of treatments is shown in Fig. 1. The mean of pigs in the farm was 50, with three annual cycles of feeding and production. The weight of the animals generally ranged from 20 kg at the beginning and 110 kg at the end of each cycle (like in the industrial farms of the region). This study was carried out for 5 years (2006 to 2010).
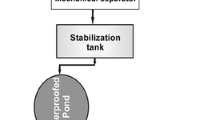
General layout of the treatments (plan view). (1) Subterranean tank, (2) fan press screw separator, (3) storing tank with an intermittent aeration disc, (4) sludge thickener, (5) sedimentation tank, (6) stopcocks, (7) intermittent horizontal subsurface flow constructed wetlands by batch mode (HSFCW), (8) reservoir tank, and (9) storage pond. (A), (B), (C), (D), (E), and (F) indicate the sampling points
2.2 Description of Mechanical Separation Techniques
The raw pig slurry from the farm was stored in a concrete subterranean tank (Fig. 1(1)), which had a mechanical shaker (GTWS-44 type, Westfalia separator, Eisele, Germany). The homogenization and oxygenation of the slurry were made in this tank by shaking for 30 min. Then, the slurry was driven by a pump through a fan press screw separator of 5 m3 h−1 (Westfalia, Germany) (Fig. 1(2)) which was used for the separation of suspended solids.
The solid phase was composted and the liquid phase of pig slurry (LPPS) was stored for 2 days in a tank of 10 m3 (Fig. 1(3)). The tank had an intermittent aeration process consisting of a five-aeration membrane system and a compressor of 30 m3 h−1 (Josval, Herraiz, Spain). After that, LPPS was pumped to a sludge thickener (Fig. 1(4)) which had a rotor screen (Biopolym, Iberica, Spain). The solids were retained by a rotating drum until they were removed from it by a scraper; this had a self-cleaning system. The water flowed from the bottom of the screen, and those particles that had not been separated by the scraper were trapped. The LPPS was stored for 2 days in a sedimentation tank 10 m3 (Fig. 1(5)). Then, LPPS was conducted to the CWs using a pump and a PVC pipe. The sedimentation tank was emptied and cleaned every time that the purification process was operated, that is every cycle of treatment. These modules worked in batch treating 10 m3 every cycle, and the hydraulic retention time (HRT) in both intermittent aeration and sedimentation tanks was 2 days.
2.3 Description of the CW and the Open Pond
Six cells of a horizontal subsurface flow constructed wetland (HSFCW) were equipped with inlet and outlet hydraulic structures in order to work with intermittent flow or batch (Fig. 1(7)). The whole volume of each cell changed from 16 m3 in the beginning of the experiment to 8 m3 in the last year, whereas the mean organic loading was 2648 g L−1 based on BOD data. The change of volume was due to the growth of a P. australis root system and the biofilm covering the substrate which progressively reduced the available volume to treat the influent in each cell. The experiment started when the aerial part of P. australis was 20 cm in height and finished when it was 2.0 m which triggered a high development of the root system and as a consequence the reduction of the available volume to treat the influent. Therefore, the hydraulic load rate varied from 16 to 8 m3.
This LPPS was discharged on the surface of coarse gravel bed without sand layer (1 m length) to avoid clogging process, and wastewater flow had a uniform horizontal distribution across the cells. The outlet structure was an orifice at the bottom of the downstream end of the cell. The outlet was 90 cm lower than the inlet to allow the hydraulic gradient. Each cell was waterproof and designed with a trapezoidal shape according to the following dimensions: 27 m length, 2.5 m width (surface) and 2 m width (bottom), 1 m depth, and 1 % slope. Each bed was excavated and lined with a plastic film (PEAD 2.0 mm) to prevent seepage. The substrate was 80 cm of limestone gravel (4–40 mm of diameter) and a surface of 20 cm of certificated sand layer. Based on preliminary studies, the density of P. australis was 7.5 plants m−2. The HSFCWs worked in batch. The duration of a single loading was equivalent to the HRT which was 4 weeks. The interval between loadings was 90 days.
A small reservoir tank to collect samples of effluents was constructed at the end of each cell. The effluent picked up in each tank was pumped to the open storage pond after 3 h, which had a volume of 100 m3, where algae species of the genera Scenedesmus grew.
The whole purification system was patented in 2012 with number of publication ES 2 363 363 B2 (Caballero-Lajarín et al. 2012).
2.4 Sampling and Analytical Methods
Samples were collected in triplicate in the different sampling points (Fig. 1). Four samplings were made every year, one per season. The total of samplings was 20 over 5 years. Every sample was placed in sterilized plastic bottles of 100 mL, labeled, and immediately cooled at 4 °C until analysis. Microbial parameters were studied in samples collected in A, E, and F sampling points in order to determine their variation before and after the phytoextraction and bioremediation treatments.
Temperature (T) and pH were determined in situ by a HANNA instrument (HI 9025) while electrical conductivity (EC) and redox potential (Eh) were determined potentiometrically also in situ by a HANNA instrument (HI 9033) (Spain). Total suspended solids (TSS) were filtered through a weighed standard glass-fiber filter and the residue retained on the filter is dried to a constant weight at 105 °C (2440-D method, APHA-AWWA-WEF 2012). Settleable solids (SS) were measured in situ by natural sedimentation in an Inhoff vessel, after 60 min (2540-F method, APHA-AWWA-WEF 2012). Total dissolved solids (TDS) were calculated according to Ayers and Wescot (1984). Chemical oxygen demand (COD) was determined by photometric determination of chromium(III) concentration after 2 h of oxidation with potassium dichromate/sulfuric acid and silver sulfate at 148 °C (Macherey-Nagel GmbH and Co. KG. Nanocolor Test, Ref 985 028/29) (DIN 38 409 - H41 - 1, DIN ISO 15 705 - H45). Biochemical oxygen demand in 5 days (BOD5) was determined by manometer OXITOP WTW equipment.
Total phosphorus (TP) was photometrically determined as molybdenum blue after acidic hydrolysis and oxidation at 120 °C (Macherey-Nagel GmbH and Co. KG. Nanocolor Test; Ref 985 055). Copper (Cu) and zinc (Zn) were determined after an acid digestion by atomic absorption spectrophotometry (A-Analyst 800 Perkin Elmer). Total nitrogen (TN) was determined by the Kjeldahl method (Duchaufour 1970), modified using 1 mL of pig slurry in the digestion. Ammonium nitrogen (NH4 +-N) was determined by steam distillation and titration with HCl 0.1 N. Organic nitrogen (ON) was calculated by the difference between TN and NH4 +-N. Nitrate (NO3 −) was measured by photometric determination with 2,6-dimethylphenol in sulfuric acid/phosphoric acid mixture (Macherey-Nagel GmbH and Co. KG. Nanocolor Test; Ref 985 064). Chloride (Cl−), bromide (Br−), sulfate (SO4 2−), sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+) ions were analyzed by high-performance liquid chromatography (HPLC) (Dionex DX500 ED40).
Microorganisms were determined by preparing serial dilutions of samples with autoclaved peptone water. Recount of mesophilic aerobic (MA) bacteria was determined by culturing each sample in two petri dishes with trypticase soy agar (TSA) at 31 °C for 72 h. Total coliforms (TC) were determined by culturing each sample in triplicate in Brilliant Green Bile Lactose Broth (BGBL) 2 % tubes at 37 °C for 24/48 h. Fecal coliforms (FC) were determined by culturing the positive total coliform tubes in BGBL 2 % tubes at 44.5 °C for 24–48 h using a sterilized inoculation loop. The presence or lack of Escherichia coli was determined by culturing the positive fecal coliforms in petri dishes with eosin methylene blue (EMB) at 37 °C for 24–48 h. After that, suspected colonies were isolated by a sterilized inoculation loop in peptone tubes and were analyzed in API 20 E gallery at 37 °C for 18–24 h. The APILAB program was used to identify the microorganism. Fecal streptococcus (FS) was determined by culturing each sample in triplicate in kanamicina-aesculina-azida (KAA) broth at 37 °C for 24–48 h and confirmation of the positive tubes in petri dishes at 37 °C for 24–48 h.
The presence or lack of Salmonella sp. and Shigella sp. was determined by a selective enrichment in selenite cystine broth, isolation in a selective solid medium, xylose lysine deoxycholate agar (XLD), and confirmation by API 20 E gallery.
The efficiency of removal for the mechanical separation, constructed wetlands, and open pond was determined by the calculation of the decrease or increase of each parameter before and after every one.
2.5 Statistical Analyses
Data normality and homoscedasticity were checked with a Kolmogorov-Smirnov test and a Levene test, respectively. The whole of studied parameters resulted nonparametric even after transformation. Data were grouped according to A, B, C, D, E, and F sampling points. Significant differences among treatments were assessed by Kruskal-Wallis (chi2—nonparametric tests) and Mann-Whitney U tests. Differences were considered significant when P < 0.05. Additionally, the percentage of removal was determined for each treatment. Experimental results were statistically analyzed using the software SPSS package for Windows, Version 19.0.
3 Results and Discussion
3.1 Physico-chemical Parameters
3.1.1 Temperature, Redox Potential, and pH
As Fig. 2a shows, T kept very homogeneous after each treatment. Substrate and roots kept the wastewater temperature higher than air temperature, between 2 and 3 °C more, in CW during the winter. This fact likely allowed the microbiological activity continually (Hiley 2003). This parameter did not show statistically significant differences among treatments (P > 0.05), although the temperature varied according to the season (Fig. 2a).
Mean and standard deviation values of effluent according to different treatments. a subterranean tank, b fan press screw separator, c intermittent aeration discs-sludge thickener, d sedimentation tank, e horizontal subsurface flow constructed wetlands, and f storage pond. Percentages indicate increase and decrease according to the different treatments and global treatments. Different letters indicate significant differences between treatments (P < 0.05) according to Kruskal-Wallis and Mann-Whitney U tests. (a) Temperature (T), (b) redox potential (Eh), (c) pH, (d) electrical conductivity (EC), (e) total suspended solids (TSS), (f) settleable solids (SS), (g) total dissolved solids (TDS), and (g) total phosphorus (TP)
Redox potential exhibited negative values after each treatment (Fig. 2b), pointing out anaerobic conditions. Similar results were described by other researchers in raw pig slurry (Moral et al. 2005; Rufete et al. 2006). The Eh values showed significant differences among treatments (P < 0.05), with increments after aeration and HSFCW because of the oxygen supply.
The average value of pH of the raw pig slurry was neutral or slightly alkaline (Fig. 2c), being similar to those shown in previous research, ranging from 6.8 to 8.3 (Massé et al. 2011; Monroy et al. 2009). The pH showed significant differences among treatments (P < 0.05). The highest pH value was observed in the open pond, which could be related to the photosynthetic activity of the microalgae (Mashauri et al. 2000; de Godos et al. 2009). Additionally, the alkaline pH favors ammonium precipitation (Phillips et al. 2000). Therefore, it could be inferred that microalgae activity triggered NH4-N removal in our open pond, as it was described by de Godos et al. (2009) and de la Noue and Basseres (1989).
3.1.2 Electrical Conductivity and Ions
Electrical conductivity was high in the raw pig slurry (Fig. 2c) as well as the soluble salt concentrations compared with other studies, which reported values ranging from 6.8 to 23.2 days m−1 (Suresh et al. 2009; Wong and Selvam 2009). Electrical conductivity (Fig. 2d) showed no significant differences among treatments (P < 0.05) from the aeration to the open pond. Usually, the high EC is due to the dietary intake of salts and high-protein feed (Moral et al. 2005).
The ion contents found were similar to those reported in previous research (Sánchez and González 2005; Carrasco 2005; Plaza 2002). In general, ion contents exhibited significant differences among treatments (P < 0.05). HSFCW and the open pond showed decreasing Ca2+ whereas SO4 2−, Cl−, Na+, K+, and Mg2+ generally increased. These variations were likely caused by interactions between the substrate and the biofilm which recover the gravels in the HSFCW (Kadlec and Knight 1996). Finally, the decreasing Ca2+ contents observed in the open pond could be connected with the microalgae activity and its immobilization (Mashauri et al. 2000). However, the other ion concentrations increased likely due to evaporation (Fig. 4a, d, e).
3.1.3 Total Suspended Solids, Settleable Solids, and Total Dissolved Solids
As a general pattern, solids dismissed after each treatment, showing statistically significant differences among treatments (P < 0.05) for TSS and SS while TDS exhibited similarities from aeration to the open pond. The highest removal of TSS was observed in the HSFCW and for SS was registered in the sedimentation tank for SS being completely removed in the HSFCW. This fact could be explained based on the precipitation in the HSFCW and in the sedimentation tank coupled with the fix to particles of the substrate.
3.1.4 Total Phosphorus, COD, and BOD5
TP was significantly and progressively removed after each treatment, especially after HSFCW (Fig. 2h). The typical mechanisms for TP removal associated to a long-term storage in CWs are adsorption and chemical precipitation with Ca+2 coupled with iron, aluminum, and organic matter fixed in the substrate (Kadlec and Knight 1996; Healy and O’ Flynn 2011). In accordance, Ca+2 contents decreased after treatments (Fig. 4f) in our study, which seems to indicate precipitation processes as an important mechanism to reduce TP. Healy and O’ Flynn (2011) reported 88 % of P removal of PO4 3−-P in vertical-flow CWs with agricultural dairy-soiled wasters. Moreover, P. australis, algae, and bacteria could absorb P to be used as nutrient, although this mechanism is described by Healy and O’ Flynn (2011) as a short-term storage in the HSFCW.
COD and BOD5 significantly decreased after each treatment (Fig. 3a, b). The main processes which contribute to COD and BOD5 decreases are volatilization, photochemical oxidation, sedimentation, adsorption, and biological degradation (Kadlec 1992). These processes mainly would take place in the HSFCW treatment, explaining the higher COD and BOD5 decrease.
Mean and standard deviation values of effluent according to different treatments. a subterranean tank, b fan press screw separator, c intermittent aeration discs-sludge thickener, d sedimentation tank, e horizontal subsurface flow constructed wetlands, and f storage pond. Percentages indicate increase and decrease according to the different treatments and global treatments. Different letters indicate significant differences between treatments (P < 0.05) according to Kruskal-Wallis and Mann-Whitney U tests. (a) Chemical oxygen demand (COD), (b) biochemical oxygen demand in 5 days (BOD5), (c) total nitrogen (TN), (d) ammonium nitrogen (NH4 +-N), (e) organic nitrogen (ON), (f) nitrate (NO3 −), (g) copper (Cu), and (h) zinc (Zn)
3.1.5 Total Nitrogen, Ammonium Nitrogen, Organic Nitrogen, and Nitrates
The 78 % of TN was in inorganic form, mainly as ammonium nitrogen; the rest of the nitrogen was in organic form (Fig. 3c, d). Similar results were found in other studies (Bonmati and Flotats 2003; Sánchez and González 2005). The different N forms mainly decreased in the HSFCWs and in the open pond. The main mechanisms referred to in the literature for nitrogen removal are nitrification, denitrification, adsorption, and absorption (Reddy and Patrick 1984). Results showed noticeable TN, NH4-N, NO, and nitrate variations in the HSFCW and in the open pond compared with the mechanical separation techniques. Therefore, we can infer that the mentioned mechanisms for N removal were more relevant both in the HSFCW and in the open pond.
Additionally, the mean annual temperature in the zone was 17 °C which would favor the N removal. Christos and Tsihrintzis (2007) observed that N precipitation was higher when temperature was over 15 °C. Furthermore, plant growth was larger and consequently microbiological activity was more intensive favoring absorption processes both in the HSFCW and in the open pond. Additionally, the pH increased until 8.6 in the open pond. This fact could trigger a higher NH4 +-N precipitation as it was mentioned before as well as volatilization as NH3 and NOx which could be caused by the pH increase coupled with the high mean annual temperature (Reddy and Patrick 1984; Vymazal et al. 1998).
Nitrates significantly increase in the HSFCW, probably due to nitrification processes owing to the release of O2 by P. australis in the rhizosphere (Huang et al. 2000). The opposite pattern was observed in the open pond where NO3 − significantly decreased likely as a consequence of simultaneous nitrification-denitrification processes and absorption by microalgae (Vymazal 2002; de Godos et al. 2009).
3.1.6 Copper and Zinc
Cu and Zn contents showed significant differences among treatments (P < 0.05). The higher removals took place in the sedimentation tank, in the HSFCW, and in the open pond. Metal removal rates in CWs depend on the type of element, ionic forms, substrate conditions, season, and plant species (Marchand et al. 2010). Karathanasis and Thompson (1993) found a positive correlation between organic matter and metal removal. We observed a high COD and BOD5 decease after the HSFCW and the open pond treatments (Fig. 3a, b). Therefore, we can infer the immobilization of Cu and Zn in the organic matter. Lema et al. (2014) also assessed the absorption onto the particulate matter as well as the filtration of the root and the uptake of these elements (Fig. 3g, h). However, other elements such as Cl−, SO4 −2, Na+ and Mg+2 increased (Fig. 4).
Mean and standard deviation values of effluent according to different treatments. a subterranean tank, b fan press screw separator, c intermittent aeration discs-sludge thickener, d sedimentation tank, e horizontal subsurface flow constructed wetlands, and f storage pond. Percentages indicate increase and decrease according to the different treatments and global treatments. Different letters indicate significant differences between treatments (P < 0.05) according to Kruskal-Wallis and Mann-Whitney U tests. (a) Chloride (Cl−), (b) bromide (Br−), (c) sulfate (SO4 −2), (d) sodium (Na+), (f) calcium (Ca+2), and (g) magnesium (Mg+2)
3.1.7 Pathogenic microorganisms
The pathogenic microorganism contents obtained (Fig. 5a–d) were in accordance with those found by Tofant et al. (2006) in the raw pig slurry. In our study, 27 % of the raw pig slurry samples (Table 1) showed the presence of both E. coli and Salmonella. However, Shigella was not detected, similar to the results found by Ros et al. (2006).
Mean and standard deviation values of effluent according to different treatments. A subterranean tank, E horizontal subsurface flow constructed wetlands, and F storage pond. Percentages indicate increase and decrease according to the different treatments and global treatments. Different letters indicate significant differences between treatments (P < 0.05) according to Kruskal-Wallis and Mann-Whitney U tests. (a) Aerobic mesophilic (MA), (b) total coliforms (TC), (c) fecal coliforms (FC), and (d) fecal streptococcus (FS)
These pathogenic microorganisms significantly decreased after the different treatments. Neralla et al. (2000), Mashauri et al. (2000), and Steer et al. (2002) described 99 % of removal for fecal coliforms after purification of pig slurry with CWs. We can infer that the pathogenic microorganism removal after HSFCW was maintained in the open pond where no contamination was supposed to happen.
3.2 Efficiency of Treatment
3.2.1 Mechanical Separation
In this research, it was found that mechanical treatments (fan press screw separator, intermittent aeration process, sludge thickener with a rotor screen, and sedimentation tank) removed mainly solids (62 % for TSS, 91 % for SS), BOD5 (59 %), COD (45 %), TP (52 %), Cu (42 %), Zn (39 %), and some ions such as SO4 2− (50 %) and Mg2+ (36 %).
Walker et al. (2010) evaluated the effectiveness of a static gravity screen-roll press separator operated in tandem with a polyacrylamide-assisted gravity belt thickener to separate solid and liquid components of liquid pig manure under production-scale conditions. Significant reductions were obtained in settleable solids (98.4 and 94.3 %), total suspended solids (98.2 and 80.9 %), total nitrogen (60.6 and 37.0 %), and phosphorus (91.7 and 70.9 %) concentrations for years 1 and 2, respectively. Sorensen and Thomsen (2005) assessed that the mechanical manure separation contributed to reduce nutrient leaching from livestock wastewaters. Moreover, it reduces its weight and volume and makes easier the handling of the manure, decreasing the cost and the potential environmental damage due to its transport.
3.2.2 Phytoextraction Through Constructed Wetlands
Based on the values of each studied parameter before and after the HSFCWs, solids were mainly removed (83 % for TSS and 100 % for SS), as well as BOD5 (71 %), COD (68 %), TP (90 %), Cu (69 %), Zn (74 %), TN (63 %), NH4-N (63 %), and ON (69 %). Knight et al. (2000) assessed the reduction of TSS by 57 % in CWs which purified livestock wastewaters, from 118 to 51 mg L−1. Brix (1997) found that the higher SS removal performances to treat urban runoff using CWs were attributed to larger surface areas, reduced water velocities, and reinforced settling and filtration by the root network. Accordingly, the cell design of the HSFCW in this study had 27 m of length, explaining the high percentage of solid removal. However, we can suppose that this percentage is so high, thanks to the efficiency of previous mechanical treatments which avoid the HSFCW saturation.
In accordance with our results, Puigagut et al. (2007) showed BOD5 minimization of 75–93 % while COD reduction of 50 % was reported at the piggery effluent in artificial plant treatment systems in the USA (Werblan et al. 1978). Kadlec and Knight (1996) assessed the P removal mainly by adsorption in the substrate and the absorption by the plant. Both physico-chemical and biochemical processes and the phytoextraction were supposed to influence very positively the P minimization in the HSFCWs. Since the P. australis tissues or the substrate was not analyzed, we could not assess the quantity of P absorbed by the plant and retained in the substrate. These issues could be the aim of further researches.
Similar researches indicated that more than 50 % of the heavy metals can be adsorbed onto the particulates of the wetland and they could be removed from the water column by sedimentation (Sheoran and Sheoran 2006). Accordingly, the declined levels of total metals observed in our study were consistent with the decrease of suspended solids, COD, and BOD5 in our study.
The increase of nitrates after HSFCW was evident (70 %). The macrophytes transport approximately 90 % of the oxygen available in the rhizosphere. This stimulates both aerobic decomposition of organic matter and growth of nitrifying bacteria (Reddy et al. 1989; Brix 1997; Scholz 2006).
According to literature, Nitrosomonas sp. oxidizes ammonia to nitrate while Nitrobacter sp. oxidizes nitrite to nitrate (Bock et al. 1986; Willers et al. 1998). Nitrification rates are influenced by factors like dissolved oxygen concentration, pH, and T. The optimum value of pH for both species ranges between 7.5 and 8.0 (Bock et al. 1986; Antoniou et al. 1990; Willers et al. 1998), very close to the mean value of pH observed in our study. Likewise, the average of T in the studied zone seemed to be also suitable for their growth. Based on NO3 − contents before and after the HSFCW and oppositely to other studies conducted by Vymazal (2002), simultaneous reactions of nitrification-denitrification could not be inferred. As a consequence, the nitrate contents increased noticeably after the HSFCW treatment. This fact could be identified as a limitation in the N removal using HSFCW.
Most ions increased after the HSFCW treatment, except for Ca+2 and Br−. Limestone was a material expected to promote the precipitation of Ca phosphates (Drizo et al. 1999). Because of this, a general decrease of Ca+2 and P was observed in our study which could be attributed to precipitation of the calcium phosphate. Other authors reported that sodium, potassium, and electrical conductivity increased with passage through the trench (Finlayson and Chick 1983), supporting our results.
Related to microorganism load, many studies point out that fecal removal efficiency is generally excellent in CWs, exceeding 95 % (Neralla et al. 2000; Steer et al. 2002). It may vary depending on the constructed wetland design, operational factors, substrate, temperature, and wastewater (Haberl et al. 1995; Potter and Karathanasis 2001). Even though we did not observe so high percentage of removal, we could assess noticeable efficiency of removal for TC (77 %), FC (64 %), and FS (70 %) as well as Salmonella, Shigella, and E. coli (100 %). These results highlight the effectiveness of HSFCW to reduce the pathogen numbers.
3.2.3 Bioremediation Using an Open Pond
Taking into account the values of the studied parameters before and after the bioremediation treatment by microalgae, the elements mainly removed were Zn (74 %), Cu (69 %), total nitrogen (60 %), ammonium nitrogen (60 %), organic nitrogen (49 %), and nitrate (67 %). The microalgae growth caused an increase of 51 % for STS and 14 % for pH. Moderate percentages of P removal were observed in the open pond which could be explained based on the buffer action of the purified pig slurry (de Godos et al. 2009). Although the highest percentages of pathogenic microorganism decrease were observed in the HSFCW, it continued in the open pond. We found 18 % decrease of MA, 2 % in TC, 16 % in FC, and 19 % in FS. Therefore, Scenedesmus sp. played an important role in the pollutant removal of the pig slurry, mainly nitrate which increased in the HSFCW. The optimal metabolic kinetics of microalgae takes place in the range of 15–30 °C (Molina-Grima 1999), similar to the conditions in our study zone.
The first microalgae-based bioremediation studies were carried out with domestic wastewaters 50 years ago in California (Oswald et al. 1957). These preliminary works showed that heterotrophic bacteria need oxygen for the degradation of the organic matter which could be supplied by microalgae. Meanwhile, bacteria provide the carbon dioxide and the nutrients (N and P) that microalgae need in the photosynthesis. In the same way, de Godos et al. (2010) studied the performance of microalgae consortia isolated from ponds of pig slurry. They assessed the microalgae positive effect in biodegrading pig slurry.
4 Conclusion
The purification system tested in this study has achieved noticeable decreases in potential pollutants from pig slurry: 89 % for TSS, 100 % for SS, 90 % for BOD5, 91 % for COD, 97 % for TP, 96 % for Cu, 92 % for Zn, 89 % for TN, 87 % for NH4 +-N, 91 % for ON, 42 % for Ca+2, 78 % for TC, 70 % for FC, 75 % FS, and 100 % for Salmonella, Shigella, and E. coli. Based on the significant differences found among treatments, it can be concluded that each module contributed to purify the pig slurry.
The mechanical treatments helped to improve the removal of potential pollutants in the HSFCWs, avoiding substrate saturation and clogging. The highest decrease of solids, organic load, and P was achieved in the HSFCWs. Conversely, the highest removal of the different forms of nitrogen was achieved in the open pond. This fact points out the effectiveness of the phytoextraction by P. australis and the bioremediation through microalgae in the pig slurry purification. However, some limitations in the nitrate removal were observed in the HSFCW module where modifications of the design should be included in order to enhance the simultaneous nitrification-denitrification processes. Additionally, the purification system should increase the efficiency of salt removal.
Nonetheless, the purification system could be considered as a suitable option to efficiently treat and manage the pig slurries and to minimize environmental and human risks.
References
AEMET. Agencia Estatal de Meteorología. Gobierno de España. (2012). In: http://www.aemet.es/ . Updated: April 2014.
Antoniou, P., Hamilton, J., Koopman, B., Jain, R., Holloway, B., Lyberatos, G., & Svoronos, S. A. (1990). Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Research, 24, 97–101.
APHA, AWWA, WEF. (2012). Standard methods for examination of water and wastewater. 22nd American Public Health Association (Eds.). Washington. 1360 pp. In: http://www.standardmethods.org. Updated: May 2014.
Ayers, R. S., & Wescot, D. W. (1984). Water quality for agriculture. Irrigation and drainage paper 29. Roma: FAO.
Bock, E., Koops, H., Harms, H. (1986). Cell biology of nitrifying bacteria. In Prosser, J. I., (ed.) Nitrification, IRL Press. pp. 17–38.
Bonmati, A., & Flotats, X. (2003). Air stripping of ammonia from pig slurry: characterization and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Management, 23, 261–272.
Brix, H. (1997). Do macrophytes play a role in constructed treatment wetlands? Water Science and Technology, 35, 11–17.
Burton, C. H. (1997). Manure management—treatment strategies for sustainable agriculture. Silsoe: Silsoe Research Institute.
Burton, C. H. (2007). The potential contribution of separation technologies to the management of livestock manure. Livestock Science, 112, 208–216.
Caballero-Lajarín, A., Faz Cano, A., Lobera Lössel, J. B. (2012). Humedal artificial y uso del mismo para la fitopurificación de efluentes líquidos. Patent: ES 2 363 363 B2.
Carpenter, S. R., Caraco, N. F., Correll, D. L., Howarth, R. W., Sharpley, A. N., & Smith, V. H. (1998). Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications, 8, 559–568.
Christos, S. A., & Tsihrintzis, V. A. (2007). Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecological Engineering, 29, 173–191.
de Godos, I., Blanco, S., García-Encina, P. A., Becares, E., & Muñoz, R. (2009). Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresource Technology, 100(19), 4332–4339.
de Godos, I., Vargas, V. A., Blanco, S., García González, M. C., Soto, R., García-Encina, P. A., Becares, E., & Muñoz, R. A. (2010). Comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresource Technology, 101, 5150–5158.
de la Noue, J., & Basseres, A. (1989). Biotreatment of anaerobically digested swine manure with microalgae. Biological Wastes, 29, 17–31.
DIN 38 409 - H41 -1 and DIN ISO 15705 –H45. German standard methods for the examination of water, wastewater and sludge.
Drizo, A., Frost, C. A., Grace, J., & Smith, K. A. (1999). Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Research, 33, 3595–3602.
Duchaufour, P. H. (1970). Precis de Pedologie. Masson. Paris. 481 pp.
Finlayson, C. M., & Chick, A. J. (1983). Testing the potential of aquatic plants to treat abattoir effluent. Water Research, 17, 415–22.
Ge, Y., Han, W., Huang, C., Wang, H., Liu, D., Chang, S. X., Gu, B., Zhang, C., Gu, B., Fan, X., Du, Y., & Chang, J. (2015). Positive effects of plant diversity on nitrogen removal in microcosms of constructed wetlands with high ammonium loading. Ecological Engineering, 82, 614–623.
Gómez-Garrido, M., Martínez-Martínez, S., Faz-Cano, A., Büyükkılıç-Yanardag, A., & Arocena, J. M. (2014). Soil fertility status and nutrients provided to spring barley (Hordeum distichon L.) by pig slurry. Chilean Journal of Agricultural Research, 74(1), 73–82.
Haberl, R., Perfler, R., & Mayer, H. (1995). Constructed wetlands in Europe. Water Science and Technology, 32, 305–315.
Healy, M. G., & O’ Flynn, C. J. (2011). The performance of constructed wetlands treating primary, secondary and dairy soiled water in Ireland (a review). Journal of Environmental Management, 92(10), 2348–2354.
Hiley, P. (2003). Performance of wastewater treatment and nutrient removal wetlands. In U. Mander & P. Jenssen (Eds.), Constructed wetlands for wastewater treatment in cold climates (reedbeds) in cold temperature climates (pp. 1–18). Southampton: WIT Press.
Hill, V. R., Pasternak, J. I., Rice, J. M., Marra, M. C., Humenik, F. J., Sobsey, M. D., Szogi, A. A., & Hunt, P. G. (1999). Economics of nitrogen and enteric microbe reductions for alternative swine waste treatment techniques. In G. B. Havenstein (Ed.), Proceedings of the animal waste management symposium (pp. 297–301). Raleigh: NCSU Animal Waste Management Field Day Committee.
Hoffman, J. P. (1998). Wastewater treatment with suspended and nonsuspended algae. Journal of Phycology, 34, 757–763.
Huang, J., Reneau, R. B., Jr., & Hagedorn, C. (2000). Nitrogen removal in constructed wetlands employed to treat domestic wastewater. Water Research, 34(9), 2582–2588.
Hunt, P. G., Szogi, A. A., Humenik, F. J., Rice, J. M., Matheny, T. A., & Stone, K. C. (2002). Constructed wetlands for treatment of swine wastewaters from an anaerobic lagoon. Transactions of ASAE, 45, 639–647.
Kadlec, R. (1992). Hydrological factors in wetland water treatment. In D. A. Hammer (Ed.), Constructed wetland for wastewater treatment: municipal, industrial and agricultural (pp. 25–29). Chelsea: Lewis Publishers.
Kadlec, R., & Knight, R. L. (1996). Treatment wetlands. Boca Raton: Lewis Publishers.
Karathanasis, A. D., & Thompson, Y. L. (1993). Substrate effects on metal retention and speciation in simulated acid mine wetlands. Bulletin of Environmental Contamination and Toxicology, 51, 421–429.
Kebede-Wheshead, E., Pizarro, E., & Mulbry, W. W. (2003). Environmental and economic aspects of recycling livestock wastes-algae production using waste products. Southern Journal of Agricultural Economics, 3, 1275–1282.
Knight, R. L., Payne, V. W. E., Jr., Borer, R. E., Clarke, R. A., Jr., & Pries, J. H. (2000). Constructed wetlands for livestock wastewater management. Ecological Engineering, 15, 41–55.
Knops, J. M. H., Bradley, K. L., & Wedin, D. A. (2002). Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecology Letters, 5(3), 454–46.
Krapaca, I. G., Deya, W. S., Roya, W. R., Smythb, C. A., Stormentc, E., & Sargenta, S. L. (2002). Impacts of swine manure pits on groundwater quality. Environmental Pollution, 120, 475–492.
Lee, B. H., & Scholz, M. (2007). What is the role of Phragmites australis in experimental constructed wetland filters treating urban runoff? Ecological Engineering, 29(1), 87–95.
Lema, E., Machunda, R., Nicholas, NJAU. (2014). Influence of macrophyte types towards agrochemical phytoremediation in a tropical environment. International Journal of Engineering Research and General Science 2(5).
Carrasco, M. L. (2005). Tesis doctoral: Utilización agronómica de purines de cerdo en brócoli y sandía en condiciones mediterráneas semiáridas. Influencia en el sistema suelo- planta. Pp. 374.
Lorimor, J., Fulhage, C., Zhang, R., Funk, T., Sheffield, R., Sheppard, D. C., Newton, G.L. (2006). Manure management strategies and technologies. In: Animal agriculture and the environment: National Center for Manure and Animal Waste Management white papers. American Society of Agricultural and Biological Engineers, St. Joseph, MI, USA.
Macherey-Nagel GmbH & Co. KG. Web: http://www.mn-net.com. Nanocolor Test; ref: 985 028/29, 985 055, 985 064.
Marchand, L., Mench, M., Jacob, D. L., & Ottem, M. L. (2010). Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: a review. Environmental Pollution, 158, 3447–3461.
Mashauri, D. A., Mulungu, D. M. M., & Abdulhussein, B. S. (2000). Constructed wetland at the University of Dar Es Salaam. Water Research, 34, 1135–1144.
Massé, D., Gilbert, Y., & Topp, E. (2011). Pathogen removal in farm-scale psychrophilic anaerobic digesters processing swine manure. Bioresource Technology, 102, 641–646.
Melse, R. W., & Verdoes, N. (2005). Evaluation of four farm scale systems for the treatment of liquid pig manure. Biosystems Engineering, 92, 47–57.
Molina-Grima, E., (1999). Microalgae mass culture methods. In: Flickinger, M. C., Drew, S.W. (Eds.), Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. Wiley.
Møller, H. B., Lund, I., & Sommer, S. G. (2000). Solid–liquid separation of livestock slurry: efficiency and cost. Bioresource Technology, 74(3), 223–229.
Monroy, F., Aira, M., & Domínguez, J. (2009). Reduction of total coliform numbers during vermicomposting is caused by short-term direct effects of earthworms on microorganisms and depends on the dose of application of pig slurry. Science of the Total Environment, 407, 5411–5416.
Moral, R., Pérez-Murcia, M. D., Pérez-Espinosa, A., Moreno-Caselles, J., & Paredes, C. (2005). Estimation of nutrient values of pig slurries in southeast Spain using easily determined properties. Waste Management, 25, 719–725.
Mulbry, W., Kebede Westhead, E., Pizarro, C., & Sikora, L. (2005). Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresource Technology, 96(4), 451–458.
Muñoz, R., & Guieysse, B. (2006). Algal-bacteria processes for the treatment of hazardous contaminants, a review. Water Research, 40(2006), 2799–2815.
Neralla, S., Weaver, R. W., Lesikar, B. J., & Persyn, R. A. (2000). Improvement of domestic wastewater quality by subsurface flow constructed wetlands. Bioresource Technology, 75, 19–25.
Oswald, W. J., Gotaas, H. B., & Golueke, C. G. (1957). Algae in wastewater treatment. Sewage and Industrial Wastes, 29, 437–455.
Phillips, V. R., Scholtens, R., Lee, D. S., Garland, J. A., & Sneath, R. W. (2000). A review of methods for measuring emission rates of ammonia from livestock buildings and slurry or manure stores. Part 1: assessment of basic approaches. Journal of Agricultural Engineering Research, 77, 355–364.
Plaza, C. (2002). Aprovechamiento agrícola del purín de cerdo en agroecosistemas semiáridos: efectos sobre suelos y plantas. Tesis Doctoral. Universidad Autónoma de Madrid.
Plaza, C., Hernández, D., García-Gil, J., & Polo, A. (2004). Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biology and Biochemistry, 36, 1577–1585.
Potter, C. L. & Karathanasis, A. D. (2001). Vegetation effects on the performance of constructed wetlands treating domestic wastewater. Proceedings of Ninth National Symposium on Individual and Small Community Sewage Systems, ASAE, Fort Worth, TX, March 2001, pp. 663–673.
Puigagut, J., Vilaseñor, J., Salas, J. J., Béceras, E., & García, J. (2007). Subsurface flow constructed wetlands in Spain for the sanitation of small communities: a comparative study. Ecological Engineering, 30, 312–9.
Reddy, K. R., & Patrick, W. H. (1984). Nitrogen transformations and loss in flooded soils and sediments. Critical Review in Environmental Control, 13, 273–309.
Reddy, K. R., Patrick, W. H., & Lindau, C. W. (1989). Nitrification–denitrification at the plant root-sediment interface in wetlands. Limnology and Oceanography, 34, 1004–1013.
Reimann, W., & Potsdam, M. S. (1991). Fest-flussig-trennung anaerob behandelter gulle. Landtechnik, 46, 11–91.
Ros, M., García, C., & Hernández, T. (2006). A full-scale study of treatment of pig slurry by composting: kinetic changes in chemical and microbial properties. Waste Management, 26, 1108–1118.
Rufete, B., Pérez-Murcia, M. D., Pérez-Espinosa, A., Moral, R., Moreno-Caselles, J., & Paredes, C. (2006). Total and faecal coliform bacteria persistence in a pig slurry amended soil. Livestock Science, 102, 211–215.
Sánchez, M., & González, J. L. (2005). The fertilizer value of pig slurry. I. Values depending on the type of operation. Bioresource Technology, 96, 1117–1123.
Scholz, M. (2006). Wetland systems to control urban runoff. Amsterdam: Elsevier.
Sheoran, A. S., & Sheoran, V. (2006). Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Minerals Engineering, 19(2), 105–116.
Sorensen, P., & Thomsen, I. K. (2005). Separation of pig slurry and plant utilization and loss of nitrogen-15-labeled slurry nitrogen. Soil Science Society of America Journal, 69, 1644–1651.
Steer, D., Fraser, D. L., Boddy, J., & Seibert, B. (2002). Efficiency of small constructed wetlands for subsurface treatment of single-family domestic effluent. Ecological Engineering, 18, 429–440.
Suresh, A., Choi, H. L., Ohb, D. I., & Moon, O. K. (2009). Prediction of the nutrients value and biochemical characteristics of swine slurry by measurement of EC—electrical conductivity. Bioresource Technology, 100, 4683–4689.
Tofant, A., Vucˇemilo, M., Pavicˇic´, Z., & Milic´, D. (2006). The hydrogen peroxide, as a potentially useful slurry disinfectant. Livestock Science, 102, 243–247.
Vymazal, J. (2002). The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecological Engineering, 18, 633–646.
Vymazal, J. (2011). Plants used in constructed wetlands with horizontal subsurface flow: a review. Hydrobiology, 674(1), 133–156.
Vymazal, J., Brix, H., Cooper, P. F., Haberl, R., Perfler, R., Laber, J. (1998). Removal mechanisms and types of constructed wetlands. In: constructed wetlands for waste-water treatment in Europe. Vymazal, J., Brix, H., Cooper, P., Green, M. B., Haberl, R. (Eds.), Leiden, pp 17–66.
Walker, P. M., Wade, C. A., & Kelley, T. R. (2010). Evaluation of a polyacrylamide assisted solid/liquid separation system for the treatment of liquid pig manure. Biosystems Engineering, 105, 241–246.
Werblan, D., Smith, R. J., Van der Valk, A. G., Davis, C. B. (1978). Treatment of waste from a confined hog feeding unit by using artificial marshes. In: Mickim, H. L., (Ed.), Proceedings of international symposium on land treatment of wastewater. Hannover, New Hampshire, USA, pp. 1–13.
Willers, H. C., Derikx, P. J. L., ten Have, P. J. W., & Vijn, T. K. (1998). Nitrification limitation in animal slurries at high temperatures. Bioresource Technology, 64, 47–54.
Wong, J. W. C., & Selvam, A. (2009). Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. Journal of Hazardous Materials, 169, 882–889.
Zhang, R. H., & Westerman, P. W. (1997). Solid-liquid separation of animal manure for odor control and nutrient management. Applied Engineering in Agriculture, 13, 657–664.
Zimno, O. R., van der Steen, N. P., & Gijzen, H. J. (2003). Comparison of ammonia volatilisation rates in algae and duckweed-based waste stabilisation ponds treating domestic wastewater. Water Research, 37, 4587–4594.
Acknowledgments
This research was possible thanks to the financial support from the Spanish Ministry of Education and Science, Projects PET2006 - 0075 and CTM 2007-65888, and the Department of Agriculture and Water, Government of Murcia Region.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caballero-Lajarín, A., Zornoza, R., Faz, A. et al. Combination of Low-Cost Technologies for Pig Slurry Purification Under Semiarid Mediterranean Conditions. Water Air Soil Pollut 226, 341 (2015). https://doi.org/10.1007/s11270-015-2606-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2606-0